Abstract
The post-translation attachment of O-linked N-acetylglucosamine, or O-GlcNAc, to serine and threonine residues of nuclear and cytoplasmic proteins is increasingly recognized as a key regulator of diverse cellular processes. O-GlcNAc synthesis is essential for cell survival and it has been shown that acute activation of pathways, which increase cellular O-GlcNAc levels is cytoprotective; however, prolonged increases in O-GlcNAcylation have been implicated in a number of chronic diseases. Glucose metabolism via the hexosamine biosynthesis pathway plays a central role in regulating O-GlcNAc synthesis; consequently, sustained increases in O-GlcNAc levels have been implicated in glucose toxicity and insulin resistance. Studies on the role of O-GlcNAc in regulating cardiomyocyte function have grown rapidly over the past decade and there is growing evidence that increased O-GlcNAc levels contribute to the adverse effects of diabetes on the heart, including impaired contractility, calcium handling, and abnormal stress responses. Recent evidence also suggests that O-GlcNAc plays a role in epigenetic control of gene transcription. The goal of this review is to provide an overview of our current knowledge about the regulation of protein O-GlcNAcylation and to explore in more detail O-GlcNAc-mediated responses in the diabetic heart.
Keywords: O-GlcNAc, O-GlcNAcylation, diabetes, cardiomyocytes
Introduction
The dramatic increase in obesity and type 2 diabetes during the past few decades constitutes a major health burden to the both developed and developing nations (Doll et al., 2002). A major contributing factor to the morbidity and mortality associated with diabetes is the overall increased incidence in cardiovascular disease as well as the greater risk of developing heart failure (Jaffe et al., 1984, Kannel and McGee, 1979). The diabetic heart shows abnormalities in intermediate metabolism, cardiac fibrosis, endothelial and vascular smooth muscle cell function, and contractile performance (Frustaci et al., 2000). The specific mechanisms underlying the adverse effects of diabetes on the heart remain poorly understood; although, it is likely to be the result of multiple factors including poorly-controlled blood glucose, insulin resistance, and dyslipidemia. Glucose toxicity arising from chronic hyperglycemia is one factor involved in mediating the detrimental effects of diabetes via the activation of a number of pathways including the polyol pathway, increased advanced glycation end products, oxidative stress, mitochondrial dysfunction and increased activity of protein kinases (Darley-Usmar et al., 2012, Laczy et al., 2011b, Yan et al., 2003). More recently, increased levels of O-linked N-acetylglucosamine (O-GlcNAc) on nuclear and cytosolic proteins have been implicated in contributing to the pathogenesis of diabetic complications in a variety of tissues and cells including the heart (Laczy, Fülöp, 2011b, Nishio et al., 1995, Torres and Hart, 1984). Protein O-GlcNAcylation has been shown to be involved in many cellular process including signal transduction, protein trafficking, protein-protein interactions, protein degradation and regulation of gene expression, which are discussed in detail in other recent reviews (Darley-Usmar, Ball, 2012, Ngoh et al., 2010, Zachara, 2012). Therefore, in this review we will first provide an overview of our current understanding of the regulation of protein O-GlcNAcylation, and explore in greater detail the contribution of O-GlcNAcylation in mediating the adverse effects in the diabetic heart.
O-GlcNAc as a response to cellular and nutrient stress
The post-translational modification of proteins by O-GlcNAc is an atypical glycosylation consisting of the O-linked attachment of a single monosaccharide (N-acetyl-D-glucosamine, O-GlcNAc) to serine and threonine residues of nuclear and cytoplasmic proteins (Torres and Hart, 1984). In contrast to classical protein glycosylation in oligosaccharide structures, O-GlcNAcylation is a dynamic process involving the reversible addition of a single O-GlcNAc moiety (Hu et al., 2010). Since O-GlcNAc modifies serine and threonine residues and can directly affect protein function, and often thought of as a metabolically regulated protein modification that is analogous to protein phosphorylation (Copeland et al., 2008). Interestingly, O-GlcNAc and phosphate moieties can compete for the same proteins residues, and proteins can also be both O-GlcNAcylated and phosphorylated. Indeed, it is increasingly evident that there is a complex interplay between phosphorylation and O-GlcNAcylation (Wang et al., 2008). The addition of UDP-GlcNAc onto target proteins is catalyzed by the UDPGlcNAc: polypeptide O-β-N-acetyl glucosaminyltransferase, or O-GlcNAc transferase (OGT); conversely, the O-β-N-acetylglucosaminidase hexosamindase C, O-GlcNAcase (OGA), is responsible to the removal of this sugar moiety from proteins. The fact that only two enzymes regulate the addition and detachment of the O-GlcNAc moiety irrespective of the target protein is an obvious contrast to protein phosphorylation, which is regulated by hundreds of specific kinases and phosphatases. It should also be noted that both OGT and OGA can themselves be O-GlcNAcylated and both also interact with other proteins to regulate downstream processes (Kreppel et al., 1997). Taken together, this suggests that O-GlcNAc may serve as a key player in signal transduction. It is of note that much of our understanding of the role of O-GlcNAc in mediating cellular function has been in the context of dysregulation of O-GlcNAc turnover in the setting of chronic disease states such as cancer, neurodegeneration, and diabetes (Hart et al., 2011); however, more recent studies have demonstrated that active synthesis of OGlcNAc is essential for cell survival and indeed that acutely augmenting cellular OGlcNAc levels is cytoprotective (Chatham and Marchase, 2010).
UDP-GlcNAc, the substrate for OGT, is synthesized by the hexosamine biosynthesis pathway (HBP), and flux through the HBP is largely regulated by the metabolism of glucose by the enzyme, glucosamine–fructose 6-phosphate aminotransferase, GFAT. Thus, it is commonly assumed that protein O-GlcNAcylation is principally regulated by glucose availability and that the increase in O-GlcNAc levels seen in a response to diabetes is therefore due principally to hyperglycemia. However, work by Zachara and coworkers clearly demonstrated that cellular stressors such as heat, free radicals, and ultraviolet (UV) radiation lead to increased O-GlcNAc levels (Zachara et al., 2004). Moreover, additional studies have reported, somewhat paradoxically, that glucose deprivation also increases O-GlcNAc levels and that this may be due at least in part to the upregulation of OGT and a concomitant downregulation of OGA (Taylor et al., 2008). Furthermore, we have recently reported that stress induced increases in O-GlcNAcylation also appear to be dependent on Ca2+ activation of calmodulin-dependant protein kinase II (CaMKII) (Zou et al., 2012). It is also increasingly clear that O-GlcNAc levels are not simply regulated by substrate availability. Thus, the increased O-GlcNAcylation associated with diabetes is likely far more complicated than first appears, and may be a result not only from increased glucose flux through the HBP, but also be due to other cellular stress responses such as oxidative stress or even reduced glucose uptake, all of, which are known to occur in diabetes.
O-GlcNAcylation, glucotoxicity, lipotoxicity, and insulin resistance
The heart preferentially uses fatty acids and lactate over glucose for oxidative energy production, and although diabetes is commonly associated with increased fatty acid utilization, it is less well appreciated that this is accompanied by decreased lactate oxidation with no change in glucose oxidation (Wang et al., 2005). Thus, any detrimental effect of glucose on the heart is likely not a consequence of alterations in oxidative metabolism and there is increasing evidence to support the notion that hyperglycemia may upregulate protein O-GlcNAcylation resulting in abnormal downstream protein signaling. For example diabetic patients and rodents exhibit increased cardiomyocyte loss and hypertrophy (Fiordaliso et al., 2001, Frustaci, Kajstura, 2000). Importantly, numerous transcription factors that mediate cell growth and survival are known to be O-GlcNAcylated, including specific protein-1 (Sp1), activator protein-1 (AP), cAMP response element binding protein-1 (CREB1), host cell factor-1 (HCF1), nuclear factor of activated T-cells (NFAT), yin yang-1 (YY1), tumor suppressor gene-53 (p53), pancreatic duodenal homeobox-1 (PDX1), forkhead box (FOXO), and myelocytomatosis oncogene cellular homolog (myc) (Zachara et al., 2006). Taken together these data suggest that O-GlcNAcylation of promoters may contribute directly to the cell survival and cell growth, which could alter cardiomyocyte function and cardiomyocyte response to stress.
As mentioned above, fatty acid oxidation is the main contributor to ATP production in cardiomyocytes, which is increased in the type 2 diabetic heart in the absence of hyperglycemia or increased circulating lipids (Buchanan et al., 2005, Wang, Lloyd, 2005). These data suggest that metabolic dysfunction occurs prior to the onset of overt diabetes and may possibly be due to other mechanisms besides the Randle Cycle. Interestingly, fatty acid transporters (FAT/CD36) are responsible for ~ 50–80% of the fatty acid uptake in the heart (Brinkmann et al., 2002, Kuang et al., 2004). FAT/CD36 translocates from the intracellular storage compartments to plasma membrane in response to stimuli such as insulin or increased cardiac work via AMPK and ACC regulatory mechanism, and thereby facilitating fatty acid oxidation (Coort et al., 2007, Luiken et al., 2003). However, we have shown that acute increases of O-GlcNAc through the activation of HBP by increased glucosamine alters cardiac metabolic pathways, specifically fatty acid oxidation by increasing plasma membrane levels of FAT/CD36 independently of AMPK or ACC activity (Laczy et al., 2011a). Also of interest, we also demonstrated that OGT is associated with FAT/CD36; in which FAT/CD36 is also O-GlcNAcylated (Laczy, Fulop, 2011a). While the downstream ramifications of FAT/CD36 O-GlcNAcylation are not known, this may be one mechanism underlying the shift in substrate utilization in diabetic hearts (Figure 1.1). It is also worth noting that while O-GlcNAcylation may represent a novel mechanism for regulating fatty acid metabolism, Weigert et al, reported that the addition of palmitate to myotubes, significant increased GFAT mRNA and protein levels as well as UDP-GlcNAc levels (Weigert et al., 2003). Thus, since diabetes is also associated with dyslipidemia, this could also contribute to the increase in O-GlcNAcylation.
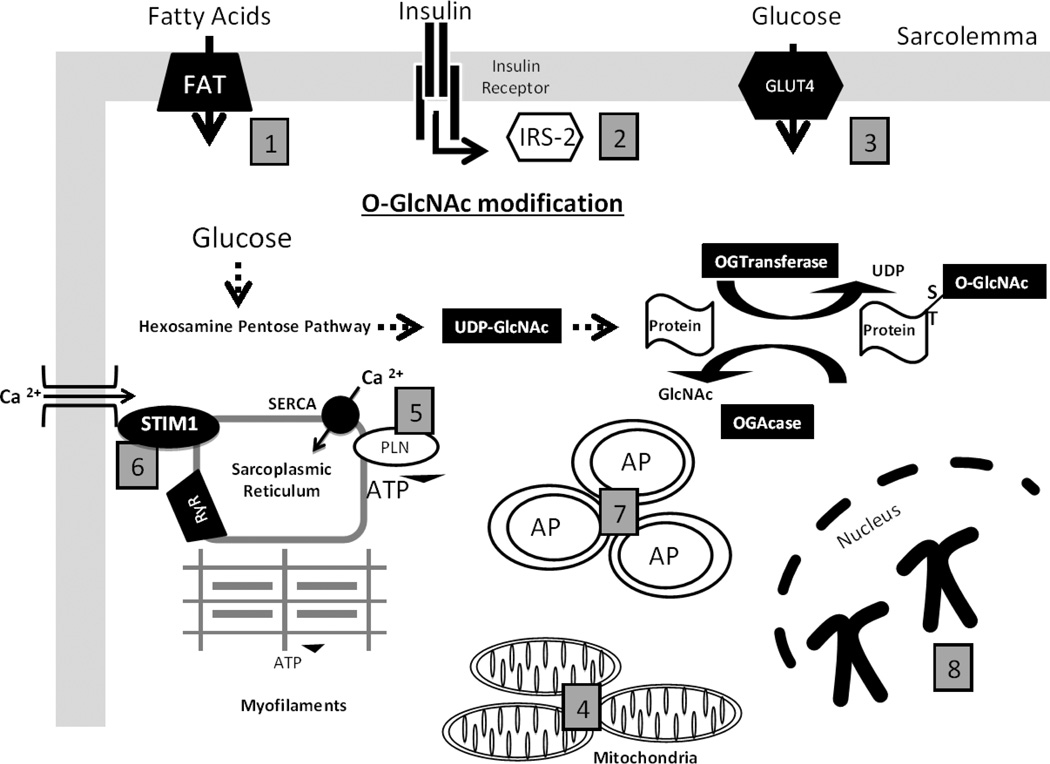
Figure 1. The roles of O-GlcNAc-mediated responses in the diabetic cardiomyocyte.
(1) Fatty Acid transporters (FAT36) is O-GlcNAc modified in the cardiomyocyte to shift substrate utilization in the diabetic heart. (2) Insulin Receptor Substrate (IRS) is O-GlcNAc modified to impair downstream insulin signaling and impair glucose transporters translocation to the membrane of the cardiomyocyte. (3) Glucose Transporter (GLUT) -4 is O-GlcNAc modified to impair glucose transport into the cardiomyocyte. (4) O-GlcNAcylation of mitochondrial proteins have been reported to inhibit mitochondrial respiratory capacity and increase mitochondrial fission, thereby impairing energy production. (5) Phospholamban (PLN) is O-GlcNAc modified to decrease Ca2+ removal by sarcroplasmic reticulum Ca2+ -ATPase (SERCA). (6) Stromal interacting molecule 1 (STIM1), a sarcroplasmic reticulum membrane protein, which regulates O-GlcNAc store operated calcium entry is O-GlcNAcylated and this may contribute to impaired cardiomyocyte Ca2+ signaling. (7) Autophagosomes (AP) are shown to represent potential O-GlcNAc regulation of autophagy, as Beclin-1 an early activator of autophagy is O-GlcNAcylated. (8) Chromosomes are representative of O-GlcNAc mediated regulation of chromatin, which are shown to modulate chromatin remodeling, gene transcription, and protein synthesis.
Systemic insulin resistance is defined as the inability of insulin to trigger appropriate glucose uptake of a cell and is a characteristic of both obesity and type 2 diabetes. While O-GlcNAc is associated with the development of insulin resistance, it is unclear whether it has a causal role in this process, although it should be noted that insulin resistance is associated with stressors such as oxidative stress and inflammation, both of which can upregulate protein O-GlcNAcylation. The upregulation of the HBP induces insulin resistance in skeletal muscle (Hawkins et al., 1997) and adipocytes (Vosseller et al., 2002); however, additional studies are warranted to elucidate O-GlcNAc mediated insulin resistance in the context of cardiomyocytes. However, O-GlcNAc appears to play a role in insulin signaling by inhibiting the phosphorylation and subsequent activation of AKT which then alters the downstream signaling cascade; this appears to occur as the insulin receptor substrate-1 (IRS-1) becomes O-GlcNAcylated rather than phosphorylated as AKT is no longer active to phosphorylate this site (Vosseller, Wells, 2002) (Figure 1.2). Interestingly, GLUT4, the glucose transporter in the heart, is also O-GlcNAc-modified (Buse et al., 2002); however, whether this modification contributes to the impaired translocation of GLUT4 to the membrane or glucose uptake remains to be determined in diabetic cardiomyocytes (Figure 1.3).
A number of studies have suggested that O-GlcNAcylation of mitochondrial proteins may also contribute to the adverse effects of hyperglycemia (Gawlowski et al., 2012, Hu et al., 2009, Makino et al., 2011) (Figure 1.4). For example, Hu et al., found that in neonatal cardiomyocytes exposed to high glucose levels mitochondrial respiratory chain complex members were shown to be O-GlcNAc modified and this was associated with with impaired activity of Complex I, III, and IV in addition to lower mitochondrial calcium and cellular ATP content. Moreover, studies have also reported that proteins such as dynamin-related protein-1 (DRP1) and optical atrophy 1 (OPA1), which play a role in regulating mitochondrial fission and fusion are also targets for O-GlcNAcylation and that this may also contribute to impaired mitochondrial function associated with diabetes (Gawlowski, Suarez, 2012, Makino, Suarez, 2011).
O-GlcNAcylation and calcium handling
Diastolic dysfunction or increased ventricular stiffness is one of the most commonly recognized functional abnormalities seen diabetic patients and is a key contributor to the development of diabetic cardiomyopathy and heart failure (Fang et al., 2004). In isolated cardiomyocytes impaired relaxation associated with prolonged action potential and decreased rate of Ca2+ removal have been observed within only a few days of STZ-induced diabetes (Ren and Davidoff, 1997); which can be mimicked by treating normal cardiomyocytes with high glucose media for only 24 hours as well as glucosamine (Ren et al., 1997). These early changes are linked to impaired sarcoplasmic reticulum Ca2+-ATPase (SERCA) function in the absence of changes in protein levels of SERCA or phospholamban (PLB) phosphorylation (Dutta et al., 2002). Interestingly cardiomyocytes exposed to high glucose also exhibit impaired Ca2+ handling and relaxation, which was associated with increased protein O-GlcNAcylation compared to untreated cardiomyocytes (Ren, Gintant, 1997). Of particular note, increasing OGT expression in neonatal cardiomyocytes, mimicked the effects of both high glucose and glucosamine on Ca2+ transients and this was associated with decreased SERCA mRNA and protein levels (Clark et al., 2003). Subsequently, it was shown that in increasing cardiac OGA expression in a murine model of STZ-induced diabetes restored SERCA protein levels and increased PLB phosphorylation to improve cardiac function (Hu et al., 2005). Taken together, these data suggest a novel regulatory mechanism by which O-GlcNAc impairs calcium cycling and excitation coupling of the cardiomyocyte that is observed in diabetes (Figure 1.5).
The effect of diabetes on SERCA protein levels may also be attributed to O-GlcNAcylation of the transcription factor Sp1. It has been shown that Sp1 transcription factors are important for the SERCA gene regulation (Brady et al., 2003) as this transcription factor is O-GlcNAcylated in the presence of high glucose (Clark, McDonough, 2003). It is thought that O-GlcNAc may possibly contribute to the reduced expression of SERCA (Walgren et al., 2003, Yang et al., 2001). However, it is important to note that O-GlcNAc can also directly affect protein-protein interactions (Yokoe et al., 2010) as acute regulation of SERCA function is through a direct protein-protein binding with PLB, which is regulated by phosphorylation of the serine (Ser) 16 by cAMP-dependant protein kinase (PKA) (Asahi et al., 1999, Kimura et al., 1997, Toyofuku et al., 1994). In the unphosphorylated state, PLB inhibits SERCA function by lowering its affinity to calcium and which is reversed by phosphorylation (Morris et al., 1991). Interestingly, the Ser 16 of PLB can also be O-GlcNAcylated, which disrupts the association of PLB to SERCA to delay the reuptake of Ca2+ by SERCA (Yokoe, Asahi, 2010). Taken together these studies support the notion that increased O-GlcNAcylation can acutely modulate SERCA function by direct modification of PLB. Therefore, inhibition PLB phosphorylation in the setting of prolonged increases in O-GlcNAcylation may lead to a transcriptional mechanism mediated by Sp1 to decrease SERCA protein. Nonetheless, in both cases the outcome is the same, namely impaired Ca2+ reuptake into the sarcoplasmic reticulum (SR) contributing to slower cardiomyocyte relaxation.
It is well established that in addition to its role in excitation contraction coupling, Ca2+ also acts as an important second messenger for diverse signals including angiotensin-II (Ang II), endothelin-1, ß-adrenergic agents, and mechanical stretch to trigger hypertrophic response of the cardiomyocyte (Molkentin, 2000). The two major Ca2+-dependent signaling pathways result in the activation of nuclear factor of activated T cell (NFAT) through calcineurin or the regulation of histone deacetylase activity through CaMK-II and/or protein kinase D. For the Ca2+-dependant regulation of nuclear histone deacetylase activity, it has been demonstrated that inositol triphosphate (IP3)-dependant release of Ca2+ from the nuclear envelope is responsible for CaM-kinase IIdependant activation of protein kinase D (Zhang et al., 2004). Marchase and colleagues demonstrated that in both neonatal and adult cardiomyocytes the Ca2+ activation of the calcineurin-NFAT axis is mediated via the non-voltage gated storeoperated calcium entry (SOCE) pathway (Hunton et al., 2002, Pang et al., 2002). Interestingly, it was also demonstrated that activation of hypertrophic signaling by α-adrenergic agonists was inhibited by the sustained HBP activation in neonatal cardiomyocytes (Hunton, Lucchesi, 2002). Moreover, the positive inotropic effects of α-adrenergic agonists, but not β-adrenergic agonists, was attenuated by the acute activation of the HBP in adult perfused rat heart (Pang et al., 2004). Subsequently, it has also been shown that increasing O-GlcNAc levels by inhibiting OGA also attenuated the Ang II induced increase in cytosolic Ca2+ (Nagy et al., 2006, Pang, Hunton, 2002), suggesting that O-GlcNAc may mediate the hypertrophic response in diabetic cardiomyocytes.
SOCE is well accepted as the predominant Ca2+ signaling pathway in non-excitable cells, and it is now widely recognized that stromal interacting molecule 1 (STIM1) plays a central role in regulating SOCE (Liou et al., 2005, Roos et al., 2005, Zhang et al., 2005). STIM1 is an endoplasmic reticulum (ER) membrane protein and, in response to IP3-induced ER Ca2+ release, it forms subplasmalemmal clusters which couples to the plasma membrane composing of a pore-forming subunit of the Ca2+ release-activated Ca2+ (CRAC) channel protein, Orai1 (Mercer et al., 2006, Peinelt et al., 2006, Xu et al., 2006, Zhang et al., 2006). The role of SOCE remains under explored in the heart; however, a few recent studies have implicated STIM1 as a potential mediator of cardiac hypertrophic signaling. We have recently shown that in the adult cardiomyocytes STIM-1 co-localizes with both SERCA and the ryanodine receptor (RyR) in the adult rat heart and that in neonatal cardiomyocytes in response to SR Ca2+ depletion, STIM1 forms puncta and interacts with Orai1 consistent with its function in non-excitable cells (Zhu, 2010). Of particular relevance to this discussion, we have also demonstrated that increasing O-GlcNAc levels either by activating the HBP with glucosamine or inhibition of OGA attenuates STIM1 puncta formation and subsequent SOCE; preliminary studies also indicate that STIM1 is a target for O-GlcNAcylation (Zhu, 2010), as shown in Figure 1.6. Thus, we postulate that O-GlcNAc modification of STIM1 is a potential mechanism to account for the hyperglycemia-mediated inhibition of α-adrenergic induced cardiomyocyte hypertrophy.
O-GlcNAc and hypertrophy in the diabetic heart
Consistent with the notion that increased O-GlcNAc levels might impair normal cardiomyocyte hypertrophic signaling, we recently demonstrated that adult cardiomyocytes isolated from type 2 diabetic db/db mice exhibited a blunted response to both Ang II and phenylephrine (Marsh et al., 2011). The sensitivity to both hypertrophic agonists was normalized by inhibition of glucose entry into the HBP with GFAT inhibitors azaserine and 6-diazo-5-L-oxonorleucine (DON); furthermore, augmenting O-GlcNAc levels in normal cardiomyocytes with either glucosamine or OGA inhibition mimicked the effects of diabetes (Marsh, Dell'italia, 2011). These data lend further credence to the notion that diabetes leads to impaired hypertrophic signaling. While hypertrophy is typically associated with adverse outcomes, it should be noted that the initial response is an acute adaptation to increased hemodynamic stress; thus, inhibition of pro-hypertrophic signaling could result in a maladaptive response. Indeed, we found that combining relatively mild models of diabetes and pressure overload induced hypertrophy in rats, which resulted in an increase in apoptosis and significantly impaired cardiac function that was not present with either stress alone (Marsh et al., 2012).
It is noteworthy that in contrast to these studies indicating that elevated O-GlcNAc levels impair cardiac hypertrophy, a recent study reported that in cardiac tissue from patients with hypertrophy and heart failure of various etiologies exhibited increased O-GlcNAc levels (Lunde et al., 2012). Moreover, others have shown that knockdown of OGT impaired subsequent hypertrophic response and accelerated the progression to heart failure in a mouse model pressure overload (Facundo et al., 2012b). Furthermore, the same group showed that OGT was not only required for normal hypertrophic signaling, but also that increased OGT expression was sufficient to activate the transcriptional machinery necessary for hypertrophy (Watson et al., 2010). These contrasting and apparently contradictory results, namely that increased O-GlcNAcylation is both necessary for normal hypertrophic signaling and impairs hypertrophic signaling, represents a paradox that is quite common in studies of O-GlcNAcylation in the heart. Although these studies provide novel insights regarding the emerging role of protein O-GlcNAcylation in regulating cardiac hypertrophy, the role of O-GlcNAc signaling is likely contextual and dependant on the setting in which the hypertrophy occurs.
The mechanisms underlying this paradoxical effect are likely to be multifactorial and could occur at the transcriptional level or directly alter downstream signal transduction pathways. Indeed, O-GlcNAc-dependent NFAT activation appears essential for hypertrophic signaling (Facundo et al., 2012a). Moreover, we recently found that O-GlcNAc blunts the induction of autophagy, a cellular response that is essential for cell survival and the hypertrophic response in the heart (Marsh, Powell, 2012). Interestingly, many of the stressors that increase O-GlcNAc and autophagy are the same such as nutrient deprivation, glucosamine, and oxidative stress. Crosstalk between autophagy and apoptosis is mediated by the competitive binding of BNIP3 (Bcl-2 adenovirus E1B 19 kDa-interacting protein 3) and Beclin-1 (autophagic marker) to Bcl-2 (an antiapoptotic marker) (Levine et al., 2008). Intriguingly, we demonstrated for the first time that both Beclin-1 and Bcl-2 are targets for O-GlcNAcylation (Marsh, Powell, 2012). While further studies are clearly warranted; these studies raise a novel concept that O-GlcNAc turnover may mediate the balance between the process of cell survival (autophagy) or cell death (apoptosis) as shown in Figure 1.7.
O-GlcNAc and epigenetic regulation
The majority of research in the O-GlcNAc field has centered on the effect of O-GlcNAcylation of transcription factors and proteins, but there is increasing evidence that the O-GlcNAc pathway is also a key regulator of gene transcription at the level of chromatin. Chromatin consists of DNA wrapped around a backbone formed by core histones (H2A, H2B, H3, H4) and bound together by linker histones (H1, H5) that stabilize the structure (Jenuwein and Allis, 2001). Tightly bound, or compacted, chromatin restricts access to DNA and consequently represses transcription, replication, and DNA repair. Compaction and relaxation of the chromatin structure is regulated by post-translational modifications of the core histones and, as the DNA sequence is not altered, this process is often referred to as epigenetic regulation. While modifications such as methylation, acetylation, phosphorylation, and sumoylation are known to regulate histones, recent evidence suggests that protein O-GlcNAcylation of histones is also a key mediator of gene transcription (Sakabe et al., 2010). The downstream effect of histone O-GlcNAcylation is still unclear although O-GlcNAc modification of H2B appears to promote ubiquitination in vitro (Fujiki et al., 2011). RNA polymerase II is also a target for O-GlcNAcylation (Comer and Hart, 2001) although the effect of this modification on transcription is not yet known.
Protein complexes can also regulate chromatin structure and gene transcription; for example, mSin3A and histone deacetylase 1 (HDAC1) are common components of repressor complexes. Interestingly, OGT interacts with mSin3A and HDAC1 to promote gene silencing (Yang et al., 2002). While the TPR domain of OGT is necessary for binding to mSin3A, both the TPR and catalytic domains are required for full repression. It is also of note that both mSin3A and HDAC1 are O-GlcNAcylated under conditions of high glucose (Yang, Zhang, 2002); the downstream effects on gene transcription are not entirely clear. OGT also interacts with other corepressors such as nuclear receptor corepressor (NCoR) and silencing mediator of retinoic acid and thyroid hormone receptor (SMRT), as a result NCoR1 and SMRT are both increased with increased cellular O-GlcNAc (Bowe et al., 2006). OGA also has intrinsic histone acetyltransferase (HAT) activity in vitro (Toleman et al., 2004) and is often part of the OGT-mSin3AHDAC1 complex (Whisenhunt et al., 2006).
Now that it has been established that chromatin proteins are O-GlcNAcylated, and that OGT and OGA participate in chromatin remodeling and gene repression, how does this affect gene transcription, protein synthesis and downstream signal transduction in diabetes? To our knowledge, no studies have yet examined the combined effect of O-GlcNAc pathway and chromatin in the non-diabetic or diabetic heart; however, there has been extensive work on the role of chromatin remodeling and epigenetic processes in the progression of pancreatic beta cell pathology in type 2 diabetes (Pinney and Simmons, 2010). In addition, pharmacological inhibition of HDAC activity triggers cardioprotection (Zhao et al., 2003), improves skeletal muscle insulin sensitivity in diabetes (Takigawa-Imamura et al., 2003) and protects against diabetic retinopathy and nephropathy (Crosson et al., 2010, Lee et al., 2007); all of these processes have been linked to protein O-GlcNAcylation (Figure 1.8).
In the non-diabetic heart, transcriptional control of pathological cardiac hypertrophy, including regulation of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) synthesis, occurs via chromatin remodeling induced by reduced activation of the repressor element 1 (RE-1)-silencing transcription factor (REST) complex (Bingham et al., 2007), also known as neuronal restrictive silencing factor (NRSF). REST is a key transcription repressor found in numerous tissues, including the heart, and interacts with corepressors such as mSin3A to form a complex that recruits HDAC1 and HDAC2, thus altering chromatin structure resulting in gene silencing of hypertrophic mediators (Kuwahara et al., 2001). As we recently demonstrated that O-GlcNAc modulates hypertrophic signaling in both diabetic and non-diabetic cardiomyocytes (Marsh, Dell'italia, 2011) and that others reported that both mSin3A and HDAC1 are O-GlcNAcylated (Yang, Zhang, 2002), it is possible that chronic elevation of O-GlcNAcylation in the diabetic heart may regulate hypertrophy upstream of gene transcription.
If O-GlcNAc, OGT and OGA do indeed regulate chromatin and gene transcription in the diabetic heart, an added layer of complexity will be added to the field of O-GlcNAc and diabetic complications. Until now, applied research in the field has primarily been focused on O-GlcNAcylation of transcription factors and signaling proteins, but we may have to consider the possibility that O-GlcNAc induces epigenetic changes in diabetic hearts. If this proves to be true, will this mean that the epigenetic abnormalities seen in diabetic hearts will be inherited by their offspring? Whether epigenetic information can be inherited is currently being debated (Migicovsky and Kovalchuk, 2011), but if this does occur, the current epidemic of metabolic syndrome and type 2 diabetes could lead to inheritance of what is now a lifestyle-induced disease, resulting in irreversible type 2 diabetes in future generations and leading to an exponential and unsustainable rise in the healthcare burden.
Conclusion
In summary, over the past decade our understanding of the role of O-GlcNAc in regulating cardiomyocyte function has grown rapidly and it is now recognized as playing a critical role in regulating numerous cardiomyocyte functions. O-GlcNAc levels are chronically elevated in response to diabetes and studies have implicated this increase in O-GlcNAcylation to many of the adverse effects of diabetes on the heart including metabolic dysregulation, impaired Ca2+ handling and abnormal hypertrophic signaling. Much of the work in this field has been focused on O-GlcNAc regulation of cellular signaling pathways and transcriptional regulation; however, there is now growing evidence to suggest that O-GlcNAcylation may also contribute to epigenetic regulation. The possibility that O-GlcNAc, OGT, and OGA may regulate chromatin and gene transcription in the diabetic heart opens up an entirely new avenue for research in the role of O-GlcNAc and diabetic complications.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute HL104549 (SAM), HL101192 and HL079364 (JCC), and the Washington State University of Office Research (SAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asahi M, Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Transmembrane Helix M6 in Sarco(endo)plasmic Reticulum Ca2+-ATPase Forms a Functional Interaction Site with Phospholamban. Journal of Biological Chemistry. 1999;274:32855–32862. doi: 10.1074/jbc.274.46.32855. [DOI] [PubMed] [Google Scholar]
- Bingham AJ, Ooi L, Kozera L, White E, Wood IC. The repressor element 1-silencing transcription factor regulates heart-specific gene expression using multiple chromatin-modifying complexes. Mol Cell Biol. 2007;27:4082–4092. doi: 10.1128/MCB.00269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe DB, Sadlonova A, Toleman CA, Novak Z, Hu Y, Huang P, et al. O-GlcNAc Integrates the Proteasome and Transcriptome To Regulate Nuclear Hormone Receptors. Mol Cell Biol. 2006;26:8539–8550. doi: 10.1128/MCB.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady M, Koban MU, Dellow KA, Yacoub M, Boheler KR, Fuller SJ. Sp1 and Sp3 transcription factors are required for trans-activation of the human SERCA2 promoter in cardiomyocytes. Cardiovascular Research. 2003;60:347–354. doi: 10.1016/s0008-6363(03)00529-7. [DOI] [PubMed] [Google Scholar]
- Brinkmann J, Abumrad N, Ibrahimi A, van der Vusse G, Glatz J. New insights into longchain fatty acid uptake by heart muscle: a crucial role for fatty acid translocase/CD36. Biochemical Journal. 2002;367:9. doi: 10.1042/BJ20020747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- Buse MG, Robinson KA, Marshall BA, Hresko RC, Mueckler MM. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am J Physiol Endocrinol Metab. 2002;283:E241–E250. doi: 10.1152/ajpendo.00060.2002. [DOI] [PubMed] [Google Scholar]
- Chatham J, Marchase RB. Protein O-GlcNacylation: A critical regulator of the cellular response to stress. Curr Signal Transduc Ther. 2010;5:10. doi: 10.2174/157436210790226492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, et al. Diabetes and the Accompanying Hyperglycemia Impairs Cardiomyocyte Calcium Cycling through Increased Nuclear O-GlcNAcylation. Journal of Biological Chemistry. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- Comer FI, Hart GW. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry. 2001;40:7845–7852. doi: 10.1021/bi0027480. [DOI] [PubMed] [Google Scholar]
- Coort S, Bonen A, van der Vusse G, Glatz J, Luiken J. Cardiac substrate uptake and metabolism in obesity and type-2 diabetes: Role of sarcolemmal substrate transporters. Molecular and Cellular Biochemistry. 2007;299:5–18. doi: 10.1007/s11010-006-9372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. American Journal of Physiology - Endocrinology And Metabolism. 2008;295:E17–E28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson CE, Mani SK, Husain S, Alsarraf O, Menick DR. Inhibition of histone deacetylase protects the retina from ischemic injury. Invest Ophthalmol Vis Sci. 2010;51:3639–3645. doi: 10.1167/iovs.09-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley-Usmar VM, Ball LE, Chatham JC. Protein O-linked β-N-acetylglucosamine: A novel effector of cardiomyocyte metabolism and function. Journal of Molecular and Cellular Cardiology. 2012;52:538–549. doi: 10.1016/j.yjmcc.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S, Paccaud F, Bovet P, Burnier M, Wietlisbach V. Body mass index, abdominal adiposity and blood pressure: consistency of their association across developing and developed countries. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26:48–57. doi: 10.1038/sj.ijo.0801854. [DOI] [PubMed] [Google Scholar]
- Dutta K, Carmody MW, Cala SE, Davidoff AJ. Depressed PKA Activity Contributes to Impaired SERCA Function and is Linked to the Pathogenesis of Glucose-induced Cardiomyopathy. Journal of Molecular and Cellular Cardiology. 2002;34:985–996. doi: 10.1006/jmcc.2002.2035. [DOI] [PubMed] [Google Scholar]
- Facundo HT, Brainard RE, Watson LJ, Ngoh GA, Hamid T, Prabhu SD, et al. O-GlcNAc Signaling is Essential for NFAT-Mediated Transcriptional Reprogramming During Cardiomyocyte Hypertrophy. Am J Physiol Heart Circ Physiol. 2012a doi: 10.1152/ajpheart.00775.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facundo HT, Brainard RE, Watson LJ, Ngoh GA, Hamid T, Prabhu SD, et al. O-GlcNAc Signaling is Essential for NFAT-Mediated Transcriptional Reprogramming During Cardiomyocyte Hypertrophy. American Journal of Physiology - Heart and Circulatory Physiology. 2012b doi: 10.1152/ajpheart.00775.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ZY, Prins JB, Marwick TH. Diabetic Cardiomyopathy: Evidence, Mechanisms, and Therapeutic Implications. Endocrine Reviews. 2004;25:543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, et al. Hyperglycemia Activates p53 and p53-Regulated Genes Leading to Myocyte Cell Death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, et al. Myocardial Cell Death in Human Diabetes. Circulation Research. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- Fujiki R, Hashiba W, Sekine H, Yokoyama A, Chikanishi T, Ito S, et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–560. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, et al. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross Talk Between O-GlcNAcylation and Phosphorylation: Roles in Signaling, Transcription, and Chronic Disease. Annual Review of Biochemistry. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest. 1997;99:2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Letters. 2010;584:2526–2538. doi: 10.1016/j.febslet.2010.04.044. [DOI] [PubMed] [Google Scholar]
- Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, et al. Adenovirus-Mediated Overexpression of O-GlcNAcase Improves Contractile Function in the Diabetic Heart. Circulation Research. 2005;96:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, et al. Increased Enzymatic O-GlcNAcylation of Mitochondrial Proteins Impairs Mitochondrial Function in Cardiac Myocytes Exposed to High Glucose. Journal of Biological Chemistry. 2009;284:547–555. doi: 10.1074/jbc.M808518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell'Italia LJ, Marchase RB. Capacitative Calcium Entry Contributes to Nuclear Factor of Activated T-cells Nuclear Translocation and Hypertrophy in Cardiomyocytes. Journal of Biological Chemistry. 2002;277:14266–14273. doi: 10.1074/jbc.M107167200. [DOI] [PubMed] [Google Scholar]
- Jaffe A, Spadaro J, Schechtman K, Roberts R, Geitman E, Sobel B. Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. AM Heart J. 1984;108:6. doi: 10.1016/0002-8703(84)90541-6. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the Histone Code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Kannel WB, McGee DL. Diabetes and cardiovascular disease: The framingham study. JAMA: The Journal of the American Medical Association. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Phospholamban Inhibitory Function Is Activated by Depolymerization. Journal of Biological Chemistry. 1997;272:15061–15064. doi: 10.1074/jbc.272.24.15061. [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Blomberg MA, Hart GW. Dynamic Glycosylation of Nuclear and Cytosolic Proteins. Journal of Biological Chemistry. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- Kuang M, Febbraio M, Wagg C, Lopaschuk GD, Dyck JRB. Fatty Acid Translocase/CD36 Deficiency Does Not Energetically or Functionally Compromise Hearts Before or After Ischemia. Circulation. 2004;109:1550–1557. doi: 10.1161/01.CIR.0000121730.41801.12. [DOI] [PubMed] [Google Scholar]
- Kuwahara K, Saito Y, Ogawa E, Takahashi N, Nakagawa Y, Naruse Y, et al. The neuron-restrictive silencer element-neuron-restrictive silencer factor system regulates basal and endothelin 1-inducible atrial natriuretic peptide gene expression in ventricular myocytes. Mol Cell Biol. 2001;21:2085–2097. doi: 10.1128/MCB.21.6.2085-2097.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laczy B, Fulop N, Onay-Besikci A, Des Rosiers C, Chatham JC. Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation. PLoS ONE. 2011a;6:e18417. doi: 10.1371/journal.pone.0018417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laczy B, Fülöp N, Onay-Besikci A, Des Rosiers C, Chatham JC. Acute Regulation of Cardiac Metabolism by the Hexosamine Biosynthesis Pathway and Protein O-GlcNAcylation. PLoS ONE. 2011b;6:e18417. doi: 10.1371/journal.pone.0018417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HB, Noh H, Seo JY, Yu MR, Ha H. Histone deacetylase inhibitors: a novel class of therapeutic agents in diabetic nephropathy. Kidney Int Suppl. 2007:S61–S66. doi: 10.1038/sj.ki.5002388. [DOI] [PubMed] [Google Scholar]
- Levine B, Sinha SC, Kroemer G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Do Heo W, Jones JT, Myers JW, Ferrell JE, Jr, et al. STIM Is a Ca2+ Sensor Essential for Ca2+-Store-Depletion-Triggered Ca2+ Influx. Current Biology. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiken JJFP, Coort SLM, Willems J, Coumans WA, Bonen A, van der Vusse GJ, et al. Contraction-Induced Fatty Acid Translocase/CD36 Translocation in Rat Cardiac Myocytes Is Mediated Through AMP-Activated Protein Kinase Signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- Lunde IG, Aronsen JM, Kvaløy H, Qvigstad E, Sjaastad I, Tønnessen T, et al. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiological Genomics. 2012;44:162–172. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, et al. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;300:R1296–R1302. doi: 10.1152/ajpregu.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh SA, Dell'italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids. 2011;40:819–828. doi: 10.1007/s00726-010-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh SA, Powell PC, Dell’Italia LJ, Chatham JC. Cardiac O-GlcNAcylatiion blunts autophagic signaling in the diabetic heart Life Sciences. 2012 doi: 10.1016/j.lfs.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JC, DeHaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, et al. Large Store-operated Calcium Selective Currents Due to Co-expression of Orai1 or Orai2 with the Intracellular Calcium Sensor, Stim1. Journal of Biological Chemistry. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migicovsky Z, Kovalchuk I. Epigenetic memory in mammals. Front Genet. 2011;2:28. doi: 10.3389/fgene.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD. Calcineurin and Beyond : Cardiac Hypertrophic Signaling. Circulation Research. 2000;87:731–738. doi: 10.1161/01.res.87.9.731. [DOI] [PubMed] [Google Scholar]
- Morris GL, Cheng HC, Colyer J, Wang JH. Phospholamban regulation of cardiac sarcoplasmic reticulum (Ca(2+)-Mg2+)-ATPase. Mechanism of regulation and site of monoclonal antibody interaction. Journal of Biological Chemistry. 1991;266:11270–11275. [PubMed] [Google Scholar]
- Nagy T, Champattanachai V, Marchase RB, Chatham JC. Glucosamine inhibits angiotensin II-induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-linked N-acetylglucosamine. American Journal of Physiology - Cell Physiology. 2006;290:C57–C65. doi: 10.1152/ajpcell.00263.2005. [DOI] [PubMed] [Google Scholar]
- Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc Signaling in the Cardiovascular System. Circulation Research. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y, Warren CE, Buczek-Thomas JA, Rulfs J, Koya D, Aiello LP, et al. Identification and characterization of a gene regulating enzymatic glycosylation which is induced by diabetes and hyperglycemia specifically in rat cardiac tissue. The Journal of Clinical Investigation. 1995;96:1759–1767. doi: 10.1172/JCI118221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Bounelis P, Chatham JC, Marchase RB. Hexosamine Pathway Is Responsible for Inhibition by Diabetes of Phenylephrine-Induced Inotropy. Diabetes. 2004;53:1074–1081. doi: 10.2337/diabetes.53.4.1074. [DOI] [PubMed] [Google Scholar]
- Pang Y, Hunton DL, Bounelis P, Marchase RB. Hyperglycemia Inhibits Capacitative Calcium Entry and Hypertrophy in Neonatal Cardiomyocytes. Diabetes. 2002;51:3461–3467. doi: 10.2337/diabetes.51.12.3461. [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJS, Koblan-Huberson M, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney SE, Simmons RA. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol Metab. 2010;21:223–229. doi: 10.1016/j.tem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Davidoff AJ. Diabetes rapidly induces contractile dysfunctions in isolated ventricular myocytes. American Journal of Physiology - Heart and Circulatory Physiology. 1997;272:H148–H158. doi: 10.1152/ajpheart.1997.272.1.H148. [DOI] [PubMed] [Google Scholar]
- Ren J, Gintant GA, Miller RE, Davidoff AJ. High extracellular glucose impairs cardiac E-C coupling in a glycosylation-dependent manner. American Journal of Physiology - Heart and Circulatory Physiology. 1997;273:H2876–H2883. doi: 10.1152/ajpheart.1997.273.6.H2876. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. The Journal of Cell Biology. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe K, Wang Z, Hart GW. {beta}-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc Natl Acad Sci U S A. 2010;107:19915–19920. doi: 10.1073/pnas.1009023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa-Imamura H, Sekine T, Murata M, Takayama K, Nakazawa K, Nakagawa J. Stimulation of glucose uptake in muscle cells by prolonged treatment with scriptide, a histone deacetylase inhibitor. Biosci Biotechnol Biochem. 2003;67:1499–1506. doi: 10.1271/bbb.67.1499. [DOI] [PubMed] [Google Scholar]
- Taylor RP, Parker GJ, Hazel MW, Soesanto Y, Fuller W, Yazzie MJ, et al. Glucose deprivation stimulates O-GlcNAc modification of proteins through upregulation of O-linked N-acetylglucosaminyltransferase. J Biol Chem. 2008;283:6050–6057. doi: 10.1074/jbc.M707328200. [DOI] [PubMed] [Google Scholar]
- Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem. 2004;279:53665–53673. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- Torres C, Hart G. Topography and polypeptide distribution of terminal Nacetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- Toyofuku T, Kurzydlowski K, Tada M, MacLennan DH. Amino acids Lys-Asp-Asp-Lys-Pro-Val402 in the Ca(2+)-ATPase of cardiac sarcoplasmic reticulum are critical for functional association with phospholamban. Journal of Biological Chemistry. 1994;269:22929–22932. [PubMed] [Google Scholar]
- Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proceedings of the National Academy of Sciences. 2002;99:5313–5318. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walgren JLE, Vincent TS, Schey KL, Buse MG. High glucose and insulin promote O-GlcNAc modification of proteins, including α-tubulin. American Journal of Physiology - Endocrinology And Metabolism. 2003;284:E424–E434. doi: 10.1152/ajpendo.00382.2002. [DOI] [PubMed] [Google Scholar]
- Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2102–H2110. doi: 10.1152/ajpheart.00935.2004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gucek M, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: Site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proceedings of the National Academy of Sciences. 2008;105:13793–13798. doi: 10.1073/pnas.0806216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, et al. O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proceedings of the National Academy of Sciences. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert C, Klopfer K, Kausch C, Brodbeck K, Stumvoll M, Häring HU, et al. Palmitate-Induced Activation of the Hexosamine Pathway in Human Myotubes. Diabetes. 2003;52:650–656. doi: 10.2337/diabetes.52.3.650. [DOI] [PubMed] [Google Scholar]
- Whisenhunt TR, Yang X, Bowe DB, Paterson AJ, Van Tine BA, Kudlow JE. Disrupting the enzyme complex regulating O-GlcNAcylation blocks signaling and development. Glycobiology. 2006;16:551–563. doi: 10.1093/glycob/cwj096. [DOI] [PubMed] [Google Scholar]
- Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochemical and Biophysical Research Communications. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, Inflammation, and RAGE. Circulation Research. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proceedings of the National Academy of Sciences. 2001;98:6611–6616. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc Transferase to Promoters by Corepressor mSin3A: Coupling Protein O-GlcNAcylation to Transcriptional Repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- Yokoe S, Asahi M, Takeda T, Otsu K, Taniguchi N, Miyoshi E, et al. Inhibition of phospholamban phosphorylation by O-GlcNAcylation: implications for diabetic cardiomyopathy. Glycobiology. 2010;20:1217–1226. doi: 10.1093/glycob/cwq071. [DOI] [PubMed] [Google Scholar]
- Zachara NE. The Roles of O-linked β-N-acetylglucosamines (o-GlcNAc) in Cardiovascular Physiology and Disease. American Journal of Physiology - Heart and Circulatory Physiology. 2012 doi: 10.1152/ajpheart.00445.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH-F, Yu Y, Safrina O, Penna A, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proceedings of the National Academy of Sciences. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Miyamoto S, Brown JH. Cardiomyocyte Calcium and Calcium/Calmodulin-dependent Protein Kinase II: Friends or Foes? Recent Prog Horm Res. 2004;59:141–168. doi: 10.1210/rp.59.1.141. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- Zhu XZLM, Marchase Susan, Richard B, Chatham John C. Inhibition of STIM1 oligomerization by protein O-Linked N- Actelylglucosamine: A novel metabolically mediated regulation of cardiomyocyte calcium overload. Circulation. 2010;122:A18259. [Google Scholar]
- Zou L, Zou C, Chatham JC. Stress induced increase in protein O-linked-N-acetylglucosamine (O-GlcNAc) levels is CaMKII dependent. The FASEB Journal. 2012;26:1127.6. [Google Scholar]