Abstract
VNI is a potent inhibitor of CYP51 and was recently shown to achieve parasitological cure of mice infected with T. cruzi in both acute and chronic stages of infection. T. cruzi is the causative parasite of Chagas disease, a neglected tropical disease. The first enantioselective chemical synthesis of VNI (at a materials cost of less than $0.10/mg) is described. Furthermore, the key enantioselective step is performed at the 10 gram scale.
Chagas disease affects more than 8 million people throughout the Americas,1 and recent evidence suggests that it is proliferating beyond these traditional geographical borders, tracking with the lengthening reach of disease vectors that favor warm climate.2 The causative parasite of Chagas – T. cruzi – infects humans in two stages. The acute stage is treated with benznidazole or nifurtimox, which have been associated with low efficacy and general toxicity. There are no established treatments for the chronic form of infection, but posaconazole is currently considered the most promising therapeutic and is in Phase II clinical trials. Unfortunately, it is widely regarded as unaffordable with a cost estimated at over $1000 per patient. The cost of treatment is an immediate consideration for drug development since Chagas is endemic to populations in low resource areas,3 and mere repurposing efforts for expensive antifungal drugs such as posaconazole are likely to have little impact. There is insufficient incentive for industrial drug development, resulting in a neglected disease status for Chagas.4,5,6,7
Posaconazole was brought to market as an antifungal therapeutic8 and has been shown to inhibit trypanosomal sterol 14α-demethylase (CYP51)9,10,11,12 similar to other small molecules,13 thereby disrupting membrane formation in T. cruzi. CYP51 has emerged as an effective drug target since all eukaryotes rely on endogenous sterol production for membrane biogenesis, and this target has been further validated in a murine model of acute Chagas infection.14 VNI15 was recently discovered to inhibit sterol synthesis in T. cruzi,13 as well as bind to CYP51 in a manner analogous to posaconazole.11 A contributing factor to the high cost for posaconazole is its long and relatively inefficient synthesis;16 however, no enantioselective synthesis of VNI has been reported. Further development of VNI, the more potent enantiomer, and its promise as a therapeutic of reasonable cost, rests in part upon the availability of a short, selective, and high yielding synthesis.17 Herein we report the first enantioselective chemical synthesis of VNI. This preparation provided the gram-scale quantities of VNI to establish its efficacy against T. cruzi in a mouse model of infection, including evidence for its effectiveness against the chronic infection.18 The preliminary studies enabled by this material also suggest that VNI toxicity is low.
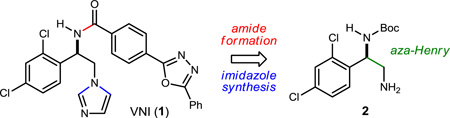
The initial quantity of VNI was obtained from a nonrenewable source and without preparative details.13 And since no preparation of VNI existed in the literature, a synthesis selective for the more potent enantiomer was needed. Key structural features of VNI include a chiral benzylic amine carbon, an amide substituent that projects a substituted biphenyl into the substrate access channel of CYP51, and an imidazole that interacts directly with the iron-heme of CYP51.12 Figure 1 outlines a modular design for the synthesis route, invoking an enantioselective aza-Henry reaction to construct the styrenyl diamine backbone of VNI in the key step. The high degree of convergency would be reflected in a short longest linear sequence (LLS).19
Figure 1.
Retrosynthetic Approach to VNI to Maximize Convergency and Modularity
N-Boc imine 3 was prepared using a standard procedure from 2,4-dichlorobenzaldehyde.20 Feasibility for the key stereochemistry-determining step was first evaluated by analysis of the enantioselective addition of commercially available nitromethane to N-Boc imine 3 using bis(amidine) catalysis (Scheme 1).21,22 Using our first generation catalyst23 to form this adduct would have required higher catalyst loading and longer reaction time, as well as the use of nitromethane as solvent. To circumvent this problem, we selected a more Brønsted basic version from our second generation of catalysts for the reaction.24 This approach delivered a disappointing 2.7:1 ratio of 4:5, the latter resulting from an unexpectedly competitive addition of adduct 4 to a second equivalent of imine 3. In principle, this 2:1 adduct could be minimized through the use of excess nitromethane; however, the ratio was improved only to the 6.5:1 level and at the cost of a drop in enantioselection (Scheme 1). As a result, commercially available bromonitromethane was investigated using the hypothesis that the bromine substituent would slow the second addition as a result of its size. In the event, the desired adduct (9) was prepared in excellent yield (90%), using only two equivalents of bromonitromethane (Scheme 2).25 This reaction was catalyzed by (+)-PBAM (6),26 which delivered the addition product in 97–99% ee for each diastereomer. Immediate treatment of the mixture with cobalt boride formed in situ,27 provided the fully reduced product (amine 2). Although adduct 9 is a mixture of diastereomers, they are homochiral at the benzylic position, thereby producing the same enantiomer after reduction to diamine 2. Formation of the imidazole was achieved by standard condensation of amine 2 with glyoxal, formalin and ammonium acetate.28 Deprotection and subsequent acylation of the amine with carboxylic acid 1229 provided milligram quantities of the desired dextrorotatory product.
Scheme 1.
Competitive Double Addition of Imine to Nitromethane
Scheme 2.
Multigram-Scale Enantioselective Synthesis of VNI for Preclinical Studies
Our first generation approach relied on chromatography to purify adduct 9, in addition to chromatographic purification in three subsequent steps. However, we first optimized the key enantioselective step, with respect to both catalyst loading and scale. Although decreasing the catalyst loading was favorable for enantioselection (Table 1, entries 1–3), a diminished yield was observed as the scale increased. This was partly due to issues associated with column chromatography on large scale. Fortunately, the catalyst could be removed with a straightforward filtration, delivering over 6 grams of pure product (Table 1, entry 4). An almost 500 fold increase in scale from initial experiments (from 0.1 mmol scale) returned favorable results (Table 1, entry 5): filtration provided 19.2 grams (nearly quantitative yield) of analytically and enantiomerically pure product using 1 mol % PBAM. The use of this highly reactive organocatalyst (>100 turnovers) in a large scale preparation to deliver the scaffold is punctuated by the ability to recover and recycle the catalyst without loss of activity.
Table 1.
Reaction Optimization of Key Bond Forming Step
 | |||||
|---|---|---|---|---|---|
| entry | cat. (mol %) |
scale (mmol) |
yieldb | eec (%) |
|
| (g) | (%) | ||||
| 1 | 10 | 1.0 | 0.37 | 90 | 95 |
| 2 | 5 | 1.0 | 0.37 | 90 | 96 |
| 3 | 2 | 5.8 | 1.99 | 83 | 96 |
| 4d | 1.3 | 14.9 | 6.05 | 98 | 97 |
| 5d | 1.0 | 47.0 | 19.2 | >98 | 98 |
All reactions were run at −20 °C for 2 d.
Yields are isolated yields.
Enantiomer ratios measured by HPLC using chiral stationary phase and are average of both diastereomers.
No column chromatography, only filtration to remove catalyst. See Supporting Information for details.
The overall synthesis route (Scheme 2) provided VNI analogs for evaluation with similar brevity. Among those is FF-VNI (13), prepared from the 2,4-difluoro-derivative of 9 (Figure 2). The halogenated aromatic ring is buried deep in the CYP51 pocket while the amide chain is projected into the substrate access channel. The exchange of both chlorines with fluorines within the VNI backbone resulted in a four-fold improvement in binding to CYP51 (T. cruzi) relative to VNI.17,30
Figure 2.
VNI Analog Preparation: FF-VNI
The final part of this study involved the preparation of gram quantities of VNI for evaluation in murine models of both acute and chronic Chagas infection. In this campaign, several chromatographic manipulations were targeted for replacement with filtration or active precipitation. The first three steps were completed by straightforward filtrations, and the first chromatography was applied to the borohydride reduction product 2. The final three steps could also be completed with a single chromatographic step of the final product, aided by precipitation steps.
The route illustrated in Scheme 2 establishes that 1) the catalyzed addition has been scaled to the 20 gram level using 1 mol % of the organocatalyst PBAM,26 providing the desired product in essentially enantiomerically pure form; 2) the overall process can be used to prepare multigram quantities of VNI; and 3) nearly all steps benefit from solid intermediates, resulting in either filtration or recrystallization for most manipulations. This synthesis is therefore an immediate platform for VNI drug development, including further structure-activity relationship studies.
In summary, VNI is prepared through total chemical synthesis using generally inexpensive materials. The salient features of the synthesis, including length (LLS = 7 steps), selectivity (>99% ee) in the key step, and nature of the intermediates (all crystalline solids) bode well for its potential scalability beyond its current multigram-scale. The current materials cost of less than $ 0.10/mg31 VNI highlights the promise of a small molecule therapeutic for a disease endemic to low resource areas.32 Furthermore, the convergency of the approach provides immediate, straightforward access to derivatives to more rapidly progress through lead optimization and into preclinical candidate selection.
Supplementary Material
Acknowledgment
The work was supported by National Institutes of Health (NIH) GM084333 (JNJ), Vanderbilt Institute of Chemical Biology (VICB) for fellowship support (MCD), VICB Pilot Project Grant 2011 (GIL), and in part by GM067871 (GIL, MRW), AI080580 and U54 MD007593 (FV).
Footnotes
Supporting Information Available Procedures (gram-scale) and analytical data for all new compounds, cost of materials analysis to produce VNI, and comparative analysis of posaconazole and VNI synthesis schemes. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Clayton J. Nature. 465:S4–S5. doi: 10.1038/nature09220. [DOI] [PubMed] [Google Scholar]
- 2.Coura JR, Vinas PA. Nature. 465:S6–S7. doi: 10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- 3.Urbina JA, Docampo R. Trends in parasitology. 2003;19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]; Buckner FS, Navabi N. Current Opinion in Infectious Diseases. 23:609–616. doi: 10.1097/QCO.0b013e3283402956. 10.1097/QCO.0b013e3283402956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton J. Nature. 465:S12–S15. doi: 10.1038/nature09224. [DOI] [PubMed] [Google Scholar]
- 5.Apt W. Drug Design Development and Therapy. 2010;4:243–253. doi: 10.2147/dddt.s8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Therapeutic development for T. brucei (human African trypanosomiasis) faces similar challenges: Smithson DC, Lee J, Shelat AA, Phillips MA, Guy RK. J. Biol. Chem. 2010;285:16771–16781. doi: 10.1074/jbc.M109.081588.
- 7.Development of serine protease inhibitors targeting cruzain remain a promising therapeutic development approach: Chen YT, Brinen LS, Kerr ID, Hansell E, Doyle PS, McKerrow JH, Roush WR. PLoS Negl Trop Dis. 2010;4:e825. doi: 10.1371/journal.pntd.0000825. Brak K, Kerr ID, Barrett KT, Fuchi N, Debnath M, Ang K, Engel JC, McKerrow JH, Doyle PS, Brinen LS, Ellman JA. J. Med. Chem. 2010;53:1763. doi: 10.1021/jm901633v.
- 8.Molina J, Martins-Filho O, Brener Z, Romanha AJ, Loebenberg D, Urbina JA. Antimicrob. Agents Chemother. 2000;44:150–155. doi: 10.1128/aac.44.1.150-155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepesheva G, Hargrove T, Kleshchenko Y, Nes W, Villalta F, Waterman M. Lipids. 2008;43:1117–1125. doi: 10.1007/s11745-008-3225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepesheva GI, Waterman MR. Mol. Cell. Endocrinol. 2004;215:165–170. doi: 10.1016/j.mce.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Lepesheva GI, Hargrove TY, Anderson S, Kleshchenko Y, Futak V, Wawrzak Z, Villalta F, Waterman MR. J. Biol. Chem. 2010;285:25582–25590. doi: 10.1074/jbc.M110.133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepesheva GI, Park H-W, Hargrove TY, Vanhollebeke B, Wawrzak Z, Harp JM, Sundaramoorthy M, Nes WD, Pays E, Chaudhuri M, Villalta F, Waterman MR. J. Biol. Chem. 2009;285:1773–1780. doi: 10.1074/jbc.M109.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepesheva GI, Ott RD, Hargrove TY, Kleshchenko YY, Schuster I, Nes WD, Hill GC, Villalta F, Waterman MR. Chem. Biol. 2007;14:1283–1293. doi: 10.1016/j.chembiol.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckner FS, Urbina JA. Int. J. Parastiol. Drugs Drug Resist. 2012 doi: 10.1016/j.ijpddr.2011.12.002. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]; Doyle PS, Chen C-K, Johnston JB, Hopkins SD, Leung SSF, Jacobson MP, Engel JC, McKerrow JH, Podust LM. Antimicrobial Agents and Chemotherapy. 2010;54:2480. doi: 10.1128/AAC.00281-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kraus JM, Tatipaka HB, McGuffin SA, Chennamaneni NK, Karimi M, Arif J, Verlinde CLMJ, Buckner FS, Gelb MH. J. Med. Chem. 2010;53:3887. doi: 10.1021/jm9013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The abbreviation ‘VNI’ refers specifically to the (R)-enantiomer the more potent inhibitor of CYP51. An X-ray crystal structure of VNI bound to CYP51 has been reported (ref. 11). VNI is the dextrorotatory (+) enantiomer.
- 16.See the Supporting Information for an outline of the synthesis reported in: Saksena AK, Girijavallabhan VM, Lovey RG, Pike RE, Wang H, Liu Y-T, Ganguly AK, Bennett F. USA: Schering Corp.; 1995. p. 82.
- 17.A synthesis of rac-1 was recently reported: Hargrove TY, Kim K, de Nazaré Correia Soeiro M, da Silva CF, da Gama Jaen Batista D, Batista MM, Yazlovitskaya EM, Waterman MR, Sulikowski GA, Lepesheva GI. Int. J. Parastiol. Drugs Drug Resist. 2012;2:178–186. doi: 10.1016/j.ijpddr.2012.06.001.
- 18.Villalta F, Dobish MC, Nde PN, Kleshchenko YY, Hargrove TY, Johnson CA, Waterman MR, Johnston JN, Lepesheva GI. J. Infect. Dis. 2012 doi: 10.1093/infdis/jit042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selected recent applications of organocatalysis to therapeutic development platforms: Coulthard G, Erb W, Aggarwal VK. Nature. 2012;489:278–281. doi: 10.1038/nature11411. Davis TA, Danneman MW, Johnston JN. Chem. Commun. (Cambridge, U. K.) 2012;48:5578–5580. doi: 10.1039/c2cc32225k. Davis TA, Johnston JN. Chem. Sci. 2011;2:1076–1079. doi: 10.1039/C1SC00061F.
- 20.Petrini M. Chem. Rev. 2005;105:3949–3977. doi: 10.1021/cr050528s. [DOI] [PubMed] [Google Scholar]; Petrini M, Torregiani E. Tetrahedron Lett. 2006;47:3501–3503. [Google Scholar]; Marianacci O, Micheletti G, Bernardi L, Fini F, Fochi M, Pettersen D, Sgarzani V, Ricci A. Chem.– Eur. J. 2007;13:8338–8351. doi: 10.1002/chem.200700908. [DOI] [PubMed] [Google Scholar]; Yin B, Zhang Y, Xu L-W. Synthesis. 2010:3583–3595. [Google Scholar]
- 21.Shen B, Makley DM, Johnston JN. Nature. 2010;465:1027–1032. doi: 10.1038/nature09125. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dobish MC, Johnston JN. Org. Lett. 2010;12:5744–5747. doi: 10.1021/ol1025712. [DOI] [PMC free article] [PubMed] [Google Scholar]; Singh A, Johnston JN. J. Am. Chem. Soc. 2008;130:5866–5867. doi: 10.1021/ja8011808. [DOI] [PubMed] [Google Scholar]; Shen B, Johnston JN. Org. Lett. 2008;10:4397–4400. doi: 10.1021/ol801797h. [DOI] [PubMed] [Google Scholar]; Singh A, Yoder RA, Shen B, Johnston JN. J. Am. Chem. Soc. 2007;129:3466–3467. doi: 10.1021/ja068073r. [DOI] [PubMed] [Google Scholar]; Hess AS, Yoder RA, Johnston JN. Synlett. 2006:147–149. [Google Scholar]
- 22.Enantioselective aza-Henry reactions selected examples: Marques-Lopez E, Merino P, Tejero T, Herrera RP. Eur. J. Org. Chem. 2009:2401–2420. Arrayas RG, Carretero JC. Chem. Soc. Rev. 2009;38:1940–1948. doi: 10.1039/b820303b. Wang CJ, Dong XQ, Zhang ZH, Xue ZY, Teng HL. J. Am. Chem. Soc. 2008;130 doi: 10.1021/ja803538x. 8606-+. Rueping M, Antonchick AP. Org. Lett. 2008;10:1731–1734. doi: 10.1021/ol8003589. Rampalakos C, Wulff WD. Adv. Synth. Catal. 2008;350:1785–1790. doi: 10.1002/adsc.200800214.
- 23.Nugent BM, Yoder RA, Johnston JN. J. Am. Chem. Soc. 2004;126:3418–3419. doi: 10.1021/ja031906i. [DOI] [PubMed] [Google Scholar]
- 24.Davis TA, Danneman MW, Johnston JN. Chem. Commun. 2012;48:5578–5580. doi: 10.1039/c2cc32225k. [DOI] [PMC free article] [PubMed] [Google Scholar]; Davis TA, Johnston JN. Chemical Science. 2011;2:1076–1079. doi: 10.1039/C1SC00061F. [DOI] [PMC free article] [PubMed] [Google Scholar]; Davis TA, Wilt JC, Johnston JN. J. Am. Chem. Soc. 2010;132:2880-+. doi: 10.1021/ja908814h. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dobish MC, Johnston JN. Org. Lett. 2010;12:5744. doi: 10.1021/ol1025712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen B, Makley DM, Johnston JN. Nature. 2010;465:1027–1032. doi: 10.1038/nature09125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis TA, Dobish MC, Schwieter KE, Chun AC, Johnston JN. Org. Synth. 2012;89:380–393. doi: 10.15227/orgsyn.089.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzman SW, Ganem B. J. Am. Chem. Soc. 1982;104:6801–6802. [Google Scholar]; Satoh T, Suzuki S, Suzuki Y, Miyaji Y, Imai Z. Tetrahedron Lett. 1969 4555-&. [Google Scholar]
- 28.Matsuoka Y, Ishida Y, Sasaki D, Saigo K. Tetrahedron. 2006;62:8199–8206. [Google Scholar]
- 29.Rostamizadeh S, Ghamkhar S. Chinese Chemical Letters. 2008;19:639–642. [Google Scholar]
- 30.Relative efficacy of CYP51 ligands is estimated through the use of several measurements in addition to Kd see refs 12, 13, 17. For this reason VNI remains the best current lead.
- 31.Unfortunately, the materials cost to produce posaconazole is unavailable, thereby preventing a direct comparison at this stage. However, the low materials cost for VNI at the gram scale bodes well for the development of an inexpensive small molecule.
- 32.Zhu C, Cook SP. J. Am. Chem. Soc. 2012;134:13577–13579. doi: 10.1021/ja3061479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.