Abstract
Objective
To investigate the effect of long zona dissection (LZD) compared with partial zona dissection (PZD) using ICSI pipettes for mechanical assisted hatching (AH) in vitrified-thawed blastocyst transfers.
Design
Prospective study.
Setting
University IVF clinic.
Patient(s)
A total of 120 women ≦ 38 years old undergone vitrified-thawed blastocyst transfers with LZD or PZD.
Intervention(s)
The culture of all pronucleate embryos to the blastocyst stage and the selection of blastocysts ≧ grade 3BB (Gardner and Schoolcraft score), followed by vitrified-thawed blastocyst transfers with LZD (n = 60) or with PZD (n = 60)
Main outcome measure(s)
Complete hatching rates, implantation rates, pregnancy rates.
Result(s)
At 5 h after thawing, complete hatching rates of blastocysts were significantly higher in LZD group compared with PZD group, 52.4 % vs. 31.8 % (P = 0.001). Implantation and clinical pregnancy rates were significantly higher in LZD group compared with PZD group, 40.9 % vs. 25.7 % and 63.0 % vs. 40.0 %, respectively (P = 0.010, P = 0.011).
Conclusion(s)
LZD using ICSI pipettes for mechanical AH improves significantly complete hatching, implantation and pregnancy rates in vitrified-thawed blastocyst transfers.
Keywords: Long zona dissection, ICSI pipettes, Assisted hatching, Vitrified-thawed blastocyst transfers
Introduction
Hatching is a critical process where the blastocyst escapes through the zona pellucida (ZP) before implantation. However, during in vitro culture or cryopreservation, the hatching of human embryos can be inhibited due to thick or hardened ZP [1]. In order to overcome these problems, a variety of assisted hatching (AH) techniques have been introduced since mechanical AH was first reported by Cohen et al in 1990 [2]. There are ZP drilling or thinning with acidified Tyrode’s solution or laser, two or three-dimensional partial zona dissection with a glass microneedle and the use of a piezo-micromanipulator [3–8]. However, the effects of AH vary according to the kind and extent of AH and its benefit is still debatable.
Recently, there have been reports providing clues about this argument. The size of AH opening is important for the complete hatching of the blastocyst because a small or moderate sized zona opening can often induce the hatching blastocyst into an ‘8’ shape and trap inner cell mass (ICM) [9]. This report could account for why the clinical outcomes of AH are various and inconsistent. Also, Miyata et al. reported on the relevance of the site of AH (20 um sized opening by laser) in vitrified-thawed human blastocysts. Complete hatching rates are significantly higher in AH group near the ICM compared with AH group at the opposite site [10]. This report implies that small sized hole is inappropriate in cleavage stage in which the site of the ICM is not confirmed and AH of the opposite side can adversely affect outcomes.
Therefore, we devised a long zona dissection (LZD) technique using ICSI pipettes that can overcome the problems related to size and site of AH and can prevent the trapping of ICM. This method does not require any new equipment and could be easily performed by an embryologist handling a micromanipulator. We report the clinical outcomes of long zona dissection using ICSI pipettes in vitrified-thawed blastocyst transfers.
Materials and methods
Patients
Inclusion criteria of patients were women aged ≦ 38 years, normal uterine cavity and fresh and ejaculated sperm. One hundred twenty patients were randomized to LZD group versus PZD group from a computer-generated random number sequence.
Blastocyst culture and selection
After the standard IVF or ICSI, all embryos were cultured to the blastocyst stage in sequential media. All blastocysts were evaluated using Gardner and Schoolcraft’s scoring system [11], and blastocysts ≧ grade 3BB were selected for vitrified-thawed blastocyst transfers.
Long or partial zona dissection using ICSI pipettes for mechanical AH
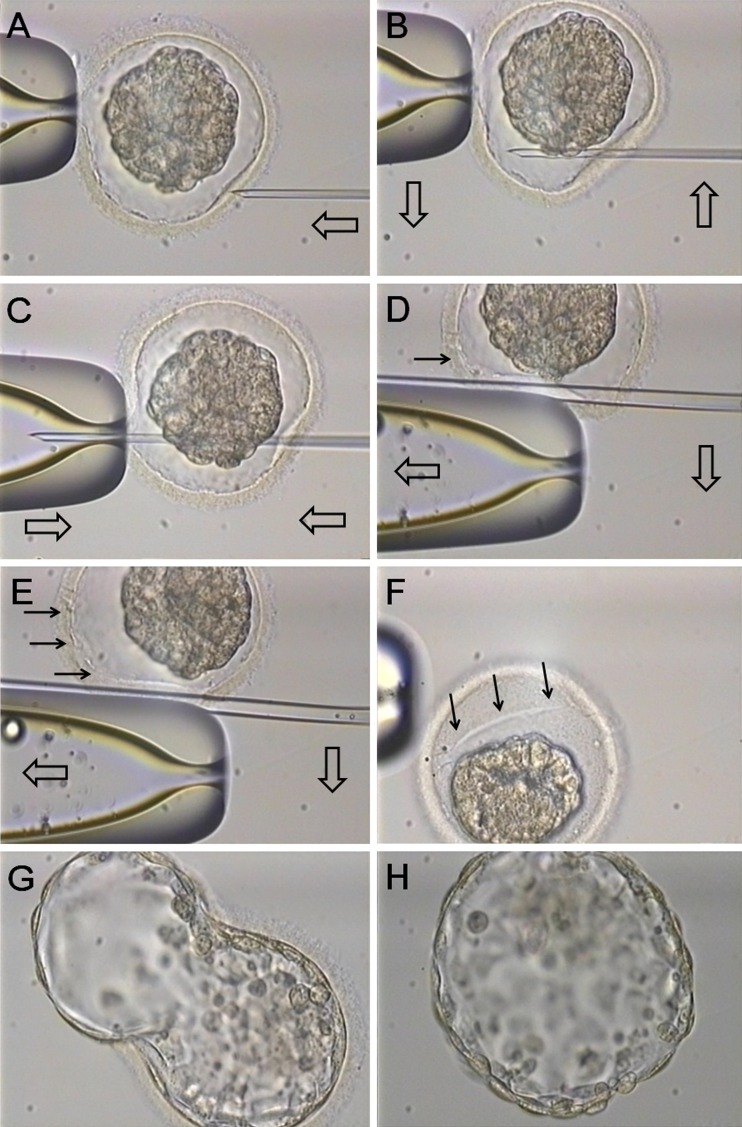
AH was performed by LZD or PZD using ICSI pipettes just after vitrification and thawing (Fig. 1). ZP was completely penetrated by the injection pipette from a 4 o’clock direction to 8 or 9 o’clock direction. Thereafter, a large split (more than 2/3 of ZP diameter) or a small split (lesser than 1/3 of ZP diameter) were created by rubbing and dissecting procedures using the edge of the holding pipette and the middle part of the injection pipette. After LZD or PZD, blastocysts were cultured. The thawed blastocysts were transferred at 5 h after thawing.
Fig. 1.
Long zona dissection using ICSI pipettes a The fixation by the holding pipette at a 9 o’clock direction after locating a shrunken blastocyst to 12 o’clock direction b The penetration by the injection pipette from a 4 o’clock direction c The penetration by the injection pipette to 8 or 9 o’clock direction d The rubbing and dissecting procedures using the edge of the holding pipette and the middle part of the injection pipette e Continuously, the rubbing and dissecting procedures f ZP with a large split after LZD g The hatching blastocyst showing a peanut-like shape h The hatched blastocyst (Filled arrows; the dissected ZP, open arrows: the handling direction of pipettes)
Endometrial preparation and blastocyst transfer
Endometrial preparation was done by administration of oral estradiol valerate. Vitrified-thawed blastocysts were transferred on cycle day 20 or 5 days after the start of luteal support.
Outcome measures and statistical analyses
Complete hatching rates of blastocysts, implantation rates, clinical pregnancy rates and ongoing pregnancy rates were investigated. Data are expressed as mean ± standard deviation unless otherwise specified.
Results
There were no significant differences in general demographic and clinical characteristics between two groups. Table 1 shows the clinical outcomes of vitrified-thawed blastocyst transfers in the LZD and PZD groups.
Table 1.
Clinical outcomes of frozen-thawed blastocyst transfers in LZD and PZD groups
| Variable | LZD group | PZD group | P value |
|---|---|---|---|
| No. of frozen-thawed cycles | 60 | 60 | NA |
| No. of frozen-thawed blastocysts (≧ grade 3BB) | 122 | 132 | NA |
| Complete hatching rates of blastocysts before cryopreservation (%)a | 4.9 % (6/122) | 6.1 % (8/132) | 0.690 |
| Complete hatching rates of blastocysts at 5 h after thawing (%)a | 52.4 % (64/122) | 31.8 % (42/132) | 0.001 |
| Average No. of transferred blastocysts | 2.0 ± 0.7 | 2.2 ± 0.8 | NS |
| Implantation rates (%) | 40.9 % (50/122) | 25.7 % (34/132) | 0.010 |
| Clinical pregnancy rates (%) | 63.0 % (38/60) | 40.0 % (24/60) | 0.011 |
| Ongoing pregnancy rates (%) | 50.0 % (30/60) | 33.3 % (20/60) | 0.064 |
NS not statistically significant, NA not assessable
aComplete hatching blastocysts mean the hatched blastocysts or the hatching blastocysts escaped ICM from ZP
Complete hatching rates of blastocysts before cryopreservation were not statistically different between both groups (P = 0.690). At 5 h after thawing, complete hatching rates were significantly higher in the LZD group compared with the PZD group, 52.4 % vs. 31.8 % (P = 0.001). The average number of transferred blastocysts were 2.0 ± 0.7 in the LZD group and 2.2 ± 0.8 in the PZD group and implantation rates were significantly higher in the LZD group compared with the PZD group, 40.9 % vs. 25.7 % (P = 0.010).
Also, clinical pregnancy rates were significantly higher in the LZD group compared with the PZD group, 63.06 % vs. 40.0 % (P = 0.011). Ongoing pregnancy rates were higher in the LZD group compared with the PZD group, 50.0 % vs. 33.3 % (P = 0.064), but were not significantly different.
Discussion
Recently, several studies have recommended AH in women with a relatively poor prognosis, including those with advanced maternal age ≧ 38, previous failed IVF cycles ≧ 2, poor embryo morphology, thickened zona or frozen-thawed embryos, but have not recommended AH in women with a relatively good prognosis [3, 12, 13]. Especially, size and site of the opening are important for the evaluation of AH because the ICM can be trapped as ‘8’ shape by partial zona dissection, the small opening or the thinning [9, 10]. If the effect of AH is evaluated in vitrified-thawed blastocysts with good quality by LZD, the results of AH may be consistent and different from previously reported results.
In human blastocysts, LZD with a large slit enhanced complete hatching rates significantly compared to a moderate slit (two-fifths of ZP diameter), 100 % vs. 71.8 %, respectively. They demonstrated that the complete hatching rate of LZD group is higher than PZD or no-AH groups and the size of hole created in the ZP is important for complete hatching because zona openings of small or moderate size can often trap the ICM. Also we have previously reported the effects of LZD using ICSI pipettes in vitrified-thawed mouse blastocysts [14]. Complete hatching rates were significantly higher in AH group compared with no AH group, 100 % vs. 50 %, respectively. These 2 reports showed that complete hatching rates of LZD group compared with PZD or no-AH groups are significantly higher in vitrified-thawed cycles as well as fresh cycles and LZD can completely overcome zona hardening.
Also, Hiraoka et al have reported the importance of the size of ZP opening by laser AH [15]. In frozen-thawed cleavage embryos that were cultured to blastocyst after thawing, implantation rates were significantly higher in the 50 % ZP opening group (100 % of ZP diameter) compared to 40 um ZP opening group and no AH group, 52 % vs. 27 % vs. 10 %, respectively. This report also showed that the size of the ZP opening is important for implantation or complete hatching.
In our study, to overcome possibility of ICM entrapping related to the size of the AH opening, the size of LZD was done by more than two thirds of ZP diameter. Frozen blastocysts were generally thawed at 9 a.m. and transferred at 2 p.m., that is to say, the complete hatching rates of blastocysts were the results investigated at 5 h after thawing. Complete hatching rates were significantly higher in the LZD group compared with PZD group, 52.4 % vs. 31.8 %, respectively. Also, the hatching blastocysts of the LZD group escaped ZP as a peanut-like shape and any hatching blastocyst did not show ‘8’ shape, even though LZD had been performed at the opposite site of ICM. Considering previous reports about LZD [9, 14], complete hatching rates might increase more over time. However, additional studies are necessary for determining how many human blastocysts are completely hatched without trapping after LZD. In our study, implantation and clinical pregnancy rates were significantly higher in the LZD group compared with control group, 40.9 % vs. 25.7 % and 63.0 % vs. 40.0 %, respectively. This means that the difference of implantation and pregnancy rates results from the difference of complete hatching rates between the 2 groups. It has been well known that zona hardening occurs in the processes of in vitro culture and cryopreservation [16]. So, AH with LZD could also improve implantation and pregnancy rates in fresh blastocyst transfers.
In conclusion, LZD using ICSI pipettes significantly improves implantation and pregnancy rates in vitrified-thawed ET cycles and highly cost effective compared with laser method. This study was limited by a small sample size and the use of frozen blastocysts, therefore further case-controlled prospective multicenter studies are needed to evaluate this technique.
Acknowledgments
This study was supported by a Biomedical Research Institute Grant (2012-11), Pusan National University Hospital
Footnotes
Capsule
Long zona dissection using ICSI pipettes for mechanical AH improves significantly hatching, implantation and pregnancy rates compared with partial zona dissection technique in vitrified-thawed blastocyst transfers.
References
- 1.Cohen J, Alikani M, Trowbridge J, Rosenwaks Z. Implantation enhancement by selective assisted hatching using zona drilling of human embryos with poor prognosis. Hum Reprod. 1992;7:685–91. doi: 10.1093/oxfordjournals.humrep.a137720. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Elsner C, Kort H, Malter H, Massey J, Mayer MP, et al. Impairment of the hatching process following IVF in the human and improvement of implantation by assisting hatching using micromanipulation. Hum Reprod. 1990;5:7–13. doi: 10.1093/oxfordjournals.humrep.a137044. [DOI] [PubMed] [Google Scholar]
- 3.Anna V, Irene B, Itziar B. Assisted hatching. In: Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Textbook of assisted reproductive techniques. UK: Informa Healthcare; 2009. pp. 181–187. [Google Scholar]
- 4.Hellebaut S, Sutter P, Dozortsev D, Onghena A, Qian C, Dhont M. Dose assisted hatching improve implantation rates after in vitro fertilization or intracytoplasmic sperm injection in all patients? A prospective randomized study. J Assist Reprod Genet. 1996;13:19–22. doi: 10.1007/BF02068864. [DOI] [PubMed] [Google Scholar]
- 5.Obruca A, Strohmer H, Sakkas D, Menezo Y, Kogosowski A, Barak Y, et al. Use of lasers in assisted fertilization and hatching. Hum Reprod. 1994;9:1723–6. doi: 10.1093/oxfordjournals.humrep.a138781. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama T, Fujuwara H, Yamada S, Tastumi K, Honda T, Fujii S. Clinical application of a new assisted hatching method using a piezo-micromanipulator for morphologically low-quality embryos in poor-prognosis infertile patients. Fertil Steril. 1999;71:1014–8. doi: 10.1016/S0015-0282(99)00131-4. [DOI] [PubMed] [Google Scholar]
- 7.Mantoudis E, Podsiadly BT, Gorgy A, Venkat G, Craft IL. A comparison between quarter, partial and total laser assisted hatching in selected infertility patients. Hum Reprod. 2001;16:2182–6. doi: 10.1093/humrep/16.10.2182. [DOI] [PubMed] [Google Scholar]
- 8.Balaban B, Urman B, Alatas C, Mercan R, Mumcu A, Isiklar A. A comparison of four different techniques of assisted hatching. Hum Reprod. 2002;17:1239–43. doi: 10.1093/humrep/17.5.1239. [DOI] [PubMed] [Google Scholar]
- 9.Lyu QF, Wu LQ, Li YP, Pan Q, Liu DE, Xia K, et al. An improved mechanical technique for assisted hatching. Hum Reprod. 2005;20:1619–23. doi: 10.1093/humrep/deh809. [DOI] [PubMed] [Google Scholar]
- 10.Miyata H, Matsubayashi H, Fukutomi N, Matsuba J, Koizumi A, Tomiyama T. Relevance of the site of assisted hatching in thawed human blastocysts: a preliminary report. Fertil Steril. 2010;94:2444–7. doi: 10.1016/j.fertnstert.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 11.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 12.Das S, Blake D, Farquhar C, Seif MM. Assisted hatching on assisted conception IIVF and ICSI. Cochrane Database Syst Rev. 2009; (2):CD001894. [DOI] [PubMed]
- 13.Practice Committee of Society for Assisted Reproductive Technology; Practice Committee of American Society for Reproductive Medicine The role of assisted hatching in in vitro fertilization: a review of the literature. A Committee opinion. Fertil Steril. 2008;90:S196–8. doi: 10.1016/j.fertnstert.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 14.Jo DH, Ko GR, Jung JH, Choi JR, Joo JK, Lee KS. Effects of the artificial shrinkage and assisted hatching before vitrification on the development of the vitrified mouse expanding blastocysts. Korean J Reprod Med. 2008;35:275–83. [Google Scholar]
- 15.Hiraoka K, Fuchiwaki M, Hiraoka K, Horiuchi T, Murakami T, Kinutani M, et al. Effect of the size of zona pellucid opening by laser assisted hatching on clinical outcome of frozen cleaved embyos that were cultured to blastocyst after thawing in women with multiple implantation failures of embryo transfer: a retrospective study. J Assist Reprod Genet. 2008;25:129–35. doi: 10.1007/s10815-008-9214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larman MG, Sheehan CB, Gardner DK. Calcium-free vitrification reduces cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes. Reproduction. 2006;131(1):53–61. doi: 10.1530/rep.1.00878. [DOI] [PubMed] [Google Scholar]