Abstract
Background
Anaplastic thyroid cancer (ATC) is a very aggressive thyroid gland malignancy with very poor prognosis. We suspect that the Notch signaling pathway, which is not active in ATC, may have a tumor suppressor function in this neoplasm. However, it remains unknown whether activation of Notch can yield therapeutic efficacies in ATC.
Method
The purpose of this study was to evaluate the effect of chrysin, a potential Notch inducer identified via high-throughput screening (HTS), on ATC both in vitro and in vivo.
Results
Chrysin treatment of ATC cells led to a dose-dependent inhibition of cellular growth. Protein and mRNA levels of Notch1 and Hes1, a down-stream Notch1 effector, were both up-regulated with treatment. Luciferase reporter assays incorporating the CBF1 binding site also confirmed the functional activity of chrysin-induced Notch1. Oral administration of chrysin suppressed the growth of ATC xenografts by an average of 59% compared with the vehicle control group (p=0.002). Additionally, calculated median time to tumor progression was 11 days for control mice and 21 days for chrysin treatment group (p=0.008). Analysis of chrysin-treated ATC tumors revealed an increase in the active intracellular domain of Notch1 protein. Activation of Notch1 in vivo was associated with the induction of cleaved Poly ADP-ribose polymerase protein, indicating that the growth inhibition was due to apoptosis.
Conclusion
The novel Notch1 activator chrysin inhibits tumor growth in ATC both in vitro and in vivo. Chrysin could be a promising therapeutic candidate for ATC, and justifies further clinical studies.
Keywords: Chrysin, tumor growth, Notch1 signaling, thyroid cancer and anaplastic thyroid carcinoma
Introduction
The Notch1 signaling pathway is highly conserved and has a very important role in regulating cell proliferation, cell death and acquisition of specific cell fate.1 Notch1 signaling is activated by proteolytic cleavages of the Notch1 receptor, which enable the release of the Notch1 intracellular domain (NICD), allowing it to translocate into the nucleus and cause downstream transcriptional activation.2,3 The aberrant gain or loss of Notch1 signaling components has been linked to multiple human disorders including cancer. Though Notch1 was first found as a proto-oncogene of T cell acute lymphoblastic leukemia,4 growing evidence supported by recent studies shows that Notch1 signaling can also have a potent tumor suppressor function in both hematological malignancies and solid tumors.5 In our previous studies, it also has been found that activation of the Notch1 signaling inhibits growth of medullary thyroid carcinoma as well as well-differentiated thyroid carcinomas.6–8
Among all types of thyroid carcinomas, anaplastic thyroid carcinoma (ATC) is characterized by the most aggressive growth features, which cause extensive local invasion and frequent distant metastasis. Though ATC makes up less than 2% of all thyroid cancer cases, it represents over half of thyroid cancer-related deaths.9 Unlike its differentiated counterparts, prognosis of ATC is extremely poor with a median overall survival of less than six months.10 Currently, there is no standardized therapeutic regimen for ATC patients, which necessitates the development for new treatment modalities.
Recently, it has been reported that Notch1 expression levels in ATC are significantly lower when compared with normal thyroid tissues. Furthermore, over-expression of Notch1 in thyroid cancer cells suppresses cell growth rates by reducing cell proliferation by 30%.11 These findings provide a promising new direction for treating ATC patients by activation of Notch1 signaling. We have previously identified 27 potential Notch activating compounds out of over 7,000 tested using a high-throughput screening method.12 Chrysin was found to be one of the most potent Notch activators among the positive hits. Chrysin is a naturally occurring compound found in honey and has been found to induce apoptosis in different malignancies including ATC.13–16 However, the associated regulatory pathways are underexplored and little is known about the in vivo drug effects of chrysin in ATC. In this study, we aimed to evaluate whether chrysin can act as a Notch1 signaling activator in ATC and determine its drug effects through both in vitro and in vivo studies.
Methods
Cell Culture
Two ATC cell lines, namely HTh7 (a kind gift from Dr. Rebecca Schweppe, University of Colorado, Denver, CO) and KAT18, were used for the in vitro studies. Both of the cell lines are derived from human ATC and have been confirmed with their unique identity by DNA profiling.17 The cell lines were maintained in standard RPMI 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) with 10% fetal bovine serum (Sigma-Aldrich, St.Louis, MO). In addition, medium for the KAT18 cells was supplemented with 1% non-essential amino acids and 1mM sodium pyruvate (Invitrogen) as previously described.16 The cells were grown at 37°C in a humidified atmosphere containing 5% CO2. Chrysin (MP Biomedicals, Solon, OH) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) at a stock concentration of 100 mM and fresh dilutions were made for each treatment.
Cell Proliferation Assay
ATC cell proliferation was measured using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. HTh7 and KAT18 cells were plated in quadruplicate on 24-well plates at a density of 20,000 cells/well and 15,000 cells/well, respectively. After incubation overnight to allow cell attachment, treatment medium with different concentrations of chrysin was added and then changed every two days for up to six days. Cell proliferation was measured on the day of cell attachment without treatment, as well as 2, 4 and 6 days after treatment. On the day of measurement, medium was removed before cells were incubated at standard conditions in 250µL of serum-free RPMI 1640 containing 0.5 mg/mL MTT for 4 hours. To dissolve the MTT formazan, 750 µL of DMSO was added to each well and mixed thoroughly. Absorbance was measured at 540nm using a spectrophotometer (µQuant; Bio-Tek Instruments, Winooski, VT).
Luciferase Reporter Assay
To determine the functional activity of Notch signaling, ATC cells were transiently transfected with luciferase constructs as previously described.7 Cells were plated onto 6-well plates in triplicate. The next day the cells were contransfected with wild-type CBF-1 luciferase reporter plasmid (a generous gift from Dr. Diane Hayward, Johns Hopkins University, Baltimore, MD) and cytomegalovirus β-galactosidase (CMV-β-gal; 0.5 µg) utilizing Lipofectamine 2000 Transfection Reagent (Invitrogen). Twenty hours after transfection, cells were treated with different concentrations of chrysin and incubated for another 48 hours. Luciferase and β-galactosidase assays (Promega, Madison, WI) were performed in accordance with the manufacturer’s instructions. In brief, after cells were harvested and lysed, luciferase levels and β-galactosidase units were measured by a Monolight 2010 Luminometer (Analytical Luminescence Laboratory, San Diego, CA) and a spectrophotometer at 420 nm (µQuant; Bio-Tek Instruments), respectively. Luciferase activity was normalized to β-galactosidase units and expressed as relative light units (RLU).
Quantitative Real-Time PCR
Total RNA was isolated from cultured cells two days after chrysin treatment using an RNeasy Plus Mini kit (Qiagen Inc., Valencia, CA). Of the total RNA isolated, 2µg was reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Sequences for each pair of PCR primers were as follows: Notch1 (forward 5’- GTCAACGCCGTAGATGACCT -3’ and reverse 5’- TTGTTAGCCCCGTTCTTCAG -3’); Hes1 (forward 5’- TTGGAGGCTTCCAGGTGGTA -3’ and reverse 5’- GGCCCCGTTGGGAATG -3’); GAPDH (forward 5’- ACCTGCCAAATATGATGAC -3’and reverse 5’-ACCTGGTGCTCAGTGTAG -3’). The quantitative real-time PCR reactions were performed on a MyiQ Thermal Cycler (Bio-Rad Laboratories). All reactions were performed in triplicate. Target gene expression was normalized to GAPDH levels in respective samples as an internal standard, and the comparative cycle threshold (ΔCt) method was used to calculate relative expression levels of target genes.
Subcutaneous Xenograft Tumor Model and Chrysin Administration
Animal studies were performed in compliance with our animal experiment protocol approved by the University of Wisconsin Madison Animal Care and Use Committee. HTh7 cells (3×106 cells per 200µL for each mouse) in Hank's balanced salt solution (Mediatech, Inc., Manassas, VA) were injected subcutaneously (s.c.) into the right flank of 6-week old male nude mice (Charles Rivers, Wilmington, MD). Mice with palpable tumors were randomly divided into two groups for equal tumor size distribution. Animals were treated every day for 21 days with either chrysin (75mg/kg) or control DMSO administrated by oral gavage. Fresh dilutions of chrysin were made with DMSO and olive oil before each treatment. The mice were weighed every four days during the study period. The volume of the xenograft tumor was measured every other day using digital calipers and calculated according to the following formula: tumor volume = length × (width)2 × π/6. The relative tumor volume was defined as the fold change of tumor volume for each time point compared to the initial volume when treatment started. The xenograft tumors were removed and snap frozen in liquid nitrogen immediately after the animals were sacrificed.
Western Blot Analysis
Total protein lysates from ATC cells and tumor xenografts were isolated as previously described.18 ATC cells were treated with different concentrations of chrysin for two days before protein isolation. Protein concentration was quantified using the BCA Protein Assay Kit (Thermo Scientific, Waltham, MA) following the manufacturer’s instructions. Denatured tissue extracts were resolved by 7.5% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked and incubated with appropriate primary antibodies overnight at 4°C. Primary antibodies were diluted as follows: 1:1000 for Notch1 (Santa Cruz Biotechnology, Santa Cruz, CA); 1:1000 for Hes1 (Abcam, Cambridge, MA); 1:2000 for β-actin; 1:3000 for cleaved poly-ADP ribose polymerase (PARP) (Cell Signaling Technology, Beverly, MA). After washing, membranes were incubated for 1.5 hours at room temperature with horseradish-peroxidase-conjugated anti-rabbit/mouse secondary antibodies. The immunoreactive bands were detected by Immunstar (Bio-Rad Laboratories), SuperSignal West Pico, or SuperSignal West Femto (Pierce Biotechnology, Rockford, IL) reagents.
Statistical Analysis
The data was analyzed using the Statistical Package for Social Sciences (SPSS for Windows, version 17.0; Chicago, IL). Unless specifically noted, continuous data were presented as mean ± SEM. Student t-test (two-sided) and One-way ANOVA were used to evaluate differences between two groups and among multiple groups, respectively. Equal to or more than 4-fold increase of tumor volume was defined as tumor progression.19 The progression-free status was estimated by the Kaplan-Meier method. Tests of survival equality were performed by the log-rank test. P <0.05 was considered statistically significant.
Results
Chrysin Inhibited Cell Growth in vitro
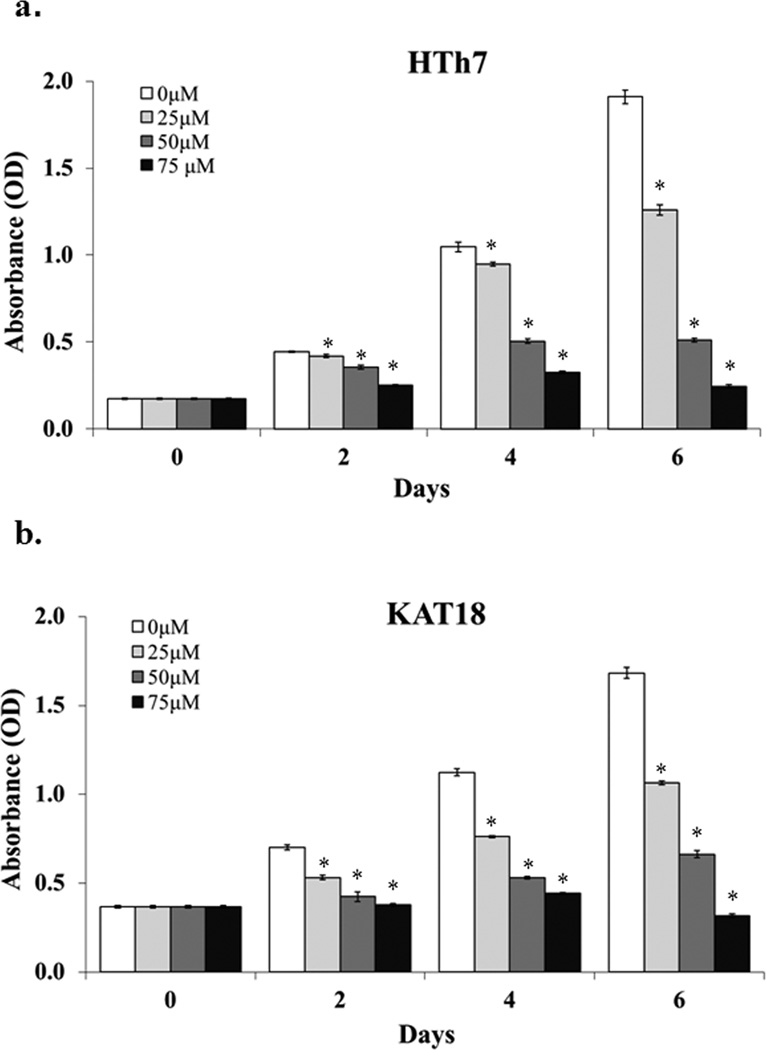
To determine the growth inhibitory effect of chrysin on ATC cells in vitro, MTT assays were carried out to measure cell viability 2, 4, 6 days after chrysin treatment of different concentrations up to 75µM. Chrysin inhibited HTh7 (Fig.1a) and KAT18 (Fig.1b) cell growth in a dose- and time-dependent manner.
Figure 1.
Chrysin inhibited ATC cell growth on a dose- and time-dependent manner. Human ATC cell lines HTh7 (a) and KAT18 (b) were treated with different doses of chrysin and cell viability was measured every two days up to six days by MTT assay. Absorbance values indicating cell viability were expressed as mean ± SEM (n=4, *p<0.05 for chrysin treatment compared with control).
Compared with the control group, 2-day chrysin treatment of 25µM resulted in a 6% reduction of cell growth in the HTh7 cell line and suppressed cell growth by 24% in KAT18. Chrysin concentrations of 25µM and 50 µM suppressed HTh7 cell growth by 34% and 73%, respectively, 6 days after treatment About 50% cell reduction was observed in both HTh7 and KAT18 cells two days after the treatment of 75µM chrysin, which suggests that a dose of 75µM exceeds the IC50. Therefore, 75µM of chrysin was not tested in the later experiments due to its toxicity to the cells. Similar growth inhibitory effects of chrysin were seen in KAT18 cells for 6-day treatment (Fig.1b).
Notch1 Pathway was Activated by Chrysin Treatment in vitro
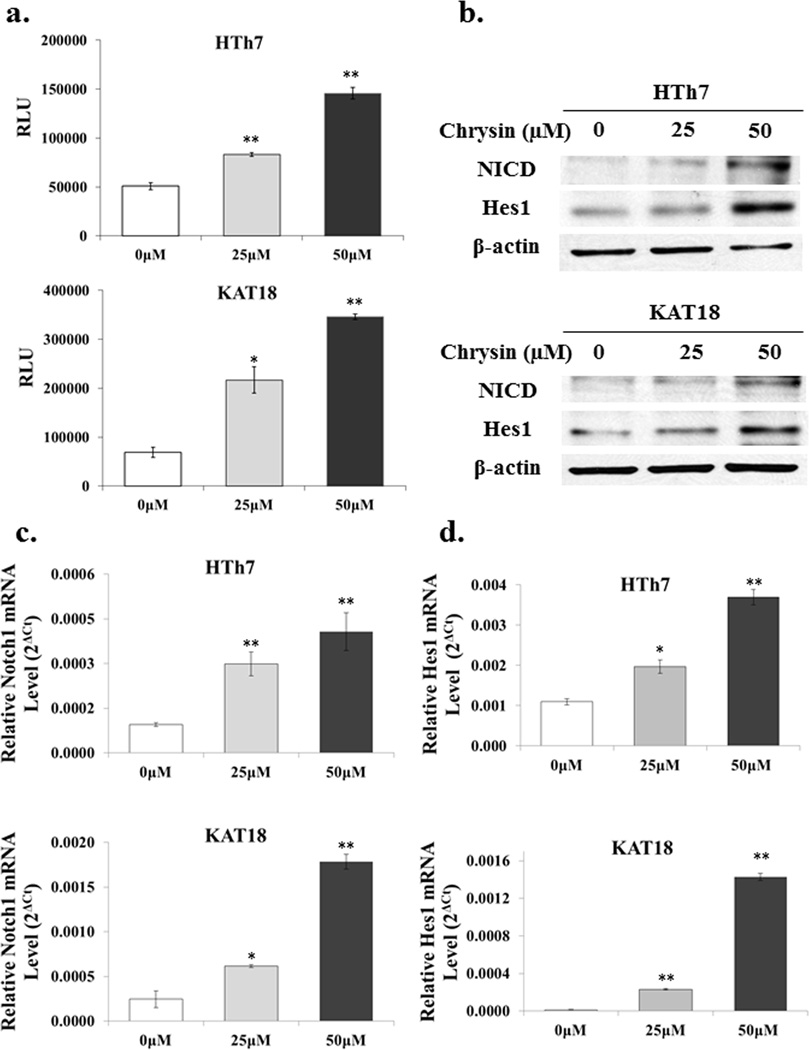
To determine whether chrysin triggers the Notch signaling cascade in an ATC context, a luciferase reporter assay incorporating the CBF1 binding site was carried out. CBF1 is the best characterized downstream effector of Notch in mammals, which, upon binding with active Notch receptors, allows generation of a transcriptional activator complex.20,21 Chrysin treatment of 25 and 50µM significantly induced luciferase activity in HTh7 and KAT18 cells, while cells without chrysin treatment showed minimal to low luciferase activity (p<0.05, Fig. 2a). The increased level of luciferase expression indicates the functional activation of the Notch pathway.
Figure 2.
Chrysin activated Notch1 signaling in both HTh7 and KAT18 cell lines. To determine whether chrysin activated the Notch pathway, ATC cells were first transiently transfected with CBF1-luciferase reporter constructs and then treated with various concentrations of chrysin for 48 hours. Luciferase activity was measured and normalized to β-galactosidase units and expressed as relative light unit (RLU). Chrysin treatment resulted in a significant increase of RLU in ATC cells (a). Total cell lysates were isolated from both HTh7 and KAT18 48 hours after chrysin treatment. Protein expression of Notch1 intracellular domain (NICD) and Hes1, a Notch1 down-stream target, were both increased with the treatment as demonstrated by Western blot (b). Additionally, relative expression levels of Notch1 mRNA were induced with chrysin treatment (c), as well as the levels of its target gene, Hes1 (d). All values were expressed as mean ± SEM (n=3, *p<0.05 and **p<0.01 compared with control).
The protein levels of NICD, an active form of Notch1, were then measured to confirm our findings. Both HTh7 and KAT18 cells revealed an obvious induction of NICD 48 hours after chrysin treatment. Hes1, a Notch1 down-stream target, was also increased with the treatment as demonstrated by Western blot (Fig. 2b). In addition, as shown by real-time PCR, chrysin treatment led to increased expression of Notch1 mRNA levels in both ATC cell lines in a dose-dependent manner (Fig.2c) as well as in mRNA levels of Hes1(Fig.2d). This evidence suggests that Notch1 signaling is activated by chrysin via transcriptional regulation.
Chrysin Suppressed ATC tumor Growth in-vivo
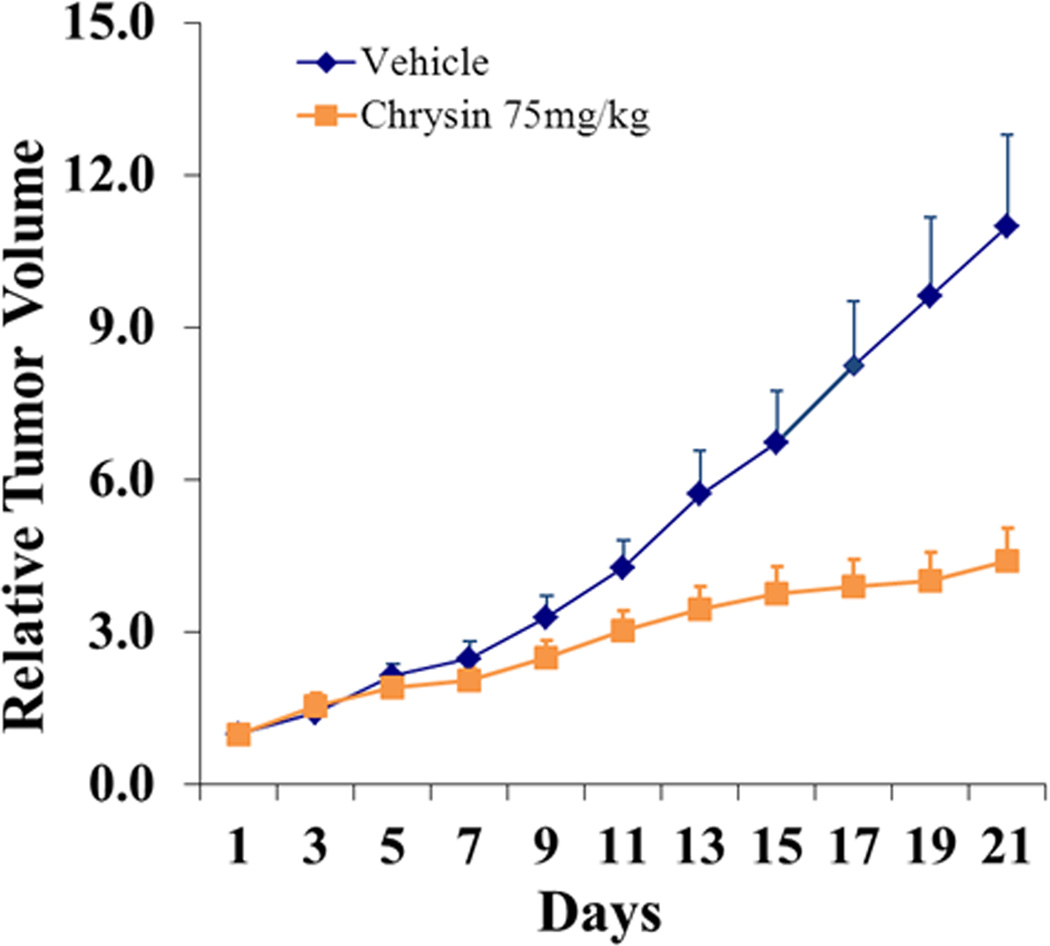
Next, we evaluated the impact of chrysin on an ATC xenograft model. Nude mice bearing subcutaneous HTh7 cell xenografts were treated with either chrysin (n=10, 75mg/kg/day) or DMSO as a vehicle control (n=8). None of the mice in the group with chrysin treatment exhibited any weight loss or overt toxicity compared to the controls. All animals survived until the end-point of the study. As shown in Fig.3, the growth of ATC xenografts was progressively suppressed in the chrysin treatment group. The tumor volume increased an average of 11-fold in the vehicle control group 21 days after DMSO treatment. Meanwhile, only a 4.4-fold induction of tumor growth was observed in chrysin-treated animals on average. Chrysin suppressed the growth of ATC xenografts by an average of 59% compared with the control group.
Figure 3.
Chrysin suppressed tumor growth in vivo. Tumor volume of HTh7 xenografts was measured every other day after treatment with chrysin of 75 mg/kg (n=10) or vehicle control (n=8). Chrysin treatment was effective to suppress tumor growth by 59% for the 21-day treatment course (p=0.002). All values were plotted as the mean of relative tumor volume with SEM.
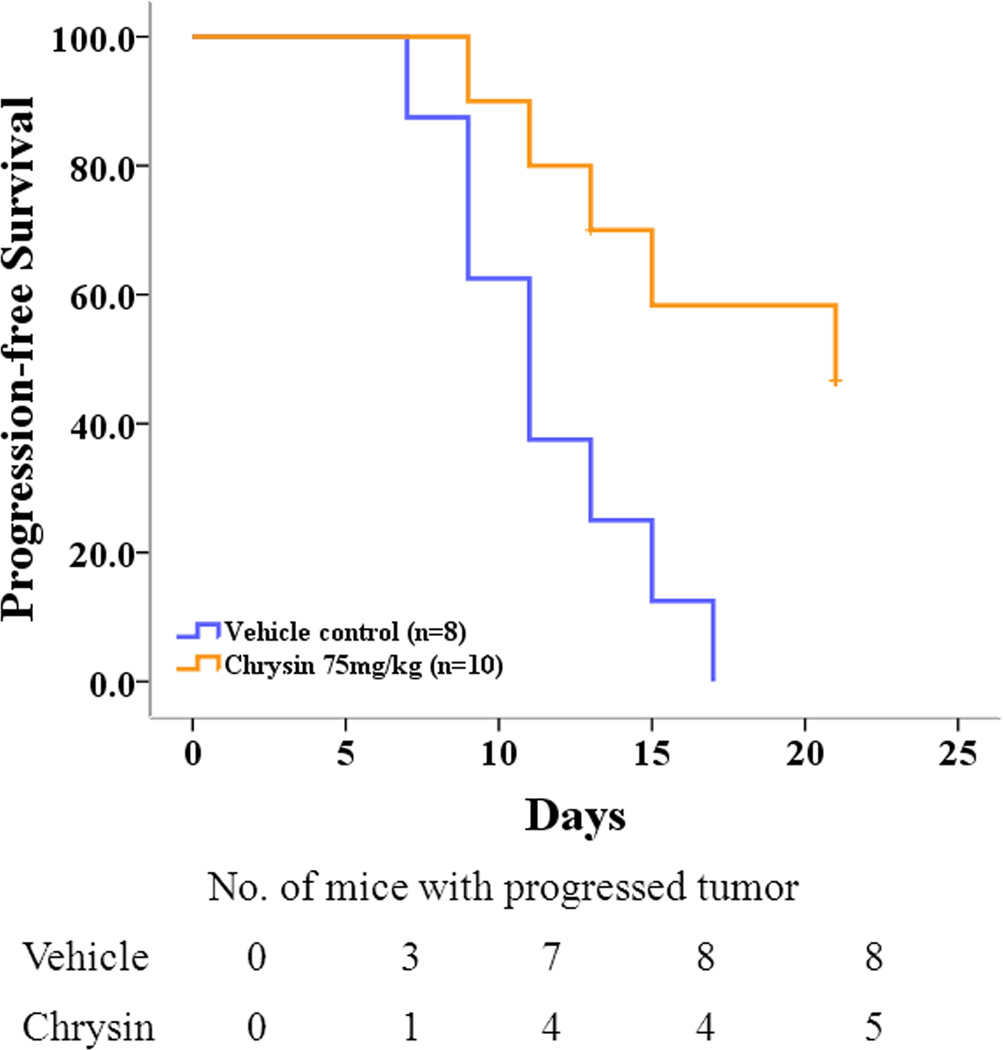
As stated in the Methods section, an equal to or more than 4-fold increase in tumor volume was defined as tumor progression. Animals treated with chrysin had smaller average tumor volumes than the control group but still showed cancer progression. By the end of the treatment course, there were 5 animals (50%) with tumor progression in the chrysin-treated group while all of the animals in the vehicle control group displayed tumor progression (Fig.4). No tumor regression was observed in either group. The calculated median time to progression was 11 days for control mice and 21 days for chrysin treatment group (p=0.008).
Figure 4.
Kaplan-Meier estimates for progression-free status in chrysin treatment group and control group. Median time to tumor progression was 11 days for control mice and 21 days for the chrysin treatment group (p=0.008, by log-rank test).
Active Notch1 Up-Regulated by Chrysin was Associated with Apoptosis in ATC Tumor Xenografts
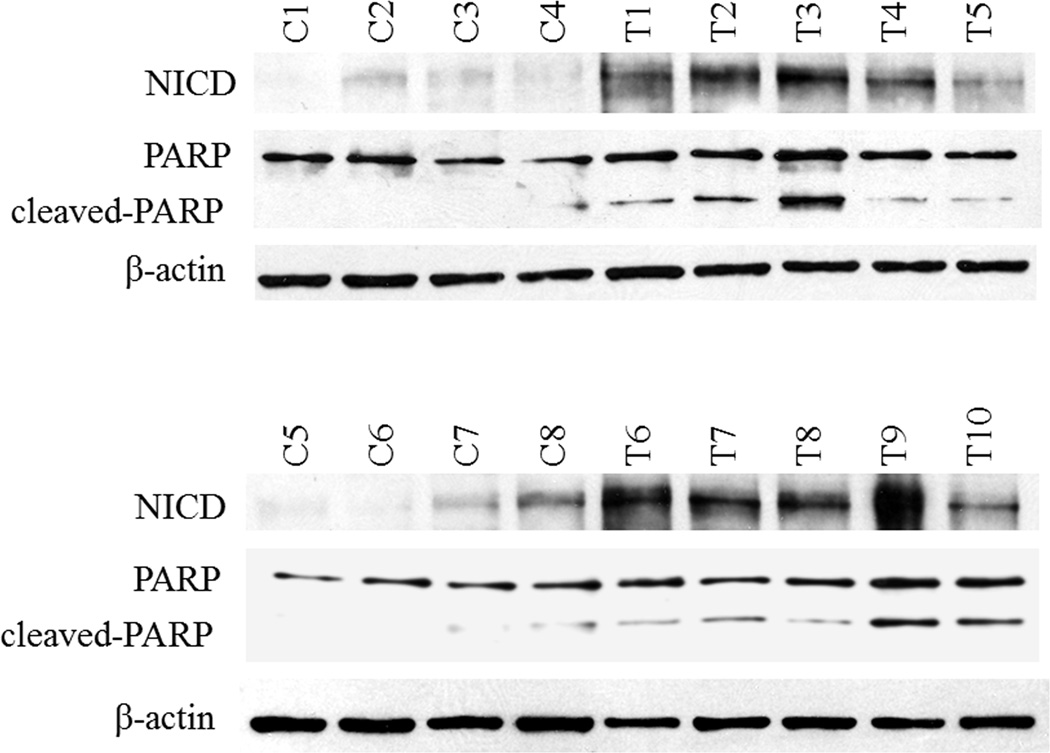
To assess whether chrysin activates Notch1 signaling in an ATC xenograft model, tumor samples from both groups were first examined for expression levels of NICD protein. NICD is the activated Notch1intracellular domain, which translocates to the nucleus and triggers downstream transcriptional activities. All the samples from the chrysin-treated group showed moderate to strong expression of NICD, while most of the samples from the control group revealed no or minimal expression. NICD profoundly increased in the treatment group, suggesting that chrysin acts as a Notch1 activator in vivo. To further investigate the possible mechanism of growth inhibition in vivo by chrysin, we examined the levels of cleaved-PARP, a product of apoptosis. Elevated expression of cleaved-PARP was found in the tumor tissue of chrysin-treated mice compared with those in the control group (Fig.5). In addition, cleaved-PARP was concomitantly increased with NICD expression in most of the samples, indicating that apoptosis induced by chrysin was associated with Notch1 activation.
Figure 5.
Activation of Notch1 in vivo was closely associated with the expression of cleaved-PARP, an end product of apoptosis. Total protein lysates were isolated from HTh7 tumor xenografts of either chrysin-treated (n=10) or vehicle-treated mice (n=8) and then analyzed by Western blot for expression of NICD (120kD) and cleaved-PARP (89kD, lower band). Increased NICD expression was seen in the treatment group together with induction of cleaved-PARP (shown in all tissue samples: “T” indicated samples from treatment group and “C” was from control group). Equal protein loading was confirmed using β-actin.
Discussion
The prognosis for ATC patients is extremely poor with no current standard treatment modality. Recently, the Notch1 pathway, which affects cell proliferation and survival, has been found to be down-regulated in ATC.11 However, little is known about therapeutic efficacies on the activation of Notch1 signaling in ATC. In this study, we evaluated the antitumor effects of chrysin, a potential Notch inducer, both in vitro and in vivo. Our data showed that chrysin inhibited cell growth and activated the Notch1 signaling pathway in ATC cells. More important, chrysin treatment resulted in over 50% tumorostatic response in vivo. In addition, the tumor xenografts from chrysin-treated mice revealed a higher expression of an apoptotic marker concomitant with Notch1 signaling activation.
Chrysin, a natural flavonoid, has emerged as an anticancer agent for different malignancies, including non-small cell lung cancer, leukemia and prostate cancer.13,22,23 Most of the anti-proliferative effects demonstrated in these studies, however, have not been shown in vivo. Thus, we sought to test chrysin on a xenograft animal model in the current study and to elucidate the molecular mechanisms involved. Chrysin treatment suppressed the growth of ATC xenografts by an average of 59% in this study. Chrysin prevented tumor progression in half of the animals. This suggests that the anti-proliferative effects of chrysin can also be achieved for cancers in vivo. Meanwhile, it remains important to improve the bioavailability of the drug either by more effective delivery or by combination with other drugs for a synergetic effect.
Additionally, chrysin was found to activate Notch1 pathway both in vitro and in vivo. The role of Notch1 has been reported as a tumor suppressor in recent studies. Notch1 loss-of-function results in spontaneous basal cell carcinoma,24 and inhibition of Notch1 signaling accelerates the development of hepatocellular carcinoma.25 For the epithelial thyroid cancers, including well-differentiated and anaplastic thyroid cancers, much lower expression of Notch1was detected in tumors compared with normal thyroid tissues. In our study, both of the ATC cell lines used had very minimal expression of active Notch1, which accurately modeled the molecular features of ATC sampled from patients. Though KAT18 had a higher proportion of endogenous Notch1 mRNA than HTh7, the basal protein level of NICD turned out to be very low. This finding was consistent with the lower mRNA and protein levels of Hes1 that were observed, suggesting that Notch1 signaling is not adequately active in HTh7 or in KAT18 cells. A significant induction of Notch1 mRNA levels as well as Notch1 intracellular domain protein levels was observed with chrysin treatment in vitro and in vivo. In addition, chrysin treatments up-regulated the down-stream targets of Notch1 signaling as evidenced by increased luciferase activity of CBF1 and expression levels of Hes1. These findings indicate that chrysin not only transcriptionally regulates Notch1 gene expression, but induces functional Notch1 protein, which is able to trigger the signaling cascade. It has been shown that overexpression of active Notch1 using DNA plasmids in either well-differentiated thyroid cancer or ATC cell causes significant growth inhibition.7,11 Therefore, it is not surprising to see the inhibitory effect on ATC by chrysin, which could be largely due to Notch1 pathway activation.
Notch1 plays a critical role in many fundamental processes including apoptosis, a programmed cell death of which most cancer cells have lost normal regulation. Notch1 regulates apoptosis through extensive networks, involving cell cycle, growth and survival pathways. As a tumor suppressor, Notch signaling has been reported to suppress growth and induce apoptosis in B cell malignancies.26 In our previous studies, it was common to see Notch1 activation by the drug treatment induce apoptosis.16,27 Several studies have reported various mechanisms regarding how Notch1 is involved in apoptotic processes, which may occur through either direct interaction with members of the IAP family or via indirect crosstalk with other signaling pathways like the p53 pathway.28,29 In this study, we demonstrated by an in vivo model that the protein level of cleaved-PARP was increased along with NICD expression in most of the samples from chrysin-treated mice, indicating that apoptosis induced by this compound was highly associated with Notch1 activation. In a recent study, PAPR1 has been identified as a novel target of Notch1 signaling to induce apoptosis. Mediated by Hes1, Notch1 interacts with PARP1 in a cell type–specific manner which allows translocation of apoptosis-inducing factor from the mitochondria to the nucleus.30 In the current study, chrysin activated Notch1 signaling and increased Hes1 expression. It was also seen to enhance the cleavage of PARP. These data imply that chrysin could induce apoptosis in ATC through Notch1 and PARP by modulating the function of the Hes1 transcriptional complex.
In conclusion, chrysin inhibits tumor growth in ATC both in vitro and in vivo, which could be mainly due to apoptosis associated with Notch1 signaling activation. These findings suggest that activation of Notch1 signaling is worthy of extensive evaluation as a treatment option for ATC patients. Additionally, novel Notch1 inducers like chrysin are promising therapeutic candidates, which justifies further preclinical and clinical studies.
Acknowledgments
We would like to thank Jacob Eide for his help in reviewing initial drafts of this paper.
Financial support: American Cancer Society Research Scholar Grant; American Cancer Society MEN2 Thyroid Cancer Professorship; NIH RO1 CA109053 and RO1 CA121115.
References
- 1.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene. 2008;27:5099–5109. doi: 10.1038/onc.2008.223. [DOI] [PubMed] [Google Scholar]
- 3.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 4.Ellisen LW, Bird J, West DC, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 5.Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it's NOTCH what you think. J Exp Med. 2011;208:1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819–39830. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 7.Xiao X, Ning L, Chen H. Notch1 mediates growth suppression of papillary and follicular thyroid cancer cells by histone deacetylase inhibitors. Mol Cancer Ther. 2009;8:350–356. doi: 10.1158/1535-7163.MCT-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook M, Yu XM, Chen H. Notch in the development of thyroid C-cells and the treatment of medullary thyroid cancer. Am J Transl Res. 2010;2:119–125. [PMC free article] [PubMed] [Google Scholar]
- 9.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S. 1985–1995 [see commetns] Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura Y, Shimizu K, Nagahama M, et al. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999;84:4043–4049. doi: 10.1210/jcem.84.11.6115. [DOI] [PubMed] [Google Scholar]
- 11.Ferretti E, Tosi E, Po A, et al. Notch signaling is involved in expression of thyrocyte differentiation markers and is down-regulated in thyroid tumors. J Clin Endocrinol Metab. 2008;93:4080–4087. doi: 10.1210/jc.2008-0528. [DOI] [PubMed] [Google Scholar]
- 12.Pinchot SN, Jaskula-Sztul R, Ning L, et al. Identification and validation of Notch pathway activating compounds through a novel high-throughput screening method. Cancer. 2011;117:1386–1398. doi: 10.1002/cncr.25652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu B, Xue J, Li Z, Shi X, Jiang BH, Fang J. Chrysin inhibits expression of hypoxia-inducible factor-1alpha through reducing hypoxia-inducible factor-1alpha stability and inhibiting its protein synthesis. Mol Cancer Ther. 2007;6:220–226. doi: 10.1158/1535-7163.MCT-06-0526. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Polier G, Kohler R, Giaisi M, Krammer PH, Li-Weber M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J Biol Chem. 2012;287:641–649. doi: 10.1074/jbc.M111.286526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landis-Piwowar KR, Milacic V, Dou QP. Relationship between the methylation status of dietary flavonoids and their growth-inhibitory and apoptosis-inducing activities in human cancer cells. J Cell Biochem. 2008;105:514–523. doi: 10.1002/jcb.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan T, Yu XM, Kunnimalaiyaan M, Chen H. Antiproliferative effect of chrysin on anaplastic thyroid cancer. J Surg Res. 2011;170:84–88. doi: 10.1016/j.jss.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunnimalaiyaan M, Ndiaye M, Chen H. Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery. 2006;140:1009–1014. doi: 10.1016/j.surg.2006.06.040. discussion 1014–1005. [DOI] [PubMed] [Google Scholar]
- 19.Jin N, Jiang T, Rosen DM, Nelkin BD, Ball DW. Dual inhibition of mitogen-activated protein kinase kinase and mammalian target of rapamycin in differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2009;94:4107–4112. doi: 10.1210/jc.2009-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh JJ, Henkel T, Salmon P, Robey E, Peterson MG, Hayward SD. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol Cell Biol. 1996;16:952–959. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronchini C, Capobianco AJ. Notch(ic)-ER chimeras display hormone-dependent transformation, nuclear accumulation, phosphorylation and CBF1 activation. Oncogene. 2000;19:3914–3924. doi: 10.1038/sj.onc.1203719. [DOI] [PubMed] [Google Scholar]
- 22.Wang HW, Lin CP, Chiu JH, et al. Reversal of inflammation-associated dihydrodiol dehydrogenases (AKR1C1 and AKR1C2) overexpression and drug resistance in nonsmall cell lung cancer cells by wogonin and chrysin. Int J Cancer. 2007;120:2019–2027. doi: 10.1002/ijc.22402. [DOI] [PubMed] [Google Scholar]
- 23.Monasterio A, Urdaci MC, Pinchuk IV, Lopez-Moratalla N, Martinez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50:90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- 24.Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 25.Viatour P, Ehmer U, Saddic LA, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963–1976. doi: 10.1084/jem.20110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zweidler-McKay PA, He Y, Xu L, et al. Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B-cell malignancies. Blood. 2005;106:3898–3906. doi: 10.1182/blood-2005-01-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenblatt DY, Cayo MA, Adler JT, et al. Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg. 2008;247:1036–1040. doi: 10.1097/SLA.0b013e3181758d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu WH, Hsiao HW, Tsou WI, Lai MZ. Notch inhibits apoptosis by direct interference with XIAP ubiquitination and degradation. EMBO J. 2007;26:1660–1669. doi: 10.1038/sj.emboj.7601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Kannan S, Fang W, Song G, et al. Notch/HES1-mediated PARP1 activation: a cell type-specific mechanism for tumor suppression. Blood. 2011;117:2891–2900. doi: 10.1182/blood-2009-12-253419. [DOI] [PMC free article] [PubMed] [Google Scholar]