Abstract
Background
Our objective was to determine the extent to which geographical core areas for gonorrhea and syphilis are located in rural areas, as compared to urban areas.
Methods
Incident gonorrhea (January 1, 2005 to December 31, 2010) and syphilis (January 1, 1999 to December 31, 2010) rates were estimated and mapped by census tract and quarter. Rurality was measured using percent rural and rural-urban commuting area (RUCA; rural, small town, micropolitan, or urban). SaTScan was used to identify spatiotemporal clusters of significantly elevated rates of infection. Clusters lasting five years or longer were considered core areas; clusters of shorter duration were considered outbreaks. Clusters were overlaid on maps of rurality and qualitatively assessed for correlation.
Results
Twenty gonorrhea core areas were identified; 65% in urban centers, 25% in micropolitan areas, and the remaining 10% were geographically large capturing combinations of urban, micropolitan, small town and rural environments. Ten syphilis core areas were identified with 80% in urban centers and 20% capturing two or more RUCAs. All ten of the syphilis core areas (100%) overlapped with gonorrhea core areas.
Conclusions
Gonorrhea and syphilis rates were high for rural parts of North Carolina; however, no core areas were identified exclusively for small towns or rural areas. The main pathway of rural STI transmission may be through the interconnectedness of urban, micropolitan, small town and rural areas. Directly addressing STIs in urban and micropolitan communities may also indirectly help address STI rates in rural and small town communities.
Keywords: spatial epidemiology, core theory, rural health, sexually transmitted infections, cluster detection
INTRODUCTION
Over thirty years ago, Rothenberg extended the concept of core transmitters of sexually transmitted infections (STIs; (1)) to include geographical core areas (2). Since then, spatial analysis and mapping of STIs has been useful for defining and describing core areas of infection (3-13), providing insight into patterns of STI transmission (8, 9, 14-17), identifying sexual networks and interrupting syphilis transmission during outbreaks (18-21).
The concept of core areas has only been evaluated in large urban areas (3, 5, 6, 8, 9, 12, 17, 22). In contrast to other high incidence areas in the United States (US), STIs in the southeast are not concentrated in urban areas only. The STI epidemic in North Carolina, typical of the southeastern US, involves both rural and urban areas of the state. In North Carolina, six of the 15 counties with the highest syphilis rates are rural, five are micropolitan, and only four are metropolitan. The 15 counties with the highest gonorrhea rates include three rural, seven micropolitan, and five metropolitan counties. Many micropolitan and metropolitan counties also support large rural areas. Yet, the structure of STI epidemics in rural areas has not been thoroughly investigated, particularly with regard to the similarities and differences with STI epidemiology in urban areas.
The applicability of core theory and geographically definable core areas to rural areas is uncertain. Core areas reflect a spatial concentration of persons with STIs and have been identified in low socioeconomic status (SES) urban neighborhoods,(9, 11, 23-25) suggesting that socio-cultural determinants of health may influence the clustered spatial pattern observed for STIs.(26) In urban areas, several socio-cultural risk factors have been associated with gonorrhea(22, 27-31) and neighborhood-level socio-cultural factors, primarily those indicative of neighborhood deprivation, explained a significant proportion of the spatial pattern of gonorrhea across both urban and rural areas of NC.(26)
In urban environments, one of the primary hypotheses for the observation of core areas of transmission is that core group members live in high population density areas of similar socio-economic status and therefore, have an increased likelihood of forming sexual partnerships with each other or members of the same sexual networks, as evidenced by the closer distance between sexual partners living in core areas compared to outside core areas (17). In rural areas, distances between persons are greater and therefore, distances between sexual partners will also be greater. Whether core groups exist in rural areas is unclear. If core groups do exist in these areas, their role in driving infection in the community is unknown.
Our objective was to determine the extent to which geographical core areas for gonorrhea and syphilis are located in rural areas, as compared to urban areas. We hypothesized that core areas would be more prominent in urban areas and less prominent in rural areas, despite relatively high incidence rates in rural areas.
MATERIALS AND METHODS
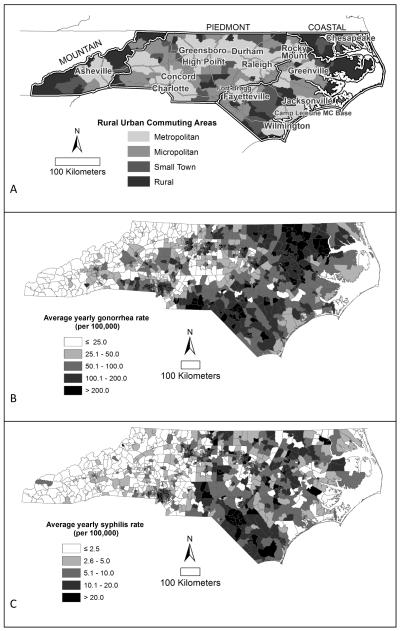
Our study area was the state of North Carolina, a rural state with nested urban areas. North Carolina can be divided into three geographic regions with distinct economic, social, and rural cultures (32): the western mountain region, the central piedmont, and the eastern coastal region (figure 1). Data were not collected specifically for this project, rather, we used rurality data provided by the US Census Bureau and the US Department of Agriculture and routinely collected STI surveillance data provided by the North Carolina Department of Health and Human Services, HIV/STD Prevention and Care Branch. The University of Toronto Research Ethics Board approved a spatial analysis of these data to assess the concept of core areas of transmission in rural and urban areas. Gonorrhea and syphilis (primary and secondary) incidence rates were mapped to understand the spatial distribution and patterns of infection. Incident gonorrhea and syphilis cases were used along with a population offset for Poisson based cluster detection.
Figure 1.
North Carolina a) rurality defined using rural-urban commuting areas (RUCAs), b) average gonorrhea rates (January 1, 2005 to December 31, 2010) and c) syphilis rates (January 1, 1999 to December 31, 2010) by census tract.
Data Sources
Measures of Rurality
Two common measures of rural for analysis are: 1) percent rural, derived from the percent of population urbanized (from the US Census Bureau, Population and Housing Characteristics, SF1-SF3), and 2) rural-urban commuting areas (RUCAs;www.ers.usda.gov/Data/RuralUrbanCommutingAreaCodes). We used RUCAs for our analysis and percent rural in a sensitivity analysis to determine if changing the definition of rural changed the interpretation of the results.
RUCAs classify the urban-rural spectrum based on population density, urbanization, and daily employment commuting distance (33). RUCAs can be particularly informative for health studies because they incorporate commuting distance for employment, which may be a good proxy for commuting distance to health services (26, 34), sexual partners, and access to resources. RUCAs can be broken down into 34 hierarchical classifications, of which we used four: rural, small town, micropolitan, and urban. Percent rural is also an important measure of rurality because it may give a more accurate representation of local environment and relative isolation.
Measures of STIs
We analyzed incident gonorrhea (January 1, 2005 to December 31, 2010) and primary and secondary syphilis (January 1, 1999 to December 31, 2010) cases geomasked (35, 36) to a location from the North Carolina State Health Department’s STI surveillance program. Data from the STI surveillance program is built on the legal requirement that physicians, other healthcare providers, and laboratories suspecting a case of syphilis or gonorrhea report the case to the local health authority. A communicable disease card (now done electronically) is completed for each suspected case of reportable infection. Each card contains limited information on the patient’s disease, report date, date of disease onset, residence, and healthcare provider at the time of diagnosis. Each card also contains limited information on demographic characteristics including gender, race, and age. Stage specific diagnoses are included with syphilis reports. Duplicate entries were removed from the database. Each county is responsible for forwarding their communicable disease cards to the state health department.
Incident gonorrhea and primary and secondary syphilis rates were estimated using census tract level population estimates for the total North Carolina population from the United States census. Rates were mapped by census tract and quarter.
Identifying Core Areas
SaTScan (37) was used to identify geographic clusters of significantly elevated gonorrhea and syphilis rates over space and time (8, 13, 38, 39). The number of cases and population at risk for each census tract was geo-located, or assigned, to the census tract centroid. Within SaTScan, a cylindrical scanning window centered on each centroid. The size of the cylinder varied continually with the circular base of the cylinder corresponding to the geographic area, and height of the cylinder corresponding to the length of time the cluster persisted. A likelihood ratio test statistic was calculated for each cylinder based on the expected and observed number of cases inside compared to outside of the cylinder. The expected number of cases under the null hypothesis was assumed to be Poisson distributed with constant risk over space and time. Monte Carlo simulation was used to determine the significance of each cluster(37, 40). A cluster with a relative rate of 2 can be interpreted as an area where the rate of infection was twice as high for those living in the cluster compared to those living outside of the cluster.
All years of data were used with a maximum cluster population size of 5% (appendix I). Statistically significant clusters were examined for persistence based on duration. Clusters lasting five consecutive years or longer were considered core areas, while clusters lasting less than five years were considered outbreak areas (8, 9). Individual level covariate case data were not available, therefore, we were not able to control for covariates during our analysis.
We qualitatively examined the spatial relationship between core areas and outbreak areas within and between diseases, and with rurality. We visually compared core and outbreak area maps for gonorrhea, syphilis, and both gonorrhea and syphilis together. Each cluster detection map was overlaid onto the RUCA map to determine where clusters occurred in relation to rural areas.
Sensitivity Analyses
We overlaid each cluster detection map with percent rural in a sensitivity analysis to determine if changing how rural was defined changed how the clustering patterns were interpreted. We were also concerned that low gonorrhea and syphilis rates in the western mountains might cause clusters in the mountains to be missed because high rates in the central piedmont and eastern coastal parts of the State might dominate the cluster detection process. Accordingly, we geographically stratified our analyses on mountain, piedmont and coastal regions in sensitivity analyses.
RESULTS
North Carolina is 37.7% metropolitan, 25.9% micropolitan, 14.3% small town, and 22.1% rural by geographic RUCA classification (figure 1), and 68.3% metropolitan, 19.4% micropolitan, 6.5% small town, and 5.8% rural by population. Overall, average gonorrhea incidence rates were low in the western mountains and highest in the rural parts of the eastern coastal region of the state (figure 1).
Gonorrhea Core Areas and Outbreaks
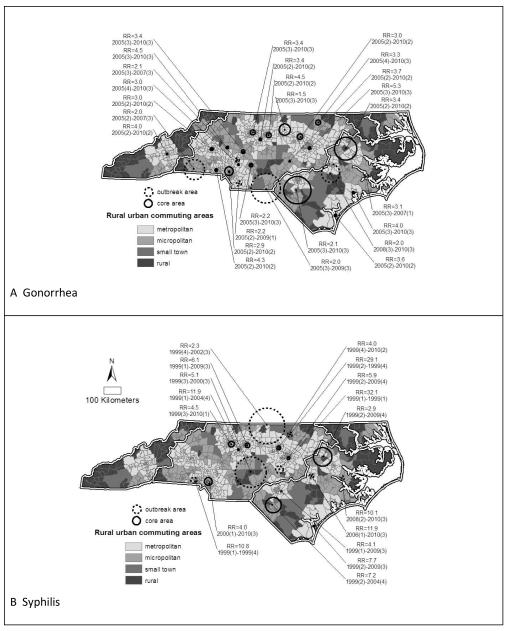
Several significant clusters of elevated gonorrhea rates were identified across North Carolina, with a higher concentration in the central piedmont region (figures 2). Twenty clusters met the criteria for core areas. Most gonorrhea core areas 65% (n=13) occurred in urban centers; 25% (n=5) were in micropolitan areas. The remaining core areas (n=2) were geographically large (1,160km2 and 1,192km2), capturing urban, micropolitan, small town and rural environments (figure 3). Both of these core areas were also much larger than core areas typically found in urban areas. No clusters occurred exclusively in small towns or rural areas.
Figure 2.
Significant clusters of high A) gonorrhea rates (2005 to 2010) and B) syphilis rates for North Carolina (1999 to 2010) overlaid on rural urban commuting areas (RUCAs).
Figure 3.
Gonorrhea (2005-2010) and syphilis (1999-2010) clusters overlaid with each other, RUCA (A), and percent rural (B).
Only six gonorrhea clusters were classified as outbreak areas, based on duration less than five years (figure 2). Two outbreak areas occurred in urban areas and one in a micropolitan area. The remainder (n=3) were geographically large outbreaks capturing combinations of rural, small town, micropolitan and urban areas (figure 2). The relative rates for core areas (median RR=3.4) were significantly greater than the relative rates for outbreak areas (median RR=2.1) of gonorrhea (p<0.01).
Syphilis Core Areas and Outbreaks
Overall, average syphilis rates were low in the western mountains and higher in the central piedmont and eastern parts of the state, with particularly high rates in the southern rural part of the state (figure 1).
Seventeen clusters of elevated syphilis rates were identified across North Carolina, concentrated predominantly in the central piedmont region (figures 2). Ten clusters met the criteria for core areas with 80% (n=8) in urban centers; 20% (n=2) were geographically large enough to capture combinations of rural, small town, micropolitan and urban areas. The large size and diversity of these remaining two core areas suggests the possibility of dynamic migratory outbreaks, rather than true core areas. Again, no core areas occurred exclusively in small towns or rural areas.
Seven syphilis clusters were identified as outbreaks (figure 2). Three outbreaks occurred in urban areas, one in a micropolitan area, one in a small town and two outbreaks were extremely large capturing combinations of rural, small town, micropolitan and urban areas (figure 2). No outbreaks occurred in rural areas. Though the relative rates for outbreak areas (median RR=10.1) were noticeably greater than the relative rates for core areas (median RR=5.3) of syphilis, the difference was not statistically significant.
Gonorrhea-Syphilis Comparison
Twice as many gonorrhea core areas (n=20) were identified compared to syphilis core areas (n=10). At the same time, all ten of the syphilis core areas (100%) overlapped with gonorrhea core areas (50%; figure 3). Seven of the ten overlapping core areas were in urban areas. The remaining three overlapping core areas included rural, small town, micropolitan and urban environments (figure 3). The remaining 10 gonorrhea core areas that did not have matching overlapping syphilis core areas, were located in the urban centers of otherwise rural areas (figures 3).
For the most part, gonorrhea and syphilis outbreaks did not co-exist (figure 3). In one case, the outer edge of a geographically large gonorrhea outbreak overlapped with a geographically large syphilis outbreak and in another case, the outer edge of a geographically large gonorrhea outbreak captured a small syphilis outbreak. However, in most cases, outbreak areas were adjacent to each other and did not touch.
The length of time clusters persisted for gonorrhea and syphilis was bimodal. That is, clusters were either short (3 consecutive years or less) or the longest (full) time period allowed by SaTScan (90% of time period: 5 consecutive years for gonorrhea and 10.5 consecutive years for syphilis). These two time periods (3 years or less, or the full time period) accounted for 85% of gonorrhea clusters and 82% of syphilis clusters. Changing the threshold for outbreaks and core areas anywhere between 3 and 10 years did not significantly change the results for syphilis. Lowering the threshold from five years to four or three years does not significantly change the core area results for gonorrhea but does decrease the number of outbreaks by half. We could not see the effect of raising the threshold for gonorrhea because there were only six years of data available and SaTSCan allows a maximium time period of 90% of the total time available for a given cluster.
Sensitivity Analyses
We qualitatively assessed the spatial correlation between gonorrhea clusters and rurality by overlaying the disease clusters on percent rural instead of RUCA and found that the core areas for both gonorrhea and syphilis still occurred primarily in urban (less than 20% rural) areas, with the exception of two geographically large, overlapping core areas that captured combinations of rural, small town, micropolitan and urban areas (figure 3).
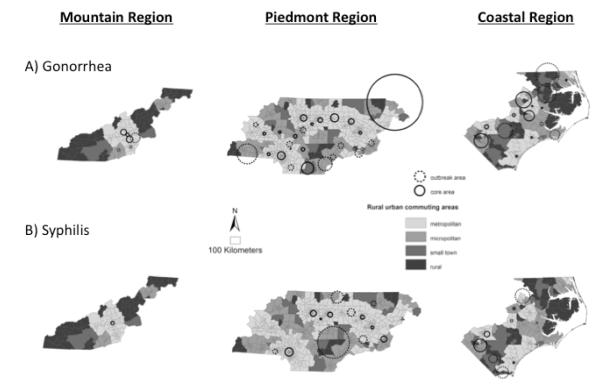
As expected, a greater number of statistically significant clusters were identified for both STIs when the analyses were stratified by region (figure 4). However, despite stratification, no clusters were identifiable in rural areas. Although no syphilis clusters were identified in the mountain region prior to stratification (figure 3), one cluster was identified in an urban area of the mountain region after stratification (figure 4).
Figure 4.
A) Gonorrhea (top row) and B) Syphilis (bottom row) clusters detected when North Carolina is stratified into Mountain, Piedmont and Coastal socio-geographic regions.
DISCUSSION
Gonorrhea and syphilis rates were high for rural parts of North Carolina (figure 1); however, the majority of core areas for both gonorrhea and syphilis still occurred in urban areas (figures 2-4). No core areas occurred exclusively in small towns or rural areas. When core areas included rural areas and small towns, they were large enough to include neighboring micropolitan and urban environments. These observations independently, and by different analytic methods, support previous findings that there can be bridging of infection from core areas to other areas by travel (16).
We hypothesize that the communities of rural North Carolina are too small and isolated for STI epidemics to persist at endemic levels and thus create core areas. As a result, the main pathway of STI transmission may be through the interconnectedness of urban, micropolitan, small town and rural areas. Core groups may exist in rural areas, with sexual network dynamics conducive to STI transmission, but this core group on its own may not be big enough to act as a reservoir of persistent infection. Consequently, infection in rural areas may be driven by core group connections to high rate core areas in nearby urban centers, micropolitans or small towns or combinations thereof.
For North Carolina, urban core areas may have a more continuous magnifying effect on rural rates in the coastal region, but a discontinuous or intermittent effect on rural rates in the mountain region. This is particularly evident in the mountains where rural rates are low even though urban cores areas were identified in the region (figures 4). RUCA classification considers rural-urban travel commuting times, however, the difference in the rural-urban connection between mountain and coastal regions is likely influenced by additional factors. For instance, the mountain region may have a more centralized urban-rural connectivity, where the mountain topography causes each rural area to be primarily connected to only one “down valley” urban core area, while in the coastal region, the flat coastal topography may facilitate rural areas being connected to multiple surrounding urban core areas. A public health implication of this finding is that controlling STIs in one vs. multiple urban core areas may need to be a critical consideration to reducing the high STI rates, especially in rural areas that are highly connected to multiple surrounding urban areas, such as in the coastal region of North Carolina.
We had 12 years of syphilis data (1999-2010) and only six years of gonorrhea data (2005-2010). We used five consecutive years as the threshold for differentiating core from outbreak for both syphilis and gonorrhea, based on previous syphilis studies in urban areas(8, 9) and given the lack of studies on gonorrhea appropriate thresholds. We still found twice as many gonorrhea core areas as syphilis core areas, despite the shorter duration of gonorrhea data and syphilis-based thresholds. The greater number of gonorrhea core areas may be due to the higher and more widespread incidence and prevalence of gonorrhea compared to syphilis.
It is still questionable whether or not five years is the optimum threshold for differentiating core areas from outbreaks for gonorrhea. Gonorrhea is a faster cycling disease than syphilis with a shorter time to reinfection, which may mean that the five-year threshold is too long for gonorrhea. At the same time, we found more core areas than outbreaks using the five-year threshold for gonorrhea.
All of the syphilis core areas fell within, or overlapped with, gonorrhea core areas. At the same time, we know of at least one syphilis outbreak of long duration (41) that met the criteria for core and so may be considered misclassified. This observation demonstrates the difficultly that can occur when differentiating outbreak areas from core areas.
Another significant limitation to our approach to classifying core versus outbreak clusters is that it is not possible to identify outbreaks that occur within core areas. That is, a core and an outbreak cannot be identified for the same place using the SaTScan detection method. The only way an outbreak can be seen within a core is if the analysis is limited to the boundaries of a core area. Two alternative methods would be to compliment the cluster detection and core/outbreak analysis with a sexual network analysis(41) or an analysis of the rate of change within core areas.
In a previous study, we compared the characteristics of clusters on a very local level (8) and found that the characteristics of cases from the core were very different from outbreak areas but very similar to non-core and non-outbreak areas. Ideally, one would conduct a similar analysis and characterize the socio-cultural demographics of core and outbreak clusters identified across North Carolina and then look for socio-demographic and correlations that could lend insight and context into the observed spatial patterns. However, the limits of surveillance data, combined with North Carolina State Health Department methods to protect case confidentiality, severely limited the gonorrhea and syphilis data we received so we could not characterize clusters. We could summarize the neighborhood characteristics of core areas, in aggregate for the state, however, we would be at high risk of falling into the trap of ecologic fallacy without the individual level case characteristics to provide context and aid interpretation.
Our methods may be of use in routine surveillance and outbreak detection of both gonorrhea and syphilis infections and may help focus more targeted surveillance, contact tracing and cases follow-up. Modern data analysis packages are being used to write programs that import surveillance data into SaTScan and routinely run spatial and temporal analyses in order to detect outbreaks regularly, for instance, every month or every quarter, for other infectious diseases already(42).
Routine spatiotemporal surveillance and analysis is particularly important for sexually transmitted infections as the core areas we identified for gonorrhea and syphilis are not isolated, single jurisdiction pockets of infection. Rather, they involve rural-urban communities, therefore requiring synchronized management to address infections, which can bounce back and forth between cases of different jurisdictions (ping-pong infections). Multi-jurisdiction coordination may be particularly important for both ‘old’ infections, such as syphilis(43), and “new” infections, such as lymphogranuloma venereum(44), where infection is maintained and controlled in an urban area, but when combined with the long distances between source contacts and cases for these infections, these previously well controlled infections may rise and spread to new and naïve areas.
From a rural public health practice perspective, even if a core group of transmitters exists locally, STI rates in the community may still be largely driven by the interconnectedness of the sexual network to, and STI rates in, surrounding urban and micropolitan areas. From an urban public health practice perspective, STI dynamics in core areas may be impacting STI rates both locally and regionally; influencing, maybe even driving, STI rates in neighbouring rural and small town communities. Consequently, directly addressing STIs in urban and micropolitan communities may also indirectly help address STI rates in rural and small town communities, presenting opportunities for mutually beneficial collaborations between communities.
The general absence of core areas of infection for gonorrhea and syphilis in rural environments supports the hypothesis of interconnected sexual networks between rural, micropolitan and urban core groups. Consequently, bridging of infection from adjacent, peripheral or distant core areas by travel of core group members may be an important mode of STI entry and transmission for rural areas. Interjurisdiction coordination of STI prevention and treatment activities may be necessary to control STIs in rural environments.
ACKNOWLEDGEMENTS
We would like to thank the North Carolina Department of Public Health for their collaboration on this project, as well as Garnet Bass from the North Carolina Rural Economic Development Center and the Rural Health Research Program at the Sheps Center in North Carolina.
This work was supported by the National Institute of Allergy and Infectious Diseases (R01 AI067913)
Funding: National Institute of Allergy and Infectious Diseases (R01 AI067913)
Appendix I. SaTScan parameterization for cluster detection
Time precision: yearly. Note: we wanted the analysis by quarter so each quarter was assigned a year before bringing the data into SaTScan.
Type of analysis: Retrospective, space-time
Probability model: Poisson
Scan for areas with: high rates
Time aggregation: quarter
Length: 1 quarter
MC replications: 999
Advanced analysis features:
Spatial window
- Maximum spatial cluster size: 5%
- Spatial window shape: circular
- Temporal window: 90% of the study period (max allowed)
Footnotes
Conflict of Interest: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dionne C Gesink, Dalla Lana School of Public Health, University of Toronto.
Ashleigh B Sullivan, Dalla Lana School of Public Health University of Toronto.
Todd Norwood, Cancer Care Ontario.
Marc L Serre, Department of Environmental Science and Engineering, University of North Carolina at Chapel Hill.
William C Miller, Department of Epidemiology, University of North Carolina at Chapel Hill.
REFERENCES
- 1.Yorke JA, Hethcote HW, Nold A. Dynamics and control of the transmission of gonorrhea. Sex Transm Dis. 1978;5(2):51–6. doi: 10.1097/00007435-197804000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg RB. The geography of gonorrhea. Empirical demonstration of core group transmission. Am J Epidemiol. 1983;117(6):688–94. doi: 10.1093/oxfordjournals.aje.a113602. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Dardet C, Marquez S, Perea EJ. Urban clusters of sexually transmitted diseases in the city of Seville, Spain. Sex Transm Dis. 1985;12(3):166–8. doi: 10.1097/00007435-198507000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Becker KM, Glass GE, Brathwaite W, et al. Geographic epidemiology of gonorrhea in Baltimore, Maryland, using a geographic information system. Am J Epidemiol. 1998;147(7):709–16. doi: 10.1093/oxfordjournals.aje.a009513. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein KT, Curriero FC, Jennings JM, et al. Defining core gonorrhea transmission utilizing spatial data. Am J Epidemiol. 2004;160(1):51–8. doi: 10.1093/aje/kwh178. [DOI] [PubMed] [Google Scholar]
- 6.Bush KR, Henderson EA, Dunn J, et al. Mapping the core: chlamydia and gonorrhea infections in Calgary, Alberta. Sex Transm Dis. 2008;35(3):291–7. doi: 10.1097/OLQ.0b013e31815c1edb. [DOI] [PubMed] [Google Scholar]
- 7.Ellen JM, Hessol NA, Kohn RP, et al. An investigation of geographic clustering of repeat cases of gonorrhea and chlamydial infection in San Francisco, 1989-1993: evidence for core groups. J Infect Dis. 1997;175(6):1519–22. doi: 10.1086/516491. [DOI] [PubMed] [Google Scholar]
- 8.Gesink DC, Sullivan AB, Miller WC, et al. Sexually transmitted disease core theory: roles of person, place, and time. American Journal of Epidemiology. 2011;174(1):81–9. doi: 10.1093/aje/kwr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gesink Law DC, Bernstein KT, Serre ML, et al. Modeling a syphilis outbreak through space and time using the Bayesian maximum entropy approach. Ann Epidemiol. 2006;16(11):797–804. doi: 10.1016/j.annepidem.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Jolly AM, Wylie JL. Gonorrhoea and chlamydia core groups and sexual networks in Manitoba. Sex Transm Infect. 2002;78(Suppl 1):i145–51. doi: 10.1136/sti.78.suppl_1.i145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law DC, Serre ML, Christakos G, et al. Spatial analysis and mapping of sexually transmitted diseases to optimise intervention and prevention strategies. Sex Transm Infect. 2004;80(4):294–9. doi: 10.1136/sti.2003.006700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahmanesh M, Gayed S, Ashcroft M, et al. Geomapping of chlamydia and gonorrhoea in Birmingham. Sex Transm Infect. 2000;76(4):268–72. doi: 10.1136/sti.76.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schleihauf E, Watkins RE, Plant AJ. Heterogeneity in the spatial distribution of bacterial sexually transmitted infections. Sexually Transmitted Infections. 2009;85(1):45–9. doi: 10.1136/sti.2008.030197. [DOI] [PubMed] [Google Scholar]
- 14.Gindi RM, Sifakis F, Sherman SG, et al. The Geography of Heterosexual Partnerships in Baltimore City Adults. Sex Transm Dis. doi: 10.1097/OLQ.0b013e3181f7d7f4. [DOI] [PubMed] [Google Scholar]
- 15.Sobela F, Pepin J, Gbeleou S, et al. A tale of two countries: HIV among core groups in Togo. J Acquir Immune Defic Syndr. 2009;51(2):216–23. doi: 10.1097/QAI.0b013e31819c170f. [DOI] [PubMed] [Google Scholar]
- 16.Wylie JL, Jolly A. Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex Transm Dis. 2001;28(1):14–24. doi: 10.1097/00007435-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Zenilman JM, Ellish N, Fresia A, et al. The geography of sexual partnerships in Baltimore: applications of core theory dynamics using a geographic information system. Sex Transm Dis. 1999;26(2):75–81. doi: 10.1097/00007435-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Michaud JM, Ellen J, Johnson SM, et al. Responding to a community outbreak of syphilis by targeting sex partner meeting location: an example of a risk-space intervention. Sex Transm Dis. 2003;30(7):533–8. doi: 10.1097/00007435-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Ogilvie GS, Taylor DL, Moniruzzaman A, et al. A population-based study of infectious syphilis rediagnosis in British Columbia, 1995-2005. Clinical Infectious Diseases. 2009;48(11):1554–8. doi: 10.1086/598997. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg D, Moseley K, Kahn R, et al. Networks of persons with syphilis and at risk for syphilis in Louisiana: evidence of core transmitters. Sex Transm Dis. 1999;26(2):108–14. doi: 10.1097/00007435-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Sena AC, Muth SQ, Heffelfinger JD, et al. Factors and the sociosexual network associated with a syphilis outbreak in rural North Carolina. Sex Transm Dis. 2007;34(5):280–7. doi: 10.1097/01.olq.0000237776.15870.c3. [DOI] [PubMed] [Google Scholar]
- 22.Jennings JM, Curriero FC, Celentano D, et al. Geographic identification of high gonorrhea transmission areas in Baltimore, Maryland. Am J Epidemiol. 2005;161(1):73–80. doi: 10.1093/aje/kwi012. [DOI] [PubMed] [Google Scholar]
- 23.Lacey CJ, Merrick DW, Bensley DC, et al. Analysis of the sociodemography of gonorrhoea in Leeds, 1989-93. BMJ. 1997;314(7096):1715–8. doi: 10.1136/bmj.314.7096.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potterat JJ, Rothenberg RB, Woodhouse DE, et al. Gonorrhea as a social disease. Sex Transm Dis. 1985;12(1):25–32. doi: 10.1097/00007435-198501000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Rice RJ, Roberts PL, Handsfield HH, et al. Sociodemographic distribution of gonorrhea incidence: implications for prevention and behavioral research. Am J Public Health. 1991;81(10):1252–8. doi: 10.2105/ajph.81.10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan AB, Gesink DC, Brown P, et al. Are neighborhood sociocultural factors influencing the spatial pattern of gonorrhea in North Carolina? Annals of epidemiology. 2011;21(4):245–52. doi: 10.1016/j.annepidem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191(Suppl 1):S115–22. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 28.Browning CR, Burrington LA, Leventhal T, et al. Neighborhood structural inequality, collective efficacy, and sexual risk behavior among urban youth. J Health Soc Behav. 2008;49(3):269–85. doi: 10.1177/002214650804900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtgrave DR, Crosby RA. Social capital, poverty, and income inequality as predictors of gonorrhoea, syphilis, chlamydia and AIDS case rates in the United States. Sex Transm Infect. 2003;79(1):62–4. doi: 10.1136/sti.79.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilmarx PH, Zaidi AA, Thomas JC, et al. Sociodemographic factors and the variation in syphilis rates among US counties, 1984 through 1993: an ecological analysis. Am J Public Health. 1997;87(12):1937–43. doi: 10.2105/ajph.87.12.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semaan S, Sternberg M, Zaidi A, et al. Social capital and rates of gonorrhea and syphilis in the United States: spatial regression analyses of state-level associations. Soc Sci Med. 2007;64(11):2324–41. doi: 10.1016/j.socscimed.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 32.North Carolina Agriculture Regional Perspectives. North Carolina Rural Economic Development Center; 2009. p. 28. [Google Scholar]
- 33.Agriculture USDo [Accessed August 2 2011];Measuring Rurality: Rural-Urban Commuting Area Codes 2005. http://www.ers.usda.gov/briefing/rurality/ruralurbancommutingareas/
- 34.Hall SA, Kaufman JS, Ricketts TC. Defining urban and rural areas in U.S. epidemiologic studies. Journal of urban health : bulletin of the New York Academy of Medicine. 2006;83(2):162–75. doi: 10.1007/s11524-005-9016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allshouse WB, Fitch MK, Hampton KH, et al. Geomasking sensitive health data and privacy protection: an evaluation using an E911 database. Geocarto Int. 2010;25(6):443–52. doi: 10.1080/10106049.2010.496496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hampton KH, Fitch MK, Allshouse WB, et al. Mapping health data: improved privacy protection with donut method geomasking. Am J Epidemiol. 2010;172(9):1062–9. doi: 10.1093/aje/kwq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulldorff M. A spatial scan statistic. Communications in Statistics: Theory and Methods. 1997;26:1481–96. doi: 10.1080/03610927708831932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aamodt G, Samuelsen SO, Skrondal A. A simulation study of three methods for detecting disease clusters. International journal of health geographics. 2006;5:15. doi: 10.1186/1476-072X-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song C, Kulldorff M. Power evaluation of disease clustering tests. International journal of health geographics. 2003;2(1):9. doi: 10.1186/1476-072X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulldorff M, Athas WF, Feurer EJ, et al. Evaluating cluster alarms: a space-time scan statistic and brain cancer in Los Alamos, New Mexico. American Journal of Public Health. 1998;88(9):1377–80. doi: 10.2105/ajph.88.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doherty IA, Adimora AA, Muth SQ, et al. Comparison of sexual mixing patterns for syphilis in endemic and outbreak settings. Sexually Transmitted Diseases. 2011;38(5):378–84. doi: 10.1097/OLQ.0b013e318203e2ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Public Health Ontario . Monthly Infectious Diseases Surveillance Report. Toronto: 2012. [Google Scholar]
- 43.Torrone EA, Bertolli J, Li J, et al. Increased HIV and primary and secondary syphilis diagnoses among young men--United States, 2004-2008. Journal of acquired immune deficiency syndromes. 2011;58(3):328–35. doi: 10.1097/QAI.0b013e31822e1075. [DOI] [PubMed] [Google Scholar]
- 44.Richardson D, Goldmeier D. Lymphogranuloma venereum: an emerging cause of proctitis in men who have sex with men. International journal of STD & AIDS. 2007;18(1):11–4. doi: 10.1258/095646207779949916. quiz 5. [DOI] [PubMed] [Google Scholar]