Abstract
Objectives
The authors assessed whether the levels and progression rates of carotid intima-media thickness (IMT) and adventitial diameter (AD) vary by menopausal stage.
Methods
249 Women (42–57 years old, premenopausal (49%) or early peri-menopausal (46%)) from the Study of Women’s Health Across the Nation were included in the current analysis. Participants were followed for up to 9 years (median=3.7 years) and had up to 5 carotid scans. Linear mixed models were used for analysis.
Results
The overall rate of change in IMT was 0.007 mm/year. Independent of age and race, progression rate of IMT increased substantially in late peri-menopausal stage (0.017 mm/year) compared to both premenopausal (0.007 mm/year) and early peri-menopausal (0.005 mm/year) stages; (P≤0.05). For AD, while the overall rate of change was negative (−0.009 mm/year), significant positive increases in the rate of change were observed in late peri-menopausal (0.024 mm/year) and postmenopausal (0.018 mm/year) stages compared to premenopausal stage (−0.032 mm/year); (P<0.05). In final models, postmenopausal stage was independently associated with higher levels of IMT and AD (P<0.05) compared to premenopausal stage.
Conclusions
During the menopausal transition, the carotid artery undergoes an adaptation that is reflected in adverse changes in IMT and AD. These changes may impact the vulnerability of the vessel to disease in older women.
Keywords: atherosclerosis, carotid intima-media thickness, epidemiology, menopause, risk factors
INTRODUCTION
The incidence rate of cardiovascular diseases (CVD), the leading cause of death in women, increases after the age of 50,1 when most women are transitioning to menopause. This suggests a possible link between the menopausal transition and the development of CVD. Whether it is menopause or age related-changes that are associated with the increase in the incidence rate of CVD remains an active research area. Studies show that earlier age at menopause,2 premature menopause, and surgically-induced menopause 3,4 are significant risk factors for CVD, suggesting an effect of menopause on CVD risk that is independent from age.
Menopause is accompanied by several physiological changes that potentially contribute to the increased risk of CVD, including cessation of ovarian estrogen secretion, changes in body fat distribution,5 and adverse changes in lipid profiles.6 Further, menopause has been linked to the metabolic syndrome, insulin resistance,7 and changes in plasma adiponectin levels.8 To better understand the potential role of menopause in the development of CVD, it is important to evaluate whether the menopausal transition is associated with the progression of subclinical measures of CVD independent of age.
Previous studies examining the relationship between the menopausal transition and subclinical CVD have been mainly cross-sectional and focused on intima-media thickness (IMT) and arterial distensibility.9–11 One such study showed that women at 5 to 8 years after menopause have larger IMT measures compared to premenopausal women.9 Further, premenopausal cardiovascular risk factors 9,10 as well as the change in pulse pressure between premenopausal and first year postmenopausal10 have been reported to be independently associated with IMT measured after menopause. In addition, compared with premenopausal women, postmenopausal women have significantly lower arterial distension.11
Previous analyses from the Study of Women’s Health Across the Nation (SWAN) have shown that late peri-menopause was associated with larger common carotid adventitial diameter (AD) compared with pre/early peri-menopause in multivariable analysis, 12 However, this analysis was limited by its cross-sectional design and lack of data on the progression rate of subclinical CVD in the different stages of the menopausal transition.
The purposes of the present study were to 1) estimate and then compare progression rates of carotid IMT and AD across the four stages of the menopausal transition (pre-, early peri, late peri-, and postmenopausal stages) and 2) to determine the independent effect of menopausal status on IMT and AD levels in women transitioning through the menopause.
METHODS
Study participants
SWAN is an ongoing, longitudinal, multi-ethnic study of the biological, physical, psychological, and social changes during the menopausal transition. The study design has been previously reported.13 In brief, between 1996 and 1997, 3,302 participants aged 42–52 years were recruited from seven designated sites (Boston, MA; Detroit, MI; Oakland, CA; Los Angeles, CA; Pittsburgh, PA; Chicago, IL; and Newark, NJ). The eligibility criteria for the SWAN study were 1) An intact uterus and at least 1 ovary, 2) not pregnant or breastfeeding, 3) at least 1 menstrual period within the past 3 months, 4) no HT use within the past 3 months.
Participants of the current study were part of an ancillary study to SWAN at the Pittsburgh site. Enrollment began between the baseline and 3rd annual visit of the SWAN study (N=257). Participants were deemed eligible if they were not pregnant. Each participant had up to 5 carotid scans (815 total scans) over a maximum of 9 years of follow-up (median=3.7 years).
For the current analysis, data were censored at the time the participant underwent hysterectomy (n=27 observations), developed stroke and/or angina (n=3 observations), or started HT (n=179 observations). The final analysis included 249 participants with a total of 606 scans.
Research protocols were approved by the institutional review board at each site and all the participants provided a written informed consent prior to enrollment.
Study measures
Ultrasound measures
IMT and AD of the right and left carotid arteries was assessed by B-mode ultrasound using a Toshiba (Toshiba American Medical Systems, Tustin, CA) SSA-270A scanner. For IMT, B-mode images were obtained from 8 locations – 4 each from the left and right carotid arteries: the near and far walls of the distal common carotid artery (1 cm proximal to the carotid bulb), the far walls of the carotid bulb, and the internal carotid artery (from the flow divider to 1cm distal to this point). IMT measures were obtained by electronically tracing the lumen-intima interface and the media-adventitia interface across a 1-cm segment; one measurement was generated for each pixel over the area, for a total of approximately 140 measures for each segment. For analyses, the mean value of the average readings at all 8 locations was used.
For AD, the distance from the adventitial-medial interface on the near wall to the medial-adventitial interface on the far wall at end-diastole was measured for both right and left common carotid artery. For analyses, the average value of the readings at both sides was used. All readings were conducted at the University of Pittsburgh Ultrasound Research Laboratory. Readers were centrally trained with a standardized protocol and recertified annually against the same set of scans to guard against reader drift. Readers were blinded to participants’ clinical information. Replicate readings were performed on 20 scans to determine the inter-reader reproducibility of subclinical measures, with an intra-class correlation of 0.98 for IMT values and 0.99 for AD.
Blood assays
A fasting blood specimen was obtained either during the early follicular phase of the menstrual cycle (day 2–5) for menstruating participants or randomly within the 90-day period of recruitment for peri- and postmenopausal women. Lipids, glucose, insulin, and CRP were assayed at the Medical Research Laboratories (Lexington, KY), certified by National Heart Lung and Blood Institute, Centers for Disease Control and Prevention Part III program. 14 Total cholesterol and triglycerides levels were analyzed using enzymatic methods on a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics, Indianapolis, Ind), and high-density lipoprotein cholesterol (HDL-c) was isolated using heparin-manganese. Low density lipoprotein cholesterol (LDL-c) was calculated using the Friedewald equation.15 Serum insulin was measured by a radioimmunoassay (DPC Coat-a-count) and monitored as part of a monthly quality assurance program. Glucose was measured with hexokinase coupled reaction (Boehringer Mannheim Diagnostics). The HOMA index was calculated from fasting insulin and glucose as (insulin (mU/Liter)* glucose (mmoles/Liter)/22.5.16 CRP was quantified using an ultra-sensitive rate immunonephelemetric method (hs-CRP on BN 100, Dade-Behring, Marburg, Germany). The CRP assay within-run coefficient of variation was 3.3%, and between-day imprecision was 4.0% at a concentration of 58.9mg/L and 6.8% at a concentration of 10.2 mg/L.17
Physical measures
Weight and height were measured annually. Body mass index (BMI) was calculated as weight/height2. Blood pressure was averaged after 2 sequential measures in the right arm with the participant seated after at least 5 minutes of rest. Physical activity was assessed via a modified Baecke Scores of Habitual Physical Activity; with higher scores indicating more physical activity.18
Demographic, socioeconomic, co-morbidity and smoking variables
Race/ethnicity was self-reported. Age and current smoking status were derived from interviews administered during each annual visit. Hypertension and use of CVD medications (any reported use of medication for a heart condition, an anticoagulant, or for blood pressure lowering in the past year) were combined into a single co-morbidity variable.
Menopausal status
Menopausal status was determined annually based on reports about frequency, regularity of menstrual bleeding and use of hormone therapy 19 as follows: 1) Premenopause: monthly bleeding with no perceived change in cycle interval in the past year, 2) Early peri-menopause: monthly bleeding with a perceived change in cycle interval, but at least one menstrual period within the past 3 months, 3) Late peri-menopause: 3 consecutive months of amenorrhea, 4) Postmenopause: 12 consecutive months of amenorrhea, 5)Surgical menopause: menopause induced by hysterectomy with/without oophorectomy, 6) Unknown: use of hormone therapy before documentation of a final menstrual period.
Statistical Analysis
AD values were normally distributed, whereas IMT values were slightly skewed. Therefore, IMT was examined with and without a log-transformation. Since results were similar, results from models using the original values of IMT were presented for ease of interpretation. Triglycerides, glucose, insulin, HOMA index, and CRP were highly skewed and were log-transformed for analyses.
The overall rates of change in IMT and AD during the study were estimated using linear mixed effect models to account for within-subject correlation and unequal intervals between measurements.20 To estimate rates of change during each stage of the menopausal transition repeated measures of each outcome (IMT and AD) were modeled as a function of four separate time variables, one for the cumulative amount of time spent in each of the four menopausal stages since baseline. The algorithm used to calculate the cumulative time spent in each menopausal stage was successfully applied in a previous analysis from the SWAN study.21 We modified the algorithm to reflect the cumulative time spent in each stage since the baseline carotid scan (see Supplemental Digital Content 1, http://links.lww.com/MENO/A38). The beta coefficients for these four time variables provided estimates of the annual change in IMT/AD for each menopausal stage. These models were adjusted for age at baseline and race. Contrasts were used to compare rates of change in IMT and AD across menopausal transition stages (4 time variables).
To determine risk factors for greater IMT and AD, maximum likelihood linear mixed effect models were used and both unadjusted and adjusted models were fitted. All included variables were time-dependent except for age and race. Variables were included in models based on p-values from univariate analysis (p<0.05) starting with those with the lowest p-values. Variables with p≥0.1 in final models were dropped. For variables that were highly correlated (e.g. BMI and waist circumference) models were tested with each of these variables separately and the model which showed a better fit was selected.
To test the independent effect of menopausal status on IMT and AD levels, effect of status (modeled as concurrent menopausal status (time-dependent)) was assessed before and after adjusting for significant risk factors identified through the current analyses. The regression coefficients for menopausal status categories indicate the differences in levels of IMT/AD compared to premenopausal status (the reference group). Risk factors were modeled using two different approaches 1) As concurrent measures (time-dependent) and 2) As baseline (to assess cross-sectional effect) and change since baseline (to assess longitudinal effect). Changes since baseline were not significantly related to IMT or AD levels. Therefore, only results using the first approach of modeling risk factors were presented. Models with smaller Akaike’s information criterion were considered better fit. In order to obtain a meaningful intercept all continuous variables were centered to their means (except for time which was computed as time since baseline).
Residual analysis and diagnostic plots were conducted to verify model assumptions. Analyses were performed with SAS v9.2 (SAS Institute, Cary, NC). All tests were 2-sided at alpha=0.05.
RESULTS
Table 1 shows the main characteristics of the study population at baseline scan. Table 2 presents statistics of subclinical measures and traditional CV risk factors at baseline.
Table 1.
Characteristics of the Study Population at the First Scan
| Variable | Total N=249 a
|
|
|---|---|---|
| Mean or n | SD or % | |
| Age, Y | 46.2 | 2.8 |
| Race | ||
| White | 176 | 70.7% |
| Black | 73 | 29.3% |
| Menopausal Status | ||
| Premenopausal | 122 | 49.0% |
| Early Peri-menopausal | 115 | 46.2% |
| Late Peri-menopausal | 4 | 1.6% |
| Postmenopausal | 8 | 3.2% |
| Co-morbidity b | ||
| Yes | 42 | 17.1% |
| No | 204 | 82.9% |
| Physical activity score c | 8.1 | 1.7 |
| Current Smoker | ||
| Yes | 37 | 15.0% |
| No | 209 | 85.0% |
Number may vary for each variable according to missing values. Data are presented as mean (SD) for continuous variables and n (%) for categorical variables.
Composite variable for high blood pressure/antihypertensive, and medication for heart, and anticoagulant
Modified Baecke Scores of Habitual Physical Activity. Higher scores indicating more physical activity
Table 2.
Subclinical Measures and Traditional Cardiovascular Risk Factors at First Scan
| Variable | Total N=249 a
|
|
|---|---|---|
| Mean or Median | SD or IQ range | |
| AD, mm | 6.7 | 0.57 |
| IMT, mm | 0.68 | 0.07 |
| BMI, Kg/m2 | 28.2 | 6.3 |
| Waist circumference, Cm | 86.5 | 14.4 |
| DBP, mm Hg | 72.1 | 9.2 |
| SBP, mm Hg | 113.3 | 15.9 |
| Cholesterol, mg/dL | 200.0 | 34.8 |
| HDL-c, mg/dL | 55.3 | 12.3 |
| LDL-c, mg/dL | 122.4 | 31.2 |
| Triglycerides, mg/dL b | 89.0 | 68.0,124.0 |
| Glucose, mg/dL b | 89.0 | 84.0,95.0 |
| Insulin, uIu/Ml b | 7.8 | 5.9,11.3 |
| HOMA Index b | 1.7 | 1.3,2.6 |
| C-Reactive Protein, mg/DL b | 1.4 | 0.8,4.1 |
Abbreviations: AD: adventitial diameter; BMI: body mass index; DBP: diastolic blood pressure; HDL-c: high density lipoprotein cholesterol; IMT: intima-media thickness; LDL-c: pressure; HDL-c: high density lipoprotein cholesterol; IMT: intima-media thickness; LDL-c: low density lipoprotein cholesterol; SBP: systolic blood pressure.
Number may vary for each variable according to missing values. Data are presented as mean (SD) unless otherwise specified.
Medians (inter-quartile (IQ) range) are presented.
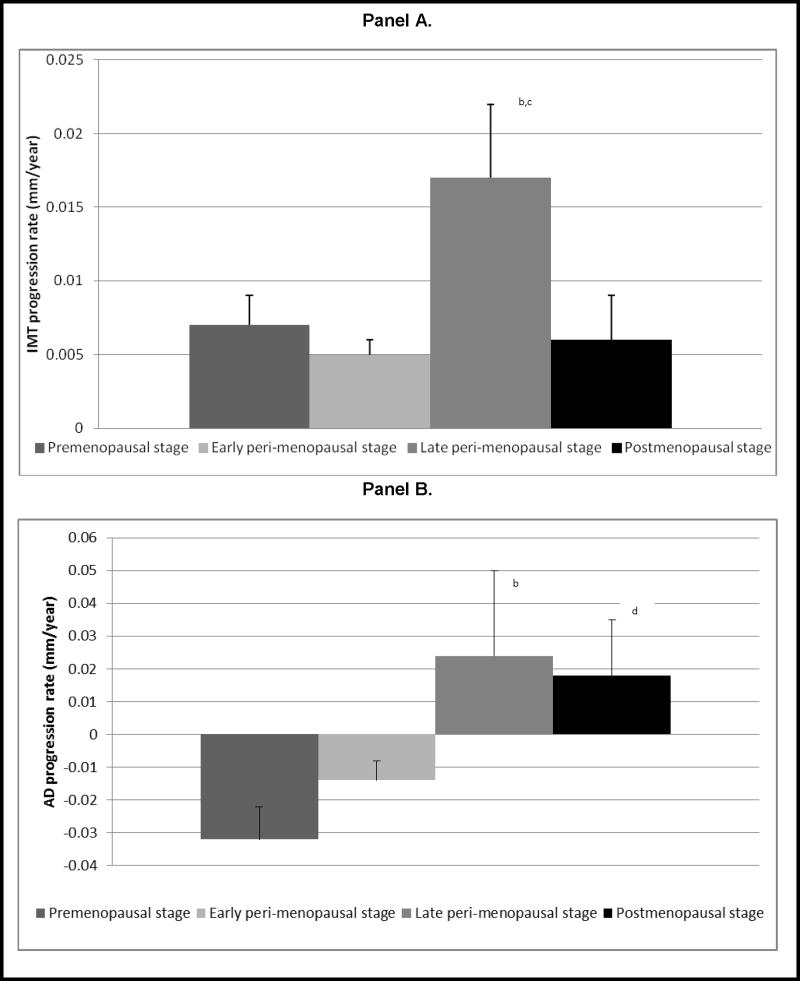
The unadjusted overall rate of change in IMT was 0.007 mm/year. When rate of change was examined in each menopausal stage adjusting for age at baseline and race, IMT increased substantially in late peri-menopausal stage (0.017 mm/year) compared to premenopausal (0.007 mm/year) and to early peri-menopausal (0.005 mm/year) stages; (P<0.05, for both comparisons) (Figure 1, panel A). For AD, while the overall rate of change was negative (−0.009 mm/year), the direction of the change rate varied by menopausal stage. Annual rate of change was negative during pre- and early peri-menopausal stages and this switched to a positive change with late peri-menopausal and postmenopausal stages. For these later time periods, increases in AD were remarkable (0.024 mm/year in late peri- and 0.018 mm/year in postmenopausal stages) and were significantly different when compared to rate of change in premenopausal stage (−0.032 mm/year) (P<0.05, for both comparisons) (Figure 1, panel B).
Figure 1.
Annual Rates of Change in Carotid IMT (Panel A) and AD (Panel B) in Pre-, Early peri-, Late peri-, and Postmenopausal Stagesa
Abbreviations: AD: adventitial diameter; IMT: intima-media thickness
a Adjusted for age at baseline and race
b Rate of change in late peri- significantly differs from that in premenopausal stage, P<0.05
c Rate of change in late peri- significantly differs from that in early peri-menopausal stage, P≤0.05
d Rate of change in postmenopausal stage significantly differs from that in premenopausal stage, P<0.05
The primary factors that were found to be significantly associated with greater IMT were higher age, BMI, waist circumference, diastolic blood pressure (DBP), systolic blood pressure (SBP), LDL-c, triglycerides, glucose, insulin, HOMA index, and CRP. With the exception of age, LDL and CRP, all previously mentioned factors in addition to lower HDL-c were found to be significantly associated with larger AD. Larger AD was found to be significantly associated with greater IMT (P <0.0001). Of these factors, higher SBP and BMI were independently associated with greater IMT and AD (P ≤0.05) adjusting for baseline age, time since baseline, race, and co-morbidity (Data not shown).
Tables 3 and 4 show the effect of menopausal status on levels of IMT and AD, respectively. Being postmenopausal was independently associated with larger levels of IMT and AD compared to being premenopausal in models adjusted for risk factors (model 2 in tables 3 and 4). No significant differences were found in levels of IMT or AD between post- and late peri-menopause status (P≥0.05).
Table 3.
Unadjusted and Adjusted Effect of Concurrent Menopausal Status on IMT Level Over Time
| IMT
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted Model | Model 1 a | Model 2 b | Model 3 c | |||||
|
| ||||||||
| β(SE) | P | β(SE) | P | β(SE) | P | β(SE) | P | |
| Intercept: | ||||||||
| Average of IMT at baseline scan, mm(SE) | 0.678(0.005) | <0.001 | 0.724(0.011) | <0.001 | 0.707(0.012) | <0.001 | 0.708(0.013) | <0.001 |
| Slope: | ||||||||
| Average annual rate of change in IMT, mm/year(SE) | 0.007(0.001) | <0.001 | 0.005(0.001) | 0.001 | 0.005(0.001) | 0.0002 | 0.006(0.001) | <0.001 |
| Menopausal Status d | ||||||||
| Premenopausal (Reference) | --- | --- | --- | --- | --- | --- | ||
| Early Peri-menopausal | −0.001(0.006) | 0.9 | −0.006(0.007) | 0.4 | −0.007(0.008) | 0.4 | ||
| Late Peri-menopausal | 0.011(0.011) | 0.3 | 0.008(0.012) | 0.5 | 0.006(0.012) | 0.6 | ||
| Postmenopausal | 0.024(0.011) | 0.03 | 0.024(0.012) | 0.04 | 0.0126(0.012) | 0.3 | ||
Abbreviations: IMT: intima-media thickness. All covariates are centered at the mean except for time since baseline
Model 1: Adjusted for age at baseline scan, race (reference: White), and co-morbidity (reference: no): High blood pressure/antihypertensive, medication for heart, anticoagulant.
Model 2: Model 1 plus current SBP, BMI, and current LDL-c.
Model 3: Model 2 plus AD at baseline.
Beta coefficients of menopausal status categories represent the differences in levels of IMT compared to premenopausal status (the reference group)
Table 4.
Unadjusted and Adjusted Effect of Concurrent Menopausal Status on AD Level Over Time
| AD
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted Model | Model 1 a | Model 2 b | Model 3 c | |||||
|
| ||||||||
| β(SE) | P | β(SE) | P | β(SE) | P | β(SE) | P | |
| Intercept: | ||||||||
| Average of AD at baseline scan, mm(SE) | 6.706(0.038) | <0.001 | 6.971(0.075) | <0.001 | 6.859(0.072) | <0.001 | 6.800(0.072) | <0.001 |
| Slope: | ||||||||
| Average annual rate of change in AD, mm/year(SE) | −0.009(0.004) | 0.02 | −0.022(0.006) | 0.0003 | −0.024(0.006) | <0.001 | −0.023(0.006) | <0.001 |
| Menopausal Status d | ||||||||
| Premenopausal (Reference) | --- | --- | --- | --- | --- | --- | ||
| Early Peri-menopausal | 0.030(0.033) | 0.4 | 0.012(0.033) | 0.7 | 0.0167(0.033) | 0.6 | ||
| Late Peri-menopausal | 0.088(0.052) | 0.09 | 0.075(0.052) | 0.1 | 0.077(0.052) | 0.1 | ||
| Postmenopausal | 0.159(0.057) | 0.006 | 0.125(0.056) | 0.03 | 0.117(0.055) | 0.04 | ||
Abbreviations: AD: adventitial diameter. All covariates are centered at the mean except for time since baseline
Model 1: Adjusted for age at baseline scan, race (reference: White), and co-morbidity (reference: no): High blood pressure/antihypertensive, medication for heart, anticoagulant.
Model 2: Model 1 plus current SBP and BMI.
Model 3: Model 2 plus IMT at baseline.
Beta coefficients of menopausal status categories represent the differences in levels of AD compared to premenopausal status (the reference group)
Because IMT and AD are significantly related to each other, we evaluated the independent effect of menopausal status on each measure while adjusting for the other. For level of IMT, adjusting for AD at baseline significantly attenuated the independent effect of menopausal status. Conversely, adjusting for IMT at baseline did not significantly impact the independent effect of menopausal status on AD level.
DISCUSSION
In the current longitudinal study, progression rates of IMT and AD differed by menopausal transition stage. This structural remodeling of the carotid artery was most evident during the late peri-menopausal stage in comparison to pre- and early peri-menopausal stages. The thickness of the carotid intima-media layer substantially increased during the late peri-menopausal stage compared with pre- and early peri-menopausal stages. Moreover, the adventitial diameter of the carotid artery decreased during pre- and early peri-menopausal stages and noticeably increased in both late peri- and postmenopausal stages compared to the premenopause.
Multivariable analyses showed that larger baseline IMT, postmenopausal status, higher BMI, and SBP were independently associated with larger AD, while larger baseline AD and higher BMI were found to be independently related to larger IMT.
The current longitudinal study demonstrates for the first time that the progression rates of IMT and AD substantially increase at the late peri-menopause stage, irrespective of age at baseline and ethnicity. These data highlight the late peri-menopause as a stage of vascular remodeling where arteries become more vulnerable. The late peri-menopause is also the stage when women are subjected to several physiological and hormonal changes that, in combination with vessel remodeling, may put older women at a higher risk of developing atherosclerosis.
Our current finding for AD is in line with previous cross-sectional analyses among SWAN Heart participants demonstrating that late peri-menopause and postmenopause are associated with larger AD.12 Although the cross-sectional analyses failed to document similar findings for IMT, our longitudinal analyses, which are more powerful, have reported thicker IMT in postmenopausal women and greater IMT progression in late peri-menopause compared to pre- and early peri-menopausal stages. In a European study of 74 early postmenopausal and 74 non-postmenopausal women comparable for age and BMI, larger common carotid luminal diameter and greater IMT were observed in the early postmenopausal group compared to a non-postmenopausal group.22 Using longitudinal data from Los Angeles Atherosclerosis study, Johnson et al reported that women transitioning from pre- to postmenopause within a 3-year period had a higher rate of IMT progression compared to women with a slower menopausal transition.23 These results suggest an accelerated progression of IMT during the peri-menopausal stage. Our results confirm these findings and extend them to a longitudinal setting.
Figure 1, panel A shows that progression rate of IMT during the postmenopausal stage appears to be less than that reported during the late peri-menopausal stage. However, this difference was not statistically significant. It was expected that there would be a similar or even greater level of IMT progression in the postmenopausal stage compared to late peri-menopausal stage. The plausible explanation for this unexpected result is likely related to the limited follow-up time contributed by the postmenopausal women compared to that contributed by the late peri-menopausal women in the current study. For 90% of the observations, the time spent in the late peri-menopausal stage during the follow-up period was 0.92 year or less while that spent in the postmenopausal stage was 0.39 year or less. Currently at SWAN visit 12, we are collecting additional time point for IMT that will be very valuable in evaluating whether the change in IMT during the postmenopausal stage would be similar or greater than that reported during the late peri-menopausal stage.
It may be surprising that the overall progression rate of AD decreased over time in the early stages of the menopause. The literature has reported inconsistent associations (positive or null) between AD and age.24,25 None of the previous studies focused on women or assessed impact of the menopausal status on age and diameter association. Our results indicate the likely reason for these inconsistent findings. While the data show a reduction in AD overall, the progression rates differ significantly by menopausal stage. AD decreased only when women were pre or early peri-menopause. When women reached late peri-menopause, the change in AD was positive. This pattern may be explained by concurrent changes in hormones, primarily estradiol (E2). Cross-sectional data from SWAN Heart revealed lower levels of E2 were associated with larger AD after adjustment for traditional cardiovascular risk factors.12 Thus, if E2 levels are influencing AD over the course of the transition, then higher levels of E2 in the pre and early-peri stages would be associated with smaller AD. It is important to remember that the trajectory of E2 is not uniformly decreasing across the menopausal transition. It has been observed that E2 may increase toward final menstrual period before it consistently decreases.26
Several mechanisms can be postulated to explain the observed significant effect of menopausal status on subclinical measures and CVD. Transitioning through menopause is accompanied by many physiological changes that make women at higher risk for the development of CVD. These changes are most likely attributed to the alterations in sex hormones during the menopausal transition. Both low sex hormone binding globulin and high free testosterone levels were found to be strongly associated with worse CVD risk profile among pre- and peri-menopausal women from SWAN.14 The increase in body weight and the alteration in body fat distribution is one of the changes related to the menopausal transition.27 In addition; alteration of the lipid profile occurs with the menopausal transition and is related to sex hormone levels.14 Previous data from SWAN showed that lipids increased exponentially around the final menstrual period, as compared with either before or after among all ethnic groups.28
IMT is a well-established index for atherosclerosis that has been associated with unfavorable cardiovascular risk profile.29 Although the subject of fewer publications, AD has also been shown as an informative measure of vascular remodeling among women. Larger AD is correlated with adverse cardiovascular risk factors,30 and associated with higher risk of coronary heart disease.31 A dilated artery may itself lead to a disturbance in blood flow as the wider artery may have less ability to dilate further in response to stimulus, which may make the artery more vulnerable to damage.
We found that current SBP and BMI were significantly associated with larger IMT and AD in final models. In addition, postmenopausal women had significantly larger IMT and AD compared to premenopausal women. Adjusting for baseline IMT did not change the independent effect of menopausal status on AD in the current study, however adjusting for baseline AD attenuated the independent effect of menopausal status on IMT. These findings suggest that arterial dilation may occur first in response to lower E2 levels.12 Wall thickening would then follow as a mechanism to normalize levels of tensile stress that would be increased when the artery dilates.22 These findings may explain the different levels of progression rate in IMT and AD observed in the postmenopausal stage as denoted in Figure 1 (Panel A, and B). Because we did not have enough follow-up time in the postmenopausal stage, it is most likely that we were not able to detect significant wall thickening in response to the significant AD dilatation.
The current study has some limitations. Approximately 40% of the study population was followed for a maximum of 2.5 years. The preponderance of pre and early peri-menopausal women who did not transition to the postmenopause during the study means that our overall results are weighted towards the early stages of the transition. Additional data from later in the menopausal transition, including early postmenopause will be valuable. However, the statistical analysis method that we used was appropriate to handle this problem by focusing on time spent at each stage rather than on how many women transitioned to a certain stage. We estimated the progression rate for each stage of the menopausal transition. Second, the sample size limited our ability to adjust for more covariates when comparing the progression rates between stages. However this should not bias the results as our findings were consistent with previous cross-sectional findings that adjusted for most of the traditional risk factors.12
This study is the first to estimate progression rates of IMT and AD at each stage of the menopausal transition in a population of women at midlife. The next step in this line of research would be to determine the exact role of endogenous sex hormones on the progression rates of IMT and AD in women transitioning through the menopause. Examination of reproductive hormones in association with vascular adaptation would shed light on possible mechanisms for the significant vascular remodeling that we have observed starting at late peri-menopausal stage of the menopausal transition.
CONCLUSIONS
Our data suggest that during the menopausal transition, and particularly during the late peri-menopause, the carotid artery may undergo an adaptation that is reflected in increases in AD followed by increases in IMT. These changes may impact the vulnerability of the vessel in the postmenopausal period. The current study highlights late peri-menopause as a time point when early intervention strategies targeting CVD might have the greatest benefit.
Supplementary Material
Acknowledgments
Financial support:
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), Department of Health and Human Services (DHHS), through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). SWAN Heart is supported by the National Heart, Lung, and Blood Institute (NHLBI) (Grants HL065581, HL065591). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand
Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, Co-PI 2001 – present; Maria Mori Brooks Co-PI 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair, Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Conflict of interest/Financial disclosure: The authors declare no conflict of interest.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 3.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lokkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53(2):226–233. doi: 10.1016/j.maturitas.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews KA, Meilahn E, Kuller LH, et al. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Powell LH, Crawford S, et al. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168(14):1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jürimäe J, Jürimäe T. Plasma adiponectin concentration in healthy pre- and postmenopausal women: relationship with body composition, bone mineral, and metabolic variables. Am J Physiol Endocrinol Metab. 2007;293(1):E42–E47. doi: 10.1152/ajpendo.00610.2006. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Lassila HC, Meilahn E, et al. Carotid atherosclerosis in premenopausal and postmenopausal women and its association with risk factors measured after menopause. Stroke. 1998;29(6):1116–1121. doi: 10.1161/01.str.29.6.1116. [DOI] [PubMed] [Google Scholar]
- 10.Matthews KA, Kuller LH, Sutton-Tyrrell K, et al. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32(5):1104–1111. doi: 10.1161/01.str.32.5.1104. [DOI] [PubMed] [Google Scholar]
- 11.Westendorp IC, Bots ML, Grobbee DE, Reneman RS, Hoeks AP, Van Popele NM, Hofman A, Witteman JC. Menopausal status and distensibility of the common carotid artery. Arterioscler Thromb Vasc Biol. 1999;19:713–717. doi: 10.1161/01.atv.19.3.713. [DOI] [PubMed] [Google Scholar]
- 12.Wildman RP, Colvin AB, Powell LH, et al. Associations of endogenous sex hormones with the vasculature in menopausal women: the Study of Women’s Health Across the Nation (SWAN) Menopause. 2008;15(3):414–421. doi: 10.1097/gme.0b013e318154b6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sowers M, Crawford S, Sternfed B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 14.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex hormone binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women’s Health Across the Nation (SWAN) Circulation. 2005;111(10):1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Kelley-Hedgepeth KA, Lloyd-Jones DM, Colvin A, Matthews KA, Johnston J, Sowers MR, Sternfeld B, Pasternak RC, Chae CU SWAN Investigators. Ethnic differences in C-reactive protein concentrations. Clin Chem. 2008;54(6):1027–1037. doi: 10.1373/clinchem.2007.098996. [DOI] [PubMed] [Google Scholar]
- 18.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev med. 1999;28(3):313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1996. Research on the menopause in the 1990s. (WHO technical report series 866) [PubMed] [Google Scholar]
- 20.Fitzmaurice GM, Laird NM, Ware JH. Applied longitdunal analysis. New York: Wiley; 2004. [Google Scholar]
- 21.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93(3):861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muscelli E, Kozàkovà M, Flyvbjerg A, et al. The effect of menopause on carotid artery remodeling, insulin sensitivity, and plasma adiponectin in healthy women. Am J Hypertens. 2009;22(4):364–370. doi: 10.1038/ajh.2009.16. [DOI] [PubMed] [Google Scholar]
- 23.Johnson BD, Dwyer KM, Stanczyk FZ, et al. The relationship of menopausal status and rapid menopausal transition with carotid intima-media thickness progression in women: a report from the Los Angeles Atherosclerosis Study. J Clin Endocrinol Metab. 2010;95(9):4432–4440. doi: 10.1210/jc.2010-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riley WA, Barnes RW, Evans GW, et al. Ultrasonic measurement of the elastic modulus of the common carotid artery. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1992;23(7):952–956. doi: 10.1161/01.str.23.7.952. [DOI] [PubMed] [Google Scholar]
- 25.Roman MJ, Saba PS, Pini R, et al. Parallel cardiac and vascular adaptation in hypertension. Circulation. 1992;86(6):1909–1918. doi: 10.1161/01.cir.86.6.1909. [DOI] [PubMed] [Google Scholar]
- 26.Hale GE, Huges CL, Burger HG, et al. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16(1):50–59. doi: 10.1097/GME.0b013e31817ee0c2. [DOI] [PubMed] [Google Scholar]
- 27.Lovejoy JC, Champagne CM, de Jonge L, et al. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32(6):949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transtion? J Am Coll Cardiol. 2009;54(25):2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128(4):262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 30.Jensen-Urstad K, Jensen-Urstad M, Johansson J. Carotid artery diameter correlates with risk factors for cardiovascular disease in a population of 55-year-old subjects. Stroke. 1999;30(8):1572–1576. doi: 10.1161/01.str.30.8.1572. [DOI] [PubMed] [Google Scholar]
- 31.Crouse JR, Goldbourt U, Evans G, et al. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1996;27(1):69–75. doi: 10.1161/01.str.27.1.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.