Abstract
Culturing multipotent adult mesenchymal stem cells as 3D aggregates augments their differentiation potential and paracrine activity. One caveat of stem cell spheroids, though, can be the limited diffusional transport barriers posed by the inherent 3D structure of the multicellular aggregates. In order to circumvent such limitations, polymeric microparticles have been incorporated into stem cell aggregates as a means to locally control the biochemical and physical properties of the 3D microenvironment. However, the introduction of biomaterials to the 3D stem cell microenvironment could alter the mechanical forces sensed by cells within aggregates, which in turn could impact various cell behaviors and overall spheroid mechanics. Therefore, the objective of this study was to determine the acute effects of biomaterial incorporation within mesenchymal stem cell spheroids on aggregate structure and mechanical properties. The results of this study demonstrate that although gelatin microparticle incorporation results in similar multi-cellular organization within human mesenchymal stem cell spheroids, the introduction of gelatin materials significantly impacts spheroid mechanical properties. The marked differences in spheroid mechanics induced by microparticle incorporation may hold major implications for in vitro directed differentiation strategies and offer a novel route to engineer the mechanical properties of tissue constructs ex vivo.
Keywords: stem cells, mesenchymal stem cells, biomaterials, polymers, microparticles, tissue engineering
1. Introduction
With an aging global population, tissue engineering strategies to repair and regenerate diseased and damaged tissues will continue to be of critical importance and at the forefront of medical research. There is, therefore, a great need for readily-available cell sources and biomaterials capable of augmenting tissue restoration, either alone or in combination with one another. To this end, mesenchymal stem cells (MSCs) have emerged as an attractive multipotent cell source for the treatment of a variety of traumatic injuries and chronic diseases. MSCs have been characterized to be included within the CD105+, CD73+, CD90+, CD34−, CD45− cell fraction of the bone marrow (Dominici et al. 2006) and, more recently, have been isolated from a number of other tissues including fat and umbilical cord (Erices et al. 2000; Zuk et al. 2001). MSCs not only possess the ability to self-renew and differentiate to a number of mesenchymal lineages in vitro and in vivo (Pittenger 2008; Pittenger et al. 1999), but these cells also secrete a cadre of potent trophic factors that contribute to tissue remodeling (Baraniak and McDevitt 2010). Furthermore, allogeneic MSCs are generally considered to be immunotolerant, thereby providing for their, off-the-shelf administration for regenerative medicine therapies.
While traditional ex vivo cell culture platforms have maintained cells in two-dimensional (2D), adherent cultures, three-dimensional (3D) aggregates of cells in suspension culture, which commonly adopt a spheroid shape, have been widely used in cancer research to mimic the tumor microenvironment (Ivascu and Kubbies 2006; Marrero et al. 2009; Ong et al. 2010; Timmins and Nielsen 2007). Scaffold-free, 3D cell culture systems are thought to more closely resemble the physiological tissue environment by enabling greater cell-cell and cell-matrix interactions than conventional monolayer culture techniques. Additionally, 3D culture is necessary to induce spontaneous differentiation in pluripotent stem cell populations (Carpenedo et al. 2007; Kurosawa 2007), and the culture of multipotent adult stem cells as 3D aggregates augments their adipogenic and osteogenic differentiation potentials and enhances the secretion of trophic factors (Baraniak and McDevitt 2011; Bartosh et al. 2010; Frith et al. 2010). Fueled by a need for the directed differentiation of stem cells to intended phenotypes, researchers have evaluated the differentiation potential of multicellular stem cell aggregates in response to soluble factors and demonstrated that diffusional barriers to the interior of aggregates may limit the efficacy of such strategies (Carpenedo et al. 2009; Sachlos and Auguste 2008). To circumvent these limitations, polymeric microparticles (with and without small molecules or growth factors) have been incorporated into stem cell aggregates and the resultant cell behavior has been characterized (Bratt-Leal et al. 2009; Bratt-Leal et al. 2011; Carpenedo et al. 2009; Carpenedo et al. 2010; Hayashi and Tabata 2011; Purpura et al. 2012).
While biomaterial incorporation within stem cell aggregates does not adversely affect cell viability or proliferation and can augment directed differentiation, the introduction of biomaterials within the stem cell microenvironment and subsequent alterations to the mechanical forces sensed by cells within aggregates has major implications for tissue morphogenesis and may affect the efficacy of in vitro and in vivo directed differentiation protocols. Mechanical stress is a critical morphogenic regulator in embryonic patterning (Davidson 2011; Gomez et al. 2010), and mechanical loading modulates regenerative processes including neovascularization and bone formation (Boerckel et al. 2011). Additionally, external mechanical forces applied in vitro regulate cell behaviors and physiology including proliferation, differentiation, extracellular matrix remodeling, and protein and gene expression profiles (Engler et al. 2006; Engler et al. 2007; Evans et al. 2009; Rowlands et al. 2008; Saha et al. 2008). As such, this study elucidates the acute effects of biomaterial incorporation within stem cell spheroids on aggregate structure and mechanical properties. The ability to modulate the 3D mechanical environment of stem cell spheroids via biomaterial presentation may afford a novel route to direct the differentiation and engineer specific cellular responses for tissue engineering and regenerative medicine applications.
2. Materials and methods
2.1 Microparticle fabrication and characterization
Gelatin microparticles (MPs) were fabricated as previously reported (Bratt-Leal et al. 2011). Briefly, a water-in-oil emulsion was created by heating 2 mL of a 10% w/w solution of gelatin B (Sigma Aldrich) in dIH2O to 55 °C before adding it drop-wise to 60 mL of corn oil and homogenizing at 5000 RPM for 5 minutes. The emulsion was then cooled at 4 °C for 10 min, and 35 mL of cold acetone was added to the solution and sonicated continuously at 12 W for one minute (Sonicator 3000, Misonix, Inc). The solution was again cooled at 4 °C for 10 minutes. The MPs were then retrieved by centrifugation at 200 xg and washed three times in acetone. Gelatin MPs were then cross-linked at room temperature with 5 mM glutaraldehyde and 0.1% w/w Tween 20 with constant stirring. After 15 hours of crosslinking, the MPs were retrieved by centrifugation and treated with 25 mM glycine in dIH2O to block residual aldehyde groups. MPs were washed three times in dIH2O and labeled with AlexaFluor 488 succinimidyl ester in a 0.1 M sodium bicarbonate solution (pH 8.3) for one hour at room temperature. Finally, fluorescently-labeled MPs were washed three times in dIH2O to remove un-conjugated dye. Microparticle average size was determined using a Multisizer 3 Coulter Counter (Beckman Coulter) equipped with a 100 m aperture with a lower detection limit of 2 m (Bratt-Leal et al. 2011). MPs were suspended in Isoton II diluent (Beckman Coulter) at a dilution which resulted in the counting of at least 10,000 events in a sample size of 1 mL.
2.2 Cell culture
2.2.1 Mesenchymal stem cell expansion
Human bone marrow-derived mesenchymal stem cells (hMSCs) were obtained from the Texas A&M College of Medicine Institute for Regenerative Medicine and expanded according to established protocols (Bartosh et al. 2010). Briefly, hMSCs were maintained on tissue culture polystyrene at sub-confluence in complete culture medium (CCM, Minimimal Essential Medium alpha (Invitrogen/GIBCO) supplemented with 16% fetal bovine serum (FBS, Atlanta Biologicals), 2 mM L-glutamine (Invitrogen/GIBCO), 100U/mL penicillin and 100 μg/mL streptomycin (Invitrogen/GIBCO). For spheroid formation, cells were detached into a single-cell suspension from tissue culture plates using 0.25% trypsin and 1 mM Ethylene Diamine Tetraacetic Acid (EDTA) in Hanks' Balanced Salt Solution (Invitrogen/GIBCO).
2.2.2 Spheroid formation and maintenance
MSC monolayers were re-suspended to obtain a single cell suspension (as detailed above) in order to generate spheroids using a previously described forced-aggregation technique (Baraniak and McDevitt 2011; Bratt-Leal et al. 2011; Ungrin et al. 2008). Briefly, 7.2 × 105 hMSCs in 0.5 mL CCM without FBS were added to Aggrewell™ 400 inserts (Stem Cell Technologies, Vancouver, CA) containing an array of 1200 microwells and centrifuged at 200 xg for 5 minutes to force cell aggregation into the wells. A second centrifugation of 50 uL of a gelatin MP solution at 200 xg for 5 min was performed immediately after cell centrifugation (resulting in a 1:8 MP:cells ratio within inserts) for samples containing MPs. Spheroids without MPs served as controls. After 18-24 hours of culture in microwells, stem cell spheroids, or mesenspheres, (consisting of approximately 600 cells/spheroid) were removed from the wells using a wide bore pipette, transferred to 100 mm bacteriological grade Petri dishes (∼1200 spheroids in 10 mL spheroid formation medium), and cultured in suspension on a rotary orbital shaker (Lab-Line Lab Rotator, Barnstead International) for up to seven days at 65±2 RPM. Media was exchanged every 3 days by allowing mesenspheres to sediment in 15mL conical tubes, aspirating the old medium, re-suspending in 10 mL fresh medium, and returning mesenspheres to Petri dishes on rotary orbital shakers.
2.3 Cell viability within spheroids
The incorporation of fluorescently-labeled MPs within mesenspheres was confirmed using an EVOS-fl digital inverted microscope (Advanced Microscopy Group). Cell viability within mesenspheres was assessed on days 2, 4, and 7 of culture using a LIVE/DEAD staining kit (Invitrogen). Samples were incubated in cell culture medium containing 1 mM calcein AM and 2 mM ethidium homodimer I at room temperature for 30 min. Mesenspheres were then washed three times with PBS and immediately imaged using fluorescent microscopy. Additionally, mesenspheres were dissociated into a single cell suspension at 37 °C using a trypsin-collagenase-dispase solution coupled with mechanical agitation. Flow cytometry for calcein AM and ethidium homodimer stained cells was performed using an Accuri C6 flow cytometer (FL1 channel for live cells and FL3 channel for dead cells) to quantitatively assess cell viability.
2.4 Spheroid whole mount analyses
The spatial location of fluorescently-labeled MPs and resultant cellular phenotype and organization within mesenspheres was examined by confocal microscopy. For whole-mount immunofluorescence, mesenspheres were washed with PBS, fixed with 10% formalin for 30 minutes, permeabilized with 2% Triton X-100 for 30 minutes, re-fixed with 10% formalin for 15 minutes, and washed again with PBS. Mesenspheres were then stained with Alexa 546 phalloidin (1:40 in PBS) overnight at 4 °C followed by nuclear counter-staining with Hoechst dye (1:100 in PBS) for 15 min at room temperature. Samples were stored in PBS at 4°C and imaged in 200 uL of phosphate buffered saline (PBS) using a Zeiss LSM 510 NLO Confocal Microscope.
2.5 Scanning electron microscopy
Cellular organization and spheroid structure were assessed using scanning electron microscopy. Mesenspheres were dehydrated in a series of graded acetone dilutions and completely dried using a Polaron E3000 critical point dryer (Quorum Technologies, Inc.). Mesenspheres were sputter coated for 120 seconds at 2.2 kV using a Polaron SC7640 sputter coater and imaged using a Hitachi S-800 scanning electron microscope (Hitachi High Technologies).
2.6 Histological analyses
For histological analyses, mesenspheres were washed twice with PBS, fixed in 10% formalin for 30 minutes, washed three times with PBS to remove formalin, paraffin processed, embedded, and sectioned at 5 um. Prior to staining, all paraffin-processed slides were de-paraffinized. Slides were stained with hematoxylin and eosin (H&E) using a Leica Autostainer XL and coverslipped. Brightfield images were captured using a Nikon 80i Upright Microscope and a SPOT Flex camera in conjunction with SPOT Advanced v.4.5 software (Diagnostic Instruments).
2.7 Spheroid mechanical analyses
Mesensphere mechanical properties were tested in a parallel-plate compression configuration using a CellScale MicroSquisher and accompanying SquisherJoy software program. The thickness of the cantilever used to test day 2 hMSC spheroids without MPs was 101.6 um, whereas a 152.4 um-thick beam was used to test day 2 spheroids containing gelatin MPs and day 7 spheroids without MPs. Day 7 mesenspheres containing MPs were tested using a 203.2 um-thick beam. All samples were tested in a PBS fluid bath (pH 7.4, containing 0.1g/L Ca++ and 0.1 g/L Mg++) warmed to 37 °C. The stainless steel upper compression plate of the cantilever was depressed until the plate made gentle contact with the top of the sample prior to compression. Samples were compressed to at least 25% engineering strain at a strain rate of 2.5 um/second, held at a constant deformation for 10 seconds, and then released at a rate of 2.5um/sec in order to record any hysteresis. Force-deformation curves were converted to engineering stress vs. engineering strain relationships using the diameter of the samples immediately prior to compression to calculate mesensphere cross-sectional area. The Young's modulus was calculated by fitting the 20% strain data point with a linear regression line. The resulting slope of the regression line was calculated as the tangent modulus of the sample (Quapp and Weiss 1998; Yamamoto et al. 1999). A minimum of 15 distinct mesenspheres, chosen at random, was examined for each condition and time point in order to determine the average Young's modulus values for the MSC aggregate populations.
2.8 Statistical analysis
Prior to performing statistical analysis, mechanical data were normalized using a Box-Cox power transformation to equalize data variance. A two-way ANOVA was calculated between the days and experimental conditions, with post-hoc Tukey analysis to determine significant differences (p< 0.05) between individual groups. Data from individual aggregates were collected for n=15 independent replicates and plotted as the mean ± standard error.
3. Results
3.1 Mesensphere formation and maintenance
Similar to previously described techniques (Baraniak and McDevitt 2011; Bratt-Leal et al. 2011), forced-aggregation of cells and microparticles into PDMS microwells using centrifugation yielded homogeneous populations of hMSC spheroids both with and without gelatin MPs following overnight incubation (18-24 hours). Prior to incorporation, gelatin MP average diameter was determined to be 3.55 ± 1.05 m (Supplemental Figure 1). No gross differences in spheroid size or morphology were observed for cell aggregates that contained or lacked MPs at the time of formation or upon extended culture for up to 7 days. All mesenspheres formed within microwells containing MPs exhibited MP incorporation after 24 hours and after 2 days of culture (Fig 1). However, evidence of gelatin MP degradation by hMSCs was suggested by the decreased fluorescent intensity of MPs within mesenspheres at day 4 and further loss of fluorescent signal from MPs within mesenspheres by day 7 (Fig 1).
Figure 1.
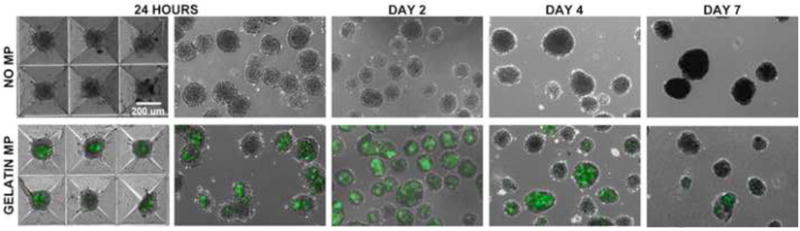
Gelatin MP incorporation and mesensphere maintenance. Gelatin MPs could be incorporated within human MSC spheroids using a forced aggregation technique and overnight incubation in PDMS microwells. Homogeneous populations of mesenspheres with and without gelatin MPs (green fluorescence) could be maintained for up to seven days of culture on a rotary orbital shaker. Decreased fluorescence intensity over time suggested degradation of gelatin MPs by MSCs over the 7 day culture period. Scale bar = 200 um.
In order to assess cell viability within mesenspheres with and without MPs, samples were collected at days 2, 4, and 7 of culture for Live/Dead and H&E staining. Fluorescent and confocal microscopy of Live/Dead stained hMSC spheroids revealed the presence of few dead cells, and no appreciable difference in cell viability was evident between mesenspheres containing gelatin MPs and those without MPs at all time points examined (Fig 2a). Quantification of ethidium homodimer and calcein AM staining using flow cytometry confirmed that MP incorporation had no significant adverse effect on cell viability within mesenspheres (Fig 2b,c,d).
Figure 2.
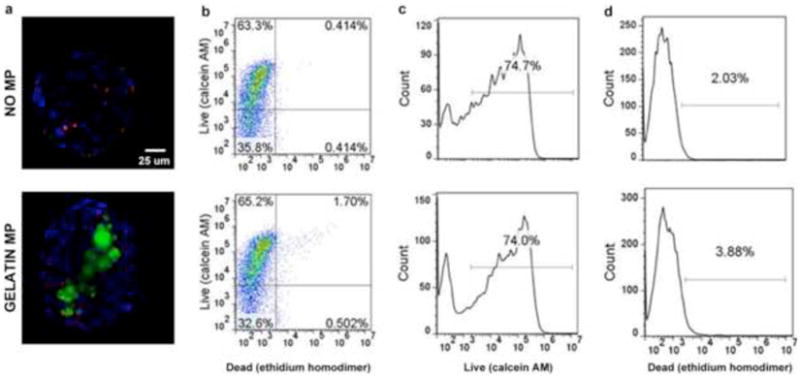
Cell viability within mesenspheres. (a) Gelatin MP incorporation had no appreciable effects on cell viability within mesenspheres. Red - ethidium homodimer (dead cells), Blue -Hoechst (nuclei), Green - gelatin MPs. Scale bar = 25 um. (b,c,d) Quantitative flow cytometry analyses of live-dead stained cells dissociated from spheroids demonstrated no appreciable differences in cell viability upon MP incorporation. Of note, the increased percentage of cells in the top right quadrant of panel (b) for gelatin MP incorporated spheroids is a result of MP autofluoresence and not indicative of a cell subpopulation positive for both stains.
3.2 Multicellular aggregate organization
The effects of MP incorporation on cellular organization within mesenspheres were assessed using different complementary forms of analysis. Confocal microscopy revealed no differences in cellular spatial organization, i.e. nuclear organization, within mesenspheres as a result of MP incorporation at days 2 or 7 of culture (Fig 3a). Evaluation of actin filament organization revealed similar cellular cytoskeletal organization with and without MP incorporation. Furthermore, Alexa 488-labeled gelatin MPs did not cluster upon incorporation and were distributed throughout mesenspheres, with no obvious accumulation radially (i.e. surface vs. interior of mesenspheres) at days 2 and 7 of culture (Fig 3a). Flow cytometry analyses of MSC size (forward scatter) and internal complexity (side scatter) demonstrated no appreciable differences in MSC morphology with MP incorporation (Fig 3b). Additionally, histological analysis of spheroid cross-sections confirmed the absence of necrotic core formation and that there were no differences in cellular organization or morphology within mesenspheres with and without gelatin MPs. Similar to previous results (Baraniak and McDevitt 2011), nuclei within all mesenspheres exhibited a randomly-organized multi-cellular pattern, and cells on the exterior appeared more circumferentially elongated than those in the interior (Fig 4). SEM imaging of spheroids demonstrated no appreciable differences in cellular organization or cell-cell contacts on spheroid surfaces or in the interior of mesenspheres as a result of MP incorporation at each time point assessed (Fig 5). Taken together, these data demonstrate that the incorporation of gelatin microparticles within mesenchymal stem cell aggregates does not alter the gross morphology of spheroid structure or multicellular organization.
Figure 3.
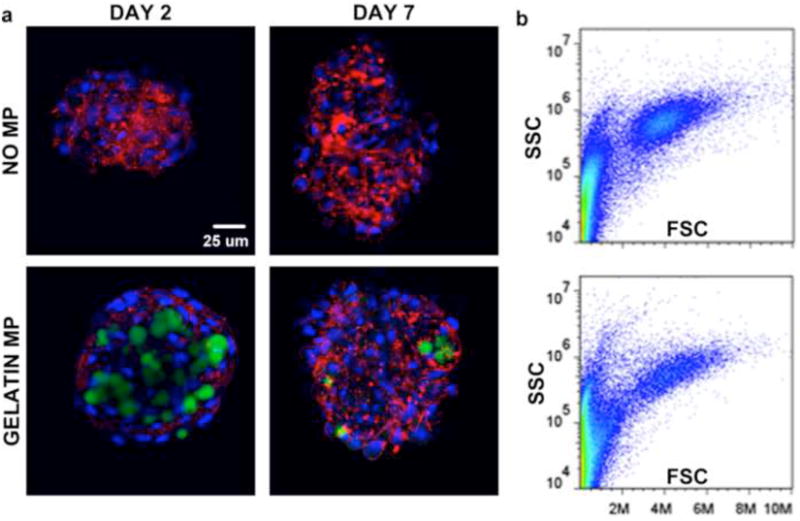
Cell cytoskeletal organization and morphology within mesenspheres. (a) Gelatin MP incorporation within mesenspheres had no appreciable effect on cell cytoskeletal organization at early and later time points. Green – gelatin MPs, Red – phalloidin (actin), Blue – Hoechst (nuclei). Scale bar = 25 um. (b) No appreciable differences in cell size (forward scatter) or internal complexity (side scatter) were noted as a result of MP incorporation.
Figure 4.
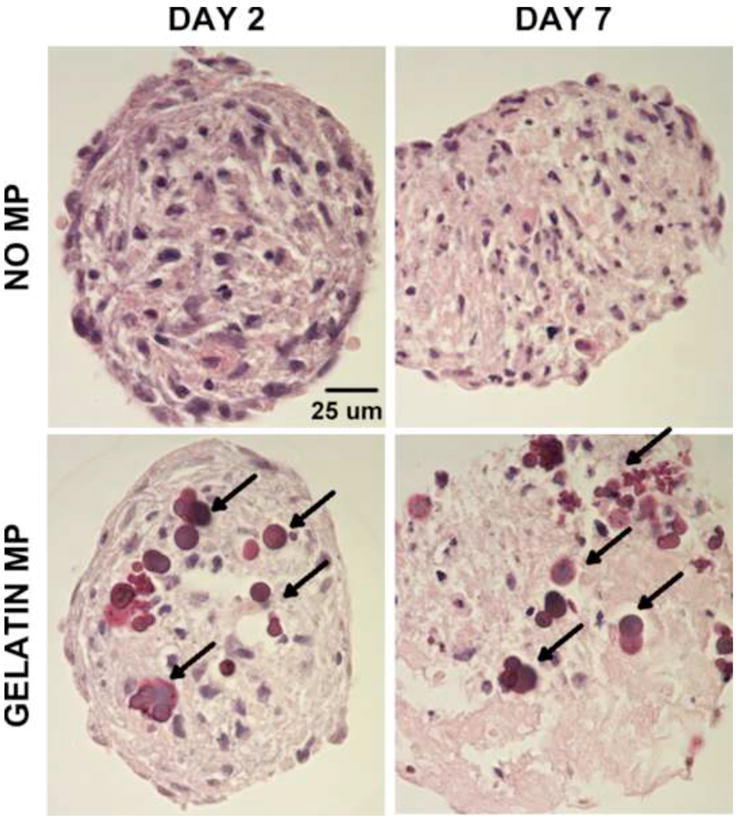
Histological assessment of mesenspheres. H&E staining demonstrated no differences in cell morphology or organization as a result of MP incorporation within MSC spheroids. Arrows – MPs. Scale bar = 25 um.
Figure 5.
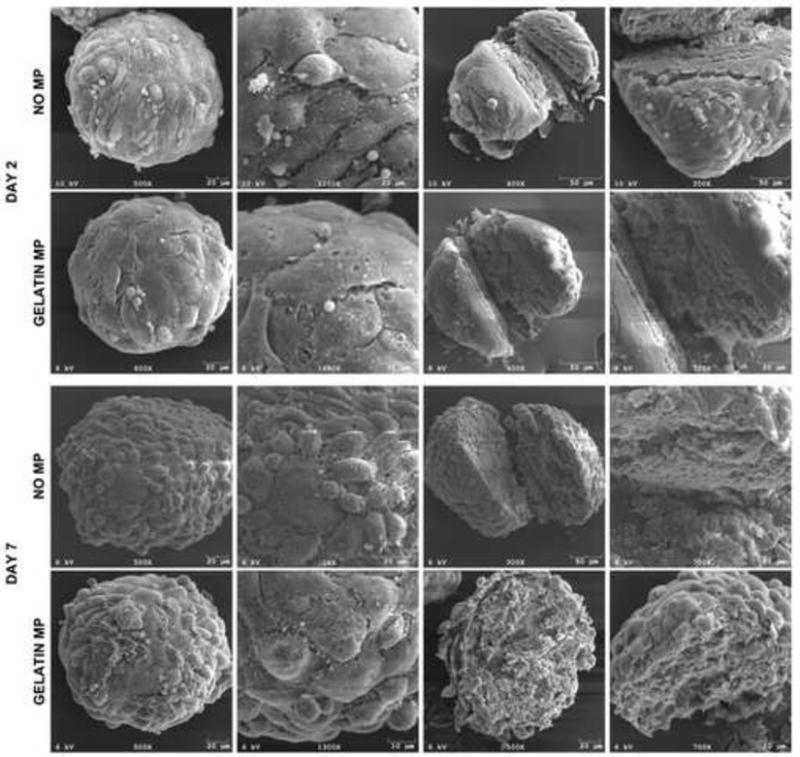
SEM analyses of mesenspheres. SEM imaging at early and later time points demonstrated no appreciable differences in cell shape, organization, or cell-cell contacts at mesensphere surfaces or in the interior of spheroids as a result of MP incorporation.
3.3 Spheroid mechanical properties
The MicroSquisher device can measure the mechanical properties of microscale tissue samples (50-2000 μm diameter) compressed between a fixed, rigid substrate and a displaceable cantilever beam. Although the individual experimental groups (+/− MPs) exhibited no contrast in spheroid mechanical properties between days 2 and 7, the stress-strain relationship between mesenspheres with or without gelatin MPs demonstrated marked differences at both time points examined (Fig 6a,b,d,e). Macroscopically, the significant difference in mechanical properties between mesenspheres with or without MPs was reflected by the need to use cantilever beams of different thickness to evaluate aggregate compression; the rigidity of the cantilever beam is varied by changing the thickness in order to accurately measure different samples that vary significantly in their stiffness. Day 2 mesenspheres containing gelatin MPs exhibited an average Young's modulus that was more than four times greater than mesenspheres that lacked MPs (190.53±50.12 Pa vs. 42.28±6.14 Pa, p< 0.05; Fig 6c). Similarly, by day 7, mesenspheres demonstrated a nearly four-fold increase in Young's modulus with MP incorporation compared to mesenspheres without MPs (240.98±23.19 Pa vs. 62.40±5.58 Pa, p< 0.05; Fig 6f). These results demonstrate that gelatin MP incorporation within MSC spheroids increases overall aggregate stiffness and that alterations in the mechanical environment are maintained over 7 days of culture, even despite the apparent degradation of at least a portion of MPs during this time.
Figure 6.
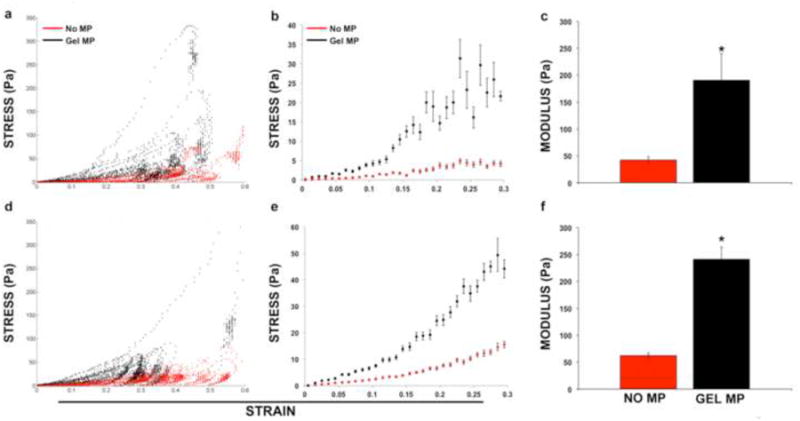
Spheroid mechanical analyses. (a,d) Individual and (b,e) averaged engineering stress-strain curves for mesenspheres (n=15) with (red) and without (black) gelatin MPs demonstrated differences in spheroid mechanics as a result of MP incorporation at day 2 (top row) and day 7 (bottom row) of culture. The average Young's modulus for mesenspheres containing MPs was significantly increased at (c) day 2 and (f) day 7 of culture compared to spheroids without MPs (*p<0.05).
4. Discussion
Tissue engineering methods to direct stem cell differentiation in vitro have traditionally relied on the controlled delivery of soluble biomolecules, such as growth factors, or the genetic modification of cells to mediate fate. However, mounting evidence suggests that environmental factors also regulate cell physiology, including lineage commitment. One such factor, cell shape, has emerged as a potent regulator of stem cell differentiation. In MSCs, cell shape has been correlated to preferential adipogenic, osteogenic, myogenic, and chondrogenic differentiation potential and has been demonstrated to be a function of actin cytoskeleton re-organization modulated by the Rho/Rock pathways (Fu et al. 2010; Gao et al. 2010; Treiser et al. 2010; Wang et al. 2011). While previous studies have elucidated the effects of biomaterial incorporation within scaffold-free 3D stem cell aggregates on cell viability, gene expression profiles, and differentiation (Bratt-Leal et al. 2009; Bratt-Leal et al. 2011; Carpenedo et al. 2009; Carpenedo et al. 2010; Hayashi and Tabata 2011; Purpura et al. 2012), the effects of MP incorporation on cell morphology within spheroids remains ill-defined.
Similar cell morphologies were evident in 3D MSC aggregates with and without adhesive biomaterial incorporation. Specifically, forward and side scatter analyses of MSCs demonstrated similarities in cell size and internal complexity (respectively) in mesenspheres with and without MPs (Fig 2b). In addition, phalloidin staining of mesenspheres demonstrated similar patterns in actin cytoskeleton organization as a result of MP incorporation (Fig 3). Finally, SEM images of mesenspheres demonstrated similar cell shapes and cell-cell contacts with and without MP incorporation (Fig 5). These results suggest that the presence of MPs within mesenspheres will not influence cell fate decisions by altering MSC shape within aggregates; however more detailed studies using quantitative measures of cell morphology and cytoskeletal organization (such as cell spreading, focal adhesion organization, and traction force generation) are needed (Yang et al. 2011).
In addition to cell shape, extracellular matrix and substrate compliance have been demonstrated to modulate cell morphology, proliferation, apoptosis, and motility, and have also been implicated in stem cell lineage commitment (Levental et al. 2007). To this end, it has been demonstrated that MSCs grown on soft matrices (mimicking the brain, for example) exhibit a neural phenotype while those grown on stiffer substrates (similar to bone) adopt an osteogenic phenotype (Engler et al. 2006; Engler et al. 2007). Additionally, MSCs maintained on substrates that mimic the mechanical properties of their native bone marrow niche remain in a quiescent state in vitro (Winer et al. 2009). Finally, three-dimensional scaffolds composed of both natural and synthetic polymers for bone tissue engineering have been shown to enhance MSC osteogenic differentiation and to modulate the extent of differentiation based on scaffold mechanical properties (Hussain et al. 2011; Mohajeri et al. 2010; Nam et al. 2011; Neuss et al. 2004; Ravindran et al. 2012; Serpooshan et al. 2010). However, in each of these studies, the presence of soluble, exogenous osteoinductive factors was still necessary to drive MSC lineage commitment.
Since microparticle incorporation within spheroids presents stem cells with an exogenous matrix to which they can attach, and an effective means of delivering growth factors in a precisely-controlled spatiotemporal manner, the current study aimed to elucidate the effects of adhesive biomaterial incorporation on aggregate mechanics. Microscale compression testing of mesenspheres demonstrated significant differences in spheroid mechanical properties upon MP incorporation (Fig 6). Mesenspheres incorporating gelatin MPs exhibited significantly greater elastic moduli over 7 days of culture compared to mesenspheres without MPs. The average Young's modulus of mesenspheres with MPs was in the 200 Pa range, comparable to that of soft tissues such as human lymph node or mammary gland (Levental et al. 2007). While the physical properties of the gelatin MPs used in this study may not lend themselves to musculoskeletal tissue engineering applications, these material parameters may be modified for such purposes. Therefore, long-term studies elucidating whether the incorporation of mechanically-tailored MPs alone (without exogenous growth factor delivery) within mesenspheres can modulate MSC physiology via alterations in cellular cytoskeletal organization (such as focal adhesion activations and microtubule and actin filament organization) (De Santis et al. 2011) and subsequently alter intracellular signaling cascades and direct cell fate are motivated by the results of this study.
5. Conclusions
While MP incorporation within stem cell spheroids has been utilized for the controlled delivery of growth factors to direct differentiation, the current study is the first to examine the influence of MP incorporation on multicellular structure and aggregate mechanics. The results of this study demonstrate that gelatin MP incorporation does not appear to radically alter cell organization within spheroids, yet it does significantly stiffen spheroids macroscopically. Stiffer spheroid microenvironments created by MP incorporation may bias stem cells to lineages generally found in stiffer tissues in vivo, such as bone. Thus, by varying material composition and mechanical properties, and thereby varying spheroid mechanics, alterations in stem cell physiology may be tailored for specific tissue engineering and regenerative medicine applications.
Supplementary Material
Supplemental Figure 1. Microparticle characterization. Coulter Counter size analyses of MPs yielded an average diameter of 3.55 ± 1.05 m.
JMBBM Highlights.
Microparticle incorporation within MSC spheroids does not alter cell or aggregate morphology
Microparticle incorporation alters aggregate mechanics, resulting in stiffer spheroids
Altered spheroid mechanics may influence in vitro directed differentiation strategies
Acknowledgments
The authors thank Drs. Ankur Singh and Andrés Bratt-Leal for valuable discussions on the design of these studies and Dr. Bratt-Leal for microparticle fabrication. Dr. Baraniak is supported by a Postdoctoral Fellowship from the American Heart Association. This work was supported by funding from the NIH (R01 GM088291, R01 EB010061) and NSF (CBET 0939511).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Priya R. Baraniak, Email: priya.r.baraniak@gatech.edu.
Marissa T. Cooke, Email: marissa.cooke@bme.gatech.edu.
Rabbia Saeed, Email: rsaeed@gatech.edu.
Melissa A. Kinney, Email: mkinney@gatech.edu.
Krista M. Fridley, Email: kfridley3@mail.gatech.edu.
Todd C. McDevitt, Email: todd.mcdevitt@bme.gatech.edu.
References
- Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniak PR, McDevitt TC. Scaffold-free Culture of Mesenchymal Stem Cell Spheroids in Suspension Preserves Multilineage Potential. Cell and Tissue Research. 2011 doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosh TJ, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107(31):13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerckel JD, et al. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc Natl Acad Sci U S A. 2011;108(37):E674–680. doi: 10.1073/pnas.1107019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt-Leal AM, et al. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25(1):43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt-Leal AM, et al. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32(1):48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenedo RL, et al. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials. 2009;30(13):2507–2515. doi: 10.1016/j.biomaterials.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenedo RL, et al. Rotary Suspension Culture Enhances the Efficiency, Yield and Homogeneity of Embryoid Body Differentiation. Stem Cells. 2007 doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- Carpenedo RL, et al. Microsphere size effects on embryoid body incorporation and embryonic stem cell differentiation. J Biomed Mater Res A. 2010;94(2):466–475. doi: 10.1002/jbm.a.32710. [DOI] [PubMed] [Google Scholar]
- Davidson LA. Embryo mechanics: balancing force production with elastic resistance during morphogenesis. Curr Top Dev Biol. 2011;95:215–241. doi: 10.1016/B978-0-12-385065-2.00007-4. [DOI] [PubMed] [Google Scholar]
- De Santis G, et al. How can cells sense the elasticity of a substrate? An analysis using a cell tensegrity model. Eur Cell Mater. 2011;22:202–213. doi: 10.22203/ecm.v022a16. [DOI] [PubMed] [Google Scholar]
- Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Engler AJ, et al. Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact. 2007;7(4):335. [PubMed] [Google Scholar]
- Erices A, et al. Mesenchymal progenitor cells in human umbilical cord blood. British journal of haematology. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- Evans ND, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater. 2009;18:1–13. doi: 10.22203/ecm.v018a01. discussion 13-14. [DOI] [PubMed] [Google Scholar]
- Frith JE, et al. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16(4):735–749. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- Fu J, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7(9):733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, et al. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28(3):564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez EW, et al. Tissue geometry patterns epithelial-mesenchymal transition via intercellular mechanotransduction. J Cell Biochem. 2010;110(1):44–51. doi: 10.1002/jcb.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011;7(7):2797–2803. doi: 10.1016/j.actbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Hussain A, et al. Magnesium Calcium Phosphate as a Novel Component Enhances Mechanical/Physical Properties of Gelatin Scaffold and Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Tissue Eng Part A. 2011 doi: 10.1089/ten.TEA.2011.0310. [DOI] [PubMed] [Google Scholar]
- Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 2006;11(8):922–932. doi: 10.1177/1087057106292763. [DOI] [PubMed] [Google Scholar]
- Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103(5):389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- Levental I, et al. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- Marrero B, et al. Generation of a tumor spheroid in a microgravity environment as a 3D model of melanoma. In Vitro Cell Dev Biol Anim. 2009;45(9):523–534. doi: 10.1007/s11626-009-9217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri S, et al. Proliferation and differentiation of mesenchymal stem cell on collagen sponge reinforced with polypropylene/polyethylene terephthalate blend fibers. Tissue Eng Part A. 2010;16(12):3821–3830. doi: 10.1089/ten.TEA.2009.0520. [DOI] [PubMed] [Google Scholar]
- Nam J, et al. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomater. 2011;7(4):1516–1524. doi: 10.1016/j.actbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuss S, et al. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22(3):405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- Ong SM, et al. Engineering a scaffold-free 3D tumor model for in vitro drug penetration studies. Biomaterials. 2010;31(6):1180–1190. doi: 10.1016/j.biomaterials.2009.10.049. [DOI] [PubMed] [Google Scholar]
- Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol. 2008;449:27–44. doi: 10.1007/978-1-60327-169-1_2. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Purpura KA, et al. Systematic engineering of 3D pluripotent stem cell niches to guide blood development. Biomaterials. 2012;33(5):1271–1280. doi: 10.1016/j.biomaterials.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quapp KM, Weiss JA. Material characterization of human medial collateral ligament. J Biomech Eng. 1998;120(6):757–763. doi: 10.1115/1.2834890. [DOI] [PubMed] [Google Scholar]
- Ravindran S, et al. Biomimetic extracellular matrix-incorporated scaffold induces osteogenic gene expression in human marrow stromal cells. Tissue Eng Part A. 2012;18(3-4):295–309. doi: 10.1089/ten.tea.2011.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands AS, et al. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295(4):C1037–1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- Sachlos E, Auguste DT. Embryoid body morphology influences diffusive transport of inductive biochemicals: a strategy for stem cell differentiation. Biomaterials. 2008;29(34):4471–4480. doi: 10.1016/j.biomaterials.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Saha K, et al. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95(9):4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpooshan V, et al. Reduced hydraulic permeability of three-dimensional collagen scaffolds attenuates gel contraction and promotes the growth and differentiation of mesenchymal stem cells. Acta Biomater. 2010;6(10):3978–3987. doi: 10.1016/j.actbio.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol Med. 2007;140:141–151. doi: 10.1007/978-1-59745-443-8_8. [DOI] [PubMed] [Google Scholar]
- Treiser MD, et al. Cytoskeleton-based forecasting of stem cell lineage fates. Proc Natl Acad Sci U S A. 2010;107(2):610–615. doi: 10.1073/pnas.0909597107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungrin MD, et al. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE. 2008;3(2):e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YK, et al. Bone Morphogenetic Protein-2-Induced Signaling and Osteogenesis Is Regulated by Cell Shape, RhoA/ROCK, and Cytoskeletal Tension. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JP, et al. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15(1):147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- Yamamoto E, et al. Mechanical properties of collagen fascicles from the rabbit patellar tendon. J Biomech Eng. 1999;121(1):124–131. doi: 10.1115/1.2798033. [DOI] [PubMed] [Google Scholar]
- Yang MT, et al. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nat Protoc. 2011;6(2):187–213. doi: 10.1038/nprot.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Microparticle characterization. Coulter Counter size analyses of MPs yielded an average diameter of 3.55 ± 1.05 m.


