Abstract
Purpose
To assess the effect of streptozotocin induced hyperglycemia on germ cell integrity, DNA ploidy and methylation status for a period of two spermatogenesis cycles in adult male Swiss albino mice.
Methods
Streptozotocin injected mice were monitored for hyperglycemia at a regular interval for a period of 36 and 72 days. The DNA integrity in epididymal spermatozoa was determined by the comet assay. Flow cytometric analysis was done in germ cells to assess the DNA ploidy. The global methylation analysis in germ cells was done by 5-methyl cytosine immunostaining.
Results
Streptozotocin administration successfully resulted in hyperglycemic response which significantly affected serum testosterone level, sperm DNA integrity and DNA ploidy at the end of 36 days. However, no changes were observed in either epididymal sperm concentration or germ cell methylation status. In contrast, at the end of 76 days, although serum testosterone level, sperm DNA integrity and DNA ploidy status were unperturbed significantly in hyperglycemic group, the epididymal sperm concentration and methylation status of preleptotene/zygotene cells were significantly altered. Importantly, an attempt to find out the association between the blood glucose levels and the abnormalities in hyperglycemic group failed to demonstrate any correlation.
Conclusions
The germ cell abnormalities observed in hyperglycemic group could be interpreted as a primary effect of streptozotocin and not due to hyperglycemia. Our results call for further evaluation of streptozotocin before its application to study the hyperglycemic responses on male germ cells.
Keywords: Diabetes mellitus, Sperm DNA integrity, Streptozotocin, Methylation
Introduction
Diabetes mellitus (DM) is a group of metabolic diseases known to cause many systemic complications including sexual dysfunction, which itself may contribute to infertility. Earlier studies have shown that patients with DM have diminished sperm quality which is possibly due to endocrine dysfunction as the alteration in testosterone level in DM is associated with steroidogenic defect in Leydig cells [1–3]. In addition, it has been shown that diabetic men have a significantly higher percentage of spermatozoa with nuclear DNA damage [2] possibly due to oxidative stress. Sperm DNA integrity is essential for accurate transmission of genetic information to the offspring. Our earlier study has demonstrated an increased risk of transgenerational genomic instability in the offspring derived from the DNA damaged sperm [4].
Methylation of genomic DNA is an epigenetic regulatory mechanism involved in controlling the transcriptional activity of genes and establishing higher order chromatin structure to maintain the genomic stability [5]. DNA methylation is catalyzed by DNA methyl transferases (DNMTs) which regulate gene expression during development. It has been shown that diabetes-induced perturbation of methyl group lead to functional hypomethylation of DNA in a tissue specific fashion [6].
The diabetogenic agent streptozotocin (STZ) is a monofunctional nitrosourea derivative, having a broad-spectrum antibiotic and cytotoxic activity which causes pancreatic beta cell loss [7]. STZ treated animals showed altered seminiferous tubules, diminished sperm characteristics, and altered seminal plasma components [8, 9]. Although, STZ in various animal studies demonstrated as a successful model for diabetes, there are concerns about potential toxic effects of this chemical due to its carcinogenic and mutagenic property [10, 11]. It remains unclear whether the responses in experimental model are due to hyperglycemia or streptozotocin itself. Hence we hypothesize that STZ negatively affects the testicular germ cell population which in turn directly or indirectly influence the germ cell DNA integrity and methylation status thereby making it an unsuitable drug for inducing hyperglycemia under experimental conditions. Based on this, the specific aims of this study were to i) investigate the influence of streptozotocin induced hyperglycemic state for a period of two spermatogenesis cycles on germ cell DNA integrity, ploidy and methylation status and ii) characterize the association between the germ cell abnormalities and blood glucose level.
Materials and methods
Animals
The animal care and handling were done according to the institutional guidelines for animal experimentation and the proposal was approved by the Institutional Ethical Committee. Eight weeks old healthy male Swiss albino mice (N = 56) were used for the experiments and they were maintained under the controlled conditions of temperature (23 ± 2 °C) and light (12 h light/dark cycle) with standard diet and water ad libitum.
Induction of hyperglycemia
Animals were randomly assigned to one of 3 experimental groups: buffer control, diabetic-36 (36 days interval, one spermatogenesis cycle) and diabetic-72 (72 days interval, two spermatogenesis cycle). The mice were fasted for a minimum period of 4 h prior to injection. Streptozotocin (STZ) (Cat No. S0130, Sigma Chemical Co., St. Louis, USA) was dissolved in Sodium citrate buffer and the solution was injected intraperitoneally at a dose of 50 mg/kg body weight to diabetic-36 and diabetic-72 group. Each animal received STZ injection for 5 days consecutively. The control group received only sodium citrate buffer. The drug was mixed with the buffer prior to injections as the drug disintegrates within 15–20 min in solution. The animals were monitored for hyperglycemia at a regular interval for a period of 36 and 72 days using a blood glucose analyzer (Accu-Chek Active, India).
Estimation of serum testosterone levels
Serum testosterone level was estimated using a commercial kit (Monobind 3725-300, USA) as per manufacturer’s instructions. The optical density (OD) was taken at 450 nm with a reference wavelength of 620–630 nm using ELISA reader (ERBA Lisa 5, Transasia Biomedicals, India).
Evaluation of epididymal sperm concentration and DNA integrity
The caudal part of the epididymis was dissected out and spermatozoa were collected in Phosphate Buffered Saline (PBS) and the sperm concentration was measured using a hemocytometer. The DNA fragmentation in spermatozoa was assessed by the neutral comet assay as described earlier [12] with minor modifications. Briefly, the spermatozoa were suspended in sterile PBS (pH 7.4) keeping the sperm density constant in order to maintain the uniform distribution of the spermatozoa during electrophoresis. The sperm suspension was mixed with equal volume of 1 % low melting agarose and layered on a slide pre-coated with 1 % normal agarose. A third coat of agarose was layered over the second layer followed by overnight incubation in lysing solution (2.5 M NaCl), 100 mM disodium EDTA, 10 mM Trizma base, 10 % DMSO, 1 % Triton X-100, 20 mM DTT (pH 10) at 4 °C. Sperm DNA unwinding was carried out by immersing the slides in electrophoresis buffer 300mM Sodium acetate and 100mM Tris base, pH >9, 20 V (VcM = 0.74 V/cm, 100 mA) for 60 min followed by neutralization of slides in 0.4 M Tris HCl buffer for 5 min. The slides were drained and immersed in chilled absolute alcohol for 30 min for dehydration and then stored in a dry area until staining.
The slides were stained using 2 μg/ml ethidium bromide, observed under a fluorescent microscope (Imager-A1, Zeiss, Germany) and images were captured using 40 X objective. The spermatozoa were differentiated from non-spermatozoal cells based on their shape size and presence of tail. Each slide was coded to avoid observer’s bias and a minimum of 50 images were captured from each slide randomly. The sperm with DNA damage attain the shape of comet with tail region consisting of fragmented DNA and head region with intact DNA. The comet evaluation of the captured images was done using Kinetic Imaging software (Komet 5.5, UK).
Germ cell ploidy analysis by DNA flow cytometry
The testes were collected in Ca++ Mg++ free PBS. A portion of each testis from either side was minced to obtain a single cell suspension. Testicular cells were fixed using chilled 70 % alcohol (diluted by Ca++ Mg++ free PBS) and stored at 4 °C. Prior to analysis, cells were washed in Hank’s Balanced Salt Solution (HBSS), and treated with 1 % pepsin at 37 °C for 7 min to digest the proteins. The cells were washed and stained with propidium Iodide (25 μg/ml, with RNAse-40 μg/ml) and DNA content was analyzed in flow cytometer (BD FACS Calibur, San Jose, USA). The data were analyzed using Cell Quest Pro software [13].
Immunohistochemical detection of 5-methyl cytosine in germ cells
Immunohistochemistry was performed for the detection of 5-methyl cytosine (5-MEC) in germ cells as reported previously [14] with minor modifications. The testicular tissues were fixed using 70 % and 95 % alcohol followed by absolute alcohol for 30 min. Tissues were transferred to xylene and then embedded in paraffin. Serial 4 μm sections were cut using Leica Reichert-Jung 2040 Autocut Microtome (USA) on frosted slides pre-coated with (3 Aminopropyl) Triethoxy-silane (APS). The paraffin sections were dewaxed using xylene for 20 min and then rehydrated by serial graded ethanol solutions and washed in tap water for 20 min. Antigen retrieval was performed in 0.5 M glycine buffer for 30 min followed by washes in Tris Buffer Solution (TBS). The inactivation of endogeneous peroxidase activity was achieved using 0.3 % H2O2 at room temperature for 30 min, followed by washes with TBS buffer. The sections were then preincubated with 25 % normal goat IgG (Cat No. X0907, DAKO Cytomation) in 1 % bovine serum albumin in TBS for 20 min to block nonspecific reaction with primary antibodies. The blocking solution was carefully removed and the sections were treated with monoclonal anti-5-methylcytosine (5-MEC) antibody, raised in mice (Cat. No. NA81, Calbiochem, USA) at a dilution of 1:50 and stored overnight at 4 °C in a humidified chamber. After washing with TBS, the sections were incubated with biotinylated antimouse IgG (Cat. No. K5007, Dako REAL Envision) whole molecule at 1:500 dilution for 1 h at 37 °C. The sections were washed and then treated with streptavidin—HRP (Horseraddish Peroxidase) conjugate (Sigma Aldrich Cat. No. S5512) at a dilution of 1:100 for 30 min. After washing with TBS, visualization of the label was achieved using a 3,3′-diaminobenzidine (DAB) solution (Cat. No. K5007, Dako REAL Envision) followed by nuclear counterstaining using hematoxylin. A negative control (omission of the primary antibody) was included on each slide.
The germ cells were identified by their characteristic nuclear shape, size and their position in the seminiferous epithelium. The number of positively stained germ cells were determined to obtain the labelling index (number of positive cells/total number of germ cells x100).
Statistical analysis
The different variables were expressed as Mean ± SEM (Standard error of mean). The data was analyzed using‘t’ test (GraphPad InStat, USA). The graphs were prepared using Microcal Origin 6.0 program.
Results
The data from the total of 48 animals were presented in this study (36 day, control, N = 10; diabetic-36, N = 18; 72 days control, N = 10, diabetic-72, N = 10). Eight animals in diabetic-72 group showed resistance to hyperglycemia hence not included for correlative analysis.
Hyperglycemic response
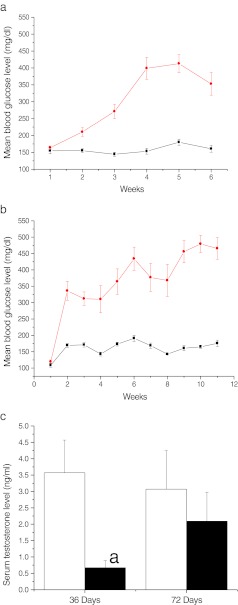
The blood glucose levels were monitored every week for a period of 36 and 72 days both in STZ treated and control animals. In 36 days control group, the mean level of glucose was 162 ± 6.26 mg/dl where as in case of the STZ treated group, the mean blood glucose level was 329 ± 13.82 mg/dl (Fig. 1a).
Fig. 1.
Streptozotocin induced hyperglycemic response a) weekly levels of blood glucose in diabetic-36 (black circle) and corresponding control (black square) group b) weekly levels of blood glucose in diabetic-72 (black circle) and corresponding control (black square). c) Serum testosterone level in diabetic (black square) and control (white square) ap < 0.05 compared to corresponding control group
At 72 days interval, the mean levels of blood glucose were 160 ± 4.26 mg/dl and 379 ± 32.13 mg/dl for control and STZ treated group respectively (Fig. 1b).
Serum testosterone level
The mean serum testosterone levels at 36 days interval were 3.57 ± 1.00 and 0.67 ± 0.22 ng/ml in control and STZ treated groups respectively and the differences were statistically significant (p < 0.05). However, at 72 days interval, although a similar trend was observed between control and STZ treated group, differences were not statistically significant (Fig. 1c).
Sperm count and DNA integrity in hyperglycemic animals
Thirty six days after STZ treatment, the mean epididymal sperm count in this group was 9.66 ± 0.64 millions/ml which was not significantly different from the control group (9.36 ± 0.98 millions/ml). Similarly, the sperm count in resistant group was not significantly different from hyperglycemic group (9.33 ± 2.3 vs 9.66 ± 0.64). In contrast, the differences were statistically significant between these groups at 72 days interval (8.39 ± 0.67 millions/ml in control and 6.42 ± 0.31 millions/ml in diabetic-72 group).
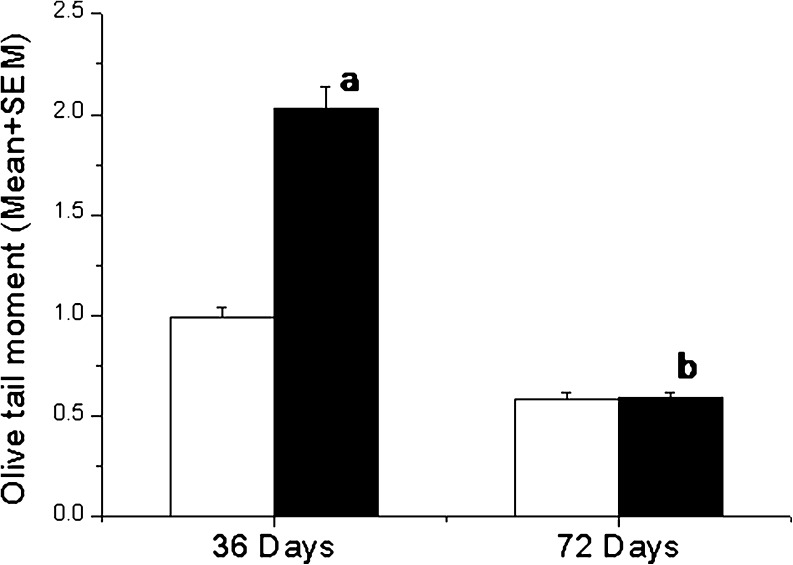
To determine whether hyperglycemic condition induces DNA damage in caudal spermatozoa, the data on olive tail moment (OTM, the product of the tail length and the fraction of total DNA in the tail which incorporates a measure of both the smallest detectable size of the migrating DNA and the number of relaxed/broken pieces product of the tail length and the fraction of total DNA in the tail) was collected. The OTM in diabetic-36 group was significantly higher in comparison to corresponding control group (p < 0.001). However, the OTM level between diabetic-36 and resistant group was not significantly different (2.05 ± 0.45 vs 1.99 ± 0.11). In contrast, the OTM reduced significantly in diabetic-72 group compared to diabetic-36 group (p < 0.001). Nevertheless, the differences between diabetic-72 and corresponding control groups were not significant (Fig. 2).
Fig. 2.
DNA integrity in hyperglycemic animals. The olive tail moment (OTM) in diabetic (black square) and control group (white square) ap < 0.001 compared to corresponding control group, bp < 0.001 versus diabetic-36 group
As OTM suggests only extent of DNA damage in a group of sperm cells, qualitative assessment of damage based on comet size was performed on a minimum of 500 spermatozoa from each animal. This provides additional information on the distribution of spermatozoa in relation to the amount of DNA damage. The number of severely damaged spermatozoa in diabetic-36 group were almost five fold higher than corresponding control group (p < 0.05). In contrast, the number of moderately damaged and severely damaged spermatozoa in diabetic-72 group were not statistically different from control which supports our OTM results (Table 1).
Table 1.
Distribution of spermatozoa in relation to the amount of DNA damage
| Group | Moderate damage | Severe damage | |
|---|---|---|---|
| 36 days | Control | 89.2 ± 4.5 | 11.78 ± 4.4 |
| STZ | 43.78 ± 15.4* | 57.71 ± 15.6* | |
| 72 days | Control | 97.06 ± 0.4 | 3.3 ± 0.54 |
| STZ | 94.01 ± 0.3 | 7.76 ± 4.0 | |
*p < 0.05 versus corresponding control group
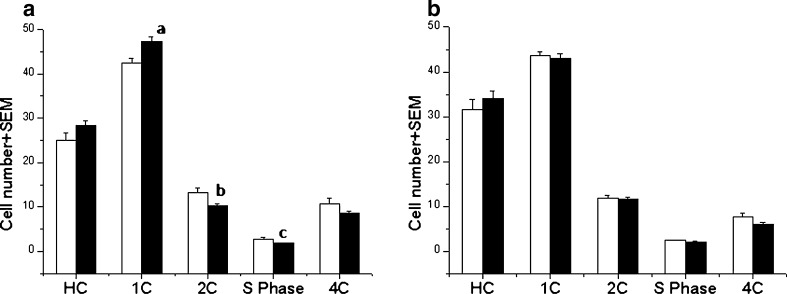
Germ cell DNA ploidy
The flow cytometric analysis of the testicular cells did not show any dramatic changes in the percentage of various cell types except some differences in the 2C, S-phase and 1C cells of diabetic-36 in comparison to control group. However, the decrease in 2C and increase in 1C cells in diabetic-36 group indicate higher turnover of cells which may be due to the marginal increase in the DNA methylation observed at this interval (Fig. 3)
Fig. 3.
Germ cell ploidy analysis by DNA flow cytometry showing percentage of various cell types HC: elongated spermatids, 1C: round spermatids, 2C: diploid cells, S-Phase: S-phase cells which includes both germ cells and non germ cell population 4C: germ cells with 4C DNA content at a) Diabetic-36 ap < 0.01 and b,cp < 0.05 compared to corresponding control group b) 72 days interval
Cytosine methylation in testicular germ cells
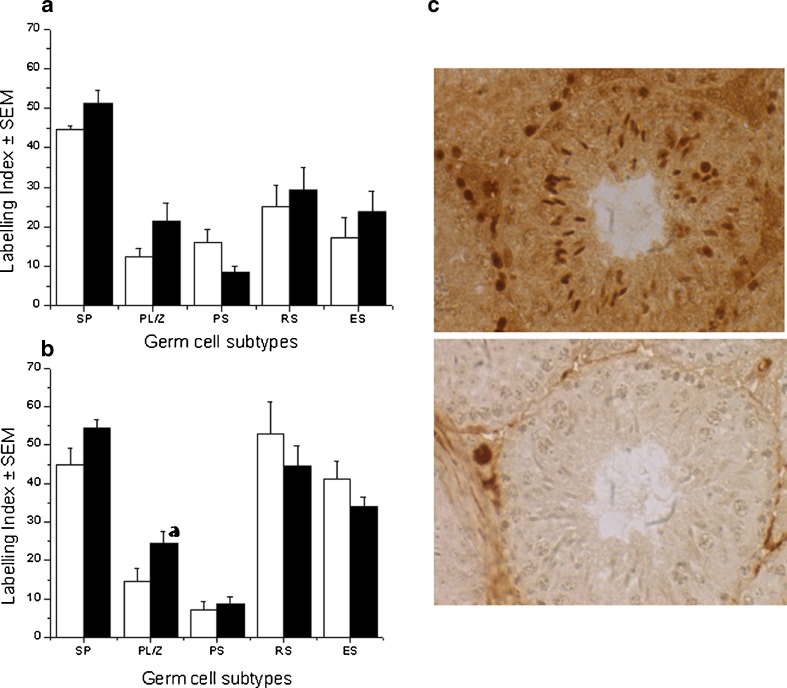
To examine whether the hyperglycemic condition for a period of 1–2 spermatogenesis cycle has any effect on global cytosine methylation in specific germ cell types, immunohistochemical detection of 5-methyl cytosine (5-MEC) was performed in control and STZ treated groups (Fig. 4a-c). In control group (36 days interval), the labeling index of 5-MEC in spermatogonia was 44.74 ± 0.76 whereas preleptotene/zygotene and pachytene spermatocytes had a labeling indices of 12.34 ± 2.06 and 16.02 ± 3.37 respectively. In case of post meiotic cells, the labeling indices were 25.11 ± 5.4 for round spermatids and 17.1 ± 5.14 for elongated spermatids. The STZ treated group also revealed similar trend although a non-significant increase in labeling indices were observed in spermatogonia, preleptotene/zygotene, round spermatids and elongated spermatids. In contrast, a non-significant decrease in the labeling index was observed in pachytene spermatocytes (Fig. 4a).
Fig. 4.
Immunolocalization of 5 methyl cytosine. Labeling index of 5 methyl cytosine (5-MEC) in testicular germ cells; diabetic (black square) and control group (white square) Spermatogonia (SP), preleptotene/zygotene (PL/Z), pachytene (PS), round spermatids (RS), and elongated spermatids (ES). a) 36 days interval and b) 72 days interval, ap < 0.05 compared to diabetic group. c) Photomicrographs showing the immunolocalization of 5 methyl cytosine (brown labeling) in testicular germ cells in diabetic-72 group (above), negative control (omission of the primary antibody) for the 5-MEC staining (below)
At 72 days interval, the trend in 5-MEC labeling was similar to that of 36 days interval in case of spermatogonia, preleptotene/zygotene spermatocytes, however, the differences between control and STZ treated group was significant in preleptotene/zygotene cells (p < 0.05). The differences between other germ cell types were not statistically significant (Fig. 4b).
Association between blood glucose level, sperm DNA integrity and methylation status
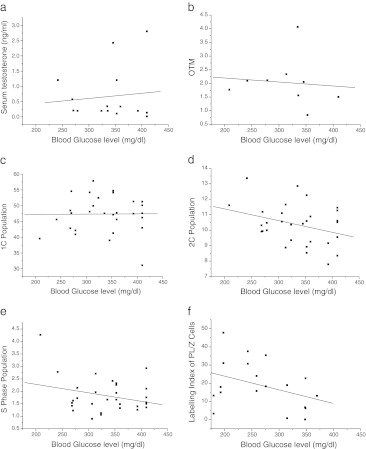
In order to determine the association between STZ induced hyperglycemia and the germ cell abnormalities observed in the present study, we performed regression analysis between blood glucose level and the parameters studied i.e. serum testosterone, DNA damage, DNA ploidy in various germ cells, and labeling index in PL/Z cells. Although, the serum testosterone level was significantly lower in diabetic-36 group, there was no correlation with blood glucose level (R2 = 0.01) (Fig. 5a). Similarly, OTM at 36 day interval failed to show any association with blood glucose level (R2 = 0.01) (Fig. 5b). Besides, the number of round spermatids (1C population), diploid (2C) cell population and S-phase cells at 36 days also did not show any correlation with blood glucose level (R2 = 0, 0.1 & 0.08 respectively) (Fig. 5c-e). As we observed a significant increase in the labeling index of preleptotene/zygotene spermatocytes in diabetic-72 group, an attempt was made to determine its relationship with blood glucose level. However, no association was evident between these groups (R2 = 0.14) (Fig. 5f).
Fig. 5.
Association between blood glucose level and a) serum testosterone level in diabetic-36 group, R2 = 0.01 b) Olive tail moment in diabetic-36 group, R2 = 0.01 c) 1C population in diabetic-36 group, R2 = 0 d) 2C population in diabetic-36 group, R2 = 0.1 e) S-phase population in diabetic-36 group, R2 = 0.08 f) Labeling index in diabetic-32 group, R2 = 0.14
Discussion
The primary objective of this study was to understand the effect of STZ induced hyperglycemia on sperm DNA integrity, germ cell ploidy and methylation status for a period of two spermatogenesis cycles which corresponds to approximately 72 days in mouse [15]. However, the results observed in this study did not demonstrate any significant association with blood glucose levels.
Administration of STZ successfully maintained hyperglycemic response until two spermatogenesis cycles. In this study, we used low dose induction protocol (50 mg/kg body weight for five consecutive days) against standard single dose protocol using 100–200 mg/kg body weight. Although, the hyperglycemic response is relatively lower in this protocol, there was no death of animals except a non-significant weight loss during 72 days of hyperglycemia. Although, the serum testosterone level declined significantly at the end of 36 days in STZ treated group, subsequent estimation on 72 day did not show any difference between control and diabetic groups. The review of earlier studies on the effect of hyperglycemia on plasma testosterone level has demonstrated conflicting results [16–19] possibly due to differences in study models and diabetes induction methods. With the decline in testosterone level at 36 days interval, we speculated an aberrant spermatogenesis in diabetic animals. However, to our surprise the epididymal sperm count was not significantly diverged from the control group. Interestingly, the sperm output was not significantly different between diabetic and resistant group. On the other hand, the histological evaluation of seminiferous tubules showed the evidence of complete spermatogenesis (data not shown). In contrast to this observation, at the end of 72 days, the sperm count declined significantly in both diabetic and resistant animals in comparison to the corresponding control group although post-meiotic germ cells were observed in most of the tubules suggesting that spermatogenesis was not completely halted in diabetic group. However, we speculate that the spermatozoa carrying increased DNA abnormalities got eliminated by apoptosis which in turn decreased the level of sperm DNA fragmentation at later time point. Overall, these observation suggest that hyperglycemia by itself did not affect the spermatogenesis although the level of testosterone dropped significantly at the end of 36 days. In insulin dependent diabetes, Leydig cell function and testosterone production decreases because of the absence of the stimulating effect of insulin on these which eventually affect sperm output and fertility because of the FSH decrease [1].
Pathophysiological dysfunctions in diabetes is associated with oxidative stress which is likely to contribute abnormalities in sperm nuclear DNA. The earlier study has reported an increase in sperm nuclear abnormalities in diabetic patients [2]. Since human studies have ethical and technical limitations to find an exact relationship between blood glucose level and sperm chromatin integrity, we assessed sperm DNA damage in controlled hyperglycemic condition for a period two spermatogenesis cycles and then correlated with blood glucose level. At the end of 36 days, the OTM in hyperglycemic group was significantly higher than control group which then declined to almost baseline control level at the end of 76 days even though a good hyperglycemic response was maintained. In addition, similar to the sperm concentration, the extent of DNA fragmentation observed in resistant group was also significantly higher than control group but comparable to hyperglycemic group. This raises a logical question, whether the DNA damage observed at the end of 36 days could be interpreted as a primary effect of STZ as this agent is known to induce oxidative stress, DNA damage and apoptosis [20].
The DNA methylation events during spermatogenesis have important implications for gamete integrity and transmission of epigenetic information to the next generation [21]. The hyperglycemic state did not show any significant difference in methylation pattern of germ cells after one or two spermatogenesis cycle except in preleptotene/zygotene cell population where labeling index was higher than control at 72 days suggesting hypermethyation. Diabetes induced perturbations of methylation has been observed in tissue-specific fashion [6] although the germ cell methylation changes are not reported till date. The changes in methylation pattern is a particularly a novel observation and supports our hypothesis that hyperglycemic condition affects germ cell methylation and may have implications on reproductive outcome. However, at this stage we cannot exclude that these changes are induced by STZ which needs to be ruled out using other diabetic models.
To validate the observations made in this study, we correlated our results with the blood glucose levels. To our surprise, the germ cell abnormalities seen in STZ treated group failed to demonstrate any association with blood glucose levels. However, it may be unreasonable to look for strong correlation since the presumed threshold of effect for glucose has been exceeded in all animals. Contrarily, the animals failed to attain hyperglycemic state also had significant level of sperm DNA fragmentation. Hence it can be reasonably postulated that abnormalities observed in this study is caused by STZ itself. While STZ is a well-known genotoxic agent in cell model, and it has been shown to causes significant oxidative impairments in the male reproductive milieu during early diabetic phase in mice [22]. The rapid decline in testosterone level and increase in sperm DNA damage during first 36 days in this study also confirms the direct effect of STZ rather than hyperglycemia induced response. The observation from an earlier study confirms the immediate action of STZ on target cells where induction of DNA damage in liver and kidney was achieved as early as 1 h and persisted up to 4 weeks [23].
Although, the present study is restricted to only two spermatogenesis cycles, our results call for further evaluation of streptozotocin before its application to study the hyperglycemic responses on male germ cells. Several attempts have been made in recent years to establish a good animal model like Ins 2Akita mouse and animals fed with high-fat diet [24, 25] which do not involve any pharmacological intervention. These approaches may provide a new and exciting model to study the pathophysiological changes associated with diabetes. In conclusion, the germ cell abnormalities observed in hyperglycemic group could be interpreted as a primary effect of streptozotocin and not due to hyperglycemia. Our results call for further evaluation of streptozotocin before its application to study the hyperglycemic responses on male germ cells.
Acknowledgment
Authors would like to acknowledge technical support from Ms. Jayalaxmi Pai and Ms. Kirthi Patil (Clinical Embryology, Kasturba Medical College, Manipal).
This work was partially supported by the intramural funding from Kasturba Medical College, Manipal to RB (Grant # PGR-012).
Footnotes
Capsule
The germ cell abnormalities observed in streptozotocin induced hyperglycemic mice suggest possible effect of the drug and not due to hyperglycemic response.
References
- 1.Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004;25:706–19. doi: 10.1002/j.1939-4640.2004.tb02845.x. [DOI] [PubMed] [Google Scholar]
- 2.Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, Lewis SE. Insulin dependent diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22:1871–7. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 3.Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE. Diabetes mellitus and sperm parameters: a brief review. J Androl. 2012;33:145–53. doi: 10.2164/jandrol.111.013193. [DOI] [PubMed] [Google Scholar]
- 4.Adiga SK, Upadhya D, Kalthur G, Bola Sadashiva SR, Kumar P. Transgenerational changes in somatic and germ line genetic integrity of first-generation offspring derived from the DNA damaged sperm. Fertil Steril. 2010;93:2486–90. doi: 10.1016/j.fertnstert.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 6.Williams KT, Garrow TA, Schalinske KL. Type I diabetes leads to tissue-specific DNA hypomethylation in male rats. J Nutr. 2008;138:2064–9. doi: 10.3945/jn.108.094144. [DOI] [PubMed] [Google Scholar]
- 7.Raketien N, Raketien ML, Nadkarmi M. Studies on the diabetogenic action of Streptozotocin. Cancer Chemother Rep. 1963;29:91–8. [PubMed] [Google Scholar]
- 8.Seethalakshmi L, Menon M, Diamond D. The effect of streptozotocin-induced diabetes on the neuroendocrine-male reproductive tract axis of the adult rat. J Urol. 1987;138:190–4. doi: 10.1016/s0022-5347(17)43042-4. [DOI] [PubMed] [Google Scholar]
- 9.Soudamani S, Malini T, Balasubramanian K. Effects of streptozotocin-diabetes and insulin replacement on the epididymis of prepubertal rats: histological and histomorphometric studies. Endocr Res. 2005;31:81–98. doi: 10.1080/07435800500229193. [DOI] [PubMed] [Google Scholar]
- 10.Weiss RB. Streptozocin: a review of its pharmacology, efficacy, and toxicity. Cancer Treat Rep. 1982;66:427–38. [PubMed] [Google Scholar]
- 11.Bolzán AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res. 2002;512:121–34. doi: 10.1016/S1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 12.Singh NP, Stephens RE. X-ray induced DNA double-strand breaks in human sperm. Mutagenesis. 1998;13:75–9. doi: 10.1093/mutage/13.1.75. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy H, Danilovich N, Morales C, Sairam MR. Qualitative and quantitative decline in spermatogenesis of the follicle-stimulating hormone receptor knockout (FORKO) mouse. Biol Reprod. 2000;62:1146–59. doi: 10.1095/biolreprod62.5.1146. [DOI] [PubMed] [Google Scholar]
- 14.Adiga SK, Ehmcke J, Schlatt S, Kliesch S, Westernströer B, Luetjens CM, Wistuba J, Gromoll J. Reduced expression of DNMT3B in the germ cells of patients with bilateral spermatogenic arrest does not lead to changes in the global methylation status. Mol Hum Reprod. 2011;17:545–9. doi: 10.1093/molehr/gar023. [DOI] [PubMed] [Google Scholar]
- 15.Clermont Y, Trott M. Duration of the cycle of the seminiferous epithelium in the mouse and hamster determined by means of 3 H-thymidine and radioautography. Fertil Steril. 1969;20:805–17. doi: 10.1016/s0015-0282(16)37153-9. [DOI] [PubMed] [Google Scholar]
- 16.Hassan AA, Hassouna MM, Taketo T, Gagnon C, Elhilali MM. The effect of diabetes on sexual behavior and reproductive tract function in male rats. J Urol. 1993;149:148–54. doi: 10.1016/s0022-5347(17)36028-7. [DOI] [PubMed] [Google Scholar]
- 17.Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl. 2006;29:482–8. doi: 10.1111/j.1365-2605.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 18.Jelodar G, Razmi N, Gholampour V. Arginase alteration in the reproductive system of alloxan-diabetic dogs. J Reprod Dev. 2007;53:317–21. doi: 10.1262/jrd.18049. [DOI] [PubMed] [Google Scholar]
- 19.Pontes DA, Fernandes GS, Piffer RC, Gerardin DC, Pereira OC, Kempinas WG. Ejaculatory dysfunction in streptozotocin-induced diabetic rats: the role of testosterone. Pharmacol Rep. 2011;63:130–8. doi: 10.1016/j.phrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Murata M, Takahashi A, Saito I, Kawanishi S. Site-specific DNA methylation and apoptosis: induction by diabetogenic streptozotocin. Biochem Pharmacol. 1999;57:881–7. doi: 10.1016/S0006-2952(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 21.Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, Sasaki H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–03. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- 22.Shrilatha B, Muralidhara Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod Toxicol. 2007;23:578–87. doi: 10.1016/j.reprotox.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Imaeda A, Kaneko T, Aoki T, Kondo Y, Nagase H. DNA damage and the effect of antioxidants in streptozotocin-treated mice. Food Chem Toxicol. 2002;40(7):979–87. doi: 10.1016/S0278-6915(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 24.O’Neill J, Czerwiec A, Agbaje I, Glenn J, Stitt A, McClure N, Mallidis C. Differences in mouse models of diabetes mellitus in studies of male reproduction. Int J Androl. 2010;33:709–16. doi: 10.1111/j.1365-2605.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 25.Mallidis C, Czerwiec A, Filippi S, O’Neill J, Maggi M, McClure N. Spermatogenic and sperm quality differences in an experimental model of metabolic syndrome and hypogonadal hypogonadism. Reproduction. 2011;142:63–71. doi: 10.1530/REP-10-0472. [DOI] [PubMed] [Google Scholar]