Abstract
Chemically-inducible rapid manipulation of small GTPase activity has proven a powerful approach to dissect complex spatio-temporal signaling of these molecular switches. However, overexpression of these synthetic molecular probes freely in the cytosol often results in elevated background activity before chemical induction, which perturbs the cellular basal state and thereby limits their wide application. As a fundamental solution, we have rationally designed and newly developed a strategy to remove unwanted background activity without compromising the extent of induced activation. By exploiting interaction between a membrane lipid and its binding protein, target proteins were translocated from one organelle to another on a time scale of seconds. This improved strategy now allows for rapid manipulation of small GTPases under a physiological state, thus enabling fine dissection of sophisticated signaling processes shaped by these molecules.
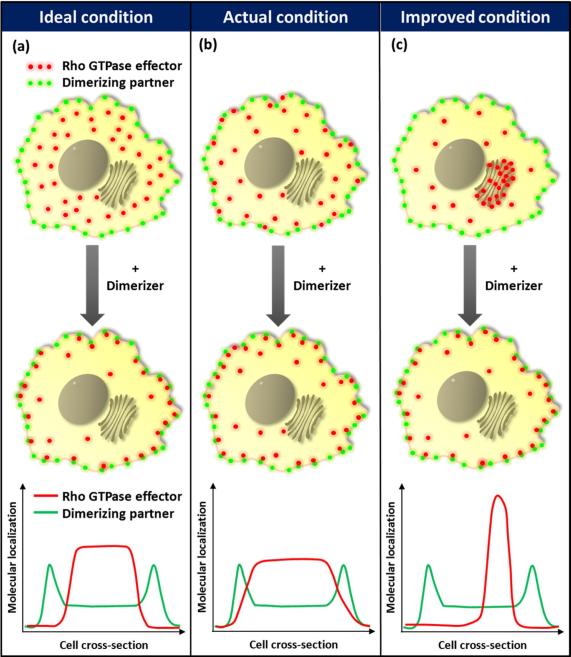
We have previously developed a series of molecular probes based on a chemically-inducible heterodimerization strategy which enabled activation or inactivation of Rho family members of small GTPases in living cells on a time scale of seconds(1, 2). This strategy has been extended to other members such as Ras(3) and Arf6(4), and also provided high spatial control where a light sensitive dimerizer was used(5, 6). In these methods, a chemical dimerizer, either rapamycin or gibberellin, induces the rapid translocation of cytosolic FKBP- or GID1- fused GTPases (or GTPase effectors) to the plasma membrane, where the respective dimerizing partner, FRB or GAI, is localized (Figure 1a)(1, 2). Thus, addition of the chemical dimerizer concentrates cytosolic effectors at the plasma membrane by a factor of five hundred to one thousand for a typical mammalian cell. This rapid and significant chemically-inducible concentration triggers activation of Rho GTPases and downstream signaling at the cell periphery, resulting in membrane ruffling and c-Jun N-terminal kinase activation(1, 2).
Figure 1.
Schematic of the present chemically-inducible heterodimerization strategy and the corresponding improved strategy. (a) Under ideal conditions, cytosolic GTPase effectors only diffuse and bind to plasma membrane dimerizing partners upon addition of dimerizer (b) The present strategy is limited by the random diffusion of cytosolic GTPase effectors to the cell cortex and plasma membrane, resulting in background activity in the absence of dimerizer (c) Background activity may be reduced by tethering GTPase effectors to cytosolic organelles to limit the random diffusion. The graphs at the bottom of each panel illustrate the localization of GTPase effectors relative to the plasma membrane-tethered dimerizing partner in the un-induced state.
SC Phua et al.
Despite its power and utility, this technique has an inherent limitation; unwanted target activation of varying extent is frequently exhibited in the absence of chemical dimerizers. This happens because cytosolic dimerizing effectors still have access to the plasma membrane as a consequence of free diffusion (Figure 1b). For useful application in spatiotemporal signalling studies, it is critical to keep such background activity as low as possible to achieve high efficiency in inducible translocation and/or activation. Therefore, we aimed to restrict the free diffusion of dimerizing effector molecules in the cytosol. One could think of confining these effector molecules inside the cell nucleus until rapamycin is added(7). However, this approach results in slower onset of target activation as the nuclear export process is an additional step before effectors can be trapped at the plasma membrane.
To avoid this additional step, we employed a binding protein for a Golgi membrane lipid to tether FKBP-fused GTPase effector molecules onto the cytoplasmic surface of the Golgi apparatus in the un-induced basal state (Figure 1c). A family of four-phosphate-adaptor proteins (FAPPs) binds to the phosphatidylinositol 4-phosphate (PI4P) lipid enriched at the trans-Golgi network through their pleckstrin homology (PH) domains(8). Specifically, FAPP1 has been reported to bind to PI4P with a dissociation constant of 230 nM(9). It may thus be expected that, while most of the FKBP effectors fused with the PH(FAPP) domain would remain on the Golgi surface at any given time, these molecules would essentially display dynamic binding behavior by dissociating from the Golgi surface and freely diffuse in the cytosol before re-associating with PI4P. When rapamycin is present, however, these FKBP effector molecules bind to rapamycin with a Kd of 0.2 nM(10), and those which are diffusing in the cytosol at a given time may subsequently get trapped by the FRB anchored at the plasma membrane. Since the FKBP.rapamycin binary complex binds to FRB (Kd = 12 nM(10)) with an approximately 20-fold higher binding affinity than the PH(FAPP).PI4P interaction, an assumption is made that the FKBP effectors are more likely to be kept at the plasma membrane than return to the Golgi surface in the presence of rapamycin.
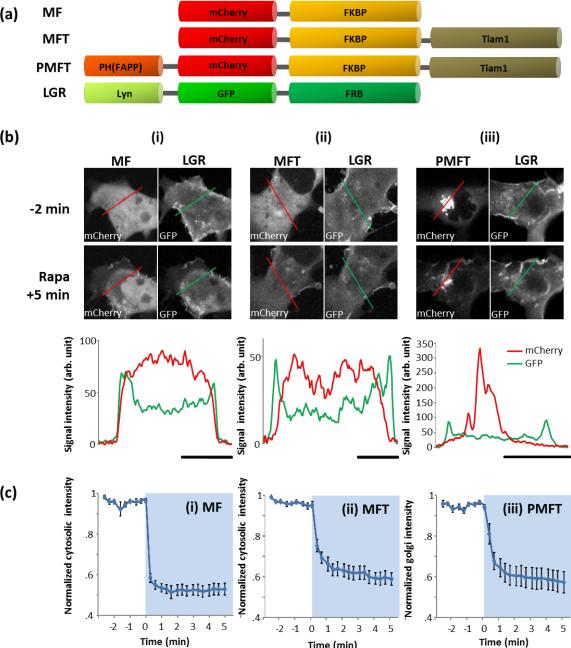
In this study, we have manipulated the activity of small GTPase Rac1 via Tiam1, a guanine nucleotide exchange factor of Rac1. To test our ideas, we first generated a fusion protein of PH(FAPP1) and FKBP-Tiam1 with a mCherry tag (PH(FAPP1)-mCherry-FKBP-Tiam1; PMFT) (Figure 2a).We then transfected PH(FAPP1)-mCherry-FKBP-Tiam1 in Cos-7 cells together with Lyn-GFP-FRB (LGR; a plasma membrane targeted FRB labelled with GFP). Line scan analysis confirmed that PH(FAPP) efficiently localized PH(FAPP1)-mCherry-FKBP-Tiam1 to the Golgi, while mCherry-FKBP(MF) and mCherry-FKBP-Tiam1(MFT) are dispersed in the cytosol (Figure 2b and Supplementary Figure 1). Localization of Tiam1 to the Golgi did not appear to have any obvious effect on the Golgi structure; and quantification of the Golgi extent(11) in all three types of cells also yielded comparable results (Supplementary Figure 2). Furthermore, subsequent addition of rapamycin induced rapid translocation of PH(FAPP1)-mCherry-FKBPTiam1 to the plasma membrane (Figure 2biii and Supplementary Figures 3, 4iii). We analyzed the translocation kinetics by measuring the mCherry fluorescent intensity at the Golgi (Figure 2ciii), finding 27.9 seconds to achieve the half maximal translocation. This kinetics is comparably fast to what was achieved by cytosolic mCherry-FKBP (11.9 seconds) and mCherry-FKBP-Tiam1 (17.5 seconds) (Figure 2ci–ii and Supplementary Figure 4ci–ii). At 5 minutes post-rapamycin addition, translocation approaches saturation and average PH(FAPP1)-mCherry-FKBP-Tiam1 Golgi signal diminished to 57% of the initial signal intensity, indicating that approximately 43% of the molecules have translocated away from the Golgi towards the plasma membrane (Figure 2ciii). In addition, PH(FAPP1)-mCherry-FKBP-Tiam1 stayed at the plasma membrane for at least 20 minutes and did not go back to the Golgi (Figure 3aiii). These data well supported our initial ideas: (1) the PH(FAPP1).PI4P interaction is sufficiently strong to localize PH(FAPP1)-mCherry-FKBP-Tiam1 at the Golgi, and (2) the 20-fold stronger binding interaction between FKBP.rapamycin complex and FRB is highly efficient in recruiting Tiam1 and overcoming the re-association of PH(FAPP1) and PI4P.
Figure 2.
Localization and translocation of PH(FAPP1)-mCherry-FKBP-Tiam1 at the Golgi (a) Schematic representation of the various FKBP-fused Tiam1 constructs (MFT and PMFT), control construct (MF), and plasma membrane tethered Lyn-GFP-FRB (LGR) construct. (b) Confocal fluorescent images showing the cellular localization of (i) MF, (ii) MFT, or (iii) PMFT with LGR at defined time points before (−) and after (+) rapamycin addition. Graph at the bottom of each column is a line scan of the respective cells prior to rapamycin addition (from respective red and green lines drawn on top panel cell images). Note that the LGR signal intensities are comparable in (i)– (iii). Scale bar beneath each graph represents 10 μm. (c) Average translocation kinetics of (i) MF, (ii) MFT or (iii) PMFT from the cytosol or Golgi (note y-axis), where light blue regions indicate presence of rapamycin. Signal intensities have been normalized against the maximum signal intensity values in each case. The average duration for MF, MFT and PMFT to reach half-maximal translocation were 11.9 s, 17.5 s and 27.9 s, respectively. S.E.M. values are indicated by error bars.
SC Phua et al.
Figure 3.
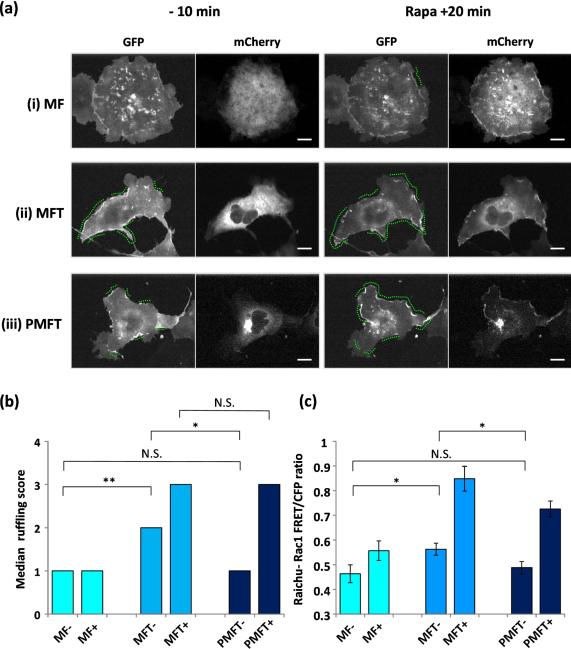
Membrane ruffling analysis and quantitative comparison of Rac1 activation levels in cells. (a) Confocal fluorescent images of cells expressing (i) MF(mCherry), (ii) MFT(mCherry) or (iii) PMFT(mCherry) with LGR (GFP) at defined time points before (−) and after (+) rapamycin addition. Green dotted lines on GFP images indicate regions of cellular edge displaying membrane ruffling activity during the 10 minutes before and 20 minutes after rapamycin addition. Scale bar indicates 10 μm. (b) Using the scoring scheme in Supplementary Table 1, cells expressing MF, MFT and PMFT were scored for membrane ruffling levels before (−) and after (+) rapamycin addition. Median membrane ruffling level for each sample is shown. P values from two-tailed Mann-Whitney U tests between different samples are also represented. (c) Rac1 activity levels represented by FRET/CFP ratios in cells expressing MF, MFT or PMFT with lyn-linker-FRB (LDR) and RaichuEV-Rac1 FRET biosensor before and after 7 minutes of rapamycin treatment. Average FRET/CFP ratios are represented. P values from two-tailed Student's T tests between different samples are also represented.
SC Phua et al.
We then compared the biological effect of the Golgi- and cytosol-localized Tiam1. Since translocation of Tiam1 to the plasma membrane promotes the local activation of Rac1 to result in membrane ruffling, we employed a semi-quantitative method to score membrane ruffling activity (see Methods and Table S1 for quantification method)(2, 12). Control cells expressing mCherry-FKBP with Lyn-GFP-FRB exhibited low levels of membrane ruffling (median =1) which did not alter after recruitment of mCherry-FKBP to the plasma membrane upon rapamycin addition (median =1, p=0.730) (Figures 3ai, 3b and Supplementary video 1). All statistical values are summarized in Table S2. In contrast, cells expressing cytoplasmic mCherry-FKBP-Tiam1 with Lyn-GFP-FRB already exhibited significantly higher levels of membrane ruffling (median=2, MF− vs. MFT− p=0.00969) in the un-induced state. The ruffling activity was further increased after rapamycin-mediated translocation of mCherry-FKBP-Tiam1 to the plasma membrane (median =3, p= 0.000275) (Figure 3aii, 3b and supplementary video 2). Importantly, the Golgi confinement of PH(FAPP1)-mCherry-FKBP-Tiam1 exhibited significantly low levels of membrane ruffling (median= 1) in the un-induced state, comparable to that of the control condition (MF− vs. PMFT−, p=0.917) and significantly lower than that of mCherry-FKBP-Tiam1 (MFT− vs. PMFT−, p= 0.0156) (Figure 3aiii and 3b). Upon rapamycin addition, PH(FAPP1)-mCherry-FKBP-Tiam1- expressing cells induced striking ruffling activity with a median score of 3, comparable to that of mCherry-FKBP-Tiam1 (MFT+ vs. PMFT+, p=0.190) (Figure 3aiii, 3b and Supplementary video 3). Collectively, these data showed that Golgi sequestration of Tiam1 almost completely removes unwanted membrane ruffling activity in the un-induced basal state while simultaneously preserving rapid and efficientmembrane ruffling activation upon rapamycin addition.
In order to validate that the observed variation in basal membrane ruffling extent were as a result of different Rac1 activation levels, we further attempted to measure the Rac1 activity in these cells using a previously reported Rac1 Förster resonance energy transfer (FRET) biosensor, RaichuEV-Rac1(13). Unfortunately, co-expression of RaichuEV-Rac1 with the heterodimerization probes system drastically compromised the expression of this molecular biosensor in Cos-7 cells, and accurate FRET measurements could not be achieved. As an alternative, we co-expressed RaichuEV-Rac1 and the heterodimerization probes in HeLa cells instead to compare the basal Rac1 activity levels in cells expressing the Golgi- and cytosol-localized Tiam1. Consistent with the membrane ruffling results, HeLa cells expressing mCherry-FKBP-Tiam1 exhibited significantly higher basal Rac1 activity than control cells expressing mCherry-FKBP, with average FRET/CFP ratios 0.56 and 0.46 respectively (MFT− vs. MF−, p=0.0243) (Figure 3c). Representative FRET/CFP cell images are shown in Supplementary Figure 5 and all statistical values are summarized in Table S3. Importantly, the basal Rac1 activity was significantly lowered in cells when Tiam1 was pre-localized at the Golgi instead, with PH(FAPP1)-mCherry-FKBP-Tiam1- expressing cells exhibiting average FRET/CFP ratio of 0.49, comparable to that of mCherry-FKBP-expressing cells (PMFT− vs. MFT−, p= 0.0407 and PMFT− vs. MF−, p= 0.558) (Figure 3c). Upon 7 minutes of rapamycin treatment, translocation of mCherry-FKBP-Tiam1 and PH(FAPP1)-mCherry-FKBP-Tiam1 to the plasma membrane resulted in a respective 1.51- and 1.49- fold increase in Rac1 activity on average (MF− vs MF+, p< 0.001; PMFT− vs. PMFT+, p<0.001), while translocation of mCherry-FKBP to the plasma membrane did not result in a significant increase in Rac1 activity (MF− vs. MF+, p= 0.0785). In all, we have clearly demonstrated that the pre-localization of Tiam1 at the Golgi via a defined PH(FAPP1).PI4P interaction have efficiently reduced Rac1 activity to a minimum under basal conditions, consequently maximizing the membrane ruffling output upon the chemical induction of heterodimerization.
As signaling networks in cells are being dissected more intricately, it is becoming increasingly clear that spatial and temporal control of signaling modules is the crux to regulating signal transduction of downstream events. This is especially true for the Rho family of small GTPases, which are principal regulators of cytoskeletal dynamics for various cellular functions including cell migration, adhesion, membrane trafficking and cytokinesis(14–16). Indeed, Rho GTPases such as Rac, Cdc42 and RhoA exhibit a highly dynamic spatiotemporal nature inside cells as revealed by fluorescent biosensors(17, 18). However, it has been a universal, inherent problem that these biosensors as well as molecular probes for manipulation of Rho GTPases exhibit background activity by either buffering or empowering physiological systems. Researchers have been circumventing these problems by carefully optimizing the expression level of the molecular probes to reduce these unwanted background signals, or performing control experiments to quantify and subtract these non-specific activities from total induced signals. Unlike these symptomatic solutions, we have improvised a spatial confinement strategy that could be adapted to reduce background signals in a variety of situations. To test the feasibility of this approach, we have demonstrated the enhanced spatiotemporal manipulation of membrane ruffling induction by a Rac1 activator. This approach relies on careful selection of two orthogonal molecular interactions occurring at two different intracellular locations: PH(FAPP).PI4P at Golgi and FKBP.rapamycin.FRB at the plasma membrane. This pre-localization scheme may be further extended to other Rho GTPases such as TC10, and additional small GTPases including K-Ras 4B, which are known to localize at the plasma membrane while having no known localization at the Golgi(19). Nevertheless, many other small GTPases (e.g. H-Ras and Cdc42) are known to localize and have functionsat the Golgi(15, 19). Therefore, the present pre-localization scheme will need to be adjusted to such circumstances for a wider use. Apart from the Golgi, additional reversible lipid-protein interactions such as PI(4,5)P2.PH(PLCq) at the plasma membrane(4) or PI3P.FYVE at endosomes(20) could be employed to pre-localize small GTPase molecular probes, which are then relocated to other organelles upon addition of chemical dimerizers for small GTPase activation.
Importantly, the spatial confinement approach described here has further expanded the spatial dynamic range of chemically-inducible manipulation of small GTPase activity. Molecules can now be rapidly translocated between organelles on a time scale of seconds under a physiological state. With this technique, spatiotemporal signalling is ready to be probed in greater depth.
METHODS
Cell culture and transfection
Cos7 or HeLa cells were cultured in DMEM (Gibco) supplemented with 10% FBS. For transfection, cells were transfected with a specified set of DNA constructs by plating them directly in a transfection solution containing DNA plasmids and FuGENE HD (Roche).
Live-cell microscopy
Live-cell fluorescence measurements were performed on a spinning-disc confocal microscope or the Axiovert135TV epi-fluorescence microscope (Zeiss). For confocal imaging, GFP and mCherry excitations were conducted with a krypton-argon laser (CVI-Melles Griot). The laser beam was fiber-coupled (OZ optics) to the spinning disk confocal unit (CSU10; Yokogawa) mounted with dual GFP-RFP dichroic mirrors (Semrock). The laser was processed with appropriate filter sets for GFP and mCherry (Chroma Technology) to capture fluorescence images with a CCD camera (Orca ER, Hamamatsu Photonics). Images were taken using a Neo Fluor 40× objective (Zeiss) mounted on an inverted Axiovert 200 microscope (Zeiss). For epi-fluorescence imaging, cells were imaged with a 63× oil objective (Zeiss) and images were collected by the QIClick chage-coupled device camera (QImaging). Imaging was driven by Metamorph 7.5 imaging software (Molecular Devices).Eighteen to twenty-four hours post transfection, live-cell, time-lapse movies were taken where confocal fluorescent images of cells were taken every 20 seconds for 30 minutes at room temperature. Rapamycin (Tecoland) was added at approximately the 10-min time point. For FRET measurements, epi-fluorescence images were taken before and after 7 minutes of rapamycin treatment. Fixed-cell microscopy
To visualize cells expressing MF, MFT or PMFT with Golgi (CFP-Golgin), ER (CFP-Cb5) and mitochondria (CFP-Tom20) markers, cells were fixed with 4% paraformaldehyde eighteen to twenty-four hours post transfection and epi-fluorescence microscopy was performed.
Quantification of Golgi extent
CFP-Golgin was co-expressed in cells expressing MF, MFT or PMFT to visualize Golgi structures in these cells. The Golgi spread was calculated by taking the width of Golgi stacks as a fraction of the nuclear perimeter (11). Values presented were based on mean values collected from 22–39 cells.
Translocation kinetics measurements
Fluorescent signal intensities of fluorescence cell images and movies were measured using Metamorph 7.5 imaging software (Molecular Devices). For quantification of MF and MFT translocation kinetics, mCherry fluorescence intensity from 3 random positions in the cytosol were measured and normalized against 3 random positions in the background. For quantification of PMFT translocation kinetics, the Golgi complex was outlined and mCherry fluorescence intensity from the highlighted region was measured; values were also normalized against a random position in the background. Translocation kinetics measurements plotted were all based on average signal intensities collected from 10 to 12 cells from 3 separate experiments. Signal intensities in Figure 2b were normalized against the highest intensity value in each case. To facilitate half maximal translocation time calculation, graphs in Figure 2c were further scaled to a range from 0 to 1 (not shown); time duration to reach half maximal translocation was then interpolated from these scaled translocation kinetics plots.
Quantification of Ruffle Activities
The semi-quantitative scoring scheme (Supplementary Table 2) was set up to score the extent of ruffling activities exhibited by cells in the time-lapse movies. Cells were visually inspected for the extent of cellular edges displaying membrane ruffles. Membrane ruffling scores were based on average scores collected from 10–12 representative cells from 3 separate experiments.
FRET measurements
To obtain average FRET/CFP ratio of each cell, cells were first outlined and average fluorescence intensity values were obtained from the outlined image regions in FRET and CFP channels. FRET efficiency of the Rac1 molecular biosensor in cells was then represented by respective average FRET/CFP ratios. FRET/CFP images shown have been subjected to background subtraction and color-coded according to the ratio range.
DNA construction
We constructed mCherry-FKBP (MF) by replacing YFP of YFP-FKBP1 with mCherry using NheI and BsrGI. To construct mCherry-FKBP-Tiam1 (MFT) we replaced YFP of YFP-FKBPTiam1(1) with mCherry using NheI and BsrGI. In order to construct PH(FAPP1)-mCherry-FKBP-Tiam1 (MFT), we performed PCR and amplified PH(FAPP1) with flanking restriction enzyme sites (NheI and AgeI). Both resultant product and MFT were digested with NheI and AgeI for the following ligation. Lyn-GFP-FRB was constructed by replacing YFP of Lyn-YFPFRB3 with GFP using NheI and BsrGI.
Statistical Analysis
Golgi extent and RaichuEV-Rac1 FRET/CFP ratios between different samples were compared using the two-tailed Student's T test. Median membrane ruffling levels between different samples were compared using the two-tailed Mann-Whitney U test.
Supplementary Material
ACKNOWLEDGEMENT
We thank T. Balla (NIH) for a FAPP-PH construct, and K. Aoki and M. Matsuda (Kyoto University) for a RaichuEV-Rac1 construct. This study was supported in part by NIH (MH084691 and GM092930 to T.I.). T.I. is a PRESTO investigator (JST, Japan). S.C.P. is a recipient of the National Science Scholarship from Agency for Science, Technology and Research (Singapore).
Footnotes
Supporting Information: This material is available free of charge via the internet at http://pubs.acs.org.
Conflict of Interest Disclosure None
REFERENCES
- 1.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat Methods. 2005;2:415–418. doi: 10.1038/nmeth763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamoto T, DeRose R, Suarez A, Ueno T, Chen M, Sun TP, Wolfgang MJ, Mukherjee C, Meyers DJ, Inoue T. Rapid and orthogonal logic gating with a gibberellin-induced dimerization system. Nat Chem Biol. 2012;8:465–470. doi: 10.1038/nchembio.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komatsu T, Kukelyansky I, McCaffery JM, Ueno T, Varela LC, Inoue T. Organelle-specific, rapid induction of molecular activities and membrane tethering. Nat Methods. 2010;7:206–208. doi: 10.1038/nmeth.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueno T, Falkenburger BH, Pohlmeyer C, Inoue T. Triggering actin comets versus membrane ruffles: distinctive effects of phosphoinositides on actin reorganization. Sci Signal. 2011;4 doi: 10.1126/scisignal.2002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeRose R, Pohlmeyer C, Umeda N, Ueno T, Nagano T, Kuo S, Inoue T. Spatio-temporal manipulation of small GTPase activity at subcellular level and on timescale of seconds in living cells. J Vis Exp. 2012;9 doi: 10.3791/3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umeda N, Ueno T, Pohlmeyer C, Nagano T, Inoue T. A photocleavable rapamycin conjugate for spatiotemporal control of small GTPase activity. J Am Chem Soc. 2011;133:12–14. doi: 10.1021/ja108258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haruki H, Nishikawa J, Laemmli UK. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 2008;31:925–932. doi: 10.1016/j.molcel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Varnai P, Balla T. Visualization and manipulation of phosphoinositide dynamics in live cells using engineered protein domains. Pflugers Arch. 2007;455:69–82. doi: 10.1007/s00424-007-0270-y. [DOI] [PubMed] [Google Scholar]
- 9.Stahelin RV, Karathanassis D, Bruzik KS, Waterfield MD, Bravo J, Williams RL, Cho W. Structural and membrane binding analysis of the Phox homology domain of phosphoinositide 3-kinase-C2alpha. J Biol Chem. 2006;281:39396–39406. doi: 10.1074/jbc.M607079200. [DOI] [PubMed] [Google Scholar]
- 10.Banaszynski LA, Liu CW, Wandless TJ. Characterization of the FKBP.rapamycin.FRB ternary complex. J Am Chem Soc. 2005;127:4715–4721. doi: 10.1021/ja043277y. [DOI] [PubMed] [Google Scholar]
- 11.Dippold HC, Ng MM, Farber-Katz SE, Lee S-K, Kerr ML, Peterman MC, Sim R, Wiharto PA, Galbraith KA, Madhavarapu S, Fuchs GJ, Meerloo T, Farquhar MG, Zhou H, Field SJ. GOLPH3 Bridges Phosphatidylinositol-4- Phosphate and Actomyosin to Stretch and Shape the Golgi to Promote Budding. Cell. 2009;139:337–351. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ihara S, Oka T, Fukui Y. Direct binding of SWAP-70 to non-muscle actin is required for membrane ruffling. J Cell Sci. 2006;119:500–507. doi: 10.1242/jcs.02767. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu N, Aoki K, Yamada M, Yukinaga H, Fujita Y, Kamioka Y, Matsuda M. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell. 2011;22(23):4647–56. doi: 10.1091/mbc.E11-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 15.Pertz O. Spatio-temporal Rho GTPase signaling - where are we now? J Cell Sci. 2010;123:1841–1850. doi: 10.1242/jcs.064345. [DOI] [PubMed] [Google Scholar]
- 16.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 17.Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 19.Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. Differential Localization of Rho Gtpases in Live Cells. The Journal of Cell Biology. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P. A Phosphoinositide Switch Controls the Maturation and Signaling Properties of APPL Endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.