SUMMARY
Ligands for the NKG2D stimulatory receptor are frequently upregulated on tumor lines, rendering them sensitive to NK cells, but the role of NKG2D in tumor surveillance has not been addressed in spontaneous cancer models. Here, we provided the first characterization of NKG2D-deficient mice, including evidence that NKG2D was not necessary for NK cell development, but was critical for immunosurveillance of epithelial and lymphoid malignancies in two transgenic models of de novo tumorigenesis. In both models, we detected NKG2D ligands on the tumor cell surface ex vivo, providing needed evidence for ligand expression by primary tumors. In a prostate cancer model, aggressive tumors arising in NKG2D-deficient mice expressed higher amounts of NKG2D ligands than did similar tumors in wild-type mice, suggesting an NKG2D-dependent immuno-editing of tumors in this model. These findings provide important genetic evidence for surveillance of primary tumors by an NK receptor.
INTRODUCTION
NKG2D is a stimulatory immunoreceptor expressed by NK cells and various T cell subsets including activated CD8+ T cells, fractions of gamma-delta, NKT cells and some activated CD4+ T cells (Groh et al., 2001; Groh et al., 1999; Jamieson et al., 2002; Raulet, 2003; Saez-Borderias et al., 2006). The receptor binds to several NKG2D ligands, including the MICA and MICB (MHC class I chain related) proteins expressed by humans but not mice (Bauer et al., 1999), and a family of proteins called Rae1 (mouse) or ULBP (human), shared by rodents and humans (Cerwenka et al., 2000; Cosman et al., 2001; Diefenbach et al., 2000), reviewed in (Raulet, 2003).
NKG2D ligands are poorly expressed by normal cells but are frequently upregulated in tumor cells (Groh et al., 1999), tumor cell lines (Cerwenka et al., 2000; Diefenbach et al., 2000; Pende et al., 2001; Pende et al., 2002), and in some infected cells (Gourzi et al., 2006; Lodoen et al., 2003; Siren et al., 2004). The mechanisms leading to ligand upregulation are under investigation (Cerwenka et al., 2000; Gasser et al., 2005; Hamerman et al., 2005). One pathway that has been implicated is the DNA damage response pathway (Gasser et al., 2005), which is frequently activated in precancerous lesions as well as advanced tumors (Bartkova et al., 2005; Gorgoulis et al., 2005). Ligand expressing cells can activate NKG2D-expressing NK cells or T cells in vitro (Bauer et al., 1999; Cerwenka et al., 2000; Diefenbach et al., 2000). Transfected tumor cell lines expressing NKG2D ligands are rejected in vivo in an NKG2D-dependent fashion (Cerwenka et al., 2001; Diefenbach et al., 2001). Whereas these findings are consistent with a role of NKG2D in tumor surveillance, there is little direct evidence for such a role. Indeed, at least some tumors may evade NKG2D surveillance (Coudert et al., 2005; Groh et al., 2002; Oppenheim et al., 2005). For instance, some cancers shed high amounts of soluble NKG2D ligands, which are believed to cause downregulation of NKG2D on the surface of lymphocytes (Groh et al., 2002).
In addition to a potential role in tumor surveillance, NKG2D has been implicated in pathogen immunity (Cosman et al., 2001; Groh et al., 2001), autoimmunity (Groh et al., 2003; Ogasawara et al., 2004) and graft rejection (Ogasawara et al., 2005). In order to address the role of NKG2D (encoded by Klrk1) in vivo, we have generated and characterized NKG2D-deficient mice. We showed that NKG2D-deficient NK cells developed normally and were defective in NKG2D recognition, yet retained activity against MHC-deficient tumor cells and bone marrow grafts. We chose two models in which mice carrying trans-oncogenes develop spontaneous, autochthonous tumors. Using NKG2D-deficient TRAMP mice, a transgenic model of prostate adenocarcinoma, and NKG2D-deficient Eμ-myc mice, a transgenic model of B cell lymphoma, we demonstrated that NKG2D plays a critical role in tumor surveillance in vivo.
RESULTS
Generation of NKG2D-deficient gene-targeted mice
By gene targeting we replaced 6 exons of the Klrk1 gene with a neo cassette in the Bruce-4 embryonic stem cell line derived from inbred C57Bl/6 (B6) mice (Supplementary Figure 1A online). By targeting B6 ES cells we ensured that the mice would have the well-characterized B6 NK gene complex, which encodes many key NK receptors, including the marker NK1.1. We generated an initial colony of mice in which the neo cassette was retained in the gene and subsequently deleted the neo cassette by crossing the mice to a B6 strain that expresses the Cre recombinase in the germline (Supplementary Fig. 1A, online). The neo-deleted mice were backcrossed to B6 mice, and Klrk1 heterozygous offspring lacking the Cre transgene were intercrossed. The experiments shown compared Klrk1−/−, Klrk1+/+, and in some cases Klrk1+/− littermates derived from intercrosses of Klrk1+/− mice from the neo-deleted or neo+ colonies as indicated.
Klrk1−/− (NKG2D-deficient) mice were born in the expected Mendelian ratio (data not shown). The mice exhibited no visible alterations in major organs or overt pathology. Therefore, NKG2D plays a dispensable role in embryonic development, despite early data showing broad expression of Rae1 transcripts in midstage embryos, especially in the central nervous system (Nomura et al., 1996).
NK cells were present in normal numbers in the spleen, bone marrow, lymph node, lung and liver of Klrk1−/− mice (Fig. 1A, B, data not shown), but lacked NKG2D surface expression (Fig. 1C), whereas cells from Klrk1+/− mice showed modestly reduced cell surface expression of NKG2D compared to wild-type mice (Fig. 1C).
Figure 1. NK cell development is preserved in NKG2D-deficient (Klrk1−/−) mice.
Analysis of NK subsets in the bone marrow (BM, n=3, panel A) and spleen (n=3–5, panel B) from Klrk1−/− (neo cassette deleted) mice and Klrk1+/+ littermate controls. The top groups in panels A and B represent gated NK1.1+CD3− cells, whereas the remaining panels represent gated CD3− cells. The numbers represent mean percentages (±SD) of cells expressing the indicated markers. (C) Representative NKG2D staining on freshly isolated NK1.1+CD3− splenic NK cells is shown for Klrk1−/− mice with the neo cassette deleted and littermate controls. Isotype control stain is shown as shaded histogram. The bone marrow analysis was repeated in one additional independent experiment and the spleen cell analysis was repeated in two additional independent experiments, with similar results.
Klrk1−/− mice had normal numbers and proportions of CD4+ and CD8+ T cells, TCRγδ T cells, NKT cells and B cells in the spleen, bone marrow and lymph nodes (Supplementary Fig. 2 online, and data not shown). The frequency of CD8+CD44+ memory T cells in the spleen was also normal (data not shown). NK subsets defined by CD11b and CD27 were not substantially different whereas various maturation markers including NK1.1, CD11b, DX5, CD122, and CD43 were expressed normally (Fig. 1A, B). The mutant mice had normal or minor differences in the expression of various stimulatory and inhibitory receptors including NK1.1, 2B4, Ly49D, Ly49C, Ly49I, Ly49F, KLRG1, Ly49G2, Ly49A, NKp46, CD94, NKG2A (Supplementary Fig. 3 online, Fig. 1, data not shown). Together, these data indicate that NKG2D expression is dispensable for normal phenotypic development of NK cells, B cells and T cells.
NKG2D-deficiency results in a higher incidence of highly malignant prostate adenocarcinomas
We investigated tumor surveillance in vivo using the well-studied TRAMP (transgenic adenocarcinoma of the mouse prostate) model of autochthonous prostate cancer, which mimics human clinical disease (Kaplan-Lefko et al., 2003). In these mice, the rat probasin promoter directs the expression of the SV40 early genes (T and t antigen) to the prostatic epithelium at puberty. In male TRAMP mice, mild to severe prostate hyperplasia develops by 12 weeks of age, followed by the appearance of severe hyperplasia and adenocarcinoma by 18 weeks of age and metastatic disease by 30 weeks of age (Huss et al., 2001; Kaplan-Lefko et al., 2003). Previous studies demonstrated heterogeneity of TRAMP tumors, which varies with genotype (Degrassi et al., 2007; Gingrich et al., 1999). Aggressive early-arising carcinomas were large and progressed rapidly to poorly differentiated (PD) lesions as defined histologically, which is indicative of poor prognosis in human prostate cancer patients. A later-arising form exhibited persistent well differentiated (WD) and moderately differentiated (MD) lesions and progressed less rapidly to PD lesions (Degrassi et al., 2007). The aggressive form was generally substantially rarer in B6 TRAMP mice than in mice of mixed FvB-B6 genotype (Gingrich et al., 1999).
For our studies, B6-Klrk1−/− mice (neo cassette-deleted) were crossed with B6-TRAMP mice and the pups were intercrossed to generate male Klrk1−/− TRAMP mice (n=43) as well as wild-type (Klrk1+/+) TRAMP littermates (n=33) for comparison. Male mice were monitored after puberty for development of prostate tumors. Animals were euthanized when they developed tumor masses as detected by palpation or were moribund. Because all TRAMP mice develop tumors, the question was whether NKG2D-deficiency results in more aggressive adenocarcinomas or alters the kinetics of tumorigenesis. Strikingly, early-arising, massive prostate tumors were rare in the wild-type mice and three times more frequent in the NKG2D-deficient littermates. Defined as those that exceeded the mean mass by >2 SEMs (i.e. >2.7 grams), the large tumors arose in 12 of the 43 Klrk1−/− mice (27.9%) compared to only 3 of the 33 wild-type mice (9.1%) (p= 0.029 by Fishers exact test) (Fig. 2A). As a result, the mean weight of prostate tissue at necropsy in the Klrk1−/− cohort significantly exceeded that of Klrk1+/+ TRAMP mice (p=0.0184 by Mann Whitney test) (Fig. 2B). Prostate carcinomas were not detected in the non-transgenic Klrk1−/− males under survey (data not shown).
Figure 2. Increased incidence of large, early prostate carcinomas in Klrk1−/− TRAMP mice.
(A) Each square represents the weight of the prostate tissue and age at necropsy of individual Klrk1+/+ TRAMP (n=33) (upper panel) or Klrk1−/− TRAMP littermates (n=43) (lower panel) mice. Large-early tumors (> 2 SEM greater than the mean weight, circled) were more frequent in Klrk1−/− mice (p=0.029 by Fishers exact test). (B) The average weights of prostate tumors at necropsy is depicted for both cohorts (p=0.0184 by Mann Whitney test).
Histological examination of tumors that arose in this analysis demonstrated that all 10 of the large tumors that we examined, 8 from Klrk1−/− mice and 2 from wild-type mice, exhibited nearly uniform PD lesions, whereas only a minority of the late-arising tumors (4/25) exhibited PD lesions, in most cases multifocally (Table 1, Supplementary Fig. 4 online, data not shown). WD lesions were detected in most of the late-arising tumors (14/25), whereas more than a quarter of the late-arising tumors from mice of either genotype were classified as phylloides-like, a non-malignant epithelial-stromal tumor (Tani et al., 2005) (Table 1). In conclusion, the histology data confirm the highly malignant status of the early-arising tumors. Thus, NKG2D-deficient mice are three times more susceptible than wild-type littermates to highly malignant, early arising prostate adenocarcinoma, demonstrating that NKG2D-mediated immune mechanisms limit the development of the most aggressive form of prostate cancer in B6-TRAMP mice.
Table 1.
Histological analysis of prostate tumors from Klrk1+/+ TRAMP (n=13) and Klrk1−/− TRAMP (n=22) mice
| Tumor Size and histology | Genotype of TRAMP mice
|
|||||
|---|---|---|---|---|---|---|
| Klrk1+/+ | Klrk1−/− | Combined | ||||
| Large tumors* | 2/13& | 8/22 | 10/35 | |||
| PD adenocarcinoma | 2/2 | 100% | 8/8 | 100% | 10/10 | 100% |
|
| ||||||
| Smaller tumors: | 11/13 | 14/22 | 25/35 | |||
| PD adenocarcinoma | 1/11 | 9.1% | 3/14 | 21.4% | 4/25 | 16.0% |
| WD adenocarcinoma | 7/11 | 63.4% | 7/14 | 50.0% | 14/25 | 56.0% |
| Phylloides (non-malignant) | 3/11 | 27.2% | 4/14 | 28.6% | 7/25 | 28.0% |
Large tumors were defined as those exceeding the mean by >2 standard errors of the mean (i.e. >2.7 g).
The entries represent the number of large or smaller tumors over the total number of tumors of each genotype examined histologically, followed by the fraction and percentage of large or smaller tumors that exhibit the indicated histology. Histological classification was based on the maximal histological grade observed for a given animal. Poorly differentiated (PD) lesions are most aggressive whereas well differentiated (WD) lesions less aggressive and Phylloides tumors are non-malignant.
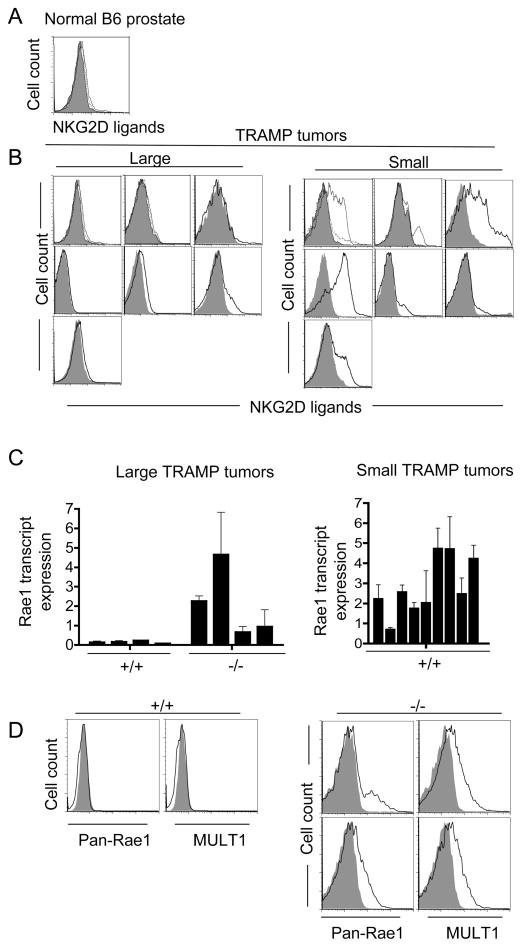
Consistent with the role of NKG2D in surveillance of prostate adenocarcinomas, an analysis of dissociated prostate tumors from a separate cohort of B6-Klrk1+/+ TRAMP mice showed common, though sporadic, expression of NKG2D ligands on the cell surface, whereas normal prostate dissociations did not express ligands (Fig. 3A, B). Interestingly, however, ligand expression was largely restricted to the smaller late-arising tumors and absent from the large early-arising ones in these wild-type mice (Fig. 3B). The results raise the possibility that large aggressive TRAMP tumors extinguish expression of NKG2D ligands as a result of NKG2D-dependent immunoselection or editing (Dunn et al., 2002). Consistent with this possibility, we observed substantially higher amounts of Rae1 transcripts in large Klrk1−/− tumors as compared to large Klrk1+/+ tumors (mean 2.12 versus 0.14 relative units, respectively, n=4 each, p=0.028) (Fig. 3C, left panel). Furthermore, both of the dissociated large Klrk1−/− tumors we examined showed cell surface expression of Rae1 and MULT1 (Fig. 3D) whereas those from Klrk1+/+ mice did not. These data suggest that NKG2D-dependent immunoselection (or editing) favors loss of NKG2D ligands on early arising, aggressive tumors. The smaller late-arising tumors, in contrast, expressed similar amounts of transcripts for NKG2D ligands whether they arose in Klrk1−/− or wild-type mice (Fig. 3C, right panel and data not shown) and displayed the ligands at the cell surface (Fig. 3B and data not shown). Despite expressing NKG2D ligands, these tumors were apparently refractory to NKG2D-mediated surveillance, suggesting that they were sequestered from NKG2D-dependent effector mechanisms or evaded them (Groh et al., 2002; Lee et al., 2004).
Figure 3. Expression of NKG2D ligands by prostate carcinomas ex vivo.
(A, B) Reduced cell surface expression of NKG2D ligands on large, early-arising B6-TRAMP prostate tumors as compared to smaller, late-arising ones. Freshly dissociated prostate tissue from a representative non-transgenic Klrk1+/+ B6 mouse (A) and sets of large and smaller tumors isolated from Klrk1+/+ B6-TRAMP mice (B) were compared. Labeled NKG2D tetramers (plain line) were used to detect all NKG2D ligands and streptavidin-PE served as a negative control (shaded histogram). In a few cases, the specificity of staining was proved by inhibition with unlabeled NKG2D tetramers (dotted line). (C) Reduced amounts of Rae1 transcripts in large (>2.7 g) Klrk1+/+ TRAMP tumors as compared to large Klrk1−/− TRAMP tumors (p=0.0286 with the Mann-Whitney test) (left panel) or smaller Klrk1+/+ TRAMP tumors (right panel). Values determined by quantitative RT-PCR with primers that detect all Rae1 isoforms were normalized to HPRT transcript amounts, and these data were normalized to the amount in nontransgenic B6 prostates (average of 5 independent B6 mice). Graphs show the mean ± SD of 2–6 separate qPCR assays for each sample. (D) Cell surface staining of Rae1 and MULT1 (solid line) on two dissociated large Klrk1−/− TRAMP tumors and a large tumor from an Klrk1+/+ TRAMP littermate. Isotype control stains are shown as shaded histograms. Gated CD45-negative (non-hematopoietic), PI-negative (live) cells were examined.
Upon necropsy, the Klrk1+/+ and Klrk1−/− mice were also screened for macroscopic metastases in liver, lung and kidney. The percentage of mice with such visible metastases was similar in Klrk1−/− and wild-type mice (approximately 21% in each), raising the possibility that NKG2D functions primarily at an early stage of tumorigenesis rather than at the stage of metastasis.
NKG2D-deficiency accelerates the progression of Eμ-myc-induced lymphomas
In order to study the role of NKG2D in the surveillance of lymphoid tumorigenesis, we used Eμ-myc transgenic mice (Adams et al., 1985), in which constitutive c-myc oncogene expression in the B cell lineage results in selective formation of B, pre-B or mixed pre-B and B lymphomas (Harris et al., 1988; Langdon et al., 1986). Genetic crosses were used to generate B6-Klrk1−/− Eμ-myc transgenic mice (n=24) and B6-Klrk1+/+ Eμ-myc transgenic littermates (n=34), which were monitored twice weekly after birth for palpable tumors and signs of illness as manifested by lymphadenopathy, tachnypea and hunched posture. Only mice showing enlarged lymphoid organs at necropsy were designated as having lymphomas. Myc-driven lymphomas arose significantly earlier in Klrk1−/− mice than in Klrk1+/+ mice, with the median time of onset occurring more than 7 weeks earlier in the Klrk1−/− mice (p=.005 using the log-rank test) (Fig. 4A). None of the nontransgenic Klrk1−/− or Klrk1+/+ mice developed lymphoma during a comparable period of monitoring (data not shown).
Figure 4. Accelerated onset of myc-driven lymphomas in Klrk1−/− mice.
(A) Kaplan-Meier representation of tumor progression among Klrk1−/− Eμ-myc transgenic (n=24) and Klrk1+/+ Eμ-myc transgenic mice (n=34). p=0.005 by the log-rank test. (B) Ex vivo analysis of lymph node cell suspensions isolated from Klrk1+/+ Eμ-myc, Klrk1−/− Eμ-myc, and non-transgenic Klrk1+/+ mice, as indicated. Dot plots show B220 and IgM expression on the blast (high forward scatter) population from independent mice which were examined after the onset of illness. The age (weeks) at necropsy is specified for each mouse as well as the percentage of cells included in the gate. The histograms show pan-Rae1 and MULT1 expression (solid line) on the gated populations indicated in each dot plot. The shaded histograms represent the staining with an isotype control antibody.
Light scatter analysis of spleen and lymph node cell samples of all the affected mice showed increased frequencies of lymphoblasts compared to nontransgenic samples, suggesting the presence of tumor cells. Tumors arising in both Klrk1+/+ (n=7) and Klrk1−/− (n=5) Eμ–myc transgenic mice expressed B220 but were heterogeneous with respect to the amount of B220 expression and IgM expression, suggesting that B, pre-B and mixed pre-B and B cell lymphomas were represented (Fig. 4B and data not shown). We detected no differences in the distribution of these tumor types when comparing this small sample of tumors from Klrk1+/+ versus Klrk1−/− mice. NKG2D ligands Rae1 and MULT1 were not expressed on non-transgenic B cells but were detected at varying amounts on all the tumor samples (Fig. 4B and data not shown). The amounts of both ligands varied from tumor to tumor in mice of both genotypes, with MULT1 expression being more common. There was no indication in this survey that expression of NKG2D ligands was selected against in Klrk1+/+ mice, despite the clear evidence that NKG2D-mediated surveillance is operative for these lymphomas. These data suggest that evasion of NKG2D-mediated surveillance by Eμ-myc induced lymphomas occurs by mechanisms that do not depend on loss of NKG2D ligands, similar to the late-arising prostate carcinomas in the TRAMP model.
NKG2D-deficiency does not affect the incidence of carcinogen-induced sarcomas
We addressed the role of NKG2D in the well-studied 3-methylcholanthrene (MCA)-induced carcinogenesis model. Klrk1−/− mice (in which the neo cassette was deleted) and Klrk1+/+ littermates were injected with 2 doses of MCA, and fibrosarcomas arose at the site of subcutaneous carcinogen application after a latency period. With a dose of 25 μg MCA, fibrosarcoma incidence was identical in Klrk1−/− and Klrk1+/+ mice (Fig. 5A, p=0.69), whereas with 5 μg MCA, the incidence was, if anything, lower in Klrk1−/− mice, though not significantly (Fig. 5B, p=0.1). Surprisingly, a separate initial analysis of Klrk1−/− mice that retained the neo cassette showed an increased incidence of MCA-induced tumors (data not shown). However, because the PGK-neo cassette inserted in a locus can profoundly repress the expression of neighboring genes (Kim et al., 1992; Pham et al., 1996; Xu et al., 1996), and many of the genes near NKG2D encode NK receptors, the results with neo-in mice are clearly unreliable compared to results in mice where the neo cassette is deleted.
Figure 5. Comparable incidence of MCA-induced fibrosarcomas in NKG2D-deficient mice and wild-type littermates.
Klrk1−/− (dashed line) and wild-type littermates (solid line) (neo cassette-deleted) were injected s.c. with 25μg (A) or 5μg (B) of 3-methylcholanthrene (MCA) and monitored for tumor development. Tumor bearing mice were defined by the presence of a mass of at least 7mm diameter growing upon 2 consecutive measurements. These data were compiled from 2 independent experiments. p values are based on the log-rank test.
Analysis of numerous freshly dissociated advanced fibrosarcomas from the neo-in or neo-deleted mice showed a great deal of heterogeneity in ligand expression regardless of Klrk1 genotype, with some tumors expressing ligands at a very low level or not at all (data not shown). Hence, there was no evidence from these studies that NKG2D selected for loss of NKG2D expression by tumor cells in this model. In conclusion, the present data do not support an important role for NKG2D in surveillance of MCA-induced sarcomas in vivo.
Klrk1−/− NK cells are defective in NKG2D function but retain other NK cell functions
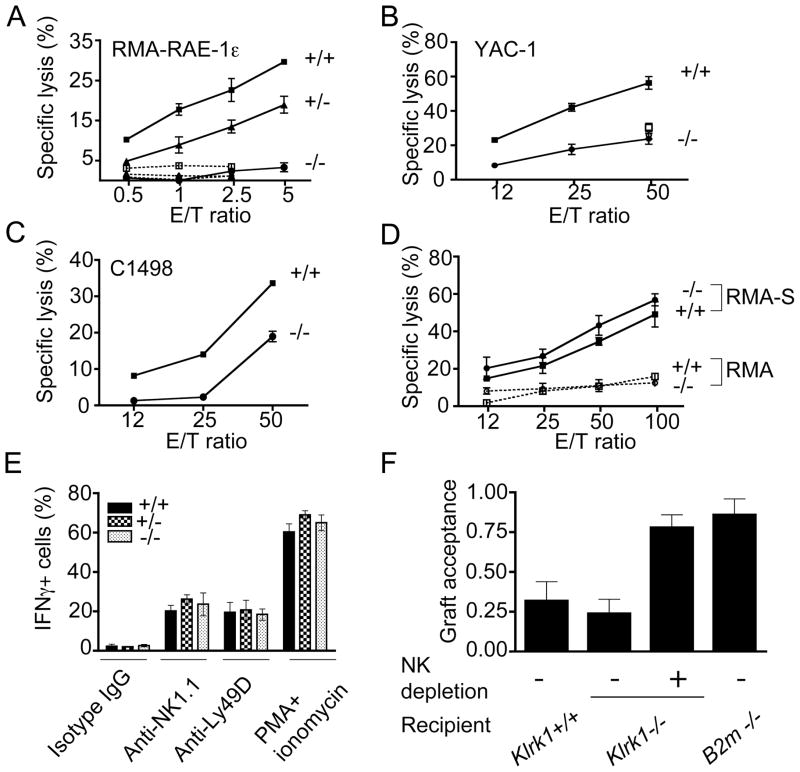
The deficient tumor surveillance in Klrk1−/− mice could arise if the mutation resulted in impaired development of NK cells, an NK cell subset, or NK cell effector function, as opposed to a selective deficiency in NKG2D function. As expected, sorted IL-2-activated NK cells from Klrk1−/− mice were devoid of lytic activity against RMA target cells transfected with the Rae1ε NKG2D ligand (Fig. 6A). Klrk1+/− NK cells, which had lower expression of NKG2D than wild-type cells, mediated reduced lysis of these target cells. In contrast, Klrk1−/− NK cells were only partially impaired in their capacity to lyse tumor cell lines that naturally express NKG2D ligands, such as YAC-1 and C1498, comparable to the activity of wild-type NK cells in the presence of NKG2D antibody (Fig. 6B, C). These data validate the findings of earlier studies suggesting that recognition by NKG2D accounts for only part of the activity of NK cells against YAC-1 and C1498 tumor cells (Jamieson et al., 2002). Moreover, NKG2D-deficient NK cells showed no defect in lysis of the MHC class I-low RMA-S cell line, which lacks NKG2D ligands and is recognized by an unknown stimulatory NK receptor other than NKG2D (Fig. 6D). These data support the conclusion that the Klrk1 mutation selectively disables recognition by NKG2D and does not alter functional recognition by other NK receptors.
Figure 6. NKG2D-deficiency does not impair NK cell functions in vitro or in vivo.
(A-C) IL-2 activated splenic NK cells (sorted NK1.1+CD3− cells in panel A, unsorted cells in panels B-C from Klrk1−/− mice fail to lyse RMA-Rae1ε target cells, and show reduced lysis of YAC-1 and C1498 target cells. Lysis in the presence of NKG2D mAb is shown for Klrk1+/+ (open squares) and Klrk1−/− (open circles) effector cells in panels A and B. (D) Normal lysis of class I-low RMAS cells by Klrk1−/− IL-2 activated NK cells. Results represent means ±SD obtained with Klrk1−/− mice (neo cassette-retained) and littermate controls. (E) Splenocytes from Klrk1−/− mice (neo cassette deleted) and littermate controls (n=5 for each genotype) were stimulated in vitro for 5 hours on plates coated with NK1.1 mAb (PK136, 40 μg/ml), Ly49D mAb (SED85, 10 μg/ml) or control mouse IgG (40 μg/ml) in the presence of Golgi-plug before staining and analysis. In parallel, NK cells were stimulated with or without a mixture of PMA and ionomycin. Intracellular IFN-γ was detected by flow cytometry on gated NK1.1+CD3− cells, except for the anti-NK1.1-stimulated cells, which were gated on DX5+CD3− cells. Results represent means +/− SD (n=5). (F) In vivo rejection of B2m−/− bone marrow cells by Klrk1−/− mice. A mixture of CFSE (5 μM) labeled BM cells from C57Bl/6-Ly5.2 and B2m−/− C57Bl/6-Ly5.1 mice was injected i.v. in irradiated Klrk1+/+ (n=4), Klrk1−/− (n=6), negative control C57Bl/6 B2m−/− (n=2), or NK-depleted Klrk1−/− (n=3) recipients. Similar results were obtained in one additional independent experiment.
We examined the responsiveness of Klrk1−/− NK cells to direct cross-linking of stimulatory receptors other than NKG2D using plate-bound antibodies specific for these receptors. Cross-linking NK1.1 or Ly49D resulted in similar IFN-γ production by Klrk1−/− and Klrk1+/+ NK cells. Klrk1−/− and Klrk1+/+ NK cells also responded similarly to pharmacological stimuli provided by PMA plus ionomycin (Fig. 6E). These data provide direct evidence that the activating functions of stimulatory NK receptors other than NKG2D are unaffected by the Klrk1 mutation.
As a measure of NK functions in vivo in NKG2D-deficient mice, we tested whether NKG2D participates in “missing self” recognition of bone marrow cells in vivo. Irradiated Klrk1+/+ or Klrk1−/− B6 mice were challenged with class I-deficient (B2m−/−), B6 strain bone marrow cells, mixed in the same inoculum with wild-type bone marrow cells that served as an internal negative control (Fernandez et al., 2005). Klrk1+/+ and Klrk1−/− recipient mice rejected the class I–deficient bone marrow grafts similarly, and depletion of NK1.1+ cells with PK136 mAb prevented rejection by Klrk1−/− mice (Fig. 6F). As expected, B2m−/− recipient mice (Klrk1+/+) failed to reject class I-deficient bone marrow grafts (Bix et al., 1991). These data indicate that NKG2D is not required for rejection of class I-deficient, B6 strain bone marrow grafts, in line with other studies suggesting no role for NKG2D in rejection of B6 bone marrow grafts by F1 mice (Ogasawara et al., 2005). Thus, the present data demonstrate that NKG2D-deficiency does not globally impair NK cell functions in vivo.
DISCUSSION
Using Klrk1 gene-targeted mice, we have provided direct genetic evidence for a role of NKG2D in surveillance of primary prostate adenocarcinoma and B lymphoma. Transgenic models of spontaneous malignancy, such as those used here, are usually considered reliable models of human cancer in comparison to high dose carcinogen models, which may not accurately mimic spontaneous human tumors in many respects (Prehn, 1975). The effect on tumor incidence is likely a direct effect of NKG2D deficiency, because we found no substantial alterations in development of NK cells or other cells, nor in NK cell functions other than those mediated by NKG2D.
Although the role of NKG2D in such models of tumorigenesis has not been previously investigated, it was previously reported that methylcolanthrene-induced tumors were more frequent when mice were treated long-term with blocking NKG2D antibodies (Smyth et al., 2005). In our studies of the MCA carcinogenesis model, however, we failed to observe an increased incidence of MCA-induced sarcomas in Klrk1−/− mice from which the neo cassette was deleted, using both standard and limiting MCA doses. The basis of the discrepancy is unknown, but one possibility is that sustained exposure to high doses of NKG2D antibodies in vivo, like exposure of NK cells to NKG2D ligands in vivo (Coudert et al., 2005; Oppenheim et al., 2005), causes generalized NK cell defects, that could impair NK cell-mediated rejection of target cells recognized via distinct NK receptors. Alternatively, long-term antibody treatment, including the presence of complexes of injected antibodies and host anti-antibodies, could alter the immune response in unpredictable ways.
Previous studies provided evidence for expression of NKG2D ligands on primary human tumors (Farag et al., 2002; Groh et al., 1999; Salih et al., 2003), but there was little data on ligand expression by primary mouse tumors. The data herein include valuable evidence that NKG2D ligands are expressed at the surface of freshly isolated primary tumor cells ex vivo in two mouse tumor systems. It was interesting that at the time of necropsy there was considerable heterogeneity in the amounts and identity (Rae1 vs MULT1) of ligands expressed by different tumors. The heterogeneity cannot be attributed mainly to selection by NKG2D-dependent mechanisms, because it was prevalent among the tumors arising in NKG2D-deficient mice. It appears likely, therefore, that the signals that regulate ligand expression are heterogeneous in advanced tumor cells or that ligands are in some cases spontaneously downregulated or shed from the surface of advanced tumor cells, as has been reported elsewhere for human tumors (Eisele et al., 2006; Holdenrieder et al., 2006; Kaiser et al., 2007).
Despite this heterogeneity of ligand expression in advanced tumors, it is likely that nascent tumors express ligands in a more consistent fashion in at least some of the tumor types we studied. It seems probable that this is true in the case of early-arising prostate carcinomas and Eμ-myc lymphomas, because in both models NKG2D surveillance had a substantial effect on the incidence or time of onset of palpable tumors, which would not be expected if many emerging tumors expressed no ligands or low amounts of ligands.
Early surveillance would be in accord with published evidence that NKG2D ligands are induced by the DNA damage response (Gasser et al., 2005), which is known to be activated at an early stage in tumorigenesis (Bartkova et al., 2005; Gorgoulis et al., 2005). Furthermore, it has been shown that oncogene-induced stress, such as that which occurs as a result of myc overexpression, activates the DNA damage response pathway (Dominguez-Sola et al., 2007; Reimann et al., 2007; Vafa et al., 2002). Upregulation of NKG2D ligands as a consequence of these and other relatively early events in tumorigenesis may sensitize emerging tumor cells to elimination by NKG2D-dependent mechanisms.
Immunoediting, also called immunoselection, is a process where immune responses select for variant tumor cells that have lost expression of specific molecules or antigens that are targeted by immune effector cells (Dunn et al., 2004). Immunoediting is therefore a likely explanation for our finding that early arising tumors from Klrk1+/+ mice lacked NKG2D ligands, whereas tumor cells from Klrk1−/− mice did not. We lack evidence, however, that the Rae1-negative tumor cells that arise in Klrk1+/+ mice are the progeny of Rae1+ tumor cells. A possible prediction of the immunoediting hypothesis is that transfer to wild-type mice of Rae1+ or MULT1+ tumors from Klrk1−/− mice should lead to rejection, whereas transfer of tumors devoid of Rae1 and MULT1 expression from Klrk1+/+ mice should not. Such a study is not currently possible, however, because we have so far been unable to successfully and reproducibly engraft primary prostate tumors procured from TRAMP mice into syngeneic hosts (N. Greenberg, unpublished data).
In contrast to the results with early-arising TRAMP tumors, expression of NKG2D did not influence NKG2D ligand expression by late-arising TRAMP tumors or Eμ-myc lymphomas, at least at the advanced stages examined. Eμ-myc lymphomas differed from late-arising TRAMP tumors, however, in that Eμ-myc lymphomas were subject to NKG2D-dependent immune surveillance, whereas the late-arising prostate carcinomas were neither delayed nor less frequent in hosts that expressed NKG2D as compared to Klrk1−/− hosts, suggesting that they were not subject to NKG2D-dependent immune surveillance.
Taken together, these data suggest that the role of NKG2D-dependent surveillance differs in the three types of tumors studied here. In the case of early-arising prostate carcinomas in TRAMP mice, many of the tumors are eliminated, and the few that are not eliminated evade surveillance by extinguishing expression of NKG2D ligands. In the case of Eμ-myc lymphomas, it appears that the emerging tumors are mostly NKG2D-sensitive, but a fraction of tumors escape NKG2D surveillance without losing NKG2D ligands. Several possible evasion mechanisms can be envisaged, including shedding of NKG2D ligands from tumor cells or other mechanisms that inactivate NKG2D+ effector cells (Groh et al., 2002; Lee et al., 2004); the loss of adhesion molecules or properties necessary for these cells to be recognized by NK cells and T cells; or rapid tumor growth that outpaces elimination by NKG2D+ cells, and which may ultimately cause local or systemic desensitization of NKG2D+ cells (Coudert et al., 2005; Oppenheim et al., 2005). The final category is represented by late-arising prostate carcinomas, which appear to be generally refractory to NGK2D-dependent surveillance. It is possible that this type of tumor is sequestered from NKG2D-dependent immune responses, locally activates inhibitory processes or regulatory cells that inactivate NKG2D+ cells (Lee et al., 2004), or expresses NKG2D ligands in a delayed fashion compared to the early-arising ones.
The data herein represent genetic evidence for a role of the NKG2D receptor in tumor surveillance. The findings add to a growing number of studies with gene targeted mice that have re-energized the immune surveillance theory, by showing roles for specific immune cells or functions in protection from cancer (Girardi et al., 2001; Shankaran et al., 2001; Smyth et al., 2000; van den Broek et al., 1996). The present findings are unique in showing the role of a specific “innate immunity receptor”, NKG2D. Considering that NKG2D is expressed by numerous T cell subsets, the relative roles of NKG2D expressed by NK cells or T cell is an important issue that will be addressed in the future. Regardless of the outcome, the data suggest that interventions to enhance the NKG2D axis of immunity and/or prevent its deregulation may have applications in cancer immunotherapy (Diefenbach et al., 2001; Jinushi et al., 2006; Zhang et al., 2006; Zhou et al., 2006). Conversely, the observation that different subtypes of prostate tumors vary in their susceptibility to NKG2D-dependent surveillance suggests that such applications will need to be tailored to specific cancer subtypes.
EXPERIMENTAL PROCEDURES
Mice
All mice were bred at the University of California, Berkeley in compliance with institutional guidelines. C57BL/6J [B6, H-2b], B6-Ly5.1 mice [catalog name, B6-Ly5.2/Cr] and Eμ-myc transgenic mice [catalog name, C57BL/6J-Tg(IghMyc)22Bri/J] were purchased from the Jackson Laboratories (Bar Harbor, ME). B6-B2m −/−-Ly5.1 mice were derived in our facilities from B6- B2m−/− mice (Zijlstra et al., 1990). C57BL/6 mice harboring a CMV-Cre transgene were generated from a CD1-Cre transgenic strain provided by Dr. A. Nagy (Samuel Lunenfeld Research Institute, Toronto Canada) by backcrossing to B6. C57BL/6 Tg-TRAMP mice were screened for the presence of the transgene by PCR as previously described (Greenberg et al., 1995). C57BL/6 Tg-Eμ-myc mice were screened for the presence of the transgene by PCR using the following primers: forward 5′-CAG CTG GCG TAA TAG CGA AGA G-3′ reverse 5′-CTG TGA CTG GTG AGT ACT CAA CC-3′. Mice were euthanized by CO2 inhalation in accord with the policies of the office of Laboratory Animal and Care (OLAC) at UC Berkeley.
Generation of NKG2D-deficient mice
Bruce-4 ES cells derived from C57BL/6 mice (Lemckert et al., 1997) were kindly provided by Dr J.D. Sedgwick (Schering–Plough Biopharma, Palo Alto, CA) and transfected with a targeting vector (pKSTKNeoLoxP) designed to delete the exons 1b to 6, encoding the cytoplasmic, transmembrane domains and partial extracellular domain of NKG2D (Supplementary Fig. 1A online). The vector contained the neo cassette and was flanked by a thymidine kinase (TK) cassette. Clones resistant to G418 and Gancyclovir were screened by Southern blotting with a probe flanking the 3′ short arm (Supplementary Figure 1B online), and two positive clones were confirmed by blotting with a 5′ probe (which hybridizes to the long arm) and a neo probe (data not shown). One targeted clone, BB9, when injected into BALB/c blastocysts, gave rise to viable chimeras that transmitted the disrupted Klrk1 allele when crossed with C57BL/6J (B6/J) females. The Klrk1+/− B6 progeny of the chimeras were crossed again back to B6/J, and then intercrossed to produce mice of all three genotypes. The same Klrk1+/− mice were separately crossed to a B6 transgenic strain that expresses Cre recombinase in the germline. Cre-transgene+, Klrk1+/− offspring were bred back to B6/J mice and Cre-transgene-negative, Klrk1+/− offspring that had deleted the neo cassette were identified by PCR and Southern-blot and interbred to produce neo-deleted, Cre-transgene-negative mice of all three Klrk1 genotypes (+/+, +/− and −/−).
Carcinogenesis
Aged-matched males were shaved and injected subcutaneously with 25μg or 5μg of 3-methylcholantrene (MCA)(Sigma Chemical Co., St Louis, Mo) dissolved completely in olive oil by heating in boiling water. Mice were examined twice weekly for at least 230 days for palpable tumors at the site of injection. Progressively growing masses of 7mm in diameter or larger were scored as tumors.
Tumor dissociation and cell staining
Solid tumors (MCA-induced fibrosarcomas and the prostate tissues of TRAMP mice) were dissected and minced in CO2 independent-medium (Gibco) containing collagenase D (1mg/ml, Roche-Applied-Science, Indianapolis) and DNAse (1μg/ml, Roche-Applied-Science, Indianapolis). The tissue was incubated with shaking at 37°C before being passed through a strainer. The suspended cells were washed twice with DMEM 10% FCS and part of the single cell suspension was used for ex vivo staining. Enlarged spleen and lymph nodes from Eμ-myc Tg mice were dissected, dissociated in RPMI-10%FCS medium and red blood cells and dead cells were removed by lympholyte treatment (Lympholyte-M, Cederlane laboratories, Ontario Canada). Surface staining was performed on freshly isolated tumor cells after a pre-incubation step with anti-CD16 mAb to saturate the Fc-receptors. Staining reagents included anti-CD45-PECY5 mAb (eBioscience), anti-pan Rae1-PE mAb (R&D Systems, Minneapolis, MN), anti-MULT1 (R&D Systems), and a distinct anti-MULT-1, kindly provided by Dr S. Jonjic (University of Rijeka, Croatia) that was biotinylated in our lab (used for studies in Fig. 4). NKG2D tetramer-biot-SAPE has been described (Diefenbach et al., 2000). Flow histograms obtained from solid tumors are shown for live cells by gating out the cells labeled with propidium iodide (PI).
Bone marrow graft rejection
Donor bone marrow graft rejection assays were performed as previously described (Fernandez et al., 2005). Briefly, a mixture of 5 × 106 cells each of CFSE-labeled B6-B2m−/−-Ly5.1 and B6 (Ly5.2) bone marrow cells was injected i.v. into mice that had been irradiated earlier in the day with 9.5 Gy from a 137Cs source. Recipient spleen cells, harvested 3 days later, were analyzed for CFSE+ Ly5.1+ (class I-deficient) and CFSE+ Ly5.2+ (nonrejection control B6 cells) by flow cytometry. Some mice were pre-depleted of NK cells by i.p. injection of anti-NK1.1 antibody (Fernandez et al., 2005). Graft acceptance corresponds to the mean (± SD) ratio of B2m−/− (Ly5.1+) to B6 (Ly5.1−) cells among CFSE+ recipient spleen cells.
Statistical analysis
Statistical comparisons were performed using the log-rank test on Kaplan-Meier curves depicting lymphomas progression, the Fisher exact test to evaluate prostate tumor incidence and the two-tailed Mann-Whitney test assuming unequal variance. P < .05 denotes significance.
Supplementary Material
Supplementary Figure 1. Generation of NKG2D-deficient (Klrk1−/−) mice.
(A) Klrk1 gene-targeting strategy. The Klrk1 locus (top) is compared to the targeting vector (middle) and the targeted locus (bottom), showing the Klrk1 exons and the location of the 3′ probe. Successful targeting substitutes Klrk1 exons 1b to 6 with a neo cassette flanked by loxP sites. (B) Tail genomic DNA was digested with BamHI and Southern blots were hybridized with the 3′probe indicated in A. The 6.2 Kb and 4.1 Kb bands correspond to the wild type (WT) and knockout (KO) alleles of Klrk1, respectively, as indicated on the maps. The 2Kb band represents the Klrk1−/− allele (KO) after deletion of the neo cassette.
Supplementary Figure 2. Lymphoid development in NKG2D-deficient mice.
Representative staining of B and T cell populations on Klrk1+/+ and Klrk1−/− splenocytes. The mean percentages (±SD) of the indicated subsets are indicated (n=3–6). The data were obtained with Klrk1−/− mice with the neo cassette deleted and littermate controls.
Supplementary Figure 3. Analysis of NK subsets in NKG2D-deficient mice.
Histograms represent gated NK1.1+CD3− splenocytes from Klrk1−/− (neo cassette deleted) and Klrk1+/+ littermate controls (n=5–8). The shaded histograms represent the corresponding isotype control staining. The numbers correspond to mean percentages (±SD) of cells expressing the indicated markers.
Supplementary Figure 4. Representative histologic analysis of prostate tumors that develop in Klrk1−/− TRAMP (−/−) and Klrk1+/+ TRAMP mice (+/+). Prostate sections stained with Hematoxilin and Eosin were analysed and graded as followed: well differentiated (WD), and poorly differentiated (PD). Magnification 10X.
Acknowledgments
We thank R. Nir-paz, A. Baciu and Steven Selvin for advice on statistical testing, P. Savage for helpful discussions, E. Treiner for help with the bone marrow graft experiments, Stipan Jonjic for anti-MULT1 antibodies, Eric Vivier for anti-NKp46 antibodies, Y. Natanzon, L. Zhang and J. Beck for technical assistance, and T. Nice, M. Whang, F. Delebecque and S. Lehar for comments on the manuscript. This work was supported by grants from the National Institute of Health and a Prostate Cancer Foundation Award to D.H.R. N. Guerra was supported by a postdoctoral fellowship from the Cancer Research Institute.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient hemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Bakker ABH, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D, Müllberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, Held W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D-ligand expressing tumor cells. Blood. 2005 doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- Degrassi A, Russo M, Scanziani E, Giusti A, Ceruti R, Texido G, Pesenti E. Magnetic resonance imaging and histopathological characterization of prostate tumors in TRAMP mice as model for pre-clinical trials. The Prostate. 2007;67:396–404. doi: 10.1002/pros.20511. [DOI] [PubMed] [Google Scholar]
- Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nature Immunology. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Sola D, Ying CY, Grandori C, Ruggiero L, Chen B, Li M, Galloway DA, Gu W, Gautier J, Dalla-Favera R. Non-transcriptional control of DNA replication by c-Myc. Nature. 2007;448:445–451. doi: 10.1038/nature05953. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, Weller M, Friese MA. TGF-{beta} and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006 doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- Farag SS, George SL, Lee EJ, Baer M, Dodge RK, Becknell B, Fehniger TA, Silverman LR, Crawford J, Bloomfield CD, et al. Postremission therapy with low-dose interleukin 2 with or without intermediate pulse dose interleukin 2 therapy is well tolerated in elderly patients with acute myeloid leukemia: Cancer and Leukemia Group B study 9420. Clin Cancer Res. 2002;8:2812–2819. [PubMed] [Google Scholar]
- Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JR, barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochtonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;6:1–6. doi: 10.1038/sj.pcan.4500296. [DOI] [PubMed] [Google Scholar]
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr, Kastrinakis NG, Levy B, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Gourzi P, Leonova T, Papavasiliou FN. A Role for activation-induced cytidine deaminase in the host response against a transforming retrovirus. Immunity. 2006;24:779–786. doi: 10.1016/j.immuni.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100:9452–9457. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8 αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T- cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med. 1988;167:353–371. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer. 2006;118:684–687. doi: 10.1002/ijc.21382. [DOI] [PubMed] [Google Scholar]
- Huss WJ, Maddison LA, Greenberg NM. Autochthonous mouse models for prostate cancer: past, present and future. Semin Cancer Biol. 2001;11:245–260. doi: 10.1006/scbi.2001.0373. [DOI] [PubMed] [Google Scholar]
- Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2006;103:9190–9195. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH, Strong RK, Groh V, Spies T. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447:482–486. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. The Prostate. 2003;55:219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- Kim CG, Epner EM, Forrester WC, Groudine M. Inactivation of the human beta-globin gene by targeted insertion into the beta-globin locus control region. Genes Dev. 1992;6:928–938. doi: 10.1101/gad.6.6.928. [DOI] [PubMed] [Google Scholar]
- Langdon WY, Harris AW, Cory S, Adams JM. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986;47:11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 Secretion and Down-Modulation of NKG2D Underlies Impaired NK Cytotoxicity in Cancer Patients. J Immunol. 2004;172:7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- Lemckert FA, Sedgwick JD, Körner H. Gene targeting in C57BL/6 ES cells. Successful germ line transmission using recipient BALB/c blastocysts developmentally matured in vitro. Nucleic Acids Research. 1997;25:917–918. doi: 10.1093/nar/25.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodoen M, Ogasawara K, Hamerman JA, Arase H, Houchins JP, Mocarski ES, Lanier LL. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Zou Z, Joh T, Takihara Y, Matsuda Y, Shimada K. Genomic structures and characterization of Rae1 family members encoding GPI-anchored cell surface proteins and expressed predominantly in embryonic mouse brain. Journal of Biochemistry. 1996;120:987–995. doi: 10.1093/oxfordjournals.jbchem.a021517. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6:938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, Lanier LL. NKG2D Blockade Prevents Autoimmune Diabetes in NOD Mice. Immunity. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, Nanni M, Biassoni R, Bottino C, Moretta A, Moretta L. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. European Journal of Immunology. 2001;31:1076–1086. doi: 10.1002/1521-4141(200104)31:4<1076::aid-immu1076>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- Pham CTN, MacIvor DM, Hug BA, Heusel JW, Ley TJ. Long-range disruption of gene expression by a selectable marker cassette. Proc Natl Acad Sci USA. 1996;93:13090–130095. doi: 10.1073/pnas.93.23.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn RT. Relationship of tumor immunogenicity to concentration of the oncogen. J Natl Cancer Inst. 1975;55:189–190. doi: 10.1093/jnci/55.1.189. [DOI] [PubMed] [Google Scholar]
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- Reimann M, Loddenkemper C, Rudolph C, Schildhauer I, Teichmann B, Stein H, Schlegelberger B, Dorken B, Schmitt CA. The Myc-evoked DNA damage response accounts for treatment resistance in primary lymphomas in vivo. Blood. 2007;110:2996–3004. doi: 10.1182/blood-2007-02-075614. [DOI] [PubMed] [Google Scholar]
- Saez-Borderias A, Guma M, Angulo A, Bellosillo B, Pende D, Lopez-Botet M. Expression and function of NKG2D in CD4(+) T cells specific for human cytomegalovirus. Eur J Immunol. 2006;36:3198–3206. doi: 10.1002/eji.200636682. [DOI] [PubMed] [Google Scholar]
- Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003 doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFN gamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Siren J, Sareneva T, Pirhonen J, Strengell M, Veckman V, Julkunen I, Matikainen S. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J Gen Virol. 2004;85:2357–2364. doi: 10.1099/vir.0.80105-0. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Thia KYT, Street SEA, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. Journal of Experimental Medicine. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani Y, Suttie A, Flake GP, Nyska A, Maronpot RR. Epithelial-stromal tumor of the seminal vesicles in the transgenic adenocarcinoma mouse prostate model. Vet Pathol. 2005;42:306–314. doi: 10.1354/vp.42-3-306. [DOI] [PubMed] [Google Scholar]
- Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–1044. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- van den Broek M, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJM, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Davidson L, Alt FW, Baltimore D. Deletion of the Ig kappa light chain intronic enhancer/matrix attachment region impairs but does not abolish V kappa J kappa rearrangement. Immunity. 1996;4:377–385. doi: 10.1016/s1074-7613(00)80251-4. [DOI] [PubMed] [Google Scholar]
- Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 2006;66:5927–5933. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- Zhou H, Luo Y, Kaplan CD, Kruger JA, Lee SH, Xiang R, Reisfeld RA. A DNA-based cancer vaccine enhances lymphocyte cross talk by engaging the NKG2D receptor. Blood. 2006;107:3251–3257. doi: 10.1182/blood-2005-10-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. Beta 2-Microglobulin deficient mice lack CD4−8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Generation of NKG2D-deficient (Klrk1−/−) mice.
(A) Klrk1 gene-targeting strategy. The Klrk1 locus (top) is compared to the targeting vector (middle) and the targeted locus (bottom), showing the Klrk1 exons and the location of the 3′ probe. Successful targeting substitutes Klrk1 exons 1b to 6 with a neo cassette flanked by loxP sites. (B) Tail genomic DNA was digested with BamHI and Southern blots were hybridized with the 3′probe indicated in A. The 6.2 Kb and 4.1 Kb bands correspond to the wild type (WT) and knockout (KO) alleles of Klrk1, respectively, as indicated on the maps. The 2Kb band represents the Klrk1−/− allele (KO) after deletion of the neo cassette.
Supplementary Figure 2. Lymphoid development in NKG2D-deficient mice.
Representative staining of B and T cell populations on Klrk1+/+ and Klrk1−/− splenocytes. The mean percentages (±SD) of the indicated subsets are indicated (n=3–6). The data were obtained with Klrk1−/− mice with the neo cassette deleted and littermate controls.
Supplementary Figure 3. Analysis of NK subsets in NKG2D-deficient mice.
Histograms represent gated NK1.1+CD3− splenocytes from Klrk1−/− (neo cassette deleted) and Klrk1+/+ littermate controls (n=5–8). The shaded histograms represent the corresponding isotype control staining. The numbers correspond to mean percentages (±SD) of cells expressing the indicated markers.
Supplementary Figure 4. Representative histologic analysis of prostate tumors that develop in Klrk1−/− TRAMP (−/−) and Klrk1+/+ TRAMP mice (+/+). Prostate sections stained with Hematoxilin and Eosin were analysed and graded as followed: well differentiated (WD), and poorly differentiated (PD). Magnification 10X.