Abstract
We report methods for the synthesis of vertex-differentiated icosahedral closo-boranes. A single B-OH vertex of the icosahedral borane [closo-B12(OH)12]2− was derivatized to prepare [closo-B12(OR)(OH)11]2− using optimized alkylation conditions and purification procedures. Several representative vertex-differentiated icosahedral closo-boranes were prepared utilizing carbonate ester and azide-alkyne click chemistries on the surface of the closo-B122− core.
Over the past decade, nanoscale carriers such as dendrimers, liposomes, and micelles have attracted much attention for their capacity to deliver multiple copies of therapeutic and diagnostic agents, potentially enhancing the clinical effectiveness of pharmaceutical agents. Nanocarriers can be constructed with or without the incorporation of a biological receptor-specific targeting moiety.1a Non-targeted nanoscale pharmaceuticals have demonstrated improved specificity for tumour cells as a result of the “enhanced permeability and retention” effect.1b The specificity of nanocarriers may be further increased by the addition of ligands (e.g., peptides, proteins, or antibodies) capable of selectively binding to receptors expressed only by target cells. Targeted nanocarriers are expected to be more effective and cause fewer side effects than conventional pharmaceuticals or non-targeted nanocarriers.
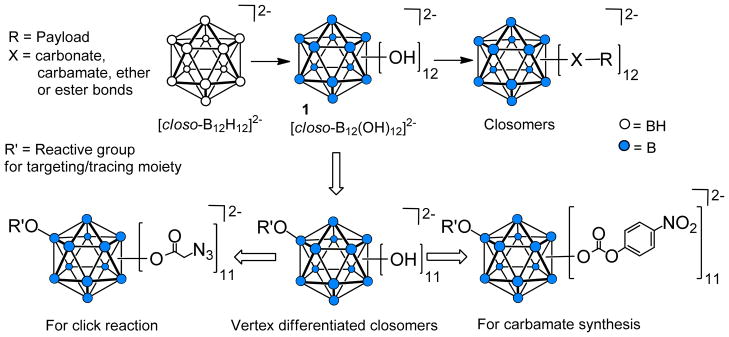
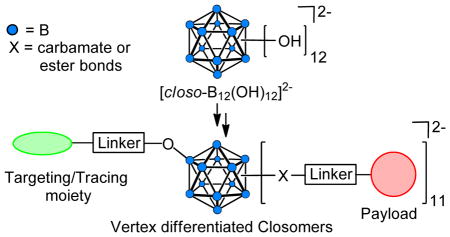
Polyhedral boranes have generated considerable interest in biomedical research in relation to their use as a 10B source in boron neutron capture therapy.2 However, a still relatively unexplored application of polyhedral boranes is their use as nanocarriers. Our group has been actively pursuing research in this area and in particular is exploring the utility of derivatives of the icosahedral dodecahydro-closo-dodecaborate dianion [closo-B12H12]2− such as [closo- (Figure 1) in synthesis of discrete nanomolecular scaffolds for targeted, high-B12(OH)12]2−, 1, payload delivery of therapeutic and diagnostic agents.3,4
Figure 1.
Schematic representation of organic synthesis on an icosahedral closo-B122− surface.
The reactivity of the B-OH vertices in 1 resembles that of alcohols and can be used to anchor up to 12 radial arms with desired functionalities on the B122− core. Previous reports from our laboratory have described syntheses of various 12-fold carbonate, carbamate, carboxylate ester, and ether derivatives, which are collectively referred to as “closomers”.5
An ideal nanocarrier for targeted drug delivery requires the presence of heterobifunctionalized linker arms within the same scaffold onto which one can install both a targeting moiety and payload molecules by employing orthogonal chemistries. We envisage utilization of the known reactions of 1 to develop a variety of novel heterobifunctionalized, monodispersed closomers useful in development of novel nanoscale targeted drug delivery systems (Figure 1). Herein, we report methods for the synthesis of vertex-differentiated closomers that will permit the addition of both a targeting moiety and multiple payload molecules.
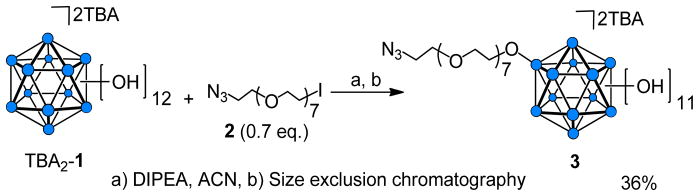
The first step in the synthesis of the vertex-differentiated closomers was reaction of a single BOH vertex in (TBA)2-1 (TBA = tetrabutylammonium) to produce the mono-alkylated closomer 3 (Scheme 1). Key to the success of this study was to obtain 3 in pure form. Reaction optimization involved trying several stoichiometric ratios of (TBA)2-1 and the alkylating bifunctionalized linker 2, employing different bases [N,N-diisopropylethylamine (DIPEA) and triethylamine (Et3N)] and varying reaction temperature and time. Under the optimized etherification conditions of 0.7 eq. of 2 and 1.0 eq. of DIPEA in acetonitrile (ACN) at 85°C for 24 h, a moderate yield of mono-alkylated product 3 (36%) was achieved. On average, two to three size-exclusion chromatographic column sequences using Lipophilic Sephadex® LH-20 were necessary to completely separate 3 from the unreacted starting material 1 and minor amounts of multi-substituted products.
Scheme 1.
Synthesis of vertex-differentiated icosahedral closo-borane scaffold 3.
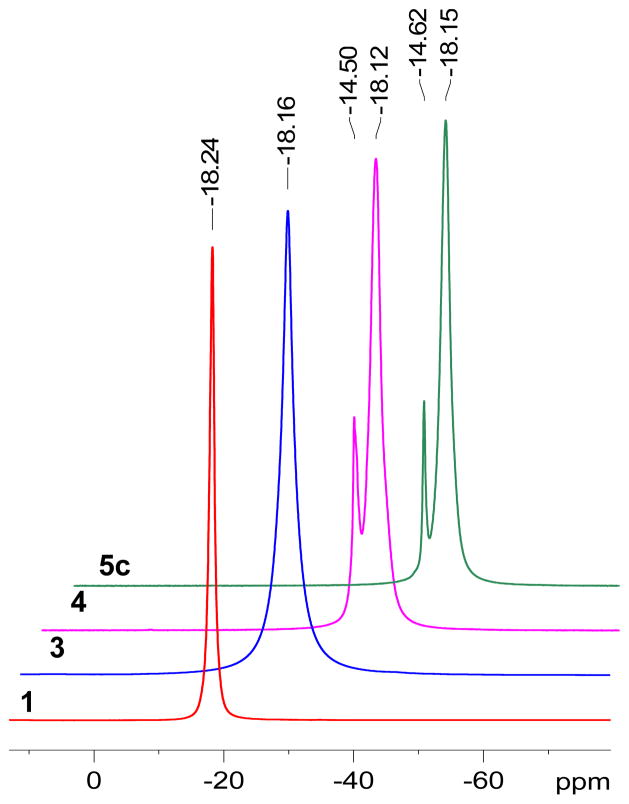
Purified product 3 was characterized by 1H, 13C and 11B NMR, HRMS and IR analysis. Whereas the 11B NMR spectrum of 1 exhibits a sharp singlet at −18.24 ppm, that of 3 showed a broadened peak at −18.16 ppm consisting of all 12 B-O vertices, with broadening due to loss of symmetry in the structure (Figure 2). The IR-spectrum of 3 also displayed a characteristic peak at 2106 cm−1 attributable to the asymmetric stretch of the azide-group (Figure 3).
Figure 2.
11B NMR (160.4 MHz, CD3CN) stacks of closomers 1, 3, 4, and 5c showing changes in chemical shifts as a result of substitution at the periphery of the cage.
Figure 3.
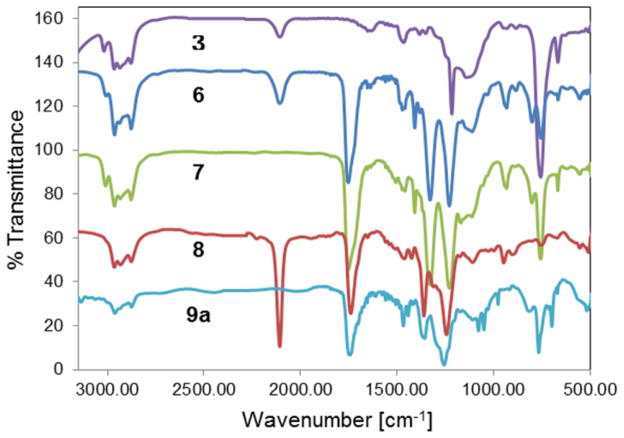
Characteristic changes in the IR spectra during synthesis of 9a.
For synthesis of 3, choice of a suitable linker is crucial. The azido group at the distal terminus of 2 (N3-OEG-I) is able to serve as a latent functional group during the multi-step synthesis, and at a later stage it can be reacted with an alkyne-terminated ligand using a Cu(I)-catalyzed variant of the Hüisgen 1,3-dipolar cycloaddition click reaction.6 The linker 2 was synthesized in four steps from octaethylene glycol (OEG).7
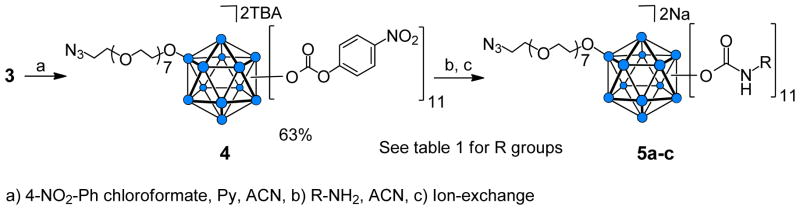
The remaining 11 hydroxyl groups on 3 were functionalized by employing closomer carbonate5e ester reactions. Reaction of 3 with 4-nitrophenyl chloroformate (60.0 eqv.) in ACN at 50°C for 15 h produced the 11-fold 4-nitrophenyl carbonate (PNPC)-substituted closomer 4 (Scheme 2). The yield was 63% following purification by size-exclusion chromatography on a Lipophilic Sephadex LH-20 column. The purified closomer 4 was characterized by 1H, 13C and 11B NMR, HRMS, and IR analysis. The 11B NMR spectrum of 4 showed two peaks, one at −14.50 ppm for the B-OR vertex and another at −18.12 ppm for the 11 B-OCOO- vertices (Figure 2). The IR spectrum of 4 also showed the presence of characteristic peaks at 2105 cm−1 and 1766 cm−1 attributable to the asymmetric stretch of the azide and carbonate groups, respectively.
Scheme 2.
Synthesis of vertex-differentiated closomers via carbonate reactions.
Reaction of 11-fold carbonate closomer 4 with various primary amines (5 eqv. per vertex) produced the 11-fold carbamate closomers 5a–c in quantitative yields (Table 1). The purified closomers 5a–c were characterized by 1H, 13C and 11B NMR, HRMS, and IR analysis. The 11B NMR spectra of 5c contained two peaks, one at −14.62 ppm for the B-OR vertex, and one at −18.15 ppm for the 11 aggregate B-OCONH- vertices (Figure 2). The 1H NMR spectra of closomers 5a–c lacked resonances in the aromatic region associated with the PNPC group, indicating successful conversion of all carbonate groups.
Table 1.
Structure of representative amines and yields of closomers 5a–c.
| Entry | R-NH2 | Yield (%) |
|---|---|---|
| 5a | n-Butylamine | 92 |
| 5b | N-Boc-ethylenediamine | 86 |
| 5c | N-Boc- 2,2′-(ethylenedioxy)diethylamine | 84 |
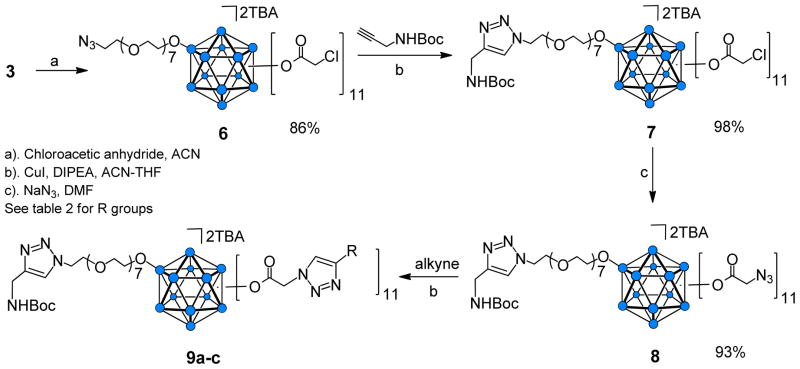
Closomer 3 was also the starting material in a series of reactions that combined closomer ester5f and click reactions to permit the generation of closomers with a variety of functionalities. First, 3 was converted to the 11-fold chloroacetate closomer 6 (Scheme 3) by reaction with chloroacetic anhydride (4 eqv. per vertex). The yield was 86% after purification by size-exclusion chromatography on a Lipophilic Sephadex LH-20 column. The purified product 6 was characterized by 1H, 13C and 11B NMR, HRMS, and IR analysis. The 1H NMR spectrum of 6 contained a characteristic peak at δ 4.1 ppm for the 22 protons assigned to the 11 Cl-CH2CO2- groups attached to the closo-B12 cage. Similar to the 11B NMR spectra of the closomer 5, two peaks were seen in the 11B NMR spectra of closomer 6, one at −14.61 ppm for the B-OR vertex and one at −18.17 ppm for the 11 B-OCO vertices.
Scheme 3.
Synthesis of vertex-differentiated closomers via azide-alkyne click reaction.
Next, a click reaction was employed to produce a Boc-protected amino-terminated linker from the lone azide group on closomer 6 (Scheme 3). Reaction of 6 with Boc-protected propargyl amine in the presence of Cu(I) and DIPEA resulted in closomer 7 in high yield. 1H NMR analysis of 7 showed a singlet at ~1.4 ppm for the nine protons specific to the Boc group. The absence of the characteristic asymmetric stretch of the azide-group at 2107 cm−1 from the IR spectrum of 7 confirmed the occurrence of the click reaction (Figure 3).
To permit addition of functionalities at the remaining 11 vertices of 7, we employed our recently reported protocol5f to prepare the 11-fold azidoacetate closomer 8. Reaction of closomer 7 with a 10-fold excess of sodium azide in dimethylformamide (DMF) at 50°C for two days produced closomer 8 in quantitative yield (Scheme 3). The 11B NMR spectrum of the azidoacetate closomer 8 did not change from that of the chloroacetate closomer 7; however, the 1H NMR spectrum showed that the characteristic peaks for the 22 protons assigned to the 11 Cl-CH2CO2- groups in closomer 7 shifted from δ 4.1 ppm to δ 3.7 ppm for the 11 N3-CH2CO2- groups in closomer 8. The IR-spectrum of 8 also exhibited a characteristic peak at 2106 cm−1 attributed to the asymmetric stretch of the azide group (Figure 3).
The 11 azido groups of closomer 8 are excellent substrates for an azide-alkyne click reaction to attach payload molecules under mild reaction conditions (Scheme 3). Several representative aryl and alkyl alkynes (Table 2) readily reacted with 8 to produce the 11-fold triazole-linked closomers 9a–c in excellent yields. All of the click products exhibited a characteristic singlet in the δ 7–8 ppm region in the 1H NMR spectra for the 12 protons of alkene-CH of the 12 triazole rings of 9a–c. The IR spectra of 9a–c also showed loss of the characteristic peak at 2106 cm−1 originally attributed to the asymmetric stretch of the azide groups in 8 (Figure 3).
Table 2.
Structures of representative
| Entry | Alkyne | Yield (%) |
|---|---|---|
| 9a | Phenylacetylene | 78 |
| 9b | 1-Hexyne | 86 |
| 9c | Propargyl acetate | 81 |
In conclusion, we have developed methods for the synthesis of vertex-differentiated closomers possessing heterobifunctionalized linker arms. Optimized mono O-alkylation and appropriate purification methods permits conversion of a single hydroxyl group in [closo-B12(OH)12]2− to the mono-alkylated closomer [closo-B12(O-OEG-N3)(OH)11]2−. The remaining 11 unreacted hydroxyl groups on the periphery of the icosahedral core can be readily transformed into highly reactive carbonate and azidoacetate closomers. The reactive end groups on each of the 11 linker arms allow for addition of suitable payload molecules via carbonate and click chemistry under very mild reaction conditions. The lone B-O-OEG vertex is spared and can be used for attachment of a targeting and or tracking moiety. This unique and versatile approach will be useful in the construction of nanomolecular targeted delivery systems capable of carrying multiple copies of therapeutic or diagnostic imaging agents.
EXPERIMENTAL SECTION
General information
Common reagents and chromatographic solvents were purchased from commercial suppliers and used without any further purification. NMR spectra were recorded on 400 and 500 MHz spectrometers. All NMR chemical shifts (δ) are reported in parts per million (ppm). The NMR peak assignments were confirmed using COSY experiments. The high-resolution mass spectrometry analysis was performed using ESI-TOF.
Synthesis of closomer 3
A mixture of (TBA)2-1 (1.15 g, 1.41 mmol) and ACN (40 mL) under stirring was allowed to reflux at 85°C. To this mixture, a solution of 2 (0.50 g, 0.99 mmol) and DIPEA (0.18 g, 1.41 mmol) in ACN (30 mL) was added slowly over 8 h at 85°C. After the addition was completed, the reaction mixture was stirred for another 15 h at 85 °C, then concentrated in the rotavap and dried in vacuo to give the crude product as yellow oil. The product was then purified by size-exclusion column chromatography (Lipophilic Sephadex® LH-20) using methanol (MeOH) as the mobile phase. The product was obtained as a colorless oil. Yield: 0.43 g (36%). IR (neat): 3366, 2935, 2106, 1465, 1215 and 754 cm−1. 1H NMR (400 MHz, CD3CN): δ 3.97 (m, 2H, -CH2-PEG), 3.67-3.50 (m, 28H, -CH2-PEG), 3.40 (t, 2H, J = 5.0 Hz, -CH2-PEG), 3.12 (m, 16H, -CH2-TBA+), 1.65 (m, 16H, -CH2-TBA+), 1.39 (m, 16H, -CH2-TBA+), 0.99 (t, J = 7.2 Hz, 24H, CH3-TBA+). 13C NMR (100.6 MHz, CD3CN): δ 71.1-70.8 (multiple peaks), 70.3, 59.2, 51.4, 24.2, 20.2, 13.6. 11B NMR (160.4 MHz, CD3CN): δ −18.16. HRMS (m/z): Calcd for C16H43B12N3O19+TBA+ [M+TBA+]− 953.6544. Found 953.6191 and Calcd for C16H43B12N3O19 [M]2− 355.6841. Found 355.6922.
Synthesis of closomer 4
A mixture of 4-nitrophenyl chloroformate (5.06 g, 25.1 mmol) and pyridine (2.15 g, 27.2 mmol) in ACN (50 mL) was stirred at 0°C for 15 min. To this mixture, a solution of 3 (0.50 g, 0.42 mmol) in ACN (20 mL) was added slowly over 0.5 h at 0°C. The reaction mixture was allowed to warm to RT and stirred for 15 h at 50°C. The mixture was then concentrated to dryness, dissolved in dichloromethane (DCM) and filtered. The filtrate was concentrated and dried in vacuo and the product was then purified by size exclusion chromatography (Lipophilic Sephadex® LH-20) using ACN as the mobile phase. The product obtained as a light yellow sticky solid. Yield: 0.80 g (63%). IR (neat): 3019, 2935, 2876, 2105, 1766, 1522, 1429, 1347, 1297, 1216 and 757 cm−1. 1H NMR (500 MHz, CD3CN): δ 8.29 (m, 22H, -CH-PNPC), 7.41 (m, 22H, -CH-PNPC), 3.62-3.58 (m, 30H, -CH2-PEG), 3.45 (m, 2H, -CH2-PEG), 3.17 (m, 14H, -CH2-TBA+), 1.69 (m, 14H, -CH2-TBA+), 1.45 (m, 14H, -CH2-TBA+), 1.05 (m, 21H, CH3-TBA+) (Lesser proton count for TBA+ is due to the exchange with H3O+ and Na+). 13C NMR (125.7 MHz, CD3CN): δ 156.7, 151.0, 145.2, 125.1, 122.4, 71.5-70.2 (multiple peaks), 58.3, 50.5, 24.8, 19.3, 12.8. 11B NMR (160.4 MHz, CD3CN): δ −14.50, −18.12. HRMS (m/z): Calcd for C93H76B12N14O63 [M]2− 1264.2193. Found 1264.2448.
General procedure for the synthesis of carbamate closomer 5
A mixture of 4 (1 eq.) and amine (60 eq.) in ACN was stirred at 65°C for 2–3 days. Progress of the reaction was monitored via mass spectrometry and 11B NMR analysis. After completion, the reaction mixture was concentrated in rotavap and dried in vacuo to obtain the crude product. The product was then purified by size exclusion chromatography (Lipophilic Sephadex® LH-20) using MeOH as the mobile phase. Purified product was passed through an ion exchange resin column to exchange TBA+ with Na+ ion.
Synthesis of carbamate closomer 5a
Closomer 5a was prepared from 4 (0.10 g, 0.03 mmol) and n-butyl amine (0.15 g, 2.00 mmol) in ACN (10 mL) using the aforementioned general procedure for carbamate synthesis. The product was obtained as a colorless oil. Yield: 55.0 mg (92%). IR (neat): 3351, 2959, 2932, 2873, 2105, 1694, 1540, 1466, 1256, 1216 and 756 cm−1. 1H NMR (400 MHz, CD3CN): δ 6.40-6.33 (bs, 11H, NH), 4.01 (m, 2H, -CH2-PEG), 3.68-3.53 (m, 28H, -CH2-PEG), 3.39 (t, 2H, J = 5.2 Hz, -CH2-PEG), 3.05 (m, 22H, -CH2-butyl), 1.48 (m, 22H, -CH2-butyl), 1.36 (m, 22H, -CH2-butyl), 0.92 (t, 33H, J = 7.2 Hz, CH3-butyl). 13C NMR (100.6 MHz, CD3CN): δ 157.1, 72.8, 71.3-71.2 (multiple peaks), 65.9, 51.6, 42.7, 42.0, 32.7, 20.9, 14.3. 11B NMR (160.4 MHz, CD3CN): δ −16.31, −18.25. HRMS (m/z): Calcd for C71H142B12N14O30+Na [M+Na]− 1825.1121. Found 1825.1139.
Synthesis of carbamate 5b
Closomer 5b was prepared from 4 (0.10 g, 0.03 mmol) and N-Boc-ethylenediamine (0.32 g, 2.00 mmol) in ACN (15 mL) using the aforementioned general procedure for carbamate synthesis. The product was obtained as a colorless oil. Yield: 80.0 mg (86%). IR (neat): 3353, 3017, 2978, 2108, 1698, 1516, 1216 and 758 cm−1. 1H NMR (400 MHz, CD3CN): δ 6.15 (bs, 22H, NH), 4.08 (m, 2H, -CH2-PEG), 3.66-3.59 (m, 28H, -CH2-PEG), 3.39 (t, 2H, J = 5.2 Hz, -CH2-PEG), 3.16 (m, 44H, -CH2-ethylenediamine), 1.42 (s, 99H, -Boc). 13C NMR (100.6 MHz, CD3CN): δ 157.5, 79.4, 71.2-70.6 (multiple peaks), 51.6, 42.3, 41.1, 28.6. 11B NMR (160.4 MHz, CD3CN): δ −16.31, −18.25. HRMS (m/z): Calcd for C104H197B12N25O52 [M]2− 1380.7379. Found 1380.7246. Calcd for C104H197B12N25O52+Na [M+Na]− 2782.4663. Found 2782.3956.
Synthesis of carbamate 5c
Closomer 5c was prepared from 4 (0.10 g, 0.03 mmol) and tert-butyl (2-(2-(2-aminoethoxy)ethoxy)ethyl) carbamate (0.50 g, 2.00 mmol) in ACN (15 mL) using the aforementioned general procedure for carbamate synthesis. The product obtained as a colorless oil. Yield: 105 mg (84%). IR (neat): 3350, 3012, 2979, 2930, 2108, 1701, 1507, 1252, 1216 and 757 cm−1. 1H NMR (400 MHz, CD3CN): δ 6.53 (bs, 11H, NH), 5.67 (bs, 11H, NH), 4.08 (m, 2H, -CH2-PEG), 3.66-3.47 (m, 118H, -CH2-PEG), 3.39 (t, 2H, J = 5.2 Hz, -CH2-PEG), 3.22 (m, 42H, -CH2-PEG), 1.43 (s, 99H, -Boc). 13C NMR (100.6 MHz, CD3CN): δ 157.1, 79.8, 71.3-70.2 (multiple peaks), 51.6, 42.10, 41.1, 28.6. 11B NMR (160.4 MHz, CD3CN): δ −14.62, −18.15. HRMS (m/z): Calcd for C148H285B12N25O74 [M]2− 1864.5215. Found 1864.4845.
Synthesis of chloroacetate closomer 6
A solution of 3 (0.43 g, 0.36 mmol) and chloroacetic anhydride (2.70 g, 15.8 mmol) in ACN (30 mL) was refluxed for 3 days in an argon atmosphere with vigorous stirring. Progress of the reaction was monitored by mass spectrometry analysis. The reaction mixture was then concentrated to dryness and purified using a size-exclusion column (Lipophilic Sephadex® LH-20) with ACN as the eluent. The product was obtained as a light brown semi-solid. Yield: 0.63 g (86%). IR (neat): 2962, 2876, 2107, 1751, 1327 and 1228 cm−1. 1H NMR (400 MHz, CDCl3): δ 4.19-4.11 (m, 22H, -OCOCH2-), 3.71-3.67 (m, 30H, -CH2-PEG), 3.41 (t, 2H, J = 5.2 Hz, -CH2-PEG), 3.18 (m, 16H, -CH2-TBA+), 1.66 (m, 16H, -CH2-TBA+), 1.45 (m, 16H, -CH2-TBA+), 1.04 (t, J = 4.0 Hz, 24H, CH3-TBA+). 13C NMR (100.6 MHz, CDCl3): δ 166.2, 71.5-70.9 (multiple peaks), 59.7, 51.5, 44.3, 24.8, 20.5, 14.4. 11B NMR (160.4 MHz, CD3CN): δ −14.61, −18.17. HRMS (m/z): Calcd for C38H54B12Cl11N3O30 [M]2− 776.0251. Found 776.0341 and Calcd for C38H54B12Cl11N3O30+Na+ [M+Na]− 1575.0405. Found 1575.0507.
Synthesis of chloroacetate closomer 7
Closomer 6 (0.48 g, 0.24 mmol), tert-butyl prop-2-yn-1-ylcarbamate (0.07 g, 0.47 mmol) and copper iodide (0.05 g, 0.24 mmol) were dissolved in a 50:50 mixture of tetrahydrofuran (THF) and ACN (12 mL). To this mixture, DIPEA (0.06 g, 0.47 mmol) was added and the reaction mixture was vigorously stirred at room temperature for 12 h under an argon atmosphere. After completion, the reaction mixture was concentrated to dryness, dissolved in ethyl acetate and filtered through a celite pad. The filtrate was concentrated and purification via size-exclusion column chromatography (Lipophilic Sephadex® LH-20) using ACN afforded the pure product as a colorless oil. Yield: 505 mg (98%). IR (neat): 2963, 2876, 1750, 1328 and 1227 cm−1. 1H NMR (400 MHz, CD3CN): δ 7.77 (s, 1H, -CH-triazole), 5.85 (bs, 1H, NH), 4.50 (t, 2H, J = 5.2 Hz, -CH2-NHBoc), 4.31 (d, 2H, J = 6 Hz, -NCH2-PEG), 4.20-4.10 (m, 10H, -OCOCH2Cl), 4.06-3.99 (m, 10H, -OCOCH2Cl), 3.95 (m, 2H, -OCOCH2Cl), 3.86 (t, 2H, J = 5.2 Hz, -CH2-PEG) 3.61-3.53 (m, 26H, -CH2-PEG), 3.48 (t, 2H, J = 5.2 Hz, -CH2-PEG), 3.10 (m, 12H, -CH2-TBA+), 1.62 (m, 12H, -CH2-TBA+), 1.45 (s, 9H, Boc), 1.36 (m, 12H, -CH2-TBA+), 0.99 (t, J = 7.2 Hz, 17H, CH3-TBA+) (Lesser proton counts for TBA+ is due to the exchange with H3O+ and Na+). 13C NMR (100.6 MHz, CD3CN): δ 166.1-165.9 (multiple peaks), 146.7, 123.8, 79.4, 73.3, 71.2-70.8 (multiple peaks), 69.8, 66.4, 59.2, 59.2, 50.8, 43.9, 43.8, 36.7, 28.5, 24.1, 20.2, 13.6. 11B NMR (160.4 MHz, CD3CN): δ −14.38, −18.12. HRMS (m/z): Calcd for C46H67B12Cl11N4O32+Na+ [M+Na]− 1730.1356. Found 1730.1433. Calcd for C46H67B12Cl11N4O32 [M]2− 853.5726. Found 853.5412.
Synthesis of azidoacetate closomer 8
In a 100 mL round bottom flask, 7 (0.48 g, 0.22 mmol) and sodium azide (1.57 g, 24.09 mmol) were mixed with 20 mL of dry DMF. This mixture was vigorously stirred at 50°C for 2 days under an argon atmosphere. The progress of the reaction was monitored by mass spectrometry analysis. After completion, the reaction mixture was filtered through a celite pad and the filtrate was concentrated to dryness. The residue was dissolved in ethyl acetate and filtered again through a celite pad. The filtrate was then collected, evaporated to dryness and purified using size-exclusion column chromatography (Lipophilic Sephadex® LH-20) with MeOH as eluent. The product was obtained as a light brown semi-solid. Yield: 0.46 g (93%). IR (neat): 2963, 2876, 2106, 1739, 1359 and 1243 cm−1. 1H NMR (400 MHz, CD3CN): δ 7.74 (s, 1H, -CH-triazole), 5.58 (bs, 1H, NH), 4.49 (t, 2H, J = 5.0 Hz, -CH2-NHBoc), 4.30 (d, 2H, J = 6 Hz, -NCH2-PEG), 3.95 (m, 2H, -OCOCH2N3), 3.88-3.82 (m, 4H, -OCOCH2N3 & -CH2-PEG), 3.79 (m, 8H, -OCOCH2N3), 3.72-3.68 (m, 10H, -OCOCH2N3), 3.63-3.47 (m, 28H, -CH2-PEG), 3.10 (m, 12H, -CH2-TBA+), 1.62 (m, 12H, -CH2-TBA+), 1.43 (s, 9H, Boc), 1.38 (m, 12H, -CH2-TBA+), 0.99 (t, 18H, J = 7.2 Hz, CH3-TBA+) (Lesser proton counts for TBA+ is due to the exchange with H3O+ and Na+). 13C NMR (100.6 MHz, CD3CN): δ 167.9-167.4 (multiple peaks), 156.7, 123.6, 79.4, 73.2, 71.0-70.8 (multiple peaks), 69.9, 66.2, 59.2, 52.1, 52.0, 50.7, 36.8, 28.5, 24.2, 20.2, 13.7. 11B NMR (160.4 MHz, CD3CN): δ −14.53, −18.08. HRMS (m/z): Calcd for C46H67B12N37O32 [M]2− 889.7980. Found 889.7671.
General procedure for the synthesis of click closomer 9
In a 25 mL round bottom flask, the azide-functionalized closomer 8 (1eq.), alkyne (5 eq. per vertex, total 55 eq.) and copper iodide (1 eq. per vertex, total 11 eq.) were dissolved in a 50:50 mixture of THF and ACN (6 mL). To this mixture, DIPEA (10 eq. per vertex, total 110 eq.) was added and the reaction mixture was vigorously stirred at room temperature for 3 days under an argon atmosphere. The progress of the reaction was monitored by mass spectrometry analysis. After completion, the reaction mixture was concentrated to dryness, dissolved in ethyl acetate and filtered through a celite pad. The filtrate was concentrated and purified via size-exclusion column chromatography (Lipophilic Sephadex® LH-20) using ACN as an eluent to afford the pure product.
Synthesis of click closomer 9a
Using the general strategy described above, 9a was synthesized from closomer 8 (100 mg, 0.04 mmol), phenylacetylene (247 mg, 2.42 mmol), copper iodide (92.3 mg, 0.48 mmol) and DIPEA (627 mg, 4.80 mmol). Pure product was obtained as a colorless sticky solid. Yield: 116 mg (78%). IR (neat): 2961, 2875, 1743, 1355 and 1255 cm−1. 1H NMR (400 MHz, CD3CN): δ 8.04 (m, 6H, -CH-triazole), 7.94 (m, 6H, -CH-triazole), 7.87-7.80 (m, 20H, -Ph), 7.37-7.20 (m, 35H, -Ph), 5.90 (bs, 1H, NH), 5.00-4.86 (m, 22H, -OCOCH2-), 4.43 (t, 2H, J = 4.8 Hz, -CH2-NHBoc), 4.28 (m, 2H, -NCH2-PEG), 3.77 (t, 2H, J = 4.8 Hz, -NCH2-PEG), 3.51-3.38 (m, 28H, -CH2-PEG), 3.07 (m, 11H, -CH2-TBA+), 1.59 (m, 11H, -CH2-TBA+), 1.40 (s, 9H, Boc), 1.37-1.30 (m, 11H, -CH2-TBA+), 0.98 (t, 17H, J = 7.2 Hz, CH3-TBA+) (Lesser proton counts for TBA+ is due to the exchange with H3O+ and Na+). 13C NMR (100.6 MHz, CD3CN): δ 166.6, 147.8, 131.7, 129.7, 128.7, 126.4, 123.0, 122.9, 73.2, 70.8-69.7 (multiple peaks), 67.2, 65.7, 66.5, 59.2, 55.1, 53.0, 50.8, 43.3, 40.7, 36.6, 28.5, 24.1, 20.1, 13.6, 12.8. 11B NMR (160.4 MHz, CD3CN): δ −14.44, −18.22. HRMS (m/z): Calcd for C134H133B12N37O32 [M]2− 1451.5574. Found 1451.5630.
Synthesis of click closomer 9b
Using the general strategy described above, 9b was synthesized from closomer 8 (100 mg, 0.04 mmol), 1-hexyne (200 mg, 2.42 mmol), copper iodide (92.3 mg, 0.48 mmol) and DIPEA (627 mg, 4.80 mmol). Pure product was obtained as a colorless viscous oil. Yield: 120.0 mg (86%). IR (neat): 2962, 2875, 1741, 1354 and 1254 cm−1. 1H NMR (400 MHz, CD3CN): δ 7.62-7.40 (m, 12H, -CH-triazole), 6.05 (bs, 1H, NH), 4.99-4.83 (m, 22H, -OCOCH2-), 4.49 (m, 2H, -CH2-NHBoc), 4.30 (m, 2H, -NCH2-PEG), 3.84 (t, 4H, -NCH2-PEG), 3.63-3.48 (m, 26H, -CH2-PEG), 3.11 (m, 4.8H, -CH2-TBA+), 2.67 (m, 22H, -CH2-Hexyne), 1.66-1.58 (m, 26H, -CH2-Hexyne, -CH2-TBA+), 1.47-1.31 (m, 40H, Boc, -CH2-Hexyne, -CH2-TBA+), 0.99 (t, 7H, J = 7.2 Hz, CH3-TBA+), 0.96-0.95 (m, 33H, -CH2-Hexyne). (Lesser proton counts for TBA+ are due to the exchange with H3O+ and Na+). 13C NMR (100.6 MHz, CD3CN): δ 166.4, 148.8, 124.0, 79.50, 79.1, 78.8, 78.5, 73.2, 71.0-70.8 (multiple peaks), 69.7, 59.2, 55.1, 52.9, 51.0, 32.3, 28.6, 25.9, 24.2, 22.9, 20.2, 14.0, 13.7. 11B NMR (160.4 MHz, CD3CN): δ −14.43, −18.21. HRMS (m/z): Calcd for C112H177B12N37O32 [M]2− 1341.7291. Found 1341.7576.
Synthesis of click closomer 9c
Using the general strategy described above, 9c was synthesized from closomer 8 (100 mg, 0.04 mmol), propargyl acetate (238 mg, 2.42 mmol), copper iodide (92.3 mg, 0.48 mmol) and DIPEA (627 mg, 4.80 mmol). Pure product was obtained as a colorless viscous oil. Yield: 120 mg (81%). IR (neat): 2961, 2876, 1742, 1354 and 1253 cm−1. 1H NMR (400 MHz, CD3CN): δ 7.82-7.71 (m, 12H, -CH-triazole), 6.02 (bs, 1H, NH), 5.17-5.15 (m, 22H, -CH2-OAc), 5.09-4.91 (m, 22H, -OCOCH2-), 4.49 (t, 2H, J = 4.4 Hz, -CH2-NHBoc), 4.31(m, 2H, -NCH2-PEG), 3.84 (t, 2H, J = 4.8 Hz, -NCH2-PEG), 3.75 (m, 2H, -CH2-PEG), 3.56-3.47 (m, 26H, -CH2-PEG), 3.11 (m, 6H, -CH2-TBA+), 2.00-1.94 (m, 33 H, -OAc), 1.63 (m, 6H, -CH2-TBA+), 1.41 (s, 9H, Boc), 1.36 (m, 6H, -CH2-TBA+), 0.98 (t, 8H, J = 7.2 Hz, CH3-TBA+) (Lesser proton counts for TBA+ is due to the exchange with H3O+ and Na+). 13C NMR (100.6 MHz, CD3CN): δ 171.3, 166.5, 143.4, 126.8, 79.5, 73.1, 70.9-70.5(multiple peaks), 69.7, 66.5, 59.2, 58.1, 55.8, 52.9, 52.9, 51.0, 43.8, 36.6, 28.5, 24.1, 20.2, 18.6, 17.3, 13.6, 12.8. 11B NMR (160.4 MHz, CD3CN): δ −14.44, −18.21. HRMS (m/z): Calcd for C101H133B12N37O54 [M]2− 1429.0017. Found 1428.9999.
Supplementary Material
Acknowledgments
This research was funded by the National Cancer Institute (Grant R21-CA114090). Authors thank Brett Meers for mass spectrometry and Pamela Cooper for editing the manuscript.
Footnotes
1H, 13C, 11B NMR and HRMS spectra of compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Danhier F, Feron O, Préat V. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]; (b) Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 2.Hawthorne MF. Angew Chem Int Ed. 1993;32:950–984. [Google Scholar]
- 3.(a) Hawthorne MF, Maderna A. Chem Rev. 1999;99:3421–3434. doi: 10.1021/cr000001+. [DOI] [PubMed] [Google Scholar]; (b) Hawthorne MF, Farha OK, Julius R, Ma L, Jalisatgi SS, Li T, Bayer MJ. ACS Symp Ser. 2005;917:312–324. [Google Scholar]
- 4.Bayer MJ, Hawthorne MF. Inorg Chem. 2004;43:2018–2020. doi: 10.1021/ic030289w. [DOI] [PubMed] [Google Scholar]
- 5.(a) Maderna A, Knobler CB, Hawthorne MF. Angew Chem Int Ed Engl. 2001;40:1661–1664. [PubMed] [Google Scholar]; (b) Li T, Jalisatgi SS, Bayer MJ, Maderna A, Khan SI, Hawthorne MF. J Am Chem Soc. 2005;127:17832–17841. doi: 10.1021/ja055226m. [DOI] [PubMed] [Google Scholar]; (c) Farha OK, Julius RL, Lee MW, Huertas RE, Knobler CB, Hawthorne MF. J Am Chem Soc. 2005;127:18243–18251. doi: 10.1021/ja0556373. [DOI] [PubMed] [Google Scholar]; (d) Lee MW, Farha OK, Hawthorne MF, Hansch C. Angew Chem Int Ed. 2007;46:3018–3022. doi: 10.1002/anie.200605126. [DOI] [PubMed] [Google Scholar]; (e) Jalisatgi SS, Kulkarni VS, Tang B, Houston ZH, Lee MW, Hawthorne MF. J Am Chem Soc. 2011;133:12382–12385. doi: 10.1021/ja204488p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Goswami LN, Chakravarty S, Lee MW, Jalisatgi SS, Hawthorne MF. Angew Chem Int Ed. 2011;50:4689–4691. doi: 10.1002/anie.201101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Grotzfeld RM, Milanov ZV, Patel HK, Lai AG, Mehta SA, Lockhart DJ. U. S. Patent Publication 0153371 A1. Conjugated Small Moleculres. 2005 Jul 14;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.