Abstract
Although there has been extensive research on small, unmyelinated fibers in the skin, little research has investigated dermal myelinated fibers in comparison. Glabrous, non-hairy skin contains mechanoreceptors that afford a vantage point for observation of myelinated fibers that have previously been seen only with invasively obtained nerve biopsies. This review discusses current morphometric and molecular expression data of normative and pathogenic glabrous skin obtained by various processing and analysis methods for cutaneous myelinated fibers. Recent publications have shed light on the role of glabrous skin biopsy in identifying signs of peripheral neuropathy and as a potential biomarker of distal myelin and mechanoreceptor integrity. The clinical relevance of a better understanding of the role of dermal myelinated nerve terminations in peripheral neuropathy will be addressed in light of recent publications in the growing field of skin biopsy.
Keywords: glabrous skin biopsy, myelinated nerve fibers, molecular architecture, Meissner corpuscles, peripheral neuropathy
Introduction
In the peripheral nervous system (PNS), nerves may be classified as small caliber unmyelinated fibers with axons encircled by a single layer of non-compact Schwann cell membrane or different caliber myelinated fibers with axons encased by multiple layers of compacted Schwann cell membrane to form compact myelin. Molecules on myelinated nerve fiber membranes are highly compartmentalized to form molecular architectures within 4 discrete compartments: the node of Ranvier, the paranode, the juxtaparanode, and the internode, each of which contains a specialized, non-overlapping set of protein constituents.1, 2 Voltage-gated sodium channels are highly concentrated at the node of Ranvier adjacent to the paranodal region, which is comprised of loops of Schwann cell membrane and interacting axolemmal protein (Contactin-associated protein, Caspr) and Schwann cell protein (Connexin 32). Both CASPR and Connexin 32 participate in Schwann cell-axon interactions and electrically isolate the nodal region. The juxtaparanodal region, the portion of myelin and the interacting axolemma adjacent to the paranode, contains voltage-gated potassium channels (Kv1.1 and 1.2) on the axolemma. The internodes between 2 nodes contain the myelin structural proteins myelin protein zero (MPZ), peripheral myelin protein 22 (PMP22), and myelin basic protein (MBP), which participate in forming the tightly-compacted myelin sheath, thereby reducing the electrical capacitance of the internode.
Diseases of the PNS (peripheral neuropathies) may predominantly perturb either myelin (de-/dysmyelinating neuropathies) or axons (axonal neuropathies). These disorders often disrupt the molecular architecture of the myelinated nerve fiber axolemma including, but not limited to, delocalization or abnormal subtype expression of voltage-gated ion channels,3, 4under-/over-expression of compact myelin proteins,5 and septate junction protein abnormalities with myelin decompaction2. In the past, morphological and molecular changes of myelin and axons in humans have been observed through sural nerve biopsies6 or occasionally through nerves taken at autopsy.7 Knowledge from these studies has significantly advanced our understanding of normal physiological functions of the PNS and the pathogenesis of peripheral nerve diseases while aiding in their diagnosis.
Recently, skin biopsy has emerged as an alternative approach to assess pathological and molecular information in the PNS. Skin biopsy is a minimally invasive procedure in which a small 2-5 mm disposable punch is used to painlessly excise an anesthetized section of skin. It has become a valuable tool in the diagnosis and monitoring of small fiber sensory neuropathy, which preferentially affects unmyelinated fibers and is most commonly sampled from the hairy skin of the leg.8 In comparison, cutaneous myelinated fibers have been much less explored, although numerous neuropathies are known to involve such distal myelinated nerve fibers. Recent studies have begun evaluating dermal myelinated fibers from glabrous skin with a variety of methods in healthy control populations9-11 and in patients with peripheral neuropathies.12-16 In this review, we will discuss the development of skin biopsy for investigation of normal and diseased dermal myelinated nerves. We highlight recent key publications to our best knowledge and discuss further applications of skin biopsy in light of such work in order to better understand the utility of the procedure and the extent of pathology on these most distal myelinated fibers.
Cutaneous neuroanatomy
Small fibers
The underlying structures of the skin include a variety of both autonomic and sensory organs innervated by axons of varying calibers that can be functionally differentiated (Fig. 1). Free nerve endings, which are found in both the dermal and epidermal layers, are small caliber fibers that convey thermal stimuli in addition to noxious stimuli. Thinly myelinated (Aδ) or unmyelinated (C) fibers branch off from the subepidermal neural plexus and either terminate in the dermis or penetrate the dermal-epidermal basement membrane and course between the keratinocyte cells of the epidermis as unmyelinated fibers.
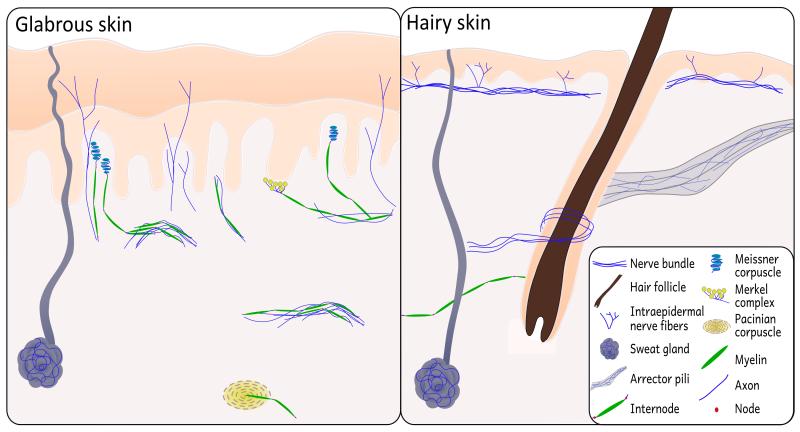
Figure 1.
Schematic of neural structures present in glabrous skin and hairy skin. Small sensory fibers branch off of dermal bundles to innervate the epidermis in both hairy and glabrous skin. In addition, autonomic nerve fiber innervation can be seen in sweat glands and arrector pili muscles. The presence of large myelinated fibers in hairy skin is largely restricted to mechanoreceptive hair follicles; glabrous skin has a comparatively high density of mechanoreceptive organs and afferent Aβ caliber myelinated fibers.
Large fibers
Mechanical sensory perception, in contrast, is conveyed by large, myelinated Aβ caliber fibers, which innervate cutaneous mechanoreceptors (Table 1). These thickly myelinated afferents in the reticular and papillary layers of the dermis have different principal sensory terminals in hairy skin compared to those in glabrous (non-hairy) skin (Fig. 1). In hairy skin, Aβ fibers innervate vascular structures like arteriovenous anastosomes (AVAs) and hair follicles that act as mechanical sensory receptors by detecting changes in hair position. Myelinated fibers are much more abundant and homogenous in glabrous skin because of the increased numbers of mechanoreceptors found only in glabrous skin (Fig. 2).9, 11
Table 1.
Innervation, location and perceived sensation of cutaneous small fiber nociceptors and large fiber mechanoreceptors
| Cutaneous sensory receptors |
Innervating axons | Location | Perceived Sensation |
|---|---|---|---|
| Free Nerve Endings | A6-thinly myelinated C - unmyelinated |
Epidermal and dermal layers of all skin |
Thermal, mechanical, and noxious stimuli |
| Meissner corpuscles | Aβ–thickly myelinated |
Dermal papillae of glabrous skin |
Light touch and low frequency vibration |
| Merkel complexes | Base of hair follicles and dermis of glabrous skin |
Prolonged touch and pressure |
|
| Ruffini corpuscles | Deep in the dermal layer of glabrous skin |
Stretching of skin and prolonged pressure |
|
| Pacinian receptors | Deep in the dermal layer of glabrous skin |
Deep pressure and high frequency vibration |
|
| Hair follicles | Dermis of hairy skin | Hair displacement |
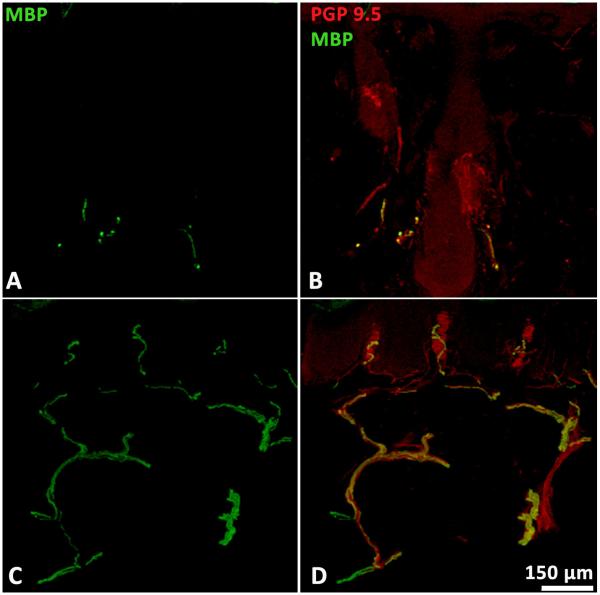
Figure 2.
Confocal images of MBP-immunoreactive myelinated fibers in hairy (A) and glabrous skin (C). In glabrous skin, fibers are prevalent and homogenously spaced as they furnish Meissner corpuscles in the dermal papillae (D). In hairy skin, MBP-ir fibers are typically much less prevalent and frequently cluster around hair follicles (B).
Mechanoreceptors
Meissner corpuscles (MCs) and Merkel complexes are 2 mechanoreceptive sensory end terminals of myelinated afferents that are located respectively in the apex and the base of dermal papillae in the subepidermal region immediately below the basement membrane. MCs respond to low-frequency stimuli and convey tactile sensations of vibration and light touch. They are found exclusively in glabrous skin in a decreasing distal-proximal gradient. Thus, higher densities are observed in the fingertip, for instance, compared to more proximal locations on the palm.17
Intrapapillary myelinated endings (IME) are the large, thickly myelinated fibers that typically furnish between 1 and 3 MCs per dermal papilla. They can be easily quantified on account of their regular vertical courses to the apex of dermal papillae, where they may innervate several relatively large corpuscles (30 × 80 μm).9 It is generally accepted that Schwann cells lose the ability to myelinate as axons enter the receptor corpuscle; however, recent studies in human18 and primate digits19 report MBP immuno-reactivity within the corpuscle. About 25% of the MCs from human digital skin contained MBP-immunoreactive nerve endings.10 Even so, they rarely appear to reach the apex of the papillae and purportedly do not actually supply the corpuscle. To the best of our knowledge, subsequent investigation of this discrepancy has not been reported in humans.
Similar large myelinated fibers can be found terminating at the base of dermal papillae in the disk-like endings of Merkel cell-neurite complexes. These slowly-adapting mechanoreceptors convey prolonged sensations of touch and pressure and are found near the bases of hair follicles in hairy skin and the bases of dermal papillae in glabrous skin. The quantification of MC afferents as an indicator of dermal fiber density has proven more suitable than those of Merkel complexes, in part because MCs are more abundant and regularly spaced with long, linear axonal paths compared to the folded, sigmoidal paths of fibers that furnish Merkel cells.9
Pacinian corpuscles and Ruffini endings are also low-threshold mechanoreceptors located in glabrous skin, yet they are typically not visualized through common procedures of skin biopsy due to their position in the deep dermis and subcutaneous tissue. Biopsies are typically collected at a relatively shallow depth in order to avoid severing larger subcutaneous blood vessels in the vicinity of these mechanoreceptors. In recent postmortem studies, however, Pacinian corpuscles and their afferents have been characterized in primates19 and in the amputated digits of humans.10
Resident Cells of the Skin
Cell types in the skin that are implicated in conveying sensory information include Merkel cells, terminal Schwann cells, and basal keratinocytes in the epidermis. Merkel cells are scattered in the basal layer of the epidermis as well as the root sheath of hair follicles.20 The function of the Merkel cell-neurite complex is not completely understood, as it is either a mechanoreceptor itself or else plays a modulatory role in the sensory function of neurons.21 Interestingly, unlike epidermal Merkel cells, those located in the outer root sheath of hair follicles express the capsaicin receptor TRPV1 (transient receptor potential vanilloid 1) and the neurotransmitter Substance P. They express multiple glutamate receptors and neuropeptide receptors22 in addition to P/Q type voltage –gated calcium channels but lack synaptic connections and neurotransmitters.20
Basal keratinocytes in the epidermis are the most plentiful cell type in the skin and have also been shown to express TRPV1 as well as the vanilloid thermoreceptors TRPV3 and TRPV4.23 Mechanical stress on keratinocyte cultures increases intracellular calcium levels which can be blocked by a TRP antagonist, suggesting a role of TRP in signal transduction.24 Keratinocytes also exhibit a trophic effect in cell culture models of sensory neurons.25 Keratinocytes may have a role in pain signal transduction as well as nerve regeneration, although the mechanism of communication with peripheral dermal nerves is still unknown; thus, direct evidence is still lacking.
Melanocytes, which produce photoprotective melanin, are also in close contact with sensory endings, and electron microscopy studies support a possible synaptic communication.26, 27 Langerhans cells are antigen-presenting cells in close proximity to Merkel cells. They do not express TRPV or TRPM (transient receptor potential melastatin) channels but are sensitive to thermal stimulation.
Terminal Schwann cells are present in the skin as modified Schwann cells that comprise the capsules of MCs. Both Schwann cells that make up the tightly wrapped compact myelin formed along dermal myelinated nerves, and those that form the noncompact single encirclement of small fibers are S100 immunoreactive. Compact myelin may be distinguished by the staining of compact myelin proteins such as Myelin basic protein (MBP). Schwann cells can also be identified using an anti-p75 nerve growth factor receptor antibody or a neural cell adhesion molecule (NCAM/CD 56), both of which allow for the evaluation of decreases in terminal Schwann cells in patients with neuropathy.28, 29
Technical considerations for skin biopsy procedure
Punch Biopsy
In myelinated fiber investigations, punch biopsy is typically applied to glabrous skin, such as the lateral aspect of the finger,30 fingertip9 or palm.17 Although the sole of the foot is also a potential site, fear of infection and prolonged recovery time are logistical concerns, particularly in patients with neuropathy. The fingertip is a highly sensitive place, and biopsies in the wrist and forearm presumably provide a lesser chance to see Meissner receptors. As a compromise, our lab prefers to do the procedure on the lateral aspect of the finger between the first and second interphalangeal joints with a small diameter punch (2 mm) positioned at least 2 to 3 mm from the border of hairy skin.30 The drawback to this method is that there is the potential for injury to the digital nerve;31 however, this has not occurred in our experience. Biopsies are collected at a depth of approximately 3-5 mm under sterile conditions after intradermal injection of local anesthetic (i.e. xylocaine or lidocaine), and at this depth, injuries to the digital nerve are unlikely. Wounds heal within 1 week without the need of sutures, and over time, only slightly visible scarring is evident. The procedure is well-tolerated by patients. Although the infection rate in glabrous skin biopsies has not been investigated on a large scale, it is most likely comparable to the relatively low rate observed in skin biopsies from the lower extremity 1.9:1000.32
Unique aspects in dealing with skin biopsies
Myelinated nerve bundles located deep in the dermis travel roughly parallel to the surface of the skin, with individual myelinated fibers branching off more superficially in the dermis where they course perpendicular to the skin’s surface to innervate mechanoreceptors and terminate in dermal papillae (Fig. 2).33 Unlike teased fiber preparations, capturing dermal fibers requires taking into account the undulating course of fibers throughout the biopsy. Combining a z-stack of images taken in small increments throughout thick sections allows three-dimensional visualization of large cutaneous structures. Thus, MCs and fibers spanning multiple internodes may be projected wholly in focus, enabling investigators to quantify the morphometrics of dermal myelinated nerves. Subsequent tissue processing procedures may be selected based on the method of visualization required to address a particular scientific question.
Skin biopsy and gene expression
Real-time polymerase chain reaction (rtPCR) has been used to evaluate expression levels of different genes in skin biopsies at the transcriptional level.30, 34, 35 Increased cytokine gene expression has also been recently demonstrated in hairy skin biopsies from patients with small fiber polyneuropathy and chronic inflammatory demyelinating polyneuropathy (CIDP).36, 37 When studying neural specific proteins such as PMP22, it is likely that the mRNA transcripts obtained are neural specific. However, this technique is limited in that mRNA expression may be largely derived from numerous non-neural cells, especially given the small fraction of neuronal tissues present in skin. Several technical caveats may limit the reliability of this approach, including the standardization of mRNA levels, secondary effects from axonal loss, and the aforementioned majority of non-neuronal cell types.
Immuno-electron microscopy (immuno-EM) is an alternative approach to quantifying protein expression in skin biopsy that allows investigators to avoid these difficulties and localize proteins to areas of compact myelin. Although technically challenging, quantification of proteins specific to myelin may be expressed in the number of conjugated particles per mm2 of myelin analyzed. Multiple proteins may be studied in the same nerve fiber by using species-specific secondary antibodies conjugated with gold particles of distinctly different sizes. This avoids any secondary effect of axonal degeneration that would decrease levels of any gene expression in neural tissues and would be difficult to exclude with real-time PCR. Using this technique, PMP22 expression has been shown to be increased and variable in patients with Charcot-Marie-Tooth disease type 1A (CMT1A) and decreased in patients with hereditary neuropathy with liability to pressure palsies (HNPP).30 There are significant technical barriers to immuno-EM that include difficulties with trimming away the large portion of non neural tissue in the skin in order to focus on myelinated nerve fascicles. Furthermore, tissue preparation procedures may be deleterious to antigens.38
Meissner corpuscle observations and suggestions as a neuropathy biomarker
Aβ fibers lose their myelin sheaths as they enter MCs and course between modified Schwann cells, outlining the lamellar structure of the corpuscle in addition to dense small fiber innervation;39 thus, MCs have been described as polyinnervated structures with nociceptive peptidergic and nonpeptidergic C fiber innervation in addition to the large Aβ afferents responsible for mechanosensation.40 A number of investigators12, 14, 16, 19, 41 have made observations of reduced densities of MCs with persisting receptors exhibiting disease-related neuropathy and marked atrophy (and less frequently, hypertrophy). Such abnormalities are described as a range of anomalies in size, orientation, and lamellar structure. MC density has been suggested to be a potential biomarker of peripheral neuropathy42 on account of the susceptible extreme distal location of corpuscles, yet typically abundant and homogenous distribution in the glabrous skin of healthy controls.9 Care must be taken in sampling, because marked reductions and inconsistencies in size and distribution have also been noted as a result of aging.43 Significant discrepancies were reported, for example, in a comparison of a 76 year-old woman (3.1 MCs/mm2), a 43 year-old man (7.3 MCs/mm2), and a 4 year-old (49.3 MCs/mm2).42 Nolano et al.9 reported the density of MCs in fingertip biopsies of healthy volunteers between the ages of 22 and 53 years (mean, 33.7 ± 9.2 years) to be 33.02 ± 13.2 MCs / mm2, with a mean density of their myelinated afferents, designated intrapapillary myelinated endings (IME), to be 59.0 ± 29.3 fibers/ mm2. Reports of gender differences in MC density may be attributed to a differences in finger surface area between men and women rather than an innate gender difference in tactile sensitivity.44 It should be noted, however, that some investigators have not seen a significant correlation between either gender and MC45 or fiber density.46 This is an important issue that warrants further investigation.
Abnormalities in myelinated Aβ fibers in more proximal sensory nerves (i.e. sural, radial, ulnar) may be detected by nerve conduction studies.47 Electrophysiologic correlations are observed in MCs by eliciting tactile responses on the fingertip with a square-wave generating vibrator and recording near nerve sensory responses.41 Correlations were found between evoked tactile responses and electrical potentials of areas with normal MC density, and conversely, no correlations were found between abnormal corpuscles and tactile responses.41 These findings support the role of MCs in detecting tactile sensations like vibration. In addition, morphological abnormalities of MCs correspond to mechanoreceptor dysfunction evidenced by reduced tactile responses recorded in the same area before biopsy in patients with Freidrich ataxia and diabetes.13 Interestingly, MC density has been reported to parallel epidermal fiber reductions in patients with a sensory neuropathy presumed to be restricted to small fibers only.48 Skin biopsies from patients with systemic sclerosis revealed a reduction of MCs and marked denervation of both sensory and autonomic fibers, indicating the previously unknown involvement of both large and small fibers.16 In spinobulbar muscular atrophy patients, there was a similar reduction in MCs and a widespread loss of small myelinated and unmyelinated autonomic and sensory fibers, even in seemingly unaffected skin.13 Sensory deficits were also evidenced in the glabrous skin biopsies of patients with Parkinson disease.49 Together, these findings illustrate the importance of reevaluating biopsies in regards to large fibers rather than quantifying small sensory fibers alone. Due to the extreme distal location of dermal myelinated fibers, more subclinical findings may surface in a number of neurological disorders.
Quantification of MC density has also been achieved in vivo by noninvasive reflectance confocal microscopy (RCM). This has been done at the distal phalanx of digit V and the thenar eminence in small samples of healthy controls, patients with HIV,50 and inherited neuropathy patients with CMT1A.51 A strong correlation was found between MC density and touch-pressure thresholds at the thenar eminence (r= −0.77; r2 = −0.59) as well as a trend toward correlations with vibration threshold.51 Densities calculated through this method appear to be significantly lower than those calculated by histology in the same location,9, 52 suggesting further work is needed to compare findings between in vivo confocal microscopy and biopsy samples to validate this method. However, its noninvasive nature makes RCM appealing as a possible sensory measure for neuropathy patients.
A large array of receptors, including those associated with nociception and growth factors, are present in myelinated and non-myelinated nerve fibers and mechanoreceptors.40, 53 Neurotrophin 3 (NT3) and brain-derived neurotrophic factor (BDNF) are trophic factors that support the health of Merkel complexes and MCs, respectively.54 Animals with genetic mutations which prevent BDNF expression have been shown to have severe developmental deficits in MC formation in digital glabrous skin.53 The lamellar cells of human MCs have been shown to express BDNF colocalized with S100 as well as the transmembrane tyrosine kinase receptor TrkB, a high-affinity receptor for BDNF.55, 56 A three-fold decrease in TrkB expression was shown to coincide with the well known reduction in corpuscle density as a result of aging.55 Furthermore, reductions of BDNF and other trophic factors have been reported in a patients with neuropathy,57 leading to an interesting question of whether the dysregulation of growth factors required for the normal function of large diameter fibers and mechanoreceptors could be correlated with cutaneous pathology from glabrous skin biopsies in patients with peripheral neuropathy.
Compartments of dermal myelinated nerve fibers and their pathogenic aberrations
Like all myelinated nerve fibers, dermal myelinated fibers are highly compartmentalized into discreet regions with unique protein compositions. Exploiting this fact with immunohistochemical staining allows for differentiation and morphological analysis of such regions as the node, paranode, juxtaparanode and internode, which appear to be similar in structure as well as composition in both the skin and in more proximal nerve bundles.30, 58 The following is a review of the efforts that have been made to characterize these compartments of dermal myelinated fibers in order to better understand normative parameters and the presence of disruptions frequently seen in a number of peripheral nerve diseases and animal models of demyelinating neuropathy.
Internodal length
During development, myelinating Schwann cells migrate along the axons, make contact with the axon, and elongate longitudinally to form individual internodes. Large diameter axons are typically wrapped by thicker myelin and form longer internodes; thus, while axons become smaller in their diameters toward their terminals, internodes become shorter.59 Data from human skin biopsy studies reflect this expected anatomical gradient.60 For instance, internodal measurements of distal fibers in the glabrous fingertip (79.1±13.8 μm)9 are shorter than those in the proximal phalanx (94.5±28.6 μm),14 also see Table 2.
Table 2.
Normative data for morphometric characteristics of dermal myelinated fibers and sural nerve fibers. Average age of patients: Provitera et al. 36.3 ± 10.3 years, Nolano et al. 33.7 ± 9.2, Saporta et al. 32.2 ± 12 years.
| Reference | Biopsy location | Section [urn] |
IME density [fibers/mm2] |
MC density [MCs/mm2] |
Internodal length [um] |
Nodal length [um] |
Diameter [um] |
|---|---|---|---|---|---|---|---|
| Provitera et al | Tip of digit III (N = 30) | 80 | – | 83.0 ± 22.5 | 3.3 ± 0.6 | 3.4 ± 0.6 | |
| Hairy skin (N = 30) |
88.6 ± 23.1 | 4.6 ± 1.1† | 3.0 ± 0.4* | ||||
| Nolano et al | Tip of digit III (N = 14) | 80 | 59.0 ± 29.3 | 33.02 ± 13.2 | 79.1 ± 13.8 | 3.5 ± 0.8 | 3.30 ± 0.50 |
| Tip of digit III, V (N = 1) |
33.6, 45.0 | ||||||
| Saporta et al | Lateral aspect of digit II (N = 12) | 60 | 14.6 ± 3.4 | 94.5 ± 28.6 | – | – | |
| Oh | Sural nerve | Teased fibers |
N/A | N/A | 200 - 1500 | – | 2-17 μn‡ |
p < 0.01
p < 0.001
range for myelinated fibers exhibiting a bimodal distribution with peaks at 5 μm and 13 μm
Provitera et al.11 compared morphometrics of glabrous skin and hairy skin, and found similarities in internodal length, yet slight anomalies were found in regards to axon diameter and nodes (e.g. slightly thicker axons and shorter nodes in glabrous skin). This is likely due to anatomical differences necessitating differing nerve courses rather than evidence of structural differences between the fibers of different skin types.11 Even so, hair follicles are the primary mode of mechanosensation in hairy skin at the depth of skin biopsies, such that sampling of myelinated fibers from hairy skin biopsies is difficult due to the relatively distant spacing of follicles, particularly in comparison to the ease of sampling such fibers innervating the much more dense population of mechanoreceptors in glabrous skin (Fig. 2).
Skin biopsy offers an exceptional opportunity to study internodal architecture in humans, as it is a minimally invasive outpatient procedure. Skin biopsy is typically sectioned perpendicular to the skin surface to reveal internodes from a similar vantage point as previously seen only with teased nerve fiber preparations of the sural nerve. Due to the shorter length of dermal internodes, even the small area of a skin biopsy section can capture multiple sequential internodes.11 For this reason, segmental demyelination can be identified using this approach as well.14
It is possible that using the finger as a biopsy site may have less pathological findings in patients who lack significant electrophysiological findings in the upper extremities. However, our experience in patients with CMT and diabetic neuropathy suggests that this site is distal enough to demonstrate abnormalities even when there are minimal exam findings in the hands.14, 61 Furthermore, it avoids the difficulty in sampling that may be encountered in cases of severe neuropathy where no fibers are present in the legs or feet. Future studies that apply this technique to demyelinating neuropathies such as CIDP or Guillain-Barré syndrome (GBS) would be informative.
A number of pathogenic mechanisms may cause shortened internodal length, including remyelination resulting from primary axonal degeneration or primary demyelination62 and developmental defects of myelinating Schwann cells in internodal lengthening.63 Differentiating the mechanism is feasible in acquired conditions, since it is known that a developmental problem is not present. Thus, shortened internodes are only related to the event of remyelination. However, this issue becomes complicated in inherited neuropathies (e.g. CMT), in which both a developmental defect and remyelination may play a role. Moreover, the chronic nature of CMT with insidious demyelination could make detection of segmental demyelination more difficult.
Our lab has investigated internodal length in patients with CMT1A using skin biopsies. We have found dermal internodes to be shortened compared to healthy controls as well as compared to patients with the primarily axonal type of CMT (type II) (Fig. 3).14 Interestingly, segmental demyelination was absent in the dermal nerves of CMT1A patients, while it was detectable in patients with CIDP by the same technique. This finding has led us to hypothesize that shortened internodes in dermal myelinated nerve fibers are caused by a developmental defect of internodal lengthening of Schwann cells in the patients with CMT1A. Because segmental demyelination is a known feature in sural nerve biopsies from patients with CMT1A, this finding in skin biopsies suggests that terminal Schwann cells of sensory nerve fibers may possess biological properties distinctive from those in proximal nerve fibers. If this is the case, this distinctive feature has likely rendered these terminal Schwann cells insensitive to segmental demyelination in CMT1A. Alternatively, chronic insidious demyelination may accumulate over time and contribute to the shortened internodes in CMT1A.14
Figure 3.
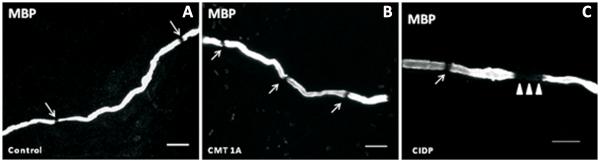
Immunohistochemical analysis of glabrous skin biopsies has been shown to reveal shortened internodal lengths in patients with CMT1A (B) and CIDP (C) compared to controls (A). Additionally, CIDP patients had signs of segmental demyelination in the paranodal regions, designated by the absence of Myelin Basic Protein staining (C, arrowheads).
Paranodal length
Each node of Ranvier is flanked by 2 hemi-paranodes, which are typically symmetric under light microscopy. After segmental demyelination, remyelination occurs to re-establish new internodes as well as new hemi-paranodes.64 The newly established internode and paranode differ in internodal length from the adjacent internode that remained intact without experiencing de-/remyelination.65 In this situation, the hemi-paranode on the remyelinated side would appear asymmetric from that on the intact side.
By immunohistochemical analysis using antibodies against the paranode-specific protein, Caspr, we have quantified the length of paranodes as well their degree of asymmetry. We found that the index of asymmetry of the hemi-paranodes was increased in the skin biopsies of patients with both CMT1A and CIDP.14 Thus, chronic, insidious segmental demyelination may have taken place in these nerve fibers. Taken together, investigating cellular compartments on dermal myelinated fibers has the potential to assist us in understanding the pathogenic processes of demyelinating neuropathies and the subsequent remodeling of myelin.
Nodal Gap
The nodal gap is a small but critical structure in the genesis and propagation of action potentials. The size of the gap directly affects the capacitance of the nodal membrane, thereby influencing the safety factor of action potential production.66, 67 Moreover, septate junctions along with paranodal myelin membrane physically insulate the node from the juxtaparanode, where voltage-gated potassium channels reside. Disruption of the paranodal membrane allows outward current of potassium channels to counteract the depolarizing inward current at the node and may result in failure of action potential propagation.68 Because axonal diameters vary minimally in dermal myelinated nerve fibers, the length of the nodal gap would reflect the area of the nodal axolemma.69 By using immunohistochemistry with antibodies against MBP, normative values have been reported.9, 11 Nodal lengths may be overestimated when they are measured with MBP staining alone, as it has been shown to poorly stain the paranodal regions flanking the node.9 Without staining the paranodes, nodal gap measurements are slightly exaggerated from true values; however, current normative data (Table 2) demonstrate that consistency between labs does not appear to be an issue, even in relatively small sample sizes. Future studies utilizing antibodies against Caspr would, however, improve accuracy, since it is specifically localized to the paranodal region.70 Future studies with larger sample sizes and assessments of reliability would enhance these data as well.
It has been well documented that the molecular architecture of nodes of Ranvier are altered in certain peripheral nerve disorders.2, 4 For instance, after segmental demyelination, voltage-gated sodium channels may spread out of the nodal region to paranodes or even juxtaparanodes.71 Subsequent remyelination will restore the sodium channel localization to the nodal region. Interestingly, during this process, heminodes may be formed, and subtypes of sodium channels may be altered, including up-regulation of Nav1.8.6, 72 Additionally, blocking this subtype has been shown to inhibit neuropathic pain in some animal models.73, 74 It is reasonable to believe that such molecular changes could also be investigated in human skin biopsies.
Axon caliber and myelin thickness
The caliber of individual axons and the thickness of their myelin sheath can be quantified using immunofluorescent staining at high power magnification (100×)9 or with the higher resolution afforded by EM or semi-thin sectioning.14 In the case of immunofluorescent staining, software was used to trace an outline of longitudinally orientated axons and myelin to calculate a G-ratio of axon diameter/fiber diameter (0.73 ± 0.04) in fingertip skin biopsies.9 In a related measure, axonal density of myelinated fibers in nerve fascicles was calculated with EM analysis of cutaneous fibers captured at a perpendicular orientation (7.73 × 10−3 ± 1.9 axons/μm2).14 Myelinated fibers become highly branched and tapered as they progress distally toward the skin, so the average diameter of dermal fibers (3.4±0.6 μm) is much smaller than what would be expected in the more proximal location of the sural nerve (≈ 5-10 μm).62
Diagnostic alternative to nerve biopsy
Nerve biopsy is invasive and often clinically unjustified without an alternative for research purposes. Skin biopsy is minimally invasive and can be repeated in different areas of the body with little discomfort to the patient, thus it is suitable for longitudinal investigations. Similar morphological parameters may be assessed as with teased fiber preparations, including detection of a number of pathogenic disease markers (summarized in Table 3).
Table 3.
Structural aberrations evident in sural nerve biopsies and skin biopsies
| Morphological abnormality |
Structural location |
Common method of detection |
Associated disorder(s) |
Sural nerve biopsy |
Skin biopsy |
|---|---|---|---|---|---|
| Tomacula | Paranodes | EM, IHC; teased nerve fiber, longitudinal sections |
HNPP | + | + |
|
| |||||
| Onion bulbs | Schwann cells | Toludine-blue staining, modified trichrome, EM; semi- thin cross sections |
CMT3 | + | - |
| basal lamina | CMT1A | + | + | ||
|
| |||||
| Segmental demyelination |
Internodes | IHC; longitudinal sections, teased fibers |
CIDP | + | + |
|
| |||||
| Shortened internodal length |
Internodes | IHC; longitudinal sections, teased fibers |
CMT1A | + | + |
|
| |||||
| Increased myelin periodicity |
Schwann cells | EM; thin section | MAG/IgM | + | + |
|
| |||||
| Inflammation and necrosis of the blood vessels |
Arteries | hematoxylin and eosin stain; cross section |
Vasculitis | + | |
|
| |||||
| Amyloid deposits | Peripheral nerves |
Alkaline congo-red stain; paraffin embedded nerve |
Amyloidosis | + | |
|
| |||||
| IgM deposits | Myelin sheath | IHC; cross sections or longitudinal sections |
Anti-MAG neuropathy |
+ | + |
|
| |||||
| Small sensory fiber loss |
Originate in DRG, epidermal termination |
IHC; longitudinal sections |
Diabetes, HIV, idiopathic |
+ | |
+ = reliable detection, - = not reliably detected, IHC = immunohistochemistry, EM = electron microscopy, HNPP = hereditary neuropathy with liability to pressure palsies, CMT = Charcot-Marie-tooth disease, CIDP = chronic inflammatory demyelinating polyneuropathy, DRG = dorsal root ganglia, MAG = myelin associated glycoprotein, IgM = Immunoglobulin M
Skin tissue can be processed similar to nerve biopsy cross sections to reveal dermal fascicles containing myelinated and unmyelinated axons. Semi-thin cross sections dyed with methylene blue and examined by light microscopy allow quantification of axonal density. Similar EM preparations as with sural nerve biopsy can be done to show myelin compaction with the ability to quantify the periodicity as well as the G-ratio of myelin thickness. In a comparative study of the 2 procedures, it was found that reductions in either large or small fibers in the sural nerve were nearly always reflected in the epidermal fiber content of the skin, although a reduction in epidermal fibers may exist while sural nerve morphometry is normal.75 This reflects the particularly susceptible terminal location of epidermal fibers. Furthermore, a recent study from Dacci et al demonstrates that skin biopsies can reveal morphological changes that are not yet detectable in sciatic nerves, thus appearing to be a more sensitive approach for detecting pathological changes.58
In other studies, Immunoglobulin M (IgM) deposits were found on dermal myelinated fibers immunoreactive for various myelin proteins (e.g. PMP22, MBP, and MAG) in patients with anti-myelin-associated glycoprotein (anti-MAG) neuropathy, suggesting the development of skin biopsy as a potential procedure for diagnosis.15, 76 Even so, the diagnostic utility of skin biopsies in myelin-related neuropathies is still in its infancy and warrants further investigation.
Expansion of skin biopsy applications
With the advance of molecular medicine, skin biopsies have been applied rapidly to more areas in neurological research. Some advances will be briefly discussed below.
Intracellular organelle trafficking
Intracellular organelles are constantly in motion, typically using microtubules as a “railway,” and many neurological diseases are related to abnormal intracellular organelle trafficking.77, 78 To examine this aspect of cell biology, one would have to use live cells from human subjects, which can be achieved by culturing cells from skin biopsies. We have recently described a new subtype of CMT, called CMT type 4J, which is caused by recessive mutations of the FIG4 gene. By taking a skin biopsy, it was possible to culture the fibroblasts of affected individuals to create an in-vitro, patient-specific model to further investigate the biology of FIG4 deficient human cells.79 As a result, it was discovered that there was excessive accumulation of vacuoles in the CMT4J fibroblasts that physically obstructed organelle trafficking.79 This finding reveals a new pathogenic mechanism of the disease and suggests the possibility of further applications of skin biopsy to studies of cellular function and pathology.
Pharmacological advancements
Glabrous skin biopsy may also prove to be a valuable indicator of drug therapy effectiveness. The dermal innervation of mouse footpads was recently used as a measure of neurotrophin-induced remyelination and consequent attenuation of behavioral and pathological signs of diabetic neuropathy in streptozocin (STZ) induced hyperglycemic mice.80 If a therapy that promoted remyelination in hyperglycemia-damaged fibers were to advance to clinical trials, glabrous skin biopsy could provide a relatively painless and affordable method of tracking responses and effectiveness on a structural level.
Several pain syndromes, including inherited erythromelalgia, idiopathic small fiber neuropathy, and paroxysmal pain syndrome have been linked to mutations in sodium channel subtype 1.7, leading to hyperexcitability.81, 82 The sodium channel dysregulation observed in neuropathy patients, in combination with theextensive role of sodium channels in other forms of pain, suggests a promising avenue for exploring new treatments for painful neuropathy. Activation of potassium channel currents by drugs such as retigabine may also provide new avenues in analgesia.83 TRPV-1 expression has also been implicated in diabetic neuropathic pain84. Most studies have been performed in rodent models, but skin biopsy could be utilized in diagnosis of ion channel subtype mutations as well as the efficacy of treatments in patients with symptoms of painful neuropathy.
Limitations of Glabrous Skin Biopsy
Although this review has focused on the expanding field of skin biopsy, there are also significant disadvantages to using glabrous skin biopsy to study histology. It is possible some patients may have such severe neuropathy that no identifiable fibers are demonstrated. This has not been found to be a deterrent in the literature or in the authors’ experience, as even patients with severe neuropathy have some dermal myelinated fiber endings which can be measured, but it significantly affects the interpretation of results. While glabrous skin has a significantly greater number of myelinated fibers than hairy skin, glabrous skin biopsies do not contain nerves large enough to study fascicular anatomy. Furthermore, there are fewer nerve fibers than a typical sural nerve, and they do not typically contain motor nerves to assist in the study of motor neuron-specific disorders. In addition, the typical amount of tissue obtained is less than that needed for protein quantification using typical scientific methods such as Western blot, which is not an issue with nerve biopsy. More work on normative data is also needed, with larger normal subject studies before these evaluations can be used for clinical diagnostic testing.
Conclusion
While a few diseases affecting the peripheral nervous system continue to be diagnosed primarily by whole nerve biopsies, many can be reliably diagnosed by electrophysiologic tests and/or genetic screening without the need to ever evaluate the molecular pathology of the nerves themselves. Evaluation of dermal myelinated fibers could provide both an inexpensive and relatively painless method of gaining a better understanding of the underlying molecular pathology of a variety of disorders causing cutaneous demyelination, mechanoreceptor atrophy, and axonal degeneration. This further application of skin biopsy can be added to ongoing evaluations of epidermal fibers, thereby expanding the assessment of neurological damage by providing a means to evaluate morphology, protein expression, and disease pathogenesis in unmyelinated and myelinated fibers during the onset and progression of peripheral neuropathies.
Acknowledgement
This work is, in part, supported by grants from NINDS K23 NS056009 (A.C. P.), NIH R01NS066927-01 (to J.L.).
Abbreviations
- PNS
peripheral nervous system
- CASPR
contactin-associated protein
- Kv
Potassium channel
- MPZ
myelin protein zero
- PMP22
peripheral myelin protein 22
- MBP
myelin basic protein
- IME
intrapapillary myelinated ending
- MC
Meissner corpuscle
- PCR
polymerase chain reaction
- CIDP
chronic inflammatory demyelinating polyneuropathy
- EM
electron microscopy
- CMT
Charcot-Marie-Tooth disease
- HNPP
hereditary neuropathy with liability to pressure palsies
- RCM
reflective confocal microscopy
- NT
neurotrophin
- BDNF
brain-derived neurotrophic factor
- Trk
tropomyosin-receptor-kinase
- GBS
Guillain-Barré syndrome
- MAG
Myelin-associated glycoprotein
- IHC
immunohistochemistry
- NaV
Sodium channel
- IgM
immunoglobulin M
- SKP
skin-derived precursor
- iPS
induced pluripotent stem cells
Reference List
- 1.Arroyo EJ, Scherer SS. On the molecular architecture of myelinated fibers. Histochem Cell Biol. 2000;113:1–18. doi: 10.1007/s004180050001. [DOI] [PubMed] [Google Scholar]
- 2.Scherer SS, Arroyo EJ. Recent progress on the molecular organization of myelinated axons. J Peripher Nerv Syst. 2002;7:1–12. doi: 10.1046/j.1529-8027.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- 3.Rasband MN, Trimmer JS, Peles E, Levinson SR, Shrager P. K+ channel distribution and clustering in developing and hypomyelinated axons of the optic nerve. J Neurocytol. 1999;28:319–331. doi: 10.1023/a:1007057512576. [DOI] [PubMed] [Google Scholar]
- 4.Rasband MN, Kagawa T, Park EW, Ikenaka K, Trimmer JS. Dysregulation of axonal sodium channel isoforms after adult-onset chronic demyelination. J Neurosci Res. 2003;73:465–470. doi: 10.1002/jnr.10675. [DOI] [PubMed] [Google Scholar]
- 5.Berger P, Niemann A, Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease) Glia. 2006;54:243–257. doi: 10.1002/glia.20386. [DOI] [PubMed] [Google Scholar]
- 6.Bai Y, Ianokova E, Pu Q, Ghandour K, Levinson R, Martin JJ, Ceuterick-de GC, Mazanec R, Seeman P, Shy ME, Li J. Effect of an R69C mutation in the myelin protein zero gene on myelination and ion channel subtypes. Arch Neurol. 2006;63:1787–1794. doi: 10.1001/archneur.63.12.1787. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Bai Y, Ianakova E, Grandis M, Uchwat F, Trostinskaia A, Krajewski KM, Garbern J, Kupsky WJ, Shy ME. Major myelin protein gene (P0) mutation causes a novel form of axonal degeneration. J Comp Neurol. 2006;498:252–265. doi: 10.1002/cne.21051. [DOI] [PubMed] [Google Scholar]
- 8.Ebenezer GJ, Hauer P, Gibbons C, McArthur JC, Polydefkis M. Assessment of epidermal nerve fibers: a new diagnostic and predictive tool for peripheral neuropathies. J Neuropathol Exp Neurol. 2007;66:1059–1073. doi: 10.1097/nen.0b013e31815c8989. [DOI] [PubMed] [Google Scholar]
- 9.Nolano M, Provitera V, Crisci C, Stancanelli A, Wendelschafer-Crabb G, Kennedy WR, Santoro L. Quantification of myelinated endings and mechanoreceptors in human digital skin. Ann Neurol. 2003;54:197–205. doi: 10.1002/ana.10615. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Suarez O, Montano JA, Esteban I, Gonzalez-Martinez T, Alvarez-Abad C, Lopez-Arranz E, Cobo J, Vega JA. Myelin basic protein-positive nerve fibres in human Meissner corpuscles. J Anat. 2009;214:888–893. doi: 10.1111/j.1469-7580.2009.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provitera V, Nolano M, Pagano A, Caporaso G, Stancanelli A, Santoro L. Myelinated nerve endings in human skin. Muscle Nerve. 2007;35:767–775. doi: 10.1002/mus.20771. [DOI] [PubMed] [Google Scholar]
- 12.Manganelli F, Iodice V, Provitera V, Pisciotta C, Nolano M, Perretti A, Santoro L. Small-fiber involvement in spinobulbar muscular atrophy (Kennedy’s disease) Muscle Nerve. 2007;36:816–820. doi: 10.1002/mus.20872. [DOI] [PubMed] [Google Scholar]
- 13.Nolano M, Provitera V, Crisci C, Saltalamacchia AM, Wendelschafer-Crabb G, Kennedy WR, Filla A, Santoro L, Caruso G. Small fibers involvement in Friedreich’s ataxia. Ann Neurol. 2001;50:17–25. doi: 10.1002/ana.1283. [DOI] [PubMed] [Google Scholar]
- 14.Saporta MA, Katona I, Lewis RA, Masse S, Shy ME, Li J. Shortened internodal length of dermal myelinated nerve fibres in Charcot-Marie-Tooth disease type 1A. Brain. 2009;132:3263–3273. doi: 10.1093/brain/awp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lombardi R, Erne B, Lauria G, Pareyson D, Borgna M, Morbin M, Arnold A, Czaplinski A, Fuhr P, Schaeren-Wiemers N, Steck AJ. IgM deposits on skin nerves in anti-myelin-associated glycoprotein neuropathy. Ann Neurol. 2005;57:180–187. doi: 10.1002/ana.20364. [DOI] [PubMed] [Google Scholar]
- 16.Provitera V, Nolano M, Pappone N, di GC, Stancanelli A, Lullo F, Crisci C, Santoro L. Distal degeneration of sensory and autonomic cutaneous nerve fibres in systemic sclerosis. Ann Rheum Dis. 2005;64:1524–1526. doi: 10.1136/ard.2005.038935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly EJ, Terenghi G, Hazari A, Wiberg M. Nerve fibre and sensory end organ density in the epidermis and papillary dermis of the human hand. Br J Plast Surg. 2005;58:774–779. doi: 10.1016/j.bjps.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Bjorklund H, Dalsgaard CJ, Jonsson CE, Hermansson A. Sensory and autonomic innervation of non-hairy and hairy human skin. An immunohistochemical study. Cell Tissue Res. 1986;243:51–57. doi: 10.1007/BF00221851. [DOI] [PubMed] [Google Scholar]
- 19.Pare M, Albrecht PJ, Noto CJ, Bodkin NL, Pittenger GL, Schreyer DJ, Tigno XT, Hansen BC, Rice FL. Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J Comp Neurol. 2007;501:543–567. doi: 10.1002/cne.21262. [DOI] [PubMed] [Google Scholar]
- 20.Boulais N, Misery L. Merkel cells. J Am Acad Dermatol. 2007;57:147–165. doi: 10.1016/j.jaad.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Mills LR, Diamond J. Merkel cells are not the mechanosensory transducers in the touch dome of the rat. J Neurocytol. 1995;24:117–134. doi: 10.1007/BF01181555. [DOI] [PubMed] [Google Scholar]
- 22.Tachibana T, Nawa T. Immunohistochemical reactions of receptors to met-enkephalin, VIP, substance P, and CGRP located on Merkel cells in the rat sinus hair follicle. Arch Histol Cytol. 2005;68:383–391. doi: 10.1679/aohc.68.383. [DOI] [PubMed] [Google Scholar]
- 23.Denda M, Tsutsumi M. Roles of transient receptor potential proteins (TRPs) in epidermal keratinocytes. Adv Exp Med Biol. 2011;704:847–860. doi: 10.1007/978-94-007-0265-3_44. [DOI] [PubMed] [Google Scholar]
- 24.Goto M, Ikeyama K, Tsutsumi M, Denda S, Denda M. Calcium ion propagation in cultured keratinocytes and other cells in skin in response to hydraulic pressure stimulation. J Cell Physiol. 2010;224:229–233. doi: 10.1002/jcp.22121. [DOI] [PubMed] [Google Scholar]
- 25.Ulmann L, Rodeau JL, Danoux L, Contet-Audonneau JL, Pauly G, Schlichter R. Trophic effects of keratinocytes on the axonal development of sensory neurons in a coculture model. Eur J Neurosci. 2007;26:113–125. doi: 10.1111/j.1460-9568.2007.05649.x. [DOI] [PubMed] [Google Scholar]
- 26.Boulais N, Misery L. The epidermis: a sensory tissue. Eur J Dermatol. 2008;18:119–127. doi: 10.1684/ejd.2008.0348. [DOI] [PubMed] [Google Scholar]
- 27.Hara M, Toyoda M, Yaar M, Bhawan J, Avila EM, Penner IR, Gilchrest BA. Innervation of melanocytes in human skin. J Exp Med. 1996;184:1385–1395. doi: 10.1084/jem.184.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinisch CM, Traxler H, Piringer S, Tangl S, Nader A, Tschachler E. Rarefaction of the peripheral nerve network in diabetic patients is associated with a pronounced reduction of terminal Schwann cells. Diabetes Care. 2008;31:1219–1221. doi: 10.2337/dc07-1832. [DOI] [PubMed] [Google Scholar]
- 29.Ebenezer GJ, McArthur JC, Thomas D, Murinson B, Hauer P, Polydefkis M, Griffin JW. Denervation of skin in neuropathies: the sequence of axonal and Schwann cell changes in skin biopsies. Brain. 2007;130:2703–2714. doi: 10.1093/brain/awm199. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Bai Y, Ghandour K, Qin P, Grandis M, Trostinskaia A, Ianakova E, Wu X, Schenone A, Vallat JM, Kupsky WJ, Hatfield J, Shy ME. Skin biopsies in myelin-related neuropathies: bringing molecular pathology to the bedside. Brain. 2005;128:1168–1177. doi: 10.1093/brain/awh483. [DOI] [PubMed] [Google Scholar]
- 31.Povlsen B. Watch out for digital nerves. BMJ. 2007;334:1236. doi: 10.1136/bmj.39241.429201.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Sole J, Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Eur J Neurol. 2010;17:903–909. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy WR, Wendelschafer-Crabb G, Polydefkis M, McArthur JC. Pathology and Quantitation of Cutaneous Innervation. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. Elsevier, Inc.; Philadelphia: 2005. pp. 869–895. [Google Scholar]
- 34.Katona I, Wu X, Feely SM, Sottile S, Siskind CE, Miller LJ, Shy ME, Li J. PMP22 expression in dermal nerve myelin from patients with CMT1A. Brain. 2009;132:1734–1740. doi: 10.1093/brain/awp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latov N. Biomarkers of CIDP in patients with diabetes or CMT1. J Peripher Nerv Syst. 2011;16(Suppl 1):14–17. doi: 10.1111/j.1529-8027.2011.00299.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee G, Xiang Z, Brannagan TH, III, Chin RL, Latov N. Differential gene expression in chronic inflammatory demyelinating polyneuropathy (CIDP) skin biopsies. J Neurol Sci. 2010;290:115–122. doi: 10.1016/j.jns.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Uceyler N, Kafke W, Riediger N, He L, Necula G, Toyka KV, Sommer C. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology. 2010;74:1806–1813. doi: 10.1212/WNL.0b013e3181e0f7b3. [DOI] [PubMed] [Google Scholar]
- 38.Koster AJ, Klumperman J. Electron microscopy in cell biology: integrating structure and function. Nat Rev Mol Cell Biol. 2003;(Suppl):SS6–10. [PubMed] [Google Scholar]
- 39.Vega JA, Garcia-Suarez O, Montano JA, Pardo B, Cobo JM. The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microsc Res Tech. 2009;72:299–309. doi: 10.1002/jemt.20651. [DOI] [PubMed] [Google Scholar]
- 40.Pare M, Elde R, Mazurkiewicz JE, Smith AM, Rice FL. The Meissner corpuscle revised: a multiafferented mechanoreceptor with nociceptor immunochemical properties. J Neurosci. 2001;21:7236–7246. doi: 10.1523/JNEUROSCI.21-18-07236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolano M, Provitera V, Saltalamacchia AM, Crisci C, Lanzillo B, Santoro L. Tactile stimulation and mechanoreceptors in sensory neuropathies. Neurol Sci. 2001;22:S31–S36. [Google Scholar]
- 42.Dyck PJ. Enumerating Meissner corpuscles: future gold standard of large fiber sensorimotor polyneuropathy? Neurology. 2007;69:2116–2118. doi: 10.1212/01.wnl.0000286934.55620.96. [DOI] [PubMed] [Google Scholar]
- 43.Bolton CF, Winkelmann RK, Dyck PJ. A quantitative study of Meissner’s corpuscles in man. Neurology. 1966;16:1–9. doi: 10.1212/wnl.16.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Peters RM, Hackeman E, Goldreich D. Diminutive digits discern delicate details: fingertip size and the sex difference in tactile spatial acuity. J Neurosci. 2009;29:15756–15761. doi: 10.1523/JNEUROSCI.3684-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruce MF. The relation of tactile thresholds to histology in the fingers of elderly people. J Neurol Neurosurg Psychiatry. 1980;43:730–734. doi: 10.1136/jnnp.43.8.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomsen NO, Mojaddidi M, Malik RA, Dahlin LB. Reduced myelinated nerve fibre and endoneurial capillary densities in the forearm of diabetic and non-diabetic patients with carpal tunnel syndrome. Acta Neuropathol. 2009 doi: 10.1007/s00401-009-0578-0. [DOI] [PubMed] [Google Scholar]
- 47.Kimura J. Nerve conduction and needle electromyography. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. Elsevier, Inc.; Philadelphia: 2005. pp. 899–969. [Google Scholar]
- 48.Nolano M, Provitera V, Stancanelli A, Saltalamacchia AM, Lanzillo B, Santoro L. Does small fiber neuropathy affect selectively small fibers? J Peripher Nerv Syst. 2005;10:67–68. [Google Scholar]
- 49.Nolano M, Provitera V, Estraneo A, Selim MM, Caporaso G, Stancanelli A, Saltalamacchia AM, Lanzillo B, Santoro L. Sensory deficit in Parkinson’s disease: evidence of a cutaneous denervation. Brain. 2008;131:1903–1911. doi: 10.1093/brain/awn102. [DOI] [PubMed] [Google Scholar]
- 50.Herrmann DN, Boger JN, Jansen C, Alessi-Fox C. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology. 2007;69:2121–2127. doi: 10.1212/01.wnl.0000282762.34274.94. [DOI] [PubMed] [Google Scholar]
- 51.Almodovar JL, Ferguson M, McDermott MP, Lewis RA, Shy ME, Herrmann DN. In vivo confocal microscopy of Meissner corpuscles as a novel sensory measure in CMT1A. J Peripher Nerv Syst. 2011;16:169–174. doi: 10.1111/j.1529-8027.2011.00342.x. [DOI] [PubMed] [Google Scholar]
- 52.Nolano M, Provitera V, Santoro L, Terme T, Herrmann DN, Neil BJ, Jansen C, Alessi-Fox C. In vivo confocal microscopy of meissner corpuscles as a measure of sensory neuropathy. Neurology. 2008;71:536–537. doi: 10.1212/01.wnl.0000324710.24747.c4. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Martinez T, Farinas I, Del Valle ME, Feito J, Germana G, Cobo J, Vega JA. BDNF, but not NT-4, is necessary for normal development of Meissner corpuscles. Neurosci Lett. 2005;377:12–15. doi: 10.1016/j.neulet.2004.11.078. [DOI] [PubMed] [Google Scholar]
- 54.Kirstein M, Farinas I. Sensing life: regulation of sensory neuron survival by neurotrophins. Cell Mol Life Sci. 2002;59:1787–1802. doi: 10.1007/PL00012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calavia MG, Feito J, Lopez-Iglesias L, de CF, Garcia-Suarez O, Perez-Pinera P, Cobo J, Vega JA. The lamellar cells in human Meissner corpuscles express TrkB. Neurosci Lett. 2010;468:106–109. doi: 10.1016/j.neulet.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 56.Montano JA, Perez-Pinera P, Garcia-Suarez O, Cobo J, Vega JA. Development and neuronal dependence of cutaneous sensory nerve formations: Lessons from neurotrophins. Microsc Res Tech. 2010;73:513–529. doi: 10.1002/jemt.20790. [DOI] [PubMed] [Google Scholar]
- 57.Anand P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog Brain Res. 2004;146:477–492. doi: 10.1016/S0079-6123(03)46030-5. [DOI] [PubMed] [Google Scholar]
- 58.Dacci P, Dina G, Cerri F, Previtali SC, Lopez ID, Lauria G, Feltri ML, Bolino A, Comi G, Wrabetz L, Quattrini A. Foot pad skin biopsy in mouse models of hereditary neuropathy. Glia. 2010 doi: 10.1002/glia.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suter U, Martini R. Myelination. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. Vol. 1. Elsevier Saunders; Philadelphia: 2005. pp. 411–431. [Google Scholar]
- 60.Saporta MA, Katona I, Lewis RA, Masse S, Shy ME, Li J. Reply: Internodal length variability of dermal myelinated fibres. Brain. 2010;133:e143. doi: 10.1093/brain/awq004. [DOI] [PubMed] [Google Scholar]
- 61.Myers MI, Artibee K, Li J, Peltier AC. Evaluation of Dermal Myelinated Fibers in Diabetes Mellitus. Ann.Neurol. 70(S15):S1–S3. 10-27-2011. Ref Type: Abstract. [Google Scholar]
- 62.Dyck PJ, Dyck JB, Engelstad J. Pathologic Alterations of Nerves. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. Elsevier, Inc.; Philadelphia: 2005. pp. 733–829. [Google Scholar]
- 63.Court FA, Sherman DL, Pratt T, Garry EM, Ribchester RR, Cottrell DF, Fleetwood-Walker SM, Brophy PJ. Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature. 2004;431:191–195. doi: 10.1038/nature02841. [DOI] [PubMed] [Google Scholar]
- 64.Griffin JW, Drucker N, Gold BG, Rosenfeld J, Benzaquen M, Charnas LR, Fahnestock KE, Stocks EA. Schwann cell proliferation and migration during paranodal demyelination. J Neurosci. 1987;7:682–699. doi: 10.1523/JNEUROSCI.07-03-00682.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hildebrand C. Myelin sheath remodelling in remyelinated rat sciatic nerve. J Neurocytol. 1989;18:285–294. doi: 10.1007/BF01190831. [DOI] [PubMed] [Google Scholar]
- 66.Koles ZJ, Rasminsky M. A computer simulation of conduction in demyelinated nerve fibres. J Physiol. 1972;227:351–364. doi: 10.1113/jphysiol.1972.sp010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rasminsky M, Sears TA. Internodal conduction in undissected demyelinated nerve fibres. J Physiol. 1972;227:323–350. doi: 10.1113/jphysiol.1972.sp010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bostock H, Rasminsky M. Potassium channel distribution in spinal root axons of dystrophic mice. J Physiol. 1983;340:145–156. doi: 10.1113/jphysiol.1983.sp014755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenbluth J. Multiple functions of the paranodal junction of myelinated nerve fibers. J Neurosci Res. 2009;87:3250–3258. doi: 10.1002/jnr.22013. [DOI] [PubMed] [Google Scholar]
- 70.Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arroyo EJ, Sirkowski EE, Chitale R, Scherer SS. Acute demyelination disrupts the molecular organization of peripheral nervous system nodes. J Comp Neurol. 2004;479:424–434. doi: 10.1002/cne.20321. [DOI] [PubMed] [Google Scholar]
- 72.Thakor DK, Lin A, Matsuka Y, Meyer EM, Ruangsri S, Nishimura I, Spigelman I. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol Pain. 2009;5:14. doi: 10.1186/1744-8069-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaida W, Klinder K, Arndt K, Weiser T. Ambroxol, a Nav1.8-preferring Na(+) channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory pain. Neuropharmacology. 2005;49:1220–1227. doi: 10.1016/j.neuropharm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95:143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 75.Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- 76.Stalder AK, Erne B, Reimann R, Renaud S, Fuhr P, Thomann S, Arnold A, Probst A, Schaeren-Wiemers N, Steck AJ. Immunoglobulin M deposition in cutaneous nerves of anti-myelin-associated glycoprotein polyneuropathy patients correlates with axonal degeneration. J Neuropathol Exp Neurol. 2009;68:148–158. doi: 10.1097/NEN.0b013e3181958187. [DOI] [PubMed] [Google Scholar]
- 77.Bronfman FC, Escudero CA, Weis J, Kruttgen A. Endosomal transport of neurotrophins: roles in signaling and neurodegenerative diseases. Dev Neurobiol. 2007;67:1183–1203. doi: 10.1002/dneu.20513. [DOI] [PubMed] [Google Scholar]
- 78.Chu CT, Plowey ED, Wang Y, Patel V, Jordan-Sciutto KL. Location, location, location: altered transcription factor trafficking in neurodegeneration. J Neuropathol Exp Neurol. 2007;66:873–883. doi: 10.1097/nen.0b013e318156a3d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X, Chow CY, Sahenk Z, Shy ME, Meisler MH, Li J. Mutation of FIG4 causes a rapidly progressive, asymmetric neuronal degeneration. Brain. 2008;131:1990–2001. doi: 10.1093/brain/awn114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience. 2007;145:303–313. doi: 10.1016/j.neuroscience.2006.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, Dib-Hajj S, Drenth JP, Waxman SG, Merkies IS. Gain of function Na(V) 1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71:26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- 82.Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, Ostman J, Klugbauer N, Wood JN, Gardiner RM, Rees M. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006;52:767–774. doi: 10.1016/j.neuron.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 83.Xu W, Wu Y, Bi Y, Tan L, Gan Y, Wang K. Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol Pain. 2010;6:49. doi: 10.1186/1744-8069-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pabbidi RM, Yu SQ, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4:9. doi: 10.1186/1744-8069-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]