Abstract
Objective
Endothelial progenitor cells (EPC) and mesenchymal stem cells (MSC) augment tissue repair, but possess slightly different properties. How the cellular phenotype affects the efficacy of this approach in renovascular disease is incompletely understood. This study tested the hypothesis that EPC and MSC protect the post-stenotic kidney by blunting different disease pathways.
Methods
Peripheral blood EPC and adipose-derived MSCs were expanded and characterized by cell surface markers (e.g. CD34/KDR, or CD44/CD90). Single-kidney hemodynamics and function were assessed in pigs after 10 weeks of renal artery stenosis (RAS) treated 4 weeks earlier with an intra-renal infusion of vehicle (n=7), EPC (RAS+EPC) or MSC (RAS+MSC) (both 10×10^6, n=6), and normal controls (n=7). Kidney disease mechanisms were evaluated ex-vivo. The ability of EPC and MSC to attenuate endoplasmic reticulum (ER) stress was also studied in isolated ER and in tubular cells co-cultured with EPC and MSC.
Results
Glomerular filtration rate in RAS was lower than controls, increased in RAS+EPC, and further improved in RAS+MSC, although both improved renal blood flow similarly. EPC prominently enhanced renal growth-factor expression and decreased oxidative-stress, while MSC more significantly attenuated renal inflammation, ER-stress, and apoptosis. Furthermore, MSC induced a greater decrease in caspase-3 and CHOP expression in cultured tubular cells through mechanisms involving cell contact
Conclusion
EPC and MSC achieve a comparable decrease of kidney injury in RAS by different mechanisms, although MSC elicited slightly superior improvement of renal function. These results support development of cell-based approaches for management of renovascular disease, and suggest cell selection based on the underlying pathophysiology of kidney injury.
Keywords: Progenitor cells, renal artery stenosis, oxidative stress, inflammation, endoplasmic reticulum stress
Introduction
Renal artery stenosis (RAS) is the major cause for secondary hypertension, and may lead to kidney ischemia and eventually end-stage kidney disease. The mechanisms responsible for renal damage include tissue inflammation and enhanced oxidative stress in the post-stenotic kidney, which result in renal fibrosis and dysfunction1, 2. Furthermore, enhanced oxidative stress or inflammatory cytokines may activate the unfolded protein response, a cellular stress response related to the endoplasmic reticulum (ER). Recently, ER stress has been recognized to play an important role in apoptosis and tissue damage3, 4, yet its involvement in renal damage in RAS has not been explored.
Tissue damage may render kidney injury irreversible in RAS. As a result, the inconsistent capability of revascularization to improve kidney function in RAS fuels the search for alternative techniques to directly repair the post-stenotic kidney. Bone-marrow derived endothelial progenitor cells (EPC), isolated and cultured from peripheral blood, have been shown to contribute to the tissue repair by eliciting formation of new blood vessels by exerting anti-inflammatory5 or antioxidant properties6, 7. We have previously demonstrated that infusion of EPC into the ischemic kidney distal to RAS improved renal function and microvascular structure8. We found that EPC directly integrate into vascular structures, and enhance renal vascular endothelial growth factor (VEGF) expression and new vessel formation8. As a result, renal fibrosis is attenuated and its function improves. Clinical studies support the notion that progenitor cells also improve cardiac function in patients with myocardial infarction9, 10. However, blood-derived EPC are technically difficult to isolate in sufficient numbers needed to achieve a therapeutic benefit, especially late outgrowth EPC that possess some endothelial cell-like characteristics.
As an alternative, mesenchymal stem cells (MSC) have a number of advantages for vascular repair. A relatively large number of MSC can be obtained from adult sources such as the bone marrow or adipose tissue. MSC are immuno-privileged, immunomodulatory, and stimulate vessel formation by paracrine mechanisms11, 12, but may have lower angiogenic potency than EPC13. Nevertheless, while late-outgrowth EPCs enhanced neovascularization after myocardial infarction better than MSC, MSC more effectively induced cardiomyogenesis and restored cardiac function14. Therefore, selection of cell type directed at specific injury targets may ensure adequate repair.
The stenotic kidney is characterized by functional deterioration secondary to substantial inflammation, fibrosis, and microvascular loss. These mechanisms may make variable levels of contributions to renal dysfunction, and thereby might offer several different therapeutic targets for cell-based therapy. However, the efficacy of EPC and MSC for kidney repair has not fully compared, and the effects of cellular phenotype on the efficacy of cell-based therapy on chronic renovascular disease remain unclear. Thus, this study was designed to test the hypothesis that EPC and MSC activate different mechanisms involved in kidney repair in RAS.
Methods
All protocols were approved by Mayo Clinic Institutional Committee of Animal Care and Use. Studies were performed in 26 female domestic pigs with initial weight 35–40kg, including 7 RAS, 6 RAS+MSC, 6 RAS+EPC, and 7 age- and body weight-matched normal control pigs. Unilateral RAS was induced under anesthesia by implantation of an irritant coil in one renal artery, as we previously reported1, 2. A telemetry catheter were secured in the femoral artery for monitoring daily blood pressure until the end of the study, as previously described1, 8, 15, 16. The degree of RAS was determined after 6 weeks of RAS using angiography, and subsequently MSC or EPC were injected into the stenotic kidney through the renal artery. Four weeks later, stenotic kidney renal blood flow (RBF) and glomerular filtration rate (GFR) were evaluated using multidetector CT (MDCT), blood samples collected for plasma renin activity (PRA) and creatinine levels, and urine samples for protein assay. A few days later, the pigs were euthanized with 20ml Sleepaway® (Fort Dodge, IA), and the kidneys dissected for evaluation of microvascular density (micro-CT), tissue fibrosis (trichrome), oxidative stress (nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase subunits p47phox and p67phox), the growth factors vascular endothelial growth factor (VEGF), its receptors Flk-1 and Flt-1, and hepatocyte growth factor (HGF), inflammation (tumor necrosis factor (TNF)-alpha, interleukin (IL)-10, IL-1β), apoptosis (Terminal deoxynucleotidyl transferase dUTP nick end labeling TUNEL, BCL-XL, and caspase3), and ER stress (GRP94, Derl3, and C/EBP homologous protein CHOP). Additional in vitro studies were performed to compare the direct effects of EPC and MSC on ER stress in cultured kidney tubular cells.
We also performed an additional pilot study in 3 animals to observe the longer effects of MSC on chronic atherosclerotic RAS for 12 weeks after cell infusion (detail methods and results are in a supplemental document).
EPC and MSC isolation
EPC were cultured from autologous mononuclear cells isolated and expanded from peripheral blood (100 mL) about 10 days (early EPC) or 3 weeks (late EPC) before delivery, as described8, 17. A previous study18 showed the synergetic effects of early and late EPC on tissue repair when. Allogeneic MSC was cultured from swine omentum fat (10g) that was digested in collagenase-H for 45min, filtered, and cultured in EGM-2 media for about 3 weeks19. The immune-modulatory properties of MSC afforded the use of allogeneic cells with little concern about rejection20. The 3rd passages of each cell type was collected and kept in cell recovery medium in −80°C for transplantation and in vitro phenotype/function analysis.
Cell characterization
Immuno-fluorescent staining was used to determine cellular phenotype 21 probing for the cell-surface markers CD34, c-kit, CD133, kinase insert Domain Receptor (KDR), CD44, CD73, CD90, CD105, CD14, CD45, and with either primary or secondary antibody alone for control. DAPI was used for nuclear staining. The capacity of MSC for tri-lineage differentiation into adipocytes, chondrocytes, and osteocytes was further characterized using an MSC Functional Identification kit (R & D Systems, MN), following manufacturer’s instruction. Additionally, conditioned culture medium was collected from both EPC and MSC, and VEGF and TNF-α levels determined using ELISA.
Cell delivery
Six weeks after induction of RAS, cells were labeled with a fluorescent membrane dye (CM-DiI), kept in 10 ml PBS (106 cells/mL), and injected slowly through a balloon placed in the renal artery proximal to the stenosis 8, 17, 22. For EPC, equal proportions of early and late cells were mixed and delivered.
Renal function
Four weeks after cell delivery, animals were anesthetized with intramuscular ketamine (20 mg/kg) and xylazine (2 mg/kg), intubated, and ventilated. Anesthesia was maintained with ketamine (0.2 mg/kg/min) and xylazine 0.03 mg/kg/min). MDCT flow studies were performed in-vivo, as previously detailed23, for assessment of RBF and GFR. Manually-traced regions of interest were selected in MDCT images in the aorta, renal cortex, and medulla and their densities tabulated. Time-density curves were used to calculate renal regional perfusion, single-kidney GFR, and RBF using previously-validated methods23, 24.
Cell tracking
A few days after completion of the in-vivo studies, the kidneys were dissected and frozen tissue cut into 5µm sections and mounted with DAPI. EPC and MSC labeled with CM-DiI were detected by red labeling and counted in each slide under a fluorescence microscope to calculate their retention rate. We have successfully employed this method before to track EPC and MSC 4 weeks after transplantation into the kidney8, 17, 19. Furthermore, frozen kidney sections from pigs infused with cells were stained with the distal tubular marker peanut agglutinin (5ug/ml, Vector) and proximal tubular marker phaseolus vulgaris erythroagglutinin (PHA-E, 5ug/ml, Vector). In 10–15 fields sampled in each section, EPC/MSC were manually counted and recorded by locations (tubular, perivascular, or interstitial).
Micro-CT
A saline-filled cannula was ligated in a segmental artery perfusing the intact end of the excised stenotic kidney, for infusion (0.8 ml/min) of an intravascular contrast agent (Microfil,) under physiological pressure. The kidney samples (2×2 cm) were scanned using micro-CT, as previously described1, 2, 16, and 3D volume images reconstructed. The images were subsequently analyzed for vascular density and spatial distribution2, 25.
Renal protein expression
Kidneys were homogenized using a standard technique, and in addition the ER was isolated using an ER isolation kit (Imgenex, CA) following manufacturer’s instructions. Standard blotting protocols were then followed, as previously described15, 25, 26, using specific antibodies against Derl3, GRP94 and CHOP (for ER extracts), and caspacs3, TNF-alpha, IL-1β, IL-10, VEGF, HGF, Flk-1, Flt-1, p47phox, and p67phox (All 1:200, Santa Cruz, CA) for kidney lysate. GAPDH was used as loading controls. Protein expression was quantified using densitometry and averaged in each group. GRP94 and CHOP expression was also localized in kidney sections using immunohistochemistry.
In vitro ER stress in cultured kidney tubular cells
ER stress was induced in-vitro in porcine kidney tubular cells (LLC-PK1, ATCC, Manassas, VA) by adding 1µM thapsigargin27 (Sigma-Aldrich, St Louise, MO) in M199 media supplied with 3% fetal bovine serum in a 24-well plate. After an 8-hrs incubation, the cell culture was continued with 1µM thapsigargin, as well as with either 1×105 EPC or 1×105 MSC added in the wells (6 wells each group) for 16hrs, while untreated tubular cells served as control. Two parallel sets of experiments were performed. First, the progenitor or stem cells and LLC-PK1 cells were separately cultured in the same well using a Millipore cell culture insert plate (PK1 cells in the bottom and progenitor cell on the inserts), which allowed exchange of only the culture media. In the second set, the progenitor or stem cells were directly mixed with the PK1 tubular cells in culture. At the end of study, EPC/MSC and LLC-PK1 cells were separated by their characteristic differential responses to trypsin; to detach, LLC-PK1 require higher concentration of trypsin (0.25%) than EPC and MSC (0.025%). Media was removed, 50µl 0.025% trypsin added to each well, and the plate monitored under a microscope until EPC/MSC detached and were collected. Plate was washed with PBS to remove EPC/MSC, and PK1 cells were then collected. All cells were then homogenized separately for Western Blotting.
Statistical Analysis
Results were expressed as mean ± SEM. Statistical comparisons of multiple groups used one-way analysis of variance (ANOVA), followed by Tukey test, and unpaired student’s t-test if applicable. In vivo data analysis between experimental periods within groups were performed using paired student’s t-test. Statistical significance was accepted for p<0.05.
Results
Cell phenotype and engraftment
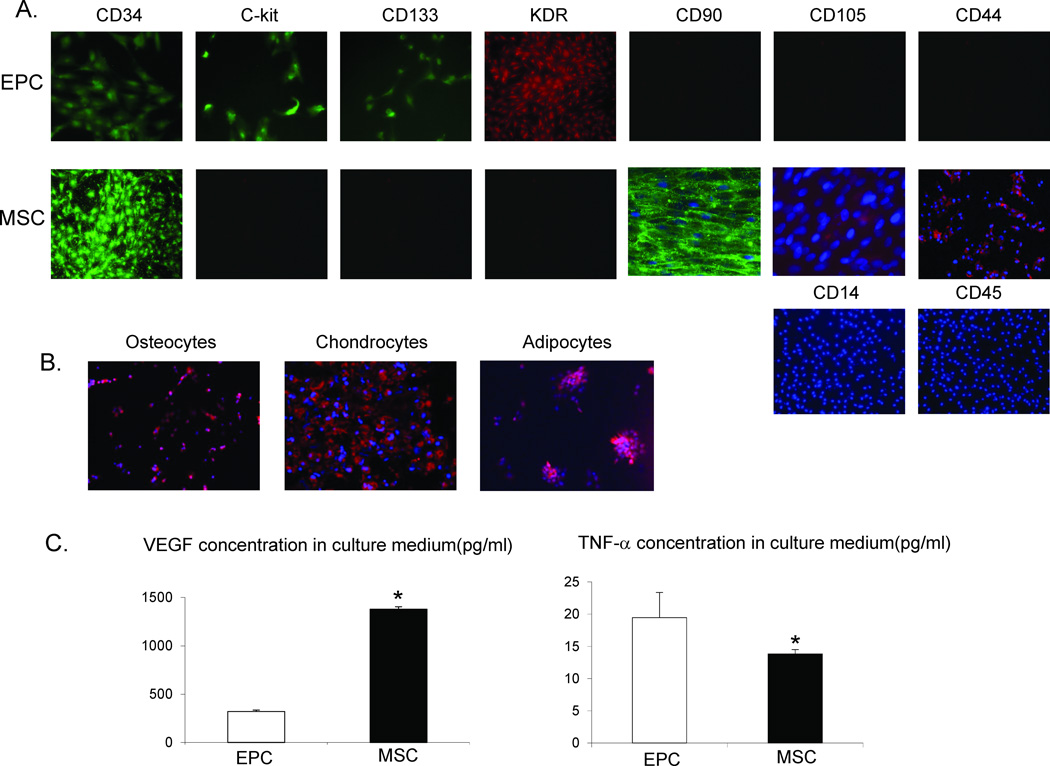
EPC expressed CD133, CD34, c-kit, and KDR, while MSC were positive for CD44 and CD90, but also expressed CD34 (Figure 1A), probably due to the use of the EGM-2 culture medium. Both EPC and MSC stained negative for CD14 and CD45. MSC were further confirmed by their capacity to transdifferentiate into adipocytes, osteocytes, and chondrocytes (Figure 1B). In conditioned media, MSC released more VEGF and less TNF-α than EPC (Figure 1C).
Figure 1.
A: Representative immunofluorescence images (20x) for surface markers of endothelial progenitor cells (EPC) and mesenchymal stem cells (MSC). B: MSC are capable of transdifferentiating into adipocytes, osteocytes, and chondrocytes (images are 20x). C: Cytokines released from the conditioned medium of EPC and MSC. MSC released greater amounts of vascular endothelial growth factor (VEGF), but lower amounts of tumor necrosis factor (TNF)-α than EPC. *p<0.05 vs. Normal
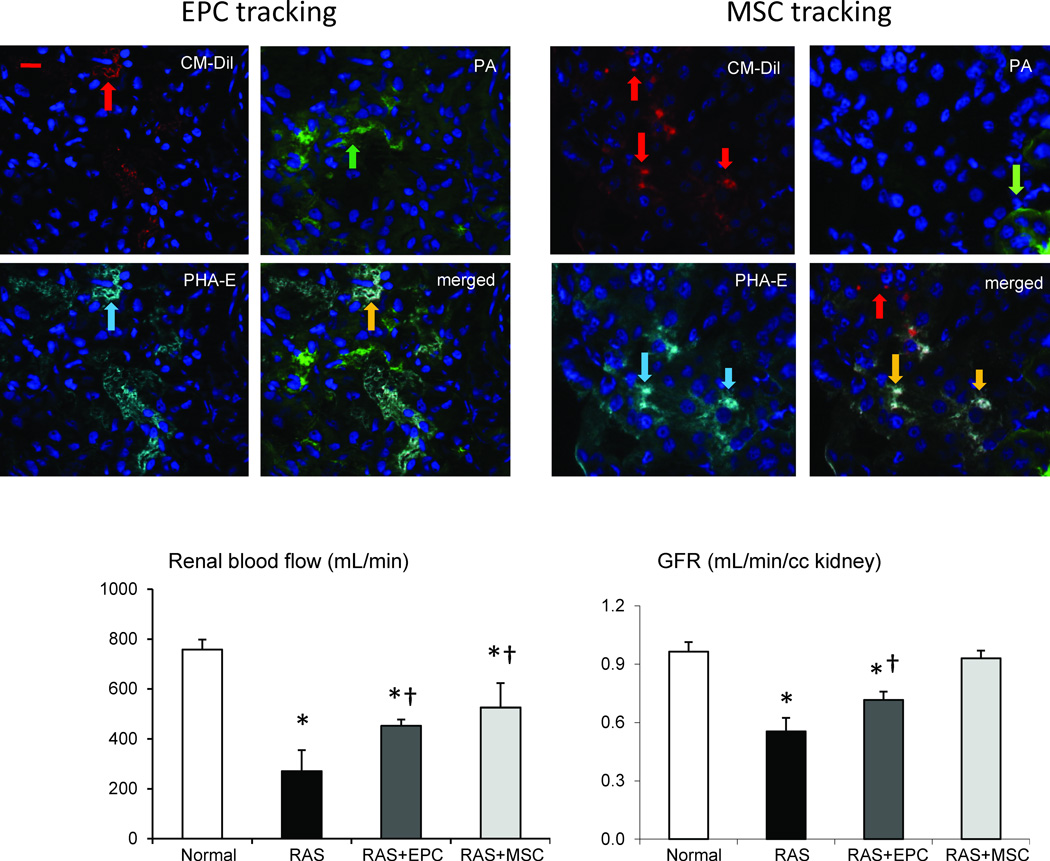
Four weeks after infusion, EPC and MSC both integrated into kidney tubular and interstitial areas, and showed a similar retention rate of around 12% (Figure 2). However, MSC were more commonly observed in the interstitium, while EPC tended to engraft in renal tubules and adventitial microvessels. We found that similar fractions of MSC integrated into tubular (48%) or interstitial (49%) compartments, while only 3% was found in perivascular regions. On the other hand, EPC integrated primarily in tubules (56%), followed by perivascular (17%) and interstitial (27%) engraftment. Interestingly, both EPC and MSC integrated only into proximal but not distal tubules (Figure 2).
Figure 2.
Top: Representative images of CM-DiI labeled (red) endothelial progenitor cells (EPC) or mesenchymal stem cells (MSC) in the post-stenotic kidneys of pigs with renal artery stenosis (RAS) 4 weeks after cell delivery. Green shows peanut agglutinin (PA, green arrow), a distal tubular marker, and cyan shows a proximal tubular marker phaseolus vulgaris erythroagglutinin (PHA-E, cyan arrow). EPC showed mainly tubular engraftment (yellow arrow), while MSC tend to integrate into both proximal tubules (yellow arrow) and interstitial area (red arrow). Bottom: Both EPC and MSC improved RBF and GFR in pigs with RAS, yet MSC more effectively restored GFR. *p<0.05 vs. Normal, †p<0.05 vs. RAS. Scale bar=200µm
Renal function
Compared to normal, after 10 weeks of RAS, all RAS pigs had similar degree of stenosis and elevated blood pressure, indicating that cell treatments had no effects on renovascular hypertension (Table 1). PRA, creatinine, and urine protein levels were similar among the groups (Table 1).
Table 1.
Systemic characteristics of pigs with renal artery stenosis (RAS), RAS treated with endothelial progenitor cells (EPC) or mesenchymal stem cells (MSC), and normal control.
| Normal | RAS | RAS+EPC | RAS+MSC | |
|---|---|---|---|---|
| Body weight (kg) | 49.5±1.5 | 56.0±3.0 | 53.6±4.0 | 51.2±4.1 |
|
Degree of stenosis(%) |
− | 70.4±5.2* | 69.3±6.0* | 74.2±8.3* |
|
Mean arterial pressure (mmHg) |
98.8±3.4 | 116.4±4.1* | 115.9±5.7* | 117.8±3.9* |
|
Plasma renin activity(ng/ml/hr) |
0.21±0.04 | 0.19±0.03 | 0.14±0.04 | 0.20±0.05 |
| Creatinine(mg/dL) | 1.4±0.06 | 1.7±0.14 | 1.5±0.15 | 1.2±0.1 |
|
Urine protein(µg/ml) |
14.4±3.7 | 21.7±4.7 | 16.8±4.1 | 20.2±5.1 |
p<0.05 vs. normal
Stenotic kidneys of RAS pigs had lower RBF and GFR compared to normal (Figure 2). EPC and MSC both improved post-stenotic RBF, although they remained lower than in normal pigs (p<0.05 vs. RAS, p<0.05 vs. Normal). EPC and MSC also both improved GFR compared to RAS, yet MSC increased GFR to levels not different than control kidneys.
Renal microvascular structure and growth factors
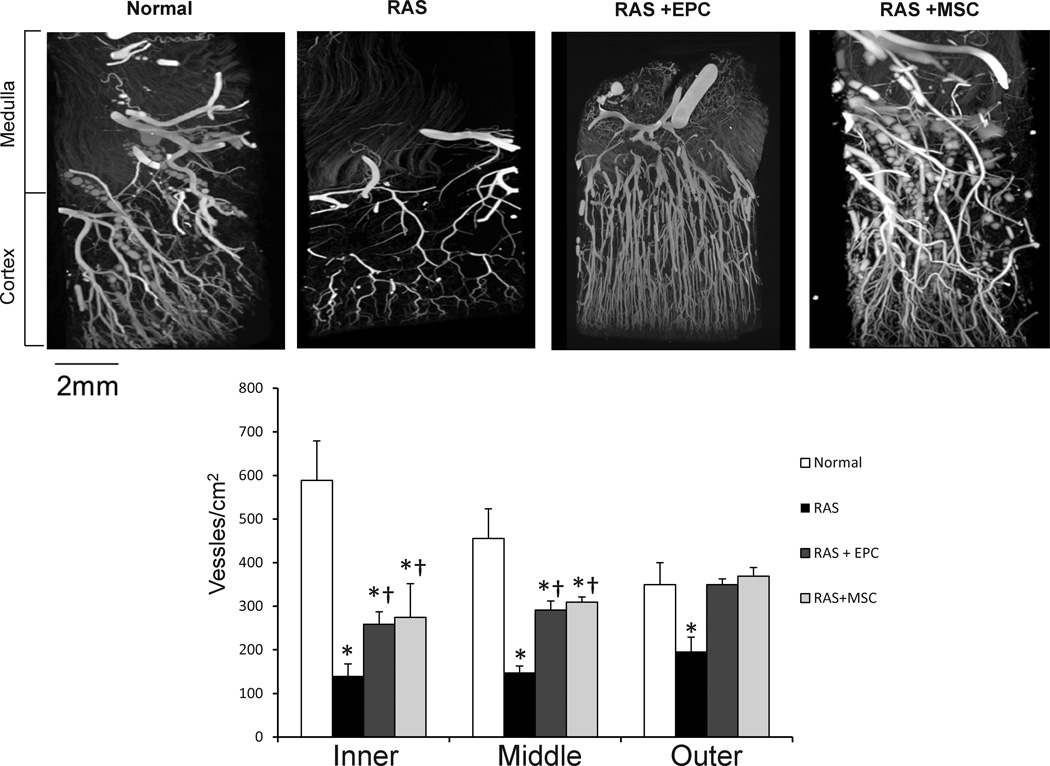
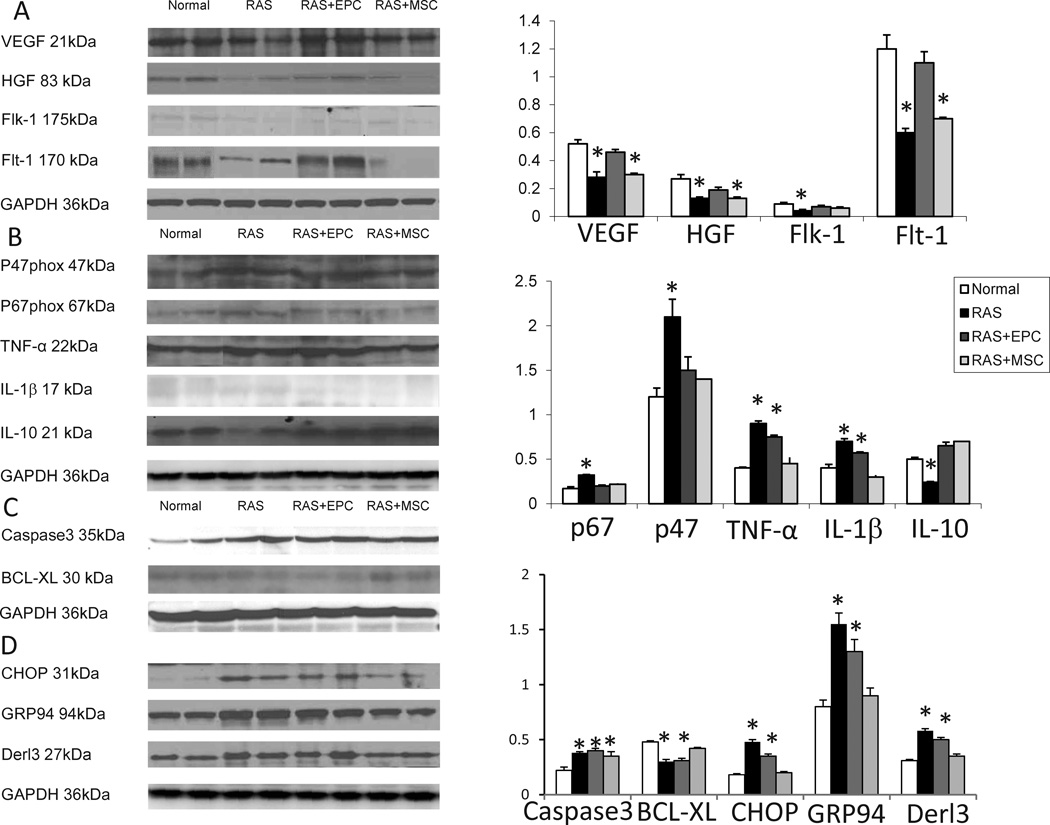
RAS pigs showed a decrease in transmural cortical microvascular density, which was improved but not normalized in the inner and middle cortex by both EPC and MSC treatments, and fully restored in the outer cortex by both (Figure 3). RAS also attenuated renal expression of the growth factors HGF, VEGF, and its receptors Flk-1 and Flt-1, most of which were improved by EPC, but not MSC (Figure 4A), with the exception of Flk-1 that was upregulated by both.
Figure 3.
Representative micro-CT images of pig kidney samples from normal, renal artery stenosis (RAS), and RAS treated with endothelial progenitor cells (EPC) or mesenchymal stem cells (MSC). Both EPC and MSC improved microvascular density in the stenotic kidney but more effectively in the outer than inner cortex. *p<0.05 vs. Normal, †p<0.05 vs. RAS. Scale bar=2mm
Figure 4.
Western blotting images and quantification of renal angiogenic (A), oxidative and inflammatory (B), and apoptosis (C) protein expression in normal, renal artery stenosis (RAS), RAS treated with endothelial progenitor cells (EPC) and mesenchymal stem cells (MSC) pigs. (D). Endoplasmic reticulum (ER) stress assessed in ER isolated from the same kidneys. *p<0.05 vs. Normal
Oxidative stress and inflammation
The expression of the NAD(P)H oxidase subunits p47phox and p67phox was upregulated in RAS, and MSC and EPC similarly downregulated their expression. The pro-inflammatory cytokine TNF-alpha and the inflammatory factor IL-1β released by activated macrophages were increased in RAS and selectively decreased in RAS+MSC, but remained elevated in RAS+EPC pigs (Figure 4B). On the other hand, the anti-inflammatory cytokine IL-10 was suppressed in RAS, but similarly restored in RAS+EPC and RAS+MSC pigs (Figure 4B).
Apoptosis and ER stress
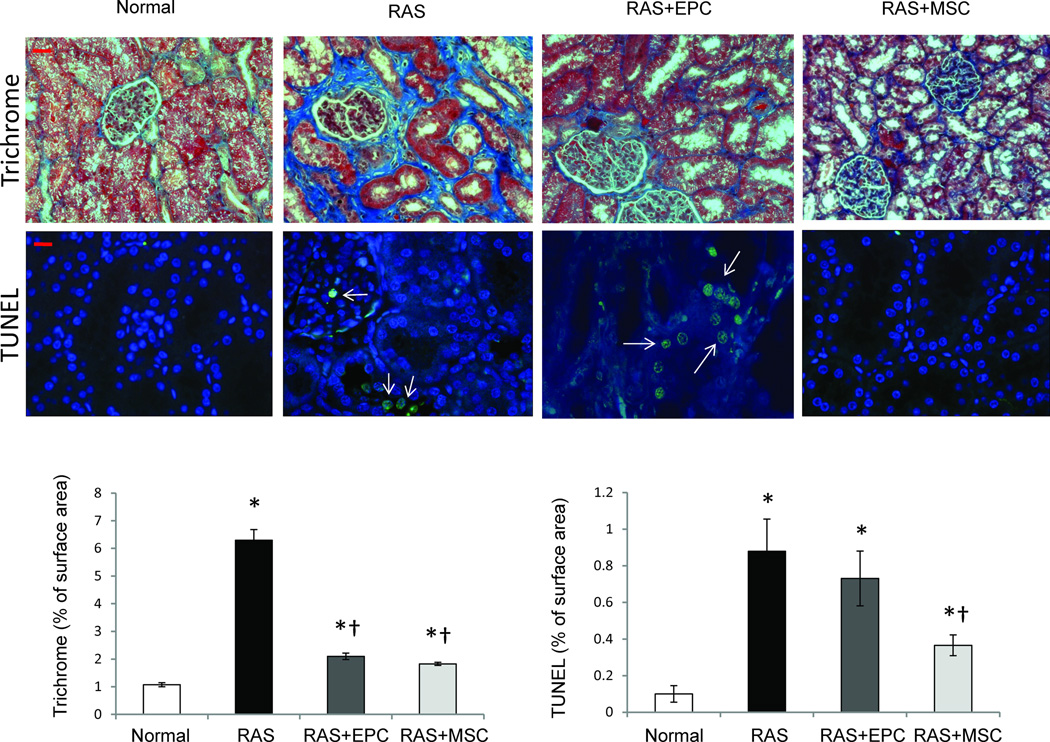
The number of renal apoptotic cells was significantly increased in RAS, and appeared to include glomerular endothelial cells, epithelial tubular cells, as well as interstitial cells (Fig 5). EPC treatment did not affect apoptosis, but the number of the apoptotic cells decreased in RAS+MSC pigs (p<0.05 vs. RAS, Figure 5). Cleaved caspase3, an effector of apoptosis, was upregulated in RAS, and unaffected by either EPC or MSC treatments (Figure 4C). However, BCL-XL, an anti-apoptotic factor, was downregulated in RAS, and normalized only in RAS+MSC. ER isolated from RAS kidneys also showed increased expression of CHOP, Derl3, and GRP94, which are involved in ER stress, and were normalized only in RAS+MSC (Figure 4D). Immunohistochemistry showed that CHOP and GRP94 were expressed in endothelial and proximal tubular cells, but not in the distal tubular cells (6C). Nevertheless, both cell types significantly decreased kidney fibrosis in the post-stenotic kidney (Figure 5).
Figure 5.
Representative images of trichrome (blue, top 20x) and TUNEL (middle, green, arrow) staining of kidneys from normal, renal artery stenosis (RAS), RAS treated with endothelial progenitor cells (EPC) and mesenchymal stem cells (MSC) pigs. EPC and MSC similarly attenuated renal fibrosis (bottom left), but MSC more efficiently prevented renal cell apoptosis (bottom right) in the stenotic kidney. *p<0.05 vs. Normal, †p<0.05 vs. RAS. Scale bar=200µm
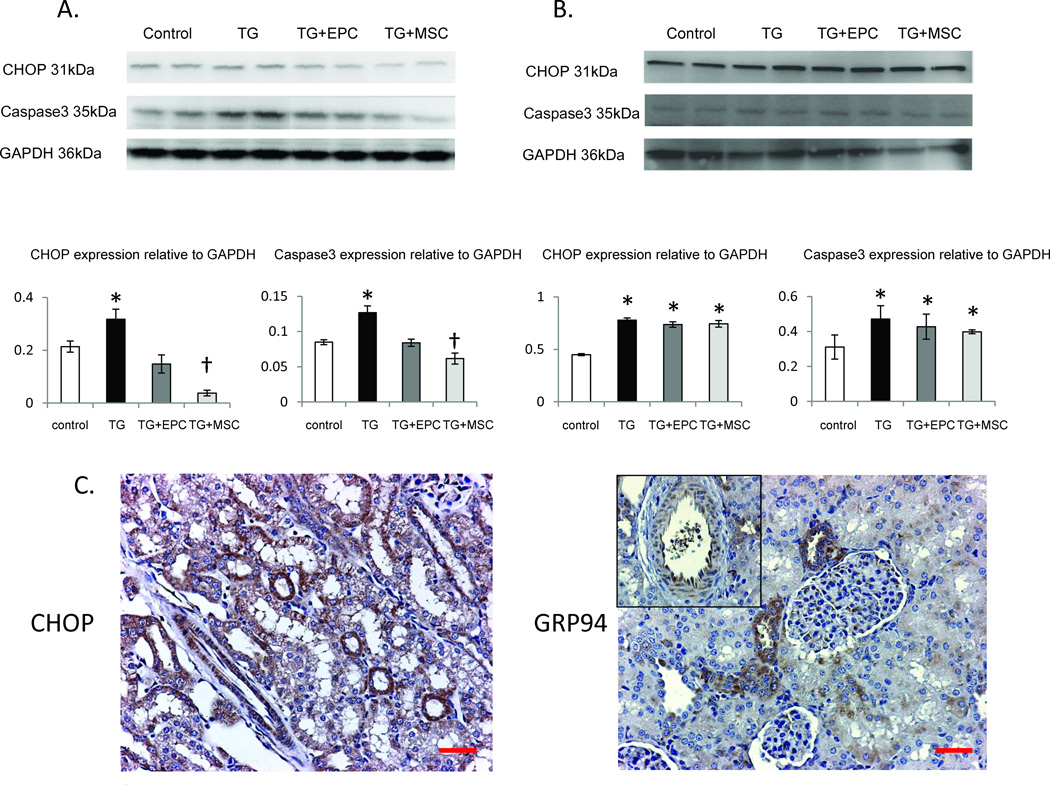
In vitro, thapsigargin-treated kidney tubular cells showed increased expression of CHOP and cleaved caspase3, which were normalized by direct co-culture with both EPC and MSC (Figure 6A). However, MSC further decreased tubular cell expression of CHOP and caspase3 compared with EPC, supporting superior protection from ER stress and apoptosis by MSC compared to EPC. Interestingly, EPC and MSC co-cultured with kidney tubular cells separated by an insert plate that allowed exchange of culture media alone, did not normalize CHOP and caspase3 (Figure 6B), suggesting that physical contact augments progenitor/stem cells rescue of tubular cells from ER stress-induced apoptosis in-vitro.
Figure 6.
Top: Western blotting images and quantification of the expression of CHOP and caspase-3 showing the effects of endothelial progenitor cells (EPC) and mesenchymal stem cells (MSC) on thapsigargin (TG)-induced endoplasmic reticulum (ER) stress and apoptosis in porcine kidney tubular cells in-vitro. Protection of kidney tubular cells from TG-induced ER stress and apoptosis required cell-cell contact (A) and was virtually absent when tubular cells were cultured separately and came in contact only with the culture medium (B). MSC induced greater decreases in CHOP and caspase-3 than EPC during co-incubation with tubular cells. C: immunohistochemistry of CHOP and GRP94 in kidney sections, localizing these ER stress indices to endothelial and proximal tubular cells. *p<0.05 vs. Control, †p<0.05 vs. TG+EPC. Scale bar=200µm
Discussion
This study demonstrated that EPC and MSC delivered into the post-stenotic kidney induced improvements in renal function that were achieved by slightly different mechanisms and to different extents. EPC elicited greater improvement in angiogenic signaling, while MSC suppressed inflammatory cytokines and ER-stress induced apoptosis to a larger extent. The greater attenuation conferred by MSC was confirmed in cultured tubular cells and augmented by direct cell contact. Importantly, in vivo both approaches effectively improved the hemodynamics of the stenotic kidney, although MSC induced a slightly greater improvement in renal function.
Progenitor and stem cells have been shown to play important roles in tissue repair 12, 28, 29, and several clinical trials of cell treatment registered at www.clinicaltrials.gov are recruiting patients with kidney diseases30. However, several important questions remain unsolved, such as possible differences between cell types in their affinity, tissue repair efficacy, and specific mechanisms of action. In the current study, we compared functional repair capacity of two well-characterized cell populations in a large animal model mimicking human ischemic kidney disease, and explored involved mechanisms.
RAS is the primary etiology underlying renovascular hypertension and may lead to end-stage kidney disease. Kidney damage distal to the stenosis is characterized by microvascular cellular loss, oxidative stress, inflammation, and interstitial fibrosis2, 31. Our previous studies showed that both EPC8, 17 and MSC19 can improve microvascular density in the stenotic kidney. The current study shows the effect of both cell types may be achieved by slightly different mechanisms in the post-stenotic kidneys. EPC may augment neovascularization via direct engraftment into vascular structures8 and/or paracrine secretion of growth factors32, 33. Indeed, we found that impaired expression of VEGF and its receptors in RAS was restored in EPC-treated but not in MSC-treated kidneys. Furthermore, a similar pattern of improvement was observed in expression of HGF, a critical growth factor in adult organ regeneration and in wound healing34. Interestingly, MSC in fact secreted more VEGF than EPC in conditioned medium in vitro. This discrepancy may imply that EPC restored renal growth factor expression not only via paracrine secretion, but may also stimulate local resident cells to express and secrete growth factors. Notably, since MSC successfully restored microvascular density without long-term upregulation of growth factors, the initial paracrine delivery of VEGF might have conceivably contributed to neovascularization. Nevertheless, the increased vascularization might have alternatively been permitted indirectly by decreased fibrosis or other mechanisms.
The affinity of EPC and MSC for renal engraftment does not seem to account for their differential effects, as their overall retention rates were very similar. However, MSC retention in the interstitial space in proximity to inflammatory cells and fibroblasts may contribute to their anti-inflammatory effects, while the greater tendency of EPC to integrate into perivascular areas may contribute to their angiogenic potential. Interestingly, both EPC and MSC integrated only into proximal but not distal tubules, likely because proximal tubular cells are more sensitive to insults and attract stem cells for repair. These observations were supported by Immunohistochemistry, showing ER stress markers expression in proximal tubular and endothelial cells, but not in distal tubules. Furthermore, inflammation plays an important role in neovascularization in ischemic tissues35, yet overexpression of inflammatory cytokines might alternatively impair angiogenesis36 and elicit oxidative stress. Our study demonstrated that MSC more effectively than EPC modulated the levels of the inflammatory cytokines TNF-α and IL-1β, which may indirectly account for the improvement in microvascular structure in RAS. IL-1β produced by activated macrophages is an important mediator of the inflammatory response, and involved in a variety of cellular activities, including proliferation, differentiation, and apoptosis37. TNF-α, an endogenous pyrogen involved in systemic inflammation, is produced chiefly by activated macrophages and is able to induce apoptotic cell death by activating death receptors38. The ability of MSC to downregulate these inflammatory mediators may thus be an important aspect of their beneficial properties.
Importantly, TNF-α may also induce cell apoptosis via ER stress by upregulating protein expression of CHOP and GRP9439, 40. The ER regulates cell function such as protein biosynthesis, folding, trafficking, and modification, functions that are sensitive to environmental insults like ischemia, oxidative stress, or inflammation, which can lead to ER stress. Cells experiencing ER stress invoke the unfolded protein response, which enhances the protein-folding capacity by activating GRP78, GRP94, and calreticulin. ER stress-induced apoptosis is mainly mediated by CHOP41, a transcription factor which induces several pro-apoptotic factors and downregulates anti-apoptotic Bcl-242, leading to enhanced oxidant injury and apoptosis. Importantly, MSC, but not EPC, downregulated CHOP, GRP94, and Derl3 expression in ER isolated from stenotic kidney cells, which may thereby increase Bcl-xl (a member of Bcl-2 family) expression in RAS. Potentiated attenuation of ER stress and apoptosis by MSC could have also contributed to decrease tissue damage and improve renal function in RAS.
Interestingly, our in vitro study showed that protection of kidney tubular cells from thapsigargin-induced ER stress and apoptosis by both MSCs and EPC was facilitated by cell-cell contact, and blunted when they were physically separated. Our results are underscored by a previous observation showing that MSCs protected chronic lymphocytic leukemia cells from fludarabine-induced apoptosis in a cell-cell contact manner43, suggesting that direct MSC or EPC delivery into an injury site might be more efficient for tissue repair than their paracrine factors. Clearly, however, the few cells observed in cell tracking could not have contact with all renal cells. Injected cells might have come across different endogenous cells during their migration in the kidney, and/or induced neighboring cells that in turn also affect adjacent cells. The salutary effects of the cells are likely mediated by essential paracrine effects, yet direct contact seems to magnify their efficacy. Further studies are needed in order to better understanding cross talk between delivered progenitor cells and kidney cells.
Study limitations
There are obvious limitations for replicating clinical conditions in relatively young animals. Also, we studied a single dose of EPCs and two doses of MSCs, found to be safe and well-tolerated. Additional studies are needed to determine the optimal dose and timing of cell delivery, and explore the combination of EPC and MSC. Furthermore, our follow-up extended up to 12 weeks after cell transfer; longer-term benefits of cell treatment remain to be determined. Nevertheless, the short-term improvement of renal function and increased vascular density might sustain kidney function and structure.
Taken together, our data demonstrated that EPC and MSC induced similar restoration of the renal microcirculation in RAS, yet employed different mechanisms. EPC may possess greater direct pro-angiogenic potency, while MSC more effectively suppress inflammatory cytokines, ER-stress, and apoptosis, which thereby recover renal structure and function. Therefore, our observations suggest that the underlying pathophysiology may need to be considered during selection of cell type targeted for kidney repair. Furthermore, our study supports rigorous development of regenerative strategies to improve the damaged kidney.
Supplementary Material
Acknowledgements
This study was partly supported by NIH grants: DK73608, DK77013, HL77131, and HL085307.
Footnotes
Xiang-Yang Zhu, (Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing)
Victor Urbieta-Caceres, (Collection and/or assembly of data, Final approval of manuscript)
James D. Krier, (Collection and/or assembly of data, Final approval of manuscript)
Stephen C. Textor, (Data interpretation, Manuscript review, Final approval of manuscript)
Amir Lerman, (Data interpretation, Manuscript review, Final approval of manuscript)
Lilach O. Lerman (Conception and design, Financial support, Administrative support, Data interpretation, Manuscript writing, Final approval of manuscript)
References
- 1.Zhu XY, Chade AR, Krier JD, et al. The chemokine monocyte chemoattractant protein-1 contributes to renal dysfunction in swine renovascular hypertension. Journal of hypertension. 2009;27:2063–2073. doi: 10.1097/HJH.0b013e3283300192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu XY, Chade AR, Rodriguez-Porcel M, et al. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 3.Liu G, Sun Y, Li Z, et al. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochemical and biophysical research communications. 2008;370:651–656. doi: 10.1016/j.bbrc.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Rao RV, Peel A, Logvinova A, et al. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS letters. 2002;514:122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuh A, Liehn EA, Sasse A, et al. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol. 2008;103:69–77. doi: 10.1007/s00395-007-0685-9. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe J, Nakamachi T, Ogawa T, et al. Characterization of antioxidant protection of cultured neural progenitor cells (NPC) against methylmercury (MeHg) toxicity. J Toxicol Sci. 2009;34:315–325. doi: 10.2131/jts.34.315. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, von Ballmoos MW, Faessler D, et al. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211:103–109. doi: 10.1016/j.atherosclerosis.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Chade AR, Zhu X, Lavi R, et al. Endothelial Progenitor Cells Restore Renal Function in Chronic Experimental Renovascular Disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 10.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 11.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 12.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 13.Aguirre A, Planell JA, Engel E. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem Biophys Res Commun. 2010;400:284–291. doi: 10.1016/j.bbrc.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 14.Dubois C, Liu X, Claus P, et al. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol. 2010;55:2232–2243. doi: 10.1016/j.jacc.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 15.Zhu XY, Daghini E, Chade AR, et al. Simvastatin prevents coronary microvascular remodeling in renovascular hypertensive pigs. J Am Soc Nephrol. 2007;18:1209–1217. doi: 10.1681/ASN.2006090976. [DOI] [PubMed] [Google Scholar]
- 16.Zhu XY, Daghini E, Chade AR, et al. Role of Oxidative Stress in Remodeling of the Myocardial Microcirculation in Hypertension. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1746–1752. doi: 10.1161/01.ATV.0000227469.40826.01. [DOI] [PubMed] [Google Scholar]
- 17.Chade AR, Zhu XY, Krier JD, et al. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem cells (Dayton, Ohio) 2010;28:1039–1047. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon CH, Hur J, Park KW, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 19.Eirin A, Zhu XY, Krier JD, et al. Adipose Tissue-Derived Mesenchymal Stem Cells Improve Revascularization Outcomes to Restore Renal Function in Swine Atherosclerotic Renal Artery Stenosis. Stem cells (Dayton, Ohio) 2012 doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comoli P, Ginevri F, Maccario R, et al. Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol Dial Transplant. 2008;23:1196–1202. doi: 10.1093/ndt/gfm740. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Weisdorf DJ, Solovey A, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulati R, Jevremovic D, Peterson TE, et al. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 23.Daghini E, Primak AN, Chade AR, et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology. 2007;243:405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 24.Krier JD, Ritman EL, Bajzer Z, et al. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. American journal of physiology. 2001;281:F630–F638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 25.Zhu XY, Rodriguez-Porcel M, Bentley MD, et al. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation. 2004;109:2109–2115. doi: 10.1161/01.CIR.0000125742.65841.8B. [DOI] [PubMed] [Google Scholar]
- 26.Zhu XY, Daghini E, Chade AR, et al. Disparate effects of simvastatin on angiogenesis during hypoxia and inflammation. Life sciences. 2008;83:801–809. doi: 10.1016/j.lfs.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuest M, Willim K, Macnelly S, et al. The transcription factor c-Jun protects against sustained hepatic endoplasmic reticulum stress thereby promoting hepatocyte survival. Hepatology. 2011 doi: 10.1002/hep.24699. [DOI] [PubMed] [Google Scholar]
- 28.Bianco P, Robey PG, Saggio I, et al. "Mesenchymal" stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther. 2010;21:1057–1066. doi: 10.1089/hum.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi YH, Kurtz A, Stamm C. Mesenchymal stem cells for cardiac cell therapy. Hum Gene Ther. 2011;22:3–17. doi: 10.1089/hum.2010.211. [DOI] [PubMed] [Google Scholar]
- 30.Perico N, Casiraghi F, Introna M, et al. Autologous Mesenchymal Stromal Cells and Kidney Transplantation: A Pilot Study of Safety and Clinical Feasibility. Clin J Am Soc Nephrol. 2010 doi: 10.2215/CJN.04950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy BI, Duriez M, Samuel JL. Coronary microvasculature alteration in hypertensive rats. Effect of treatment with a diuretic and an ACE inhibitor. Am J Hypertens. 2001;14:7–13. doi: 10.1016/s0895-7061(00)01212-7. [DOI] [PubMed] [Google Scholar]
- 32.Westenbrink BD, Lipsic E, van der Meer P, et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 33.Shintani S, Kusano K, Ii M, et al. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S123–S128. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- 34.Banquet S, Gomez E, Nicol L, et al. Arteriogenic therapy by intramyocardial sustained delivery of a novel growth factor combination prevents chronic heart failure. Circulation. 2011;124:1059–1069. doi: 10.1161/CIRCULATIONAHA.110.010264. [DOI] [PubMed] [Google Scholar]
- 35.Ren G, Dewald O, Frangogiannis NG. Inflammatory mechanisms in myocardial infarction. Curr Drug Targets Inflamm Allergy. 2003;2:242–256. doi: 10.2174/1568010033484098. [DOI] [PubMed] [Google Scholar]
- 36.Brechot N, Gomez E, Bignon M, et al. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One. 2008;3:e3950. doi: 10.1371/journal.pone.0003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo DD, Fielding C, Phillips A, et al. Interleukin-1 beta regulates proximal tubular cell transforming growth factor beta-1 signalling. Nephrol Dial Transplant. 2009;24:2655–2665. doi: 10.1093/ndt/gfp208. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Guo R, Chen P, et al. TNF induces caspase-dependent inflammation in renal endothelial cells through a Rho- and myosin light chain kinase-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F316–F326. doi: 10.1152/ajprenal.00089.2009. [DOI] [PubMed] [Google Scholar]
- 39.Greene MW, Ruhoff MS, Burrington CM, et al. TNFalpha activation of PKCdelta, mediated by NFkappaB and ER stress, cross-talks with the insulin signaling cascade. Cellular signalling. 2010;22:274–284. doi: 10.1016/j.cellsig.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. The Journal of biological chemistry. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 41.Katsoulieris E, Mabley JG, Samai M, et al. alpha-Linolenic acid protects renal cells against palmitic acid lipotoxicity via inhibition of endoplasmic reticulum stress. European journal of pharmacology. 2009;623:107–112. doi: 10.1016/j.ejphar.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Chiribau CB, Gaccioli F, Huang CC, et al. Molecular symbiosis of CHOP and C/EBP beta isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis. Mol Cell Biol. 2010;30:3722–3731. doi: 10.1128/MCB.01507-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurtova AV, Balakrishnan K, Chen R, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114:4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.