Abstract
Physical activity has been linked to better cognitive function in older adults, especially for executive control processes. Researchers have suggested that temporal processing of durations less than 1 second is automatic and engages motor processes, while timing of longer durations engages executive processes. The purpose of this study was to determine whether a higher level of physical activity is associated with better reproduction performance in older adults, especially for durations in the “cognitive” range (i.e. longer than 1 s). Older right-handed adults completed a temporal reproduction task with five target durations (300, 650, 1000, 1350, and 1700 ms). Physical activity level was assessed via estimation of VO2 peak using a self-report activity scale. Results indicated that higher physical activity level was associated with better timing accuracy and that this effect was dependent on target duration. Namely, the relationship between physical activity and timing accuracy was strongest at the longest durations. Therefore, greater physical activity in older adults may have specific benefits linked to better executive functions.
Keywords: Temporal reproduction, Physical Activity, Cognition, Older Adults, Neuro-protection
Introduction
Many central nervous system changes occur with age, including loss of overall brain volume, declines in neurotransmitters, and a reduction in neuroplasticity (for a recent review see Seidler et al., 2010). Within the peripheral nervous system, aging causes a degradation of sensory receptors as well as a loss of muscle mass and coordination (Smith, Betancourt, & Sun, 2005). These consequences of aging contribute to cognitive and motor impairments and loss of independence (Albert et al., 1995; Andrews-Hanna et al., 2007; Reuter-Lorenz & Lustig, 2005; Seidler et al., 2010). However, many age-related structural and performance declines can be mitigated with increased physical activity (Colcombe et al., 2006).
Participation in physical activity, for instance, has been shown to preferentially enhance cognitive processes sensitive to age-related decline. For example, Colcombe and Kramer (2003) completed a meta-analysis showing that executive function has the largest activity-related effect size, followed by inhibitory control, spatial, and then speeded tasks. A similar meta-analysis by Etnier and colleagues (2006) showed a small, but positive relationship between physical activity and cognitive performance in older adults. Cross-sectional studies have also found a link between physical activity and cognition. Individuals who report higher levels of physical activity show better performance on executive function tasks than those who report lower levels of activity (Eggermont, Milberg, Lipsitz, Scherder & Leveille, 2009; Hillman et al., 2006). Furthermore, structural magnetic resonance imaging has revealed increases in both white and gray matter as a result of aerobic exercise training, particularly in regions sensitive to age-related structural decline, such as the prefrontal and temporal cortices (Colcombe et al., 2006). Critically, these brain regions are also associated with processes linked to inhibition and long-term memory. In summary, the literature suggests that physical activity has the largest impact on executive processing in older adults, while tasks involving simpler “motor” processes, such as finger tapping or simple reaction time are less affected (Colcombe & Kramer, 2003).
Timing is a necessary component for many motor and cognitive processes, such as determining the order of events, coordinating and sequencing movements, speech generation and recognition, as well as many other functions (Lewis & Miall, 2006). Moreover, it is sensitive to age-related changes (Baudouin, Vanneste, Pouthas, & Isingrini, 2006; Block, Zakay, & Hancock, 1998; Lustig & Meck, 2001; Meck & Malapani, 2004), especially when the timing tasks require attending to multiple stimuli (Baudouin, Vanneste, Isingrini, et al., 2006; Lustig & Meck, 2001) or working memory (Block et al., 1998; Craik & Hay, 1999; Vanneste, Perbal, & Pouthas, 1999). However, to date, we are unaware of any work that has explored therole of physical activity on temporal reproduction ability (i.e., one’s ability to reproduce a previously presented temporal duration via a sequence of tones or other stimuli) in older adults.
Perhaps the most popular theory of timing performance, scalar expectancy theory predicts that variability in timing performance changes proportionally with changes in duration (Gibbon, Church, & Meck, 1984); thus, a scalar timer should produce coefficient of variation (CV: standard deviation divided by the mean) values that are constant across durations. However, a unitary scalar mechanism may not fully explain timing performance across both the milliseconds and seconds ranges. For example, we found violations of scalar timing in temporal reproduction across milliseconds and seconds in young adult participants (Bangert, Reuter-Lorenz, & Seidler, 2011). Lewis and Miall (2003), propose that these two duration ranges may be controlled by different timing systems. Timing in the milliseconds range may be controlled by motor regions (e.g., posterior and superior regions of the cerebellum), especially when continuous rhythmic outputs are required; timing of discrete supra-second intervals may rely on executive processes such as working memory and attention (Ivry & Richardson, 2002; Jueptner et al., 1995; Schubotz, Friederici, & von Cramon, 2000; Smith, Taylor, Lidzba, & Rubia, 2003) that engage prefrontal (e.g. dorsolateral prefrontal cortex; DLPFC) and parietal cortical regions. Indeed, disruption of the DLPFC with transcranial magnetic stimulation impairs one’s ability to reproduce supra-second durations but not sub-second durations (Koch, Oliveri, & Caltagirone, 2009; Koch, Oliveri, Torriero, & Caltagirone, 2003). Therefore, temporal processing of durations in the seconds range may benefit most from participation in physical activity (Colcombe & Kramer, 2003; Etnier et al., 2006).
The purpose of the present study was to investigate whether the extent to which older adults participate in physical activity is associated with their temporal reproduction performance involving sub-and supra-second intervals. We hypothesized that physical activity level would have little impact on older adults’ milliseconds-range timing (i.e., “motor” timing) but would be associated with better performance in the supra-second range (i.e., “cognitive” timing).
Methods
Participants
All older adult participants (N = 30) were between the ages of 60 and 85 years and had a Mini Mental State Exam score (MMSE; Folstein, Folstein, and McHugh, 1975) greater than or equal to 26. They were recruited from the community and received monetary compensation for their participation. All participants were right-handed, as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Two older adults were omitted from data analyses. One was excluded due to experimenter error during data collection and one due to failure to complete the task. This left a total of 28 (13 male; 15 female) older adult participants for analysis. This final group had a mean age of 73.1 (SD = 5.0) and a mean MMSE score of 28.8 (SD= 1.3). The study was approved by the University of Michigan Institutional Review Board and all participants gave informed consent prior to participation.
Procedure
The experiment required approximately 2.5 hours to complete. Each participant provided basic demographic information at the start of the experiment. They completed the physical activity scale (PA-R; Jackson et al., 1990) along with a report of their height and weight which allowed for the estimation of each individual’s VO2 peak. All participants then completed the temporal reproduction task.
Temporal Reproduction Task
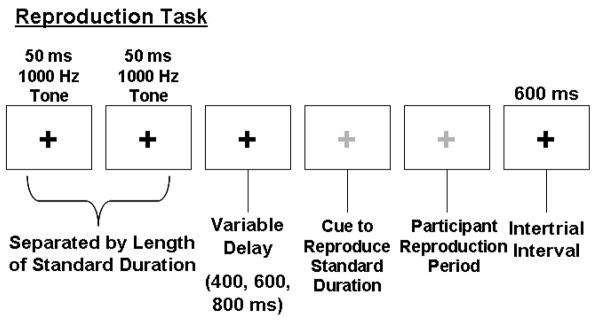
The task was implemented using E-Prime software on a Dell Optiplex GX150 computer. Tones were presented binaurally via Koss UR-29 headphones. Temporal reproduction performance was investigated across five target durations (300, 650, 1000, 1350, and 1700 ms), with each target duration presented in a block of trials. Presentation order of the durations was randomized. On each trial, individuals focused on a black fixation cross while listening to a pair of 50 ms, 1000 Hz tones. Each pair of tones was separated by an empty interval lasting the length of one of the specified durations. After presentation of the target duration and a variable delay (400, 600 or 800 ms), the fixation cross turned green, cuing the participant to reproduce the interval with two right-index finger taps on the space bar of the keyboard. During the task, participants were instructed not to count or produce any movements aside from those required to perform the task. An example of a single trial is provided in Figure 1. For each target duration participants completed 1 block of 7 runs; each run consisted of 12 trials. During the first run, participants received non-numeric visual feedback about their performance after every trial. This run was considered practice, enabled participants to develop a stable representation of the target duration, and was eliminated from later analysis of the data. A feedback screen was presented at the end of every remaining run that provided non-numeric feedback about average performance across the entire run.
Figure 1.
Illustration of the temporal reproduction task. Although shown in grey here, the fixation cross turned green in the actual experiment to cue participants to reproduce the interval.
Questionnaire assessment of VO2 peak
A questionnaire allowing for the estimation of each individual’s VO2 peak (a common measure of cardiorespiratory fitness) based upon Jackson et al.’s (1990) PA-R was used to estimate VO2 peak (mL/kg). The VO2 peak estimate is based on an equation accounting for body mass index, age, and a self-report of physical activity for the month preceding the test session. Note that slightly different equations are specified for men versus women to account for differences in body composition. Body mass index was assessed by determining height (cm) and weight (Kg), while recent physical activity level was classified on a 0–10 scale ranging from sedentary (no activity) to very active. Similar questionnaires have been validated for use in older adults (Mailey et al., 2010) and are related to several measures of cognitive function as well as brain structure (McAuley et al., 2011).1
Data Analysis
Following the computation of VO2 peak, sex differences in lung capacity, body volume, and fat content were controlled for by converting VO2 peak scores within each gender subgroup to Z scores; the resulting standardized fitness (Z-VO2) scores allowed us to evaluate VO2 peak, eliminating any specific mean differences based on gender.
For the temporal reproduction data, any trials where a participant responded before the appearance of the cue or the reproduction fell ± 2.5 standard deviations beyond his or her mean were excluded from analysis. The mean number of trials removed at each duration was as follows: 300ms, 5.6; 650ms, 4.6; 1000ms, 3.3; 1350ms, 2.6; 1700ms, 2.0.
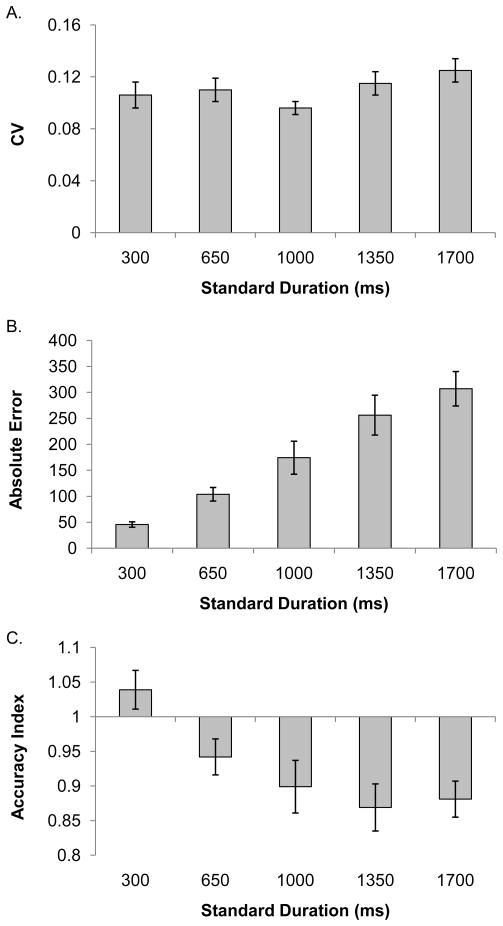
Repeated measures ANCOVAs were run for each of the dependent variables that we investigated for the temporal reproduction task, using duration (5) as a within subjects factor and standardized fitness score as a covariate. Dependent variables for each analysis included measures of temporal reproduction variability and accuracy. These included the CV, absolute error (|reproduced duration – target duration|), and accuracy index (mean subjective reproduction divided by the objective target duration). The accuracy index allows for an estimate of how close subjective reproductions were to the actual durations and enables comparisons across durations to be made on the same scale. An accuracy index value of 1 indicates perfect reproduction. Values below 1 indicate that the duration was under-reproduced, while values greater than 1 indicate that the duration was over-reproduced. In cases where the sphericity assumption was violated, the Huynh-Feldt correction was applied. When we found significant interactions between standardized fitness score and duration we conducted post-hoc correlations at each individual target duration. Our a priori predictions were that increased physical activity level would be associated with better performance on all of our dependent measures, therefore, we used a one-tailed corrected p-value (p = .02) to correct for multiple comparisons in all of our post-hoc analyses. These correlations are reported in Table 1. Figure 2 shows participants’ mean scores for each dependent measure at each standard duration.
Table 1.
Correlations between standardized fitness score and accuracy index and absolute error scores at each standard duration
| Absolute Error | |||||
|---|---|---|---|---|---|
| 300 | 650 | 1000 | 1350 | 1700 | |
| Z-VO2 peak | −.116 | −.398 | −.111 | −.308 | −.539* |
| Accuracy Index | |||||
| 300 | 650 | 1000 | 1350 | 1700 | |
|
| |||||
| Z-VO2 peak | −.007 | .536* | .155 | .464* | .611* |
p < .05, one-tailed, Bonferroni-corrected
Figure 2.
Mean performance scores for each dependent variable at each standard duration. Panel A shows the mean CV values. Panel B shows the mean absolute error scores. Panel C shows the mean accuracy index values. The error bars represent ± 1 standard error.
Results
The mean VO2 peak for the sample was 27.00 mL/kg (SD = 16.9; Range = 56-53.5), while the mean VO2 peak for males was 33.92 mL/kg (SD = 15.18; Range = 8.38– 48.2) and 21.0 mL/kg (SD = 16.46; Range = .56–53.5) for females. These values suggest that the sample overall was relatively low-fit, with the average value for the entire sample falling below the 50th percentile for men and women over the age of 60 years, based on the American College of Sports Medicine (ACSM) guidelines (2010).
Physical Activity Effects on Temporal Reproduction
For the analysis of CV we found no interaction between duration and fitness, p = .541 nor an effect of fitness, p = .430. However, there was a significant main effect of duration, F(3.46, 90.05) = 2.63, p=.047, ηp2= .09, MSE = .001, with the smallest CV (see Figure 2) found for the 1000 ms duration.
Turning to the accuracy measures, we found an interaction between duration and standardized fitness score for absolute error, F(3.55, 92.41)= 3.10, p = .024, ηp2= .11, MSE = 17942.91. Table 1 shows that the only significant correlation between fitness score and absolute error emerged at the 1700 ms duration, suggesting that higher levels of physical activity were associated with lower errors for the longest duration in the target duration set. We also found main effects for fitness, F(1, 26) = 6.55, p = .017, ηp2=.20, MSE = 42019.63 (higher fitness score was associated with lower error), and duration, F(3.55, 92.41) = 20.11, p < .001, ηp2= .44, MSE = 17942.91, with higher error found at the longer durations. Similarly, for.the accuracy index we found an interaction between fitness score and duration, F(4, 104)= 3.29, p = .014, ηp2= .11, MSE = .02, as well as main effects of fitness, F(1, 26) = 8.06, p = .009, ηp2= .24, MSE = .07 and duration, F(4, 104)= 7.89, p < .001, ηp2= .23, MSE = .02. In general, the accuracy index was higher for individuals with higher fitness scores and decreased as duration length increased. Post-hoc correlations (see Table 1) indicated that higher fitness was significantly associated with higher accuracy index scores at the 650 ms, 1350 ms and the 1700 ms durations. As shown in Figure 2, older adults tended to make shorter reproductions for all durations longer than 300 ms. Therefore the larger accuracy index scores linked with increased fitness are indicative of more accurate performance for these durations.
Discussion
Aging and Fitness
The goal of the current investigation was to evaluate whether physical activity is associated with timing performance in older adults, especially for longer durations thought to rely more heavily on executive processes. Despite the lack of physical activity effects on timing variability, physical activity was associated with timing accuracy with the strongest relationships emerging at the longest durations. Therefore, it appears that physical activity may influence mechanisms that enable people to develop accurate duration representations, but not the consistency with which they reproduce those durations.
We also found main effects of duration on all of the dependent measures. This effect on CV suggests a violation of scalar variability (Gibbon et al., 1984) and replicates a performance pattern that we have previously identified in young adults (Bangert et al., 2011). Namely, the effect appears driven by a very short CV at the 1000 ms duration followed by increasing CVs for durations longer than 1 second. This pattern is consistent with arguments that there may be a shift in the mechanisms responsible for timing when moving from sub- to supra-second durations (Lewis & Miall, 2003, 2006).
For timing accuracy, the relationship between physical activity and performance appeared strongest for longer durations, however it was not clear that 1000 ms served as a clear transition between “motor” and “cognitive” timing (Lewis & Miall, 2003). Instead, our findings suggest that some duration in the range of 650 ms may serve as a more meaningful transition point, given that we also discovered a relationship between accuracy index for the 650 ms duration and standardized fitness score. This parallels claims by other researchers who have suggested that a transition may actually occur around 500 ms (see Grondin 2001 for a review; Karmarkar and Buonomano, 2007; Michon, 1985). Regardless of where the transition between “motor” and “cognitive” timing may occur, our findings suggest that higher physical activity is linked to better timing performance in older adults at longer durations thought to rely more heavily on working memory and attentional control processes. Note that aging and Alzheimer’s disease have been linked to poorer timing of supra-second durations, with declines in controlled attention and working memory posed as explanations for why these declines may be especially pronounced at longer durations (Bangert & Balota, in press; Rakitin, Scarmeas, Li, Malapani, & Stern, 2006; Rakitin, Stern, & Malapani, 2005; Vanneste et al., 1999). A multitude of research supports the positive relationship between physical activity and performance on tasks of memory and executive function (Colcombe & Kramer, 2003; Eggermont et al., 2009; Erickson et al., 2011). Therefore, it stands to reason that, in older adults, supra-second durations benefit most heavily from activity-related improvements in executive control mechanisms (Block et al., 1998; Colcombe & Kramer, 2003; Craik & Hay, 1999; Vanneste et al., 1999). Fitness did not appear to be associated with reproduction accuracy of the shortest duration, where executive processes are thought to play less of a role.
Although the mechanisms underlying the benefits of physical fitness on tasks requiring executive functions remain elusive, research with rats indicates that daily physical activity may slow the aging of the central nervous system through maintenance of neurotransmitters (i.e. BDNF; brain-derived neurotrophic factor) and growth of new neurons (Neeper, Gomezpinilla, Choi, & Cotman, 1995; van Praag, Kempermann, & Gage, 1999). Similarly, Gunstad et al. (2008) have shown that higher BDNF serum level in the body is significantly associated with better performance on select cognitive tasks (MMSE and Boston Naming test). In addition, other researchers have found a similar relationship between exercise, cognitive function, and BDNF (Anderson-Hanley et al., 2012; Baker et al., 2010; Erickson et al., 2011; Knaepen et al., 2010), which may suggest that BDNF is produced during exercise and therefore helps to maintain cognitive function. However, more research is needed to determine the specific relationship between temporal reproduction, BDNF and fitness.
Moreover, there appears to be a positive link between physical activity, cognitive function and overall cerebrovascular health in older (postmenopausal) women (Eskes et al., 2010). Increased physical activity is also associated with enhanced hippocampal blood flow in older adults (Burdette et al., 2010) and changes in brain structure and function. Colcombe and colleagues (2006) found structural changes in response to increased aerobic fitness over the course of a 6-month intervention in both gray and white matter areas of the frontal and temporal brain cortices, suggesting that increasing fitness may be associated with increased brain volume in older humans as well as enhanced central nervous system health with age. Similarly, the hippocampus is specifically influenced by cardiorespiratory fitness (Erickson et al., 2009; 2011). Combined, this research suggests that physical exercise may result in brain functional and structural changes that may preserve executive function in humans. While the current experiment is unable to determine the cause of fitness benefits for temporal reproduction accuracy at longer durations, future work should examine the relationship between fitness, BDNF-level and brain structure changes on timing.
Caveats
One limitation of the current approach is the use of a self-report scale to estimate VO2 peak instead of a direct physical assessment via a graded exercise test. However, research by Mailey and colleagues (2010) has shown that non-exercise estimates of cardio-respiratory fitness in older adults based on equations using sex, age, body mass index, resting heart rate, and self-reported physical activity can have high validity. Similarly, McAuley and colleagues (2011) recently reported that equation estimates of VO2 are not only equally related to other more conventional means of assessing VO2, including the Rockport 1-mile walk test and graded exercise tests, but are also significantly related to several measures of cognitive function and hippocampal volume in older adults.
It is also important to note that our study was cross-sectional--fitness was not manipulated. Rather, it was assessed based on participants’ self-reported current level of physical activity. Furthermore, as previously noted, our older participants tended to fall in the low range of overall fitness level (American College of Sports Medicine, 2010), when compared to others in the same age/gender group. Moreover, approximately 61% of our sample reported participating in 1 hour or less of physical activity per week, which is well below the 150 minutes per week recommended for adults (American College of Sports Medicine, 2010). Nevertheless, we observed interesting and reliable physical activity-related effects on temporal reproduction performance for this older- age sample, implying that if more highly-fit individuals were investigated, even more robust performance differences might emerge. Future research should include direct measures of cardiorespiratory fitness (i.e., Graded Exercise Testing) as well as a group of older adults that classify as more highly fit. Moreover, it would be helpful to evaluate timing performance within participants after an exercise intervention, to evaluate how temporal reproduction performance changes as physical activity level increases. We recognize that there are additional individual difference measures, such as musical experience that may impact performance. Therefore, an assessment of musical ability would also be worthwhile to include in future investigations of the influence of fitness on timing ability in older adults.
Conclusions
In conclusion, higher physical activity is associated with better timing accuracy in older adults, especially for longer durations. These findings lend further credence to the idea that physical activity may protect older adults against declines in cognitive processes, such as attention and working memory, which support timing of longer durations. These findings open the door for investigations of whether physical activity may also help special populations (i.e. Alzheimer’s disease) whose timing may suffer due to declines in executive control processes (Bangert & Balota, in press) or whose timing deficits affect their cognitive activities of daily living (Horenea et al., 2009).
Acknowledgments
Supported by NIH AG024106 (RS), NIA Training Grant AG000030, a National Science Foundation Graduate Research Fellowship and a Rackham Graduate School pre-doctoral fellowship (ASB).
Footnotes
The present data set was collected prior to the publication of this validation in older adults.
References
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8. Baltimore, MD: Wolters Kluwer; 2010. [DOI] [PubMed] [Google Scholar]
- Albert MS, Jones K, Savage CR, Berman L, Seeman T, Blazar D, Rowe JW. Predictors of cognitve change in older persons: MacArthur studies of successfull aging. Psychology and Aging. 1995;10(4):578–589. doi: 10.1037/0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- Anderson-Hanley C, Arciero PJ, Brickman AM, Nimon JP, Okuma N, Westen SC, Merz ME, Pence BD, Woods JA, Kramer AF, Zimmerman EA. Exergaming and older adult cognition: a cluster randomized clinical trial. American Journal of Preventative Medicine. 2012;42(2):109–119. doi: 10.1016/j.amepre.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CS, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of Neurology. 2010;67(1):71–99. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert AS, Balota DA. Keep up the pace: Declines in simple repetitive timing differentiate healthy aging from the earliest stages of Alzheimer’s disease. Journal of the International Neuropsychology Society. doi: 10.1017/S1355617712000860. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert AS, Reuter-Lorenz PA, Seidler RD. Dissecting the Clock: Understanding the mechanisms of timing across tasks and temporal intervals. Acta Psychologica. 2011;1(136):20–34. doi: 10.1016/j.actpsy.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin A, Vanneste S, Isingrini M, Pouthas V. Differential involvement of internal clock and working memory in the production and reproduction of duration: A study on older adults. Acta Psychologica. 2006;121(3):285–296. doi: 10.1016/j.actpsy.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Vanneste S, Pouthas V, Isingrini M. Age-related changes in duration reproduction: Involvement of working memory processes. Brain and Cognition. 2006;62(1):17–23. doi: 10.1016/j.bandc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Block RA, Zakay D, Hancock PA. Human aging and duration judgments: A meta-analytic review. Psychology and Aging. 1998;13(4):584–596. doi: 10.1037/0882-7974.13.4.584. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Karmarkar UR. How do we tell time? Neuroscientist. 2002;8(1):42–51. doi: 10.1177/107385840200800109. [DOI] [PubMed] [Google Scholar]
- Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Rejeski JW. Using network science to evaluate exercise-associated brain changes in older adults. Frontiers in Aging Neuroscience. 2010;2:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Erickson KI, Scalf PE, Kim JS, Parkash R, McAuley E, Elvsky S. Aerobic Exercise Training Increases Brain Volume in Aging Humans. Journal of Gerontology: Medical Sciences. 2006;61A(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Hay JF. Aging and judgments of duration: Effects of task complexity and method of estimation. Attention Perception and Psychophysics. 1999;61(3):549–560. doi: 10.3758/BF03211972. [DOI] [PubMed] [Google Scholar]
- Eggermont LHP, Milberg WP, Lipsitz LA, Scherder EJA, Leveille SG. Physical activity and executive function in aging: The MOBILIZE Boston Study. Journal of the American Geriatrics Society. 2009;57:1750–1756. doi: 10.1111/j.1532-5415.2009.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, Kramer AF. Aerobic Fitness is Associated With Hippocampal Volume in Elderly Humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Parkash RS, Basak C, Szabo AN, Chaddock L, Kramer AF. Exercise Training Increases Size of the Hippocampus and improves memory. Proceedings of the National Academy of Science. 2011 doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes GA, Longman S, Brown AD, McMorris CA, Langdon KD, Hogan DB, Poulin M. Conribution of physical fitness, cerebrovascular reserve and cognitive stimulation on cognitive function in post-menopausal women. Frontiers in Aging Neuroscience. 2010;13(2) doi: 10.3389/fnagi.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier J, Nowell P, Landers D, Sibley B. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984 May;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grondin S. From physical time to the first and second moments of psychological time. Psychological Bulletin. 2001;127(1):22–44. doi: 10.1037/0033-2909.127.1.22. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Benitez A, Smith J, Glickman E, Spitznagel MB, Alexander T, Murray L. Serum brain-derived neurotrophic factor is associated with cognitive function in healthy older adults. Journal of Geriatriatric Psychiatry and Neurology. 2008;21(3):166–170. doi: 10.1177/0891988708316860. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Motl RW, Pontifex MB, Posthuma D, Stubbe JH, Boomsma DI, de Geus EJC. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Health Psychology. 2006;25(6):678–687. doi: 10.1037/0278-6133.25.6.678. [DOI] [PubMed] [Google Scholar]
- Horenea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory Fitness and Preserved Medial Temporal Lobe Volume in Alzheimer Disease. Alzheimer Disease & Associated Disorder. 2009;23(3):188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Richardson TC. Temporal control and coordination: The multiple timer model. Brain and Cognition. 2002;48(1):117–132. doi: 10.1006/brcg.2001.1308. [DOI] [PubMed] [Google Scholar]
- Jackson AS, Blair SN, Mahar MT, Weir LT, Ross RM, Stuteville JE. Prediction of functional aerobic capacity without exercise testing. Medicine and Science in Sports and Exercise. 1990;22(6):863–870. doi: 10.1249/00005768-199012000-00021. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Rijntjes M, Weiller C, Faiss JH, Timmann D, Mueller SP, Diener HC. Localization of a cerebellar timing process using PET. Neurology. 1995;45(8):1540–1545. doi: 10.1212/wnl.45.8.1540. [DOI] [PubMed] [Google Scholar]
- Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Medicine. 2010;40(9):765–801. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1525):1907–1918. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Caltagirone C. Underestimation of time perception after repetitive transcranial magnetic stimulation. Neurology. 2003;60(11):1844–1846. doi: 10.1212/wnl.60.11.1844. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Current Opinion in Neurobiology. 2003;13(2):250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Remembering the time: a continuos clock. Trends in Cogntive Science. 2006;10(9):401–406. doi: 10.1016/j.tics.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Lustig C. Grandfather’s Clock: Attention and Interval Timing in Older Adults. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. 261–288. [DOI] [Google Scholar]
- Lustig C, Meck WH. Paying attention to time as one gets older. Psychol Science. 2001;12(6):478–484. doi: 10.1111/1467-9280.00389. [DOI] [PubMed] [Google Scholar]
- Mailey EL, White SM, Wójcicki TR, Szabo AN, Kramer AF, McAuley E. Construct validation of a non-exercise measure of cardiorespiratory fitness in older adults. Biomed Cent Public Health. 2010;10(59) doi: 10.1186/1471-2458-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E, Szabo AN, Mailey EL, Erickson KI, Voss MW, White SM, Kramer AF. Non-Exercise Estimated Cardiorespiratory Fitness: Associations with Brain Structure, Cognition, and Memory Complaints in Older Adults. Mental Health and Physical activity. 2011;4:5–11. doi: 10.1016/j.mhpa.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Malapani C. Neuroimaging of interval timing. Cogn Brain Res. 2004;21(2):133–137. doi: 10.1016/j.cogbrainres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Michon JA, Jackson JL, editors. Time, mind, and behavior. Berlin: Springer Verlag; 1985. pp. 20–54. [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109–109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness- Edinburgh Inventory. Neuropsychol. 1971;9(1):97–112. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Scarmeas N, Li TN, Malapani C, Stern Y. Single-dose levodopa administration and aging independently disrupt time production. J Cogn Neurosci. 2006;18(3):376–387. doi: 10.1162/jocn.2006.18.3.376. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Stern Y, Malapani C. The effects of aging on time reproduction in delayed free-recall. Brain Cogn. 2005;58(1):17–34. doi: 10.1016/j.bandc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Ageing and temporal processing of durations within the psychological present. Eur J Cogn Psychol. 2001;13(4):549–565. doi: 10.1080/09541440042000322. [DOI] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15(2):245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, Friederici AD, von Cramon DY. Time perception and motor timing: A common cortical and subcortical basis revealed by fMRI. Neuroimage. 2000;11(1):1–12. doi: 10.1006/nimg.1999.0514. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Lipps DB. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721–733. doi: 10.1016/J.Neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Taylor E, Lidzba K, Rubia K. A right hemispheric frontocerebellar network for time discrimination of several hundreds of milliseconds. Neuroimage. 2003;20(1):344–350. doi: 10.1016/s1053=8119(03)00337-9. [DOI] [PubMed] [Google Scholar]
- Smith R, Betancourt L, Sun Y. Molecular endocrinology and physiology of the aging central nervous system. Endocr Rev. 2005;26(2):203–250. doi: 10.1210/er.2002-0017. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Perbal S, Pouthas V. Estimation of duration in young and aged subjects: The role of attentional and memory processes. L’Année Psychologique. 1999;99(3):385–414. doi: 10.3406/psy.1999.28514. [DOI] [Google Scholar]