Abstract
Chronic alcohol use disorders (AUD) have been shown to interact with normal age-related volume loss to exacerbate brain atrophy with increasing age. However, chronic cigarette smoking, a highly comorbid condition in AUD, and its influence on age-related brain atrophy has not been evaluated. We performed 1.5T quantitative MRI in non-smoking controls (nsCON; n=54), smoking light drinking controls (sCON, n=34), and 1-week-abstinent, treatment-seeking non-smoking alcohol dependent individuals (nsALC, n=35) and smoking ALC (sALC, n=43), to evaluate the independent and interactive effects of alcohol dependence and chronic smoking on regional cortical and subcortical brain volumes, emphasizing the brain reward/executive oversight system (BREOS),. nsCON and sALC showed greater age-related volume losses than nsALC in the dorsal prefrontal cortex (DPFC), total cortical BREOS, superior parietal lobule and putamen. nsALC and sALC demonstrated smaller volumes than nsCON in most cortical ROIs. sCON had smaller volumes than nsCON in the DPFC, insula, inferior parietal lobule, temporal pole/parahippocampal region and all global cortical measures. nsALC and sALC had smaller volumes than sCON in the DPFC, superior temporal gyrus, inferior and superior parietal lobules, precuneus and all global cortical measures. Volume differences between nsALC and sALC were observed only in the putamen. Alcohol consumption measures were not related to volumes in any ROI for ALC; smoking severity measures were related to corpus callosum volume in sCON and sALC. The findings indicate that consideration of smoking status is necessary for a better understanding of the factors contributing to regional brain atrophy in AUD.
Keywords: Brain volume, magnetic resonance imaging, cigarette smoking, alcohol dependence, brain reward system
INTRODUCTION
Regional gray matter (GM) and white matter (WM) volume loss (i.e., atrophy) associated with alcohol use disorders (i.e., alcohol abuse or dependence; AUD) is one of the most consistently replicated findings in magnetic resonance-based morphometric studies [see (Buhler and Mann, 2011; Durazzo and Meyerhoff, 2007) for review]. Magnetic resonance (MR) findings suggest that the clinical syndrome of AUD places a burden on the morphological integrity of brain tissue that results in accumulated injury over time. Specifically, AUD over time has been shown to interact with normal age-related GM and WM volume loss to exacerbate brain atrophy with increasing age. Pfefferbaum and colleagues observed greater age-related atrophy in the prefrontal, frontotemporal, parietal, parieto-occipital, and total cortical GM in treatment-seeking AUD relative to controls (Pfefferbaum et al., 1992). In a follow-up analysis of the same cohort (Pfefferbaum et al., 1997) participants were divided into younger (38 ± 5 years of age) and older (53 ± 6 years of age) groups, with comparable age divisions for controls. The older AUD cohort demonstrated significantly smaller cortical GM volume than the younger AUD cohort only in prefrontal regions, and the largest magnitude of cortical volume loss in older AUD participants relative to older controls was confined to the prefrontal GM. In a treatment-naïve AUD cohort, Fein et al. (Fein et al., 2010) reported greater age-related volume loss in the AUD cohort (19–50 years of age) relative to age-equivalent controls in multiple subregions of the prefrontal, temporal and parietal cortices as well as the global cortical GM. Taken together, AUD may interact with normal GM and WM volume loss over lifetime to exacerbate age-related atrophic changes, with the most prominent atrophy apparent in the anterior frontal subregions.
The volumetric and other neurobiological abnormalities observed in AUD are likely related to a complex interplay among age, premorbid/genetic factors, comorbid disorders, severities of alcohol and other substance use/abuse and environmental conditions (Durazzo and Meyerhoff, 2007; Meyerhoff and Durazzo, 2008; Tessner and Hill, 2010). Consideration of premorbid and/or comorbid participant characteristics and conditions is necessary to better understand the factors contributing to the substantial variability in the type and magnitude of morphological and neurocognitive abnormalities exhibited in AUD across the lifespan. Chronic cigarette smoking is the most common comorbidity in AUD, with a prevalence between 60 to 90 percent (Durazzo and Meyerhoff, 2007; Room, 2004). Chronic smoking was associated with morphological abnormalities (e.g., cortical thinning, cortical atrophy, WM microstructural abnormalities) in anterior frontal brain regions, in studies with AUD and non-alcohol/substance abusing samples (Durazzo and Meyerhoff, 2007; Durazzo et al., 2010a; Durazzo et al., 2011a; Liao et al., 2011; Liao et al., 2010; Zhang et al., 2011). Additionally, in MR studies with healthy elderly cohorts, chronic smoking during lifetime was associated with the lower GM density of the posterior cingulate and precuneus (Almeida et al., 2008), and greater volume loss over a 2-year interval in the posterior cingulate region, inferior parietal lobule, parahippocampal gyrus, fusiform gyrus, lingual gyrus, precuneus and middle temporal gyrus (Durazzo et al., In Press). Thus, in elderly individuals, chronic smoking appears to be associated with abnormalities in regions that also exhibit morphological abnormalities in the incipient stages of Alzheimer’s disease (AD) (Krueger et al., 2010). Correspondingly, a history of chronic cigarette smoking, particularly heavy smoking (> 1 pack/day) during middle age, is strongly linked to significantly increased risk for development of AD (Cataldo and Glantz, 2010; Rusanen et al., 2010). However, since chronic cigarette smoking was not considered in previous MR studies reporting greater age-related atrophy reported in AUD, despite its high comorbidity with AUD, it is unknown if smoking influenced the level of atrophy observed.
Emerging clinical neuroscience research strongly suggests the development and persistence of AUD and other substance use disorders involves neurobiological abnormalities that are largely localized to specific subregions of the frontal and mesial temporal lobes, limbic system and striatum, collectively referred to here as the “brain reward/extended oversight system” (BREOS) (Haber and Knutson, 2010; Makris et al., 2008a). The transition from maladaptive use to chronic abuse/dependence (the latter typifying those seeking AUD treatment) is associated with enduring, plastic changes in neuronal and glial tissue in cortical regions of the BREOS that subserve “top-down” inhibitory control/executive functions (George and Koob, 2010; Volkow et al., 2011b). Compromised integrity of top-down regulatory BREOS components is linked to impaired regulation of dorsal and ventral striatum and limbic regions involved in evaluation of reward/saliency, motivation/drive, conditioning/habits and emotional expression (George and Koob, 2010; Heatherton and Wagner, 2011). Impaired BREOS function is also related to dysfunction in traditional neurocognitive abilities involving executive functions, learning and memory, working memory, processing speed and visuospatial skills (Durazzo and Meyerhoff, 2007; Gazzaley and D’Esposito, 2007). Top-down cortical BREOS components are composed of heteromodal association and paralimbic cortex and include the anterior cingulate cortex (ACC), dorsal/dorsolateral prefrontal cortex (DPFC), orbitofrontal cortex (OFC), and insula (Baler and Volkow, 2006; Makris et al., 2008b; Volkow et al., 2011a). The top-down BREOS regions and their connectivity with other cortical/subcortical nuclei constitute the neurobiological substrate that enables adaptive goal-related behavior (Crews and Boettiger, 2009; Durazzo et al., 2010b; Potenza et al., 2011). These top-down cortical BREOS components overlap considerably with GM regions that showed significant age-related atrophy in previous MR studies of AUD.
In recent analyses, we compared morphometrics of cortical and subcortical BREOS components in 1-week-abstinent ALC who later relapsed versus those who maintained abstinence for approximately one year after treatment (Durazzo et al., 2011b). In that study, both ALC groups showed smaller volumes of BREOS components and total cortical GM than non-smoking light drinking controls. However, we did not specifically consider the influence of smoking status, evaluate for age-related atrophy, include a group of smoking controls, or conduct a full whole brain region-of-interest (ROI) analysis. Therefore, the primary goal of this study was to determine the influence of chronic smoking on age-related atrophy in treatment-seeking ALC in early recovery (i.e., at 7 ± 4 days of abstinence). We compared four groups on regional cortical and subcortical GM volumes: non-smoking light drinking controls (nsCON), smoking light drinking controls (sCON), non-smoking ALC (nsALC) and smoking ALC (sALC) to evaluate the independent/main and interactive effects of AUD and chronic smoking on regional brain structure.
We hypothesized that chronic cigarette smoking, similar to chronic excessive alcohol consumption, adds a significant burden to the structural integrity of the brain parenchyma in ALC and CON over time. We predicted groups would be ranked with respect to the magnitude of age-related volume losses (i.e., the degree of the slopes of age regressed on volume) as follows: sALC > nsALC > sCON > nsCON. We expected the greatest age-related volume loss localized to top-down BREOS regions (DPFC, OFC, ACC and insula). Volumes of these regions were expected to follow the same group order.
MATERIALS AND METHODS
Participants
Thirty-five nsALC (five females) and 43 sALC (four females), between 28 and 71 years of age, were recruited from VA Medical Center Substance Abuse Day Hospital and the Kaiser Permanente Chemical Dependence Recovery Program in San Francisco. Participants provided written informed consent prior to study according to the Declaration of Helsinki and underwent procedures that were approved by the University of California San Francisco and the San Francisco VA Medical Center. Primary inclusion criteria for ALC participants were fluency in English, DSM-IV diagnosis of alcohol dependence or alcohol abuse at the time of enrollment, consumption of greater than 150 standard alcohol-containing drinks (i.e., 13.6 grams of ethanol/drink) per month for at least 8 years prior to enrollment for men, or consumption of greater than 80 drinks/month for at least 6 years prior to enrollment for women. The alcohol dependent participants were involved in outpatient treatment at the time of assessment. Fifty-four nsCON (six females) and 34 sCON (four females) were recruited from the local community. All nsCON smoked less than 20 cigarettes during lifetime and no smoking within 10 years of study. CON were fluent in English and had no history of any DSM-IV Axis I disorder or biomedical condition that may have adversely affected brain neurobiology (see Table 1).
Table 1.
Participant Demographics, Clinical and Laboratory Measures
| Measure | nsCON (n = 54) | sCON (n = 34) | nsALC (n = 35) | sALC (n = 43) |
|---|---|---|---|---|
| Agea | 46.3 (8.5) | 47.7 (9.4) | 52.3 (10.0) | 49.0 (8.8) |
| Educationb | 16.0 (2.3) | 15.0 (1.9) | 14.3 (2.3) | 13.8 (2.0) |
| % Caucasian | 74 | 72 | 77 | 75 |
| % Male | 89 | 88 | 86 | 93 |
| 1-year average drinks/monthc | 13 (15) | 23 (24) | 325 (182) | 401 (150) |
| Lifetime average drinks/monthd | 15 (13) | 28 (16) | 164 (106) | 275 (151) |
| Fagerstrom Test for Nicotine Dependence | NA | 5.1 (1.5) | NA | 5.1 (2.0) |
| Total cigarettes/day | NA | 19 (8) | NA | 21 (6) |
| Total lifetime years smoking | NA | 24 (12) | NA | 27 (11) |
| % with medical comorbidity | NA | NA | 51 | 55 |
| % with psychiatric comorbidity | NA | NA | 43 | 40 |
| % with substance comorbidity | NA | NA | 23 | 17 |
| Body mass index e | 25.6 (4.3) | 26.3 (3.8) | 29.2 (5.6) | 25.8 (3.8) |
| Prealbumin (mg/dl) | 32.0 (1.5) | N/A | 28.0 (1.4) | 28.4 (1.2) |
| Aspartate aminotransferase (i.u.)f | 24.7 (13.6) | N/A | 38.8 (28.5) | 38.2 (21.1) |
| Alanine aminotransferase (i.u.)g | 29.3 (17.5) | N/A | 48.9 (36.0) | 41.3 (27.6) |
| Gamma glutamyltransferase (i.u.)h | 26.1 (6.4) | N/A | 88.0 (59.7) | 82.1 (55.6) |
Note: Mean (standard deviation). NA: not applicable. N/A: not available. i.u.: institutional unit.
nsALC > sALC, sCON, nsCON.
nsCON > sCON, nsALC, sALC.
nsALC, sALC > nsCON, sCON; sALC > nsALC.
nsALC, sALC > nsCON, sCON; sALC > nsALC.
nsALC> nsCON, sALC.
nsALC, sALC > nsCON.
nsALC, sALC > nsCON.
nsALC, sALC > nsCON (p <.05 for all comparisons).
Exclusion criteria for ALC participants were history of the following: dependence on any substance other than alcohol or nicotine in the 5 years immediately prior to enrollment, any intravenous drug use in the 5 years immediately prior to study enrollment, current opioid agonist therapy, intrinsic cerebral masses or vascular malformations, HIV/AIDS, cerebrovascular accident, myocardial infarction, uncontrolled chronic hypertension, type-1 diabetes or insulin dependent conditions, moderate or severe COPD, non-alcohol related seizures, significant exposure to known neurotoxins, demyelinating and neurodegenerative diseases, documented Wernicke-Korsakoff syndrome, alcohol-induced persisting dementia, any penetrating head trauma, and closed head injury with loss of consciousness > 10 minutes. Exclusion criteria also included history of schizophrenia-spectrum disorders, bipolar disorder, PTSD, obsessive-compulsive disorder, and panic disorder. Hepatitis C, type-2 diabetes, hypertension, and unipolar mood disorder (major depression and/or substance-induced mood disorder) were permitted in the ALC cohort given their high prevalence in AUD (Mertens et al., 2003; Stinson et al., 2005). For ALC, approximately 90% of participants inclusion/exclusion criteria were verified via electronic medical records and the remaining 10% by self-report. No participant tested positive for illicit substances (cannabinoids, cocaine, amphetamines, opioids or PCP) before assessment.
MR and Clinical Assessment
For all ALC participants, MR procedures were conducted approximately 7 ± 4 days after their last alcoholic drink. MR procedures were typically completed within 48 hours of the clinical assessments for all participants.
Clinical Measures
All ALC participants completed the Structured Clinical Interview for DSM-IV Axis I Disorders, Version 2.0 and CON were administered the accompanying screening module. Semi-structured interviews for lifetime alcohol consumption (Lifetime Drinking History; LDH) and substance use (in-house questionnaire assessing substance type, and quantity and frequency of use)] were administered to all participants. From the LDH, average number of alcoholic drinks per month over 1 year prior to enrollment, average number of drinks per month over lifetime, duration of heavy drinking (i.e., drinking more than 100 drinks per month in males and 80 drinks per month in females) were calculated. Premorbid verbal intelligence was estimated with the American National Adult Reading Test (AMNART). Participants also completed standardized questionnaires assessing depressive Beck Depression Inventory (BDI) and anxiety symptomatology [State-Trait Anxiety Inventory, form Y-2, STAI], and nicotine dependence via the Fagerstrom Tolerance Test for Nicotine Dependence (FTND) (see (Durazzo et al., 2011b) for references for the above measures). For nsCON, nsALC and sALC, laboratory measures of basic nutritional status (pre-albumin) and hepatocellular dysfunction [aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT)] were typically obtained within 48 hours of the MR scan.
Magnetic Resonance Acquisition and Processing
MR data was acquired on a 1.5T Siemens Vision. A magnetization-prepared rapid gradient echo (TR/TE/TI = 9.7/4/300 ms, 15° flip angle, 1×1 mm2 in-plane resolution, and 1.5-mm-thick coronal partitions) was use to acquire 3D T1-weighted images for morphological segmentation. See Gazdzinski and colleagues (Gazdzinski et al., 2005) for details.
The publicly available Freesurfer (v4.5) volumetric segmentation and cortical surface reconstruction methods (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004; Fischl et al., 1999) were used to obtain regional cortical and subcortical GM volumes and intracranial volume (ICV) (all in mm3). Individual participant T1-weighted images were visually inspected by the Authors (TCD, DP, CA) to ensure proper segmentation/parcellation. In the case of gross segmentation/parcellation errors, the quality of the T1-weighted image was re-inspected for movement artifacts, aliasing, signal dropout, etc. If no major image artifact issues were apparent, the case was resubmitted to the pipeline and reinspected. In the case of faulty segmentation/parcellation, WM and/or GM of the native images were manually edited (using control points, WM and/or pial edits as needed) and resubmitted through the processing pipeline, and again evaluated for segmentation/parcellation errors (for a general overview of procedures see http://adni.loni.ucla.edu/wp-content/uploads/2010/12/ADNI_UCSF_Freesurfer-Overview-and-QC.pdf). Only those participants with accurate segmentation/parcellation of the ROIs evaluated in this study were included.
Spatial normalization to a template cortical surface allowed automatic parcellation of the cortical surfaces into 34 anatomical regions of interest (ROI) per hemisphere. Volumes were obtained for all 34 bilateral cortical ROIs, 7 subcortical nuclei (hippocampus, amygdala, thalamus, putamen, globus pallidus, nucleus accumbens, cerebellum), brainstem, and corpus callosum subdivisions (anterior, mid anterior, central, mid posterior, posterior). The composite measures of total cortical GM and total WM volumes were computed in FreeSurfer v4.5. Freesurfer labels that were combined to form the BREOS ROIs were as follows: ACC – rostral and caudal; DPFC - rostral and caudal middle frontal and superior frontal gyri; OFC – medial and lateral; insula – standard Freesurfer label. For the ACC, DPFC and OFC, a surface-area-weighted average volume was calculated from the parcellation units that constituted each region (Durazzo et al., 2011a). A total cortical BREOS GM ROI was formed from the surface-area-weighted average of the ACC, DPFC, OFC and insula. Surface-area-weighted volume averages were also calculated for non-BREOS heteromodal/paralimbic cortices, including the inferior frontal gyrus (IFG: pars opercularis + pars triangularis + pars orbitalis), superior temporal gyrus (superior temporal gyrus + banks of superior temporal gyrus), inferior parietal lobule (inferior parietal lobe + supramarginal gyrus), superior parietal lobe (standard FreeSurfer label), precuneus (standard FreeSurfer label), isthmus of posterior cingulate (standard FreeSurfer label), and the temporal pole/parahippocampal region. Total corpus callosum volume was calculated by summing the values for each subdivision. These additional frontal, temporal and parietal cortical subregions were chosen as they are all heteromodal association or paralimbic areas involved in higher-order neurocognitive functions (e.g., attentional reorienting/shifting, learning and memory) and in regulation of drive and emotion (Kolb and Whishaw, 2009; Mesulam, 1997). Additionally, the parahippocampal region, entorhinal cortex, precuneus and posterior cingulate are regions that show significant volume loss during the incipient stages of AD (Krueger et al., 2010), and exhibit greater atrophy in elders with a history of chronic smoking (Almeida et al., 2008; Durazzo et al., In Press). Measurement of volumes in these additional ROIs permitted testing the region-specific hypothesis of greater age-related volume loss in cortical BREOS components. The pattern of group differences for each ROI volume was highly similar for the left and right hemispheres; therefore, they were combined to form one bilateral ROI. Volume measures for all ROIs were converted to z-scores based on values from the 54 nsCON.
Statistical Analyses
Primary Analyses
To test our primary hypothesis of ranked magnitude of age-related volume loss across study groups (i.e., sALC > nsALC > sCON > nsCON), a ALC status (ALC vs. CON) x smoking status (smokers vs. non-smokers) x age multivariate analysis of covariance (MANCOVA; covaried for intracranial volume) and follow up pairwise t-tests were individually conducted for cortical, subcortical GM and WM and global ROIs: Analysis 1 - Cortical ROIs: ACC, DPFC, insula, OFC, IFG, superior temporal gyrus, inferior parietal lobule, superior parietal lobe, precuneus, isthmus of posterior cingulate, temporal pole/parahippocampal region; Analysis 2 - Subcortical Nuclei/GM ROIs: hippocampus, amygdala, thalamus, caudate, putamen, globus pallidus, nucleus accumbens, cerebellum; Analysis 3 - WM ROIs: brainstem and corpus callosum subdivisions; Analysis 4 - Global Measures: total cortical BREOS GM, total non-BREOS GM, association/paralimbic cortex, total cortical GM, total lobar WM.
Secondary Analyses
Given the significantly higher 1-year average drinks per month and lifetime average drinks per month in sALC compared to nsALC (see Table 1), all volumetric comparisons among these groups were controlled for these variables. sALC and nsALC did not significantly differ on the frequency of medical, psychiatric and substance abuse comorbidities (see Table 1) or benzodiazepine usage for withdrawal symptomatology (approximately 14% in total ALC sample); however, comparisons between nsALC and sALC were controlled for these variables to determine if they were independently associated with regional brain volumes. Additionally, body mass index (BMI) was used as a covariate in Analyses 1-4 group comparisons given its associations with regional brain volumes (Taki et al., 2008). Groups were also compared on ICV with a separate univariate analysis, as this measure may reflect the influence of premorbid factors on brain function in those with AUD (Schottenbauer et al., 2007). Finally, groups were compared on volumes of all other brain ROIs (e.g., precentral gyrus, postcentral gyrus, lateral occipital lobe) that were not included in Analyses 1-4.
Correction for Multiple Comparisons
Although we had a priori predictions, alpha level for main effects, interactions and pairwise t-tests for Analysis 1 were adjusted for multiple comparisons based on the 11 ROIs and the average inter-correlation for volumes for these ROIs for all groups combined (r = 0.57) (Sankoh et al., 1997): the adjusted alpha level for Analysis 1 tests was p ≤ .018. For Analysis 2, alpha level for main effects, interactions and pairwise t-tests were adjusted for multiple comparisons based on the 8 ROIs and the average inter-correlation for volumes for these ROIs for all groups combined (r = 0.58), resulting in an adjusted alpha level of p ≤ .021. Alpha levels for Analyses 3 and 4, and secondary analysis comparing groups on brain volumes not included in Analyses 1-4, used the more conservative p ≤ .018. Effect sizes for pairwise comparisons were calculated via Cohen’s d (Cohen, 1988).
Associations between Regional Volumes, Alcohol Consumption and Smoking Measures
Associations between regional volumes and alcohol consumption in the combined ALC group were examined with linear regression (semi-partial coefficients), controlling for age. Associations among regional volumes and smoking variables in sALC and sCON were examined with linear regression (semi-partial coefficients reported), controlling for age and lifetime average drinks/month. These analyses were not corrected for multiplicity in order to explore any potentially meaningful structure-function relationships across neurocognitive measures.
RESULTS
Participant Demographics and Clinical Laboratories
All ALC met DSM-IV criteria for alcohol dependence (96% with physiological dependence) at study enrollment. Groups were equivalent on sex and ethnic frequencies. nsALC were significantly older than nsCON, sCON and sALC (p < .05), who were not significantly different on age. nsCON had a higher level of formal education than sCON, nsALC and sALC (p < .05) and sCON had higher education than sALC (p < .05). nsALC and sALC had a higher 1-year average and lifetime average drinks/month than nsCON and sCON (p < .01); nsCON and sCON were not significant different on these measures. sALC had a higher 1-year average number of drinks/month and lifetime average drinks/month than nsALC (p < .01). nsALC showed a higher BMI than nsCON and sALC (p < .05), with a trend for higher BMI than sCON (p = .07). There were no significant differences between sCON and sALC on smoking/cigarette consumption measures. nsALC and sALC had higher AST, ALT and GGT levels than nsCON (both p < .05), but these groups were equivalent on prealbumin level (See Table 1). In ALC, 11/78 (14%) participants were taking a benzodiazepine to mitigate withdrawal symptomatology. The frequency of benzodiazepine usage was equivalent between nsALC and sALC.
Analysis 1 - Cortical ROI Volumes
Main effects and interactions
MANCOVA yielded significant ALC status x smoking status x age interactions for the DPFC [F (3, 161) = 5.67, p = .017] and superior parietal lobule [F (3, 161) = 7.03, p = .008]. This three-way interaction indicated significant group differences for the slopes (i.e., non-parallel slopes) that fit volume change against age in these ROIs. For the DPFC, nsCON and sALC showed greater age-related volume losses than nsALC (both p < .018), and sCON tended to have a greater age-related volume loss than nsALC (p = .07). Similarly for the superior parietal lobule, nsCON and sALC showed greater age-related volume losses than nsALC (both p < .018). No significant age-related atrophic changes were observed in the other cortical ROIs. ALC status x smoking status interactions were observed for the DPFC [F (2, 161) = 8.01, p = .008] and superior parietal lobule DPFC [F (2, 161) = 8.31, p = .005]. ALC status main effects were apparent for the insula volume [F (1, 161) =11.05, p = .005], with trends for the DPFC (p = .028), inferior frontal gyrus (p = .026), and superior temporal gyrus volumes (p = .029). No other significant main effects or interactions were observed for cortical ROIs (see Table 2). Pairwise comparisons among the four groups were conducted to further parse these main effects and interactions.
Table 2.
Regional Cortical GM Volumes
| Region of Interest | nsCON n = 54 | sCON n = 34 | nsALC n = 35 | sALC n = 47 | P-values | Effect Size (Cohen’s d) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALC Main Effect | Smoke Main Effect | ALC X Smoke | ALC X Age | Smoke X Age | 3-way | nsCON vs. sCON | nsCON vs. nsALC | nsCON vs. sALC | sCON vs. nsALC | sCON vs. sALC | nsALC vs. sALC | |||||
| ACC | −0.03 (0.91) | −0.39 (0.96) | −0.79 (0.95) | −0.44 (0.88) | NS | NS | NS | NS | NS | NS | 0.40 | 0.81# | 0.43 | 0.42 | 0.03 | 0.40 |
| DPFC | −0.01 (0.97) | −0.56 (0.96) | −1.47 (1.04) | −1.05 (0.95) | 0.028 | NS | 0.005* | NS | NS | 0.017* | 0.57# | 1.45# | 1.08# | 0.91# | 0.51# | 0.43 |
| OFC | −0.06 (0.90) | −0.44 (0.98) | −0.79 (1.03) | −0.68 (0.96) | NS | NS | NS | NS | NS | NS | 0.38 | 0.72# | 0.63# | 0.35 | 0.24 | 0.12 |
| Insula | −0.03 (1.01) | −0.62 (1.01) | −1.13 (1.07) | −0.57 (1.04) | 0.001* | 0.10 | 0.085 | 0.019 | 0.085 | 0.07 | 0.59# | 1.06# | 0.76# | 0.49 | 0.17 | 0.33 |
| IFG | −0.08 (0.86) | −0.48 (0.86) | −0.91 (0.91) | −0.54 (0.84) | 0.026 | NS | NS | NS | NS | NS | 0.46 | 0.92# | 0.54# | 0.48 | 0.08 | 0.40 |
| STG | −0.08 (0.88) | −0.04 (0.89) | −0.80 (0.93) | −0.79 (0.87) | 0.029 | NS | NS | NS | NS | NS | 0.05 | 0.79# | 0.80# | 0.84# | 0.85# | 0.01 |
| IPL | −0.04 (0.92) | −0.56 (0.92) | −1.06 (0.96) | −1.15 (0.90) | NS | NS | NS | NS | NS | NS | 0.57# | 1.08# | 1.21# | 0.53# | 0.64# | 0.10 |
| SPL | −0.06 (0.91) | −0.31 (0.91) | −0.87 (0.96) | −0.79 (0.89) | NS | NS | 0.004* | NS | NS | 0.008* | 0.28 | 0.86# | 0.76# | 0.59# | 0.51# | 0.13 |
| Precuneus | −0.04 (0.91) | −0.10 (0.92) | −0.75 (0.96) | −0.57 (0.91) | NS | NS | 0.055 | NS | NS | 0.072 | 0.06 | 0.76# | 0.59# | 0.69# | 0.52# | 0.19 |
| Isthmus cingulate | −0.03 (1.06) | − −0.14 (1.06) | −0.29 (1.12) | −0.29 (1.04) | NS | NS | NS | NS | NS | NS | 0.10 | 0.24 | 0.24 | 0.13 | 0.14 | 0.00 |
| PH region | −0.02 (1.03) | −0.70 (1.03) | −0.82 (1.09) | −0.73 (1.01) | NS | 0.058 | NS | NS | 0.021 | NS | 0.66# | 0.75# | 0.70# | 0.11 | 0.04 | 0.08 |
| Total BREOS | −0.10 (0.95) | −0.63 (0.95) | −1.47 (1.00) | −1.06 (0.93) | 0.021 | NS | 0.005* | NS | NS | 0.017* | 0.56# | 1.43# | 1.03# | 0.88# | 0.46# | 0.44 |
| Non- BREOS cortical GM | −0.05 (0.56) | −0.33 (0.56) | −0.78 (0.59) | −0.69 (0.55) | 0.073 | NS | 0.088 | NS | NS | NS | 0.51# | 1.28# | 1.16# | 0.79# | 0.65# | 0.16 |
| Total Cortical GM | −0.12 (0.90) | −0.67 (0.89) | −1.22 (0.95) | −1.20 (0.88) | 0.012* | 0.55 | 0.019 | 0.090 | 0.038 | 0.033 | 0.61# | 1.20# | 1.23# | 0.60# | 0.61# | 0.01 |
p ≤ .018;
p ≤ .018 for pairwise t-test;
p-values > 0.10 listed as NS; IFG: inferior frontal gyrus; IPL: inferior parietal lobule; PH: parahippocampal; SPL: superior parietal lobule; STG: superior temporal gyrus; mean (standard deviation).
Pairwise comparisons
Both nsALC and sALC had significantly smaller volumes than nsCON in all ROIs except the ACC and isthmus of the cingulate (all p < .018). The magnitude of differences (i.e., effect size) in the anterior frontal regions and insula between nsCON and nsALC were consistently and unexpectedly larger than those between nsCON and sALC. In contrast, the magnitude of differences for the temporal and parietal subregions of nsALC and sALC compared to nsCON were similar. nsALC had smaller DPFC, insula, superior temporal gyrus and precuneus than sCON, while sALC had smaller DPFC, superior temporal gyrus and inferior parietal lobule than sCON (all p < .018). sCON showed smaller volumes than nsCON in the insula, inferior parietal lobule and parahippocampal region (all p < .018). There were no statistically significant volume differences between nsALC and sALC in any ROI; however, nsALC had numerically smaller ROIs than sALC, most notably in the ACC, DPFC and IFG (see Table 2).
Analysis 2 - Subcortical Nuclei/GM ROI Volumes
Main effects and interactions
No three-way interactions were observed. With the exception of the putamen, there were no significant main effects or interactions for any of the subcortical ROIs. The putamen showed a significant smoking status x age interaction [F (2, 160) = 5.83, p = .017], with nsCON (p = .015), sCON (p = .008) and sALC (p = .010) showing greater age-related atrophic changes than nsALC. Main effects were observed for alcohol status [F (1, 160) = 6.14, p = .014] and smoking status [F (1, 160) = 6.78, p = .010] for putamen volume.
Pairwise comparisons
nsALC and sALC showed smaller volumes than nsCON in the hippocampus and amygdala and smaller globus pallidus than sCON. nsALC exhibited smaller thalami, putamen and nucleus accumbens than nsCON, smaller amygdala volumes than sCON and smaller putamen volumes than sALC. sALC had smaller globus pallidus volumes than sCON (all p < .021). No significant differences between nsCON and sCON were observed in subcortical ROIs (see Table 3).
Table 3.
Regional Subcortical GM Volumes
| Region of Interest | nsCON n = 54 | sCON n = 34 | nsALC n = 35 | sALC n = 47 | P-values | Effect Size (Cohen’s d) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALC Main Effect | Smoke Main Effect | ALC X Smoke | ALC X Age | Smoke X Age | 3- way | nsCON vs. sCON | nsCON vs. nsALC | nsCON vs. sALC | sCON vs. nsALC | sCON vs. sALC | nsALC vs. sALC | |||||
| Hippo | −0.04 (1.01) | −0.47 (1.10) | −0.86 (1.15) | −0.87 (1.07) | 0.036 | NS | NS | NS | NS | NS | 0.39 | 0.73# | 0.77# | 0.35 | 0.37 | 0.01 |
| Amygdala | −0.03 (1.12) | −0.46 (1.12) | −1.02 (1.17) | −0.80 (1.10) | NS | NS | NS | NS | NS | NS | 0.38 | 0.87# | 0.70# | 0.50# | 0.31 | 0.20 |
| Thalamus | −0.05 (0.95) | −0.20 (0.96) | −0.66 (1.00) | −0.30 (1.10) | NS | NS | NS | NS | NS | NS | 0.16 | 0.63# | 0.22 | 0.47 | 0.07 | 0.37 |
| Caudate | −0.04 (0.93) | −0.14 (0.93) | −0.19 (0.98) | −0.01 (0.90) | NS | NS | NS | NS | NS | NS | 0.11 | 0.16 | 0.03 | 0.06 | 0.14 | 0.20 |
| Putamen | −0.08 (0.92) | −0.31 (0.92) | −0.69 (0.96) | −0.16 (0.90) | 0.014* | 0.010* | 0.083 | 0.038 | 0.017* | NS | 0.25 | 0.65# | 0.09 | 0.40 | 0.17 | 0.57# |
| Globus pallidus | −0.06 (0.85) | −0.01 (0.86) | −0.58 (0.90) | −0.35 (0.84) | NS | NS | NS | NS | NS | NS | 0.04 | 0.58# | 0.34 | 0.65# | 0.41# | 0.26 |
| Nucleus Accumbens | −0.06 (0.96) | −0.39 (0.96) | −0.70 (1.00) | −0.36 (0.93) | NS | NS | NS | NS | NS | NS | 0.34 | 0.65# | 0.31 | 0.32 | 0.03 | 0.35 |
| Cerebellum | −0.04 (0.96) | −0.20 (0.96) | −0.37 (1.00) | −0.26 (0.93) | NS | NS | NS | NS | NS | NS | 0.16 | 0.34 | 0.23 | 0.18 | 0.06 | 0.12 |
p ≤ .021;
p ≤ .021 for pairwise t-test;
p-values > 0.10 listed as NS; mean (standard deviation).
Analysis 3 - WM ROI Volumes
Main effects, interactions and pairwise comparisons
MANCOVA indicated no significant findings for the corpus callosum subdivisions or brainstem.
Analysis 4 - Global Volume Measures
Main effects and interactions
MANCOVA indicated a significant alcohol status x smoking status x age interaction for total BREOS cortical GM [F (3, 161) = 5.86, p = .017] (see Figure 1) and a trend for a three-way interaction for total cortical GM (p = .033). Similar to the DPFC volume, nsCON and sALC also showed greater age-related atrophic changes on these measures than nsALC (both p < .018; see Figure 1 for pattern). An ALC status x smoking status interaction was seen for the total BREOS cortical GM [F (2, 161) = 8.12, p = .005], and an alcohol status main effect was observed for total cortical GM [F (1, 161) = 6.40, p = .012], with a trend for total non-BREOS cortical GM (p = .07). No significant main effects or interactions were found for total lobar WM.
Figure 1.
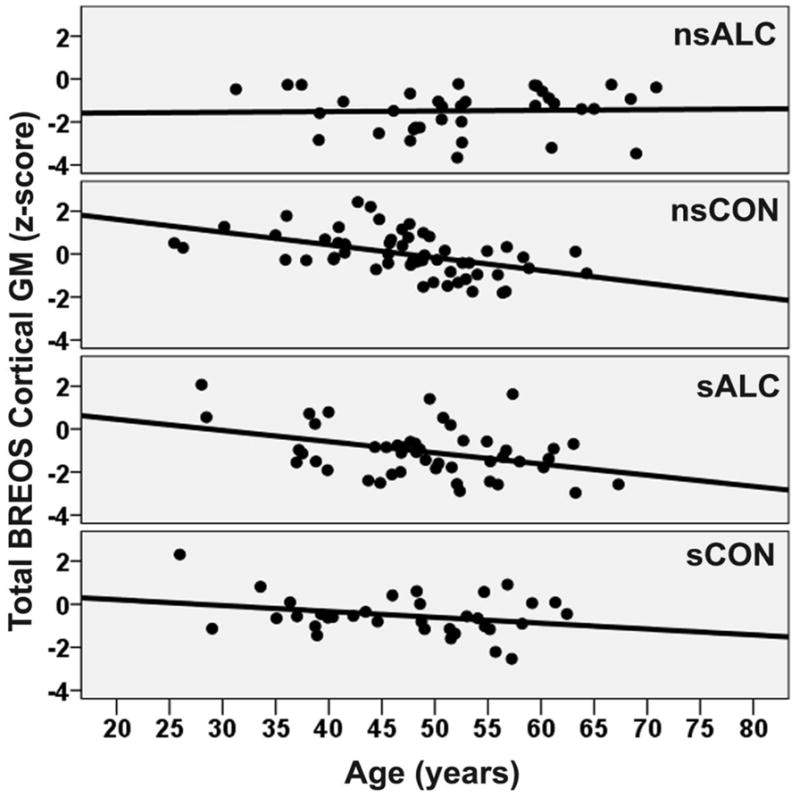
Group Age Related Atrophy for the Total BREOS Cortical Gray Matter.
nsALC: non-smoking alcohol dependent; nsCON: non-smoking control; sALC: smoking alcohol dependent; sCON: smoking control.
Pairwise comparisons
sCON, nsALC and sALC displayed significantly smaller volumes than nsCON for the total BREOS cortical GM, total non-BREOS cortical GM and total cortical GM (all p < .018). Both nsALC and sALC showed smaller volumes than sCON for total BREOS cortical GM, total non-BREOS cortical GM and total cortical GM (all p < .018).
Secondary Analyses
In comparisons between nsALC and sALC, alcohol consumption measures (e.g., lifetime average drinks/month, 1-year average drinks/month, months heavy drinking, etc), medical, psychiatric and substance abuse comorbidities, and benzodiazepine usage were not significant predictors of brain volume in any ROI. There were no significant group differences in ICV, and BMI was not a significant predictor of volume in any ROI. No significant main effects, interactions or pairwise differences were observed for ROIs not included in Analyses 1-4.
Correlations between Regional Brain Volumes and Smoking/Cigarette Consumption Variables
In the ALC group, no alcohol consumption measure was significantly related to volume in any ROI after controlling for age, consistent with the above secondary analyses. In sALC, lifetime years of smoking (controlling for age and lifetime average drinks/month) was inversely related to corpus callosum volume in the anterior (r = −0.32, p = .036) and posterior (r = −0.35, p = .021) subdivisions, as well as the total corpus callosum volume (r = −0.33, p = .027). In sCON, greater pack years (controlling for age and lifetime average drinks/month) were negatively related to mid posterior (r = −0.43, p = .030) and total corpus callosum volumes (r = −0.39, p = .042).
DISCUSSION
The primary findings in these middle-aged, primarily Caucasian male control and 1-week-abstinent ALC cohorts were as follows: 1) nsCON and sALC showed greater age-related volume losses than nsALC in the DPFC, total BREOS cortical GM, superior parietal lobule and putamen; 2) nsALC and sALC demonstrated significantly smaller volumes than nsCON in the majority of individual cortical ROIs and global cortical measures (i.e., total BREOS cortical GM, total non-BREOS cortical GM and total cortical GM), and nsALC showed smaller volumes than nsCON in six of eight subcortical GM nuclei; the corresponding effect sizes for these group differences between ALC and nsCON were generally strong; 3) sCON had smaller volumes than nsCON in the DPFC, insula, inferior parietal lobule, temporal pole/parahippocampal region and all global cortical measures, with moderate effect sizes for these group differences; 4) nsALC and sALC demonstrated smaller volumes than sCON in the DPFC, superior temporal gyrus, inferior and superior parietal lobules, precuneus and all global cortical measures; 5) no statistically significant volume differences between nsALC and sALC were apparent in any ROI, except the putamen; 6) volumes of primary sensory or motor cortex did not differ between groups; and 7) measures of alcohol consumption were not related to volumes in any ROI in ALC, while measures of smoking severity were related to corpus callosum volume in both sCON and sALC.
The predicted order of age-related volume loss across study groups (i.e., sALC > nsALC > sCON > nsCON) was not observed in the BREOS or any other ROI. Unexpectedly, nsALC showed less age-related volume loss than nsCON in the DPFC, total BREOS, superior parietal lobule and putamen and nsALC also tended to have less age-related volume loss than sCON in the DPFC; nsALC demonstrated equivalent age-related volume loss to sALC, sCON and nsCON in all other ROIs, and sALC, sCON and nsCON showed equal age-related atrophy across all ROIs. These findings are incongruent with previous reports of greater age-related atrophy in alcohol dependent individuals than controls (smoking status in study groups not considered), particularly in anterior frontal regions (Fein et al., 2010; Pfefferbaum et al., 1992; Pfefferbaum et al., 1997). Since ICV, alcohol consumption measures, medical, substance and psychiatric comorbidities were not related to regional volumes in the ALC group, the factors associated with less age-related regional atrophy in nsALC relative to nsCON and sALC are unclear. Extended longitudinal study is necessary to elucidate the functional relevance of the smaller age-related atrophic changes in nsALC.
Although nsALC showed less age-related atrophic changes in several regions, group volumes in the insula, DPFC, inferior parietal lobule and all global cortical measures (i.e., total cortical BREOS GM, total non-BREOS GM and total cortical GM), evidenced a stair-step pattern where nsCON > sCON > sALC ≈ nsALC. There were no significant group differences in primary sensory or motor cortices. In six of eight subcortical GM nuclei, nsALC demonstrated smaller volumes than nsCON, but sALC only showed smaller subcortical GM volumes than nsCON in the hippocampus and amygdala; sCON did not differ from nsCON in any subcortical ROI. These findings indicate that smoking status was related to volumes of several cortical ROIs in CON; however, smoking status in ALC (as evidenced by ALC status x smoking status x age interactions) was related only to the magnitude of age-related atrophic changes in the DPFC, total cortical BREOS GM, superior parietal lobule and putamen. The only ROI that was larger in sALC than nsALC was the putamen, which has a particularly high density of nicotinic acetlycholinergic receptors in mammals (Quik and Kulak, 2002).
The current volumetric findings showed a different pattern than our recent study (Durazzo et al., 2011a) with these cohorts of nsCON, nsALC and sALC on measures of regional cortical thickness. For regional cortical thickness, sALC had thinner cortex than nsALC, who showed thinner cortex than nsCON (i.e., sALC < nsALC < nsCON) in the ACC, insula, total cortical BREOS GM and total frontal lobe. Cortical thickness is suggested to be genetically and phenotypically distinct from volume and cortical surface area (Kremen et al., 2010; Panizzon et al., 2009; Winkler et al., 2010). The different patterns demonstrated by nsCON, nsALC and sALC on cortical thickness measures suggest the phenotype of chronic cigarette smoking is associated with singular effects on measures of regional brain volumes and cortical thickness in AUD. The volume differences between sCON and nsCON in this study are consistent with previous MR reports of lower GM density or volume in smoking controls in the DPFC, superior/inferior parietal lobule, and parahippocampal region (Almeida et al., 2008; Brody et al., 2004; Gallinat et al., 2006; Zhang et al., 2011). sCON volumes were intermediate to nsCON and the ALC groups in multiple cortical ROIs. Therefore, combining smoking and non-smoking CON into one control group significantly expand the range of the morphological measure (e.g., volume), thereby increasing the variance of the control group, which may reduce power to detect morphological differences in comparisons to AUD and other clinical cohorts.
The overall findings for sCON, nsALC and sALC indicated that the cortical volume loss for these groups was prominent in the paralimbic and neocortical association ROIs of the BREOS, as well as a generalized pattern of cortical atrophy, as evidenced by their lower total cortical GM relative to nsCON. sCON, nsALC and sALC showed smaller volumes than nsCON in top-down BREOS components that subserve working memory, information processing, problem solving/reasoning, self-monitoring, inhibition and anticipation of consequences of current behavior (George and Koob, 2010; Heatherton and Wagner, 2011). These higher-order cognitive abilities have been shown to be deficient in cohorts of both chronic smokers and those with AUD (Durazzo and Meyerhoff, 2007; Durazzo et al., 2010a, 2012), suggesting that the measured top-down BREOS ROIs are critical components of circuits that subserve these functions. Additionally, sCON and both ALC groups had smaller volumes than nsCON in the parahippocampal region and inferior parietal lobule, which are involved in learning and attentional regulation; these regions also show significant atrophy in the early stages of Alzheimer’s disease (Krueger et al., 2010).
Alcohol consumption measures, medical, substance abuse and psychiatric comorbidities, and benzodiazepine usage were not associated with regional volumes in the nsALC and sALC, and relationships between smoking severity and volumes in sCON and sALC were confined to the corpus callosum. There were no significant differences between the four groups on ICV. These findings, together with the unexpected group order in magnitude of age-related volume losses suggests that: 1) the cortical and subcortical GM volume findings for sCON, nsALC and sALC were likely influenced by comorbid or environmental factors (e.g., nutrition, exercise, general health) not accounted for in this study, and/or 2) the patterns demonstrated by sCON, nsALC and sALC in these ROIs were, at least partially, influenced by latent genetic or premorbid factors that were operational prior to the onset of smoking and/or hazardous drinking. If the observed cross-sectional volume abnormalities in these cohorts are indeed at least partially mediated by premorbid factors, then they may serve as a risk factor for the initiation of chronic smoking and/or AUD (Fineberg et al., 2010; Tessner and Hill, 2010).
In conclusion, the current findings indicate that consideration of smoking status is necessary for a better understanding of the factors contributing to age-related morphological abnormalities in AUD. Importantly, sCON demonstrated significantly smaller volumes than nsCON in several ROIs involved in higher-order neurocognitive functions. The results from this and previous MR studies [see (Durazzo et al., 2010a) for review] strongly argue against combining smoking and non-smoking CON into one cohort as they may not represent the same population. Merging sCON and nsCON into a single group will likely significantly increase the morphological heterogeneity of reference groupin comparisons and obscure the actual magnitude of morphological abnormalities demonstrated by those with an AUD or other substance use disorder in morphometric comparisons. This study did not have sufficient numbers of females to assess for the potential influence of sex; in future studies, it is important to determine if sex is related to the above findings. Given that both chronic smoking and hazardous alcohol consumption during midlife are associated with increased risk for Alzheimer’s disease [see (Durazzo and Meyerhoff, 2007; Durazzo et al., 2012) and references therein], extended longitudinal assessment of sCON, nsALC and sALC is indicated to evaluate the potential impact of the observed atrophic changes on neurocognition and psychiatric/psychological functioning.
Acknowledgments
This work was supported by the National Institutes of Health [NIDA DA24136 to T.C.D and NIAAA AA10788 to D.J.M.] and by the use of resources and facilities at the San Francisco Veterans Administration Medical Center. We thank Mary Rebecca Young, Kathleen Altieri, Ricky Chen, and Drs. Peter Banys and Ellen Herbst of the Veterans Administration Substance Abuse Day Hospital, and Dr. David Pating, Karen Moise and their colleagues at the Kaiser Permanente Chemical Dependency Recovery Program in San Francisco for their valuable assistance in recruiting participants. We also wish to extend our gratitude to the study participants, who made this research possible.
Footnotes
The authors have no disclosures or conflicts of interest to report.
References
- Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:92–98. doi: 10.1097/JGP.0b013e318157cad2. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol ClinExpRes. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Cataldo JK, Glantz SA. Smoking cessation and Alzheimer’s disease: facts, fallacies and promise. Expert Rev Neurother. 2010;10:629–631. doi: 10.1586/ern.10.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Durazzo T, Insel PS, Weiner MW. Greater regional brain atrophy rate in healthy elders with a history of cigarette smoking. Alzheimer’s & Dementia; The Alzheimer’s Disease Neuroimaging Initiative A. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. 2010a;7:3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122:105–111. doi: 10.1016/j.drugalcdep.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Meyerhoff DJ. Chronic cigarette smoking in alcohol dependence: associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addict Biol. 2011a doi: 10.1111/j.1369-1600.2011.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J Stud Alcohol Drugs. 2010b;71:278–289. doi: 10.15288/jsad.2010.71.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical Thickness, Surface Area, and Volume of the Brain Reward System in Alcohol Dependence: Relationships to Relapse and Extended Abstinence. Alcohol ClinExpRes. 2011b;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Barakos J. Age-related gray matter shrinkage in a treatment naive actively drinking alcohol-dependent sample. Alcohol ClinExpRes. 2010;34:175–182. doi: 10.1111/j.1530-0277.2009.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatic parcellation of the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol ClinExpRes. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D’Esposito M. Unifying Prefrontal Cortex Function: Executive Control, Neural Networks, and Top-Down Modulation. In: Miller BL, editor. The Human Frontal Lobes: Functions and Disorders. The Guilford Press; New York: 2007. pp. 187–206. [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and biobehavioral reviews. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Fundamentals of Human Neuropsychology. 6. Worth Publishers; New York, NY: 2009. [Google Scholar]
- Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, Franz CE, Lyons MJ, Pacheco J, Perry ME, Stevens A, Schmitt JE, Grant MD, Seidman LJ, Thermenos HW, Tsuang MT, Eisen SA, Dale AM, Fennema-Notestine C. Genetic and environmental influences on the size of specific brain regions in midlife: the VETSA MRI study. Neuroimage. 2010;49:1213–1223. doi: 10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger CE, Dean DL, Rosen HJ, Halabi C, Weiner M, Miller BL, Kramer JH. Longitudinal Rates of Lobar Atrophy in Frontotemporal Dementia, Semantic Dementia, and Alzheimer’s Disease. Alzheimer Dis Assoc Disord. 2010;24:43–48. doi: 10.1097/WAD.0b013e3181a6f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Tang J, Deng Q, Deng Y, Luo T, Wang X, Chen H, Liu T, Chen X, Brody AL, Hao W. Bilateral fronto-parietal integrity in young chronic cigarette smokers: a diffusion tensor imaging study. PLoS One. 2011;6:e26460. doi: 10.1371/journal.pone.0026460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol. 2010 doi: 10.1111/j.1369-1600.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, Kim BW, Blood AJ, Evins AE, Seidman LJ, Iosifescu DV, Lee S, Baxter C, Perlis RH, Smoller JW, Fava M, Breiter HC. Cortical thickness abnormalities in cocaine addiction--a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron. 2008a;60:174–188. doi: 10.1016/j.neuron.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008b;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens JR, Lu YW, Parthasarathy S, Moore C, Weisner CM. Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: comparison with matched controls. Arch Intern Med. 2003;163:2511–2517. doi: 10.1001/archinte.163.20.2511. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Anatomic Principles in Behavioral Neurology and Neuropsychology. In: Feinberg TE, Farah MJ, editors. Behavioral Neurology and Neuropsychology. McGraw-Hill; New York: 1997. [Google Scholar]
- Meyerhoff DJ, Durazzo TC. Proton magnetic resonance spectroscopy in alcohol use disorders: a potential new endophenotype? Alcohol ClinExpRes. 2008;32:1146–1158. doi: 10.1111/j.1530-0277.2008.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct Genetic Influences on Cortical Surface Area and Cortical Thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol ClinExpRes. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol ClinExpRes. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Kulak JM. Nicotine and nicotinic receptors; relevance to Parkinson’s disease. Neurotoxicology. 2002;23:581–594. doi: 10.1016/s0161-813x(02)00036-0. [DOI] [PubMed] [Google Scholar]
- Room R. Smoking and drinking as complementary behaviours. Biomed Pharmacother. 2004;58:111–115. doi: 10.1016/j.biopha.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Rusanen M, Kivipelto M, Quesenberry CP, Jr, Zhou J, Whitmer RA. Heavy Smoking in Midlife and Long-term Risk of Alzheimer Disease and Vascular Dementia. Arch Intern Med. 2010 doi: 10.1001/archinternmed.2010.393. [DOI] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schottenbauer MA, Momenan R, Kerick M, Hommer DW. Relationships among aging, IQ, and intracranial volume in alcoholics and control subjects. Neuropsychology. 2007;21:337–345. doi: 10.1037/0894-4105.21.3.337. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008;16:119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychol Rev. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences. 2011a doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction Circuitry in the Human Brain. Annu Rev Pharmacol Toxicol. 2011b doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


