Abstract
Purpose
To study the effect of addition of zinc to human semen sample prior to cryopreservation on post-thaw sperm quality and function.
Methods
Semen samples were collected from men attending university infertility clinic for semen analysis (n=109). Liquefied semen samples were cryopreserved in glycerol-egg yolk- citrate medium with or without the prior addition of zinc (100 μM) and stored in liquid nitrogen. After 10 days, the semen samples were thawed to assess the outcome. Sperm motility, DNA integrity, mitochondrial potential and the ability of spermatozoa to undergo capacitation and acrosome reaction was assessed in post-thaw samples.
Results
Semen samples cryopreserved after addition of zinc had a significantly higher percentage of sperm with intact DNA (p<0.001), mitochondrial function (p<0.001) and progressive motility (p<0.01) compared to the semen samples cryopreserved without zinc supplementation. Apart from this, ability to undergo capacitation and acrosome reaction in vitro was significantly higher in semen samples cryopreserved with zinc (p<0.0001 and p<0.001 respectively).
Conclusions
Addition of zinc to semen samples prior to cryopreservation helps in preventing the freeze-thaw-induced sperm DNA damage and loss of sperm function.
Keywords: Zinc, Semen cryopreservation, Sperm DNA integrity, Mitochondrial potential, Capacitation, Acrosome reaction
Introduction
Semen cryopreservation is an integral part of infertility treatment and fertility preservation field. The commonly employed semen cryopreservation techniques have certain limitations. The reduced motility, membrane damage, ultrastructural changes [1], DNA damage [2] and loss of mitochondrial function [3] are the most common detrimental effects of freeze-thaw process on spermatozoa. The limitations in the current methods necessitate the research on development of alternative techniques to improve the quality of cryopreserved spermatozoa.
Sperm DNA integrity is critical for successful transmission of correct genetic material to the next generation. There is substantial evidence available to prove that the cryopreservation causes oxidative stress to spermatozoa [4–6]. The lack of cytoplasmic free radical scavengers and high polyunsaturated fatty acid (PUFA) content in plasma membrane make human spermatozoa highly sensitive to free radical assault. It is known that the lipid peroxides generated from the oxidation of unsaturated fatty acids targets DNA to induce DNA fragmentation, base modifications and chromatin cross-linking [7–9]. Earlier studies have shown that sperm DNA damage has adverse effects on fertilization potential of the spermatozoa [10], pregnancy rate and live birth rate [11]. Therefore, protecting the spermatozoa from cryopreservation-induced DNA damage and loss of sperm functional competence is clinically relevant in assisted reproductive technology (ART). Taking this into consideration, the most common approach followed hitherto, was to protect the sperm DNA during freeze-thaw process by using free radical scavengers along with the cryopreservation medium [12, 13].
Zinc is a micronutrient required for the action of more than 200 metallo-enzymes. It plays a vital role in normal growth and development of the human body [14]. Deficiency of zinc can impair spermatogenesis and decrease serum testosterone levels [15]. Additionally, zinc helps in stabilization of polymeric macromolecules such as RNA, DNA and protein. Earlier studies have demonstrated that zinc present in the prostatic secretion provides stability to human sperm chromatin [16, 17]. Therefore, the present study was undertaken to assess whether addition of zinc to the semen sample prior to cryopreservation can protect the human spermatozoa from freeze-thaw-induced DNA damage and preserve the post-thaw sperm functional competence.
Materials and methods
Study subjects
Men attending the university infertility clinic for routine semen evaluation (n=109) were recruited for the study. The study was approved by Institutional Ethics Committee.
Semen analysis
After 2–7 days of sexual abstinence, the semen samples produced by masturbation were collected in a sterile container and allowed to liquefy at room temperature. The sperm count was assessed using Makler’s chamber (Sefi Instruments, Israel). Rest of the microscopic analysis was done as described in WHO manual [18].
Cryopreservation and thawing
The liquefied ejaculates were divided into two aliquots; zinc was added to one aliquot and the other aliquot served as control. The samples were kept at room temperature for 10 min after which they were mixed with equal volume of cryoprotective (glycerol-egg yolk-citrate buffered) medium. The aliquots were transferred to screw-top cryovials (Nunc, Denmark) and stored in liquid nitrogen for 10 days as described earlier [19]. The samples were removed from liquid nitrogen, subjected to rapid thawing and washed in Earle’s Balanced Salt Solution (EBSS, Sigma Aldrich, USA, Cat. No. M5017) supplemented with 0.1 % Human serum albumin (HSA, Sigma Aldrich, USA, Cat. No. A1653).
Elucidation of optimum zinc concentration
A stock solution (1 mg/ml) of Zinc sulfate (MERCK, India, Cat. No. 7446-19-7) was prepared in Milli Q water. Liquefied ejaculates from normozoospermic men (n=10) were supplemented with different concentrations of zinc (0, 10, 50, 100, 250, 500 and 1000 μM) and then mixed with equal volume of glycerol-egg yolk-citrate buffered cryoprotective media. The samples were stored in liquid nitrogen and thawed after 10 days. Post-thaw semen samples supplemented with 100 μM zinc had significantly higher percentage of total motility and progressive motility when compared to control (p<0.01 and p<0.05 respectively). In addition, the percentage of spermatozoa with intact DNA in post-thaw samples was almost two times higher in zinc supplemented group compared to control (~40 % v/s ~22 % respectively) (Table 1). Therefore, 100 μM of zinc sulfate was used for further studies.
Table 1.
Sperm motility and DNA damage in normozoospermic semen samples (n=10) cryopreserved after adding different concentration of zinc to liquefied semen samples
| Concentration of zinc sulphate (μM) | Percentage Motility (Mean±SEM) | Sperm with intact DNA (%) (Mean±SEM) | |
|---|---|---|---|
| Total motility | Progressive motility | ||
| 0 | 46.7±2.20 | 29.7±1.86 | 22.00±5.41 |
| 10 | 49.4±1.76 | 29.0±1.81 | 26.32±2.96 |
| 50 | 52.6±1.83 | 32.2±1.28 | 32.00±6.40 |
| 100 | 57.0±1.89 b | 38.2±1.70a c | 40.67±3.04 |
| 250 | 55.0±1.83 | 36.5±1.67 | 31.17±7.56 |
| 500 | 48.8±1.84 | 29.5±1.87d | 29.83±6.55 |
| 1000 | 47.3±2.13 | 27.6±2.10e | 26.83±8.45 |
ap<0.05, bp<0.01 v/s Control; cp<0.01 v/s 10 μM; dp<0.05 v/s 100 μM; ep<0.01 v/s 100 μM
Sperm chromatin dispersion (SCD) test
SCD was performed as described by Fernandez et al. [20] with minor modifications [13]. Briefly, the fresh and frozen-thawed semen samples were mixed with 1 % low melting point agarose maintained at 37 °C. Approximately 150 μl of this mixture was layered on a slide pre-coated with 0.65 % of normal melting point agarose after which a cover slip was carefully placed and the gel was allowed to solidify. The slides were then immersed in freshly prepared acid denaturation solution (0.08 N HCl) for 7 min at room temperature in dark. Proteins were removed by incubating the slides in lysing solution 1 (0.4 M Tris, 20 mM DTT, 1 % SDS, 50 mM EDTA, pH 7.5) for 20 min followed by incubation in lysing solution 2 (0.4 M Tris, 2 M NaCl, pH 7.5) for 15 min at room temperature. Slides were washed in Tris buffer (0.4 M Tris, pH 7.5) for 2 min, serially dehydrated in graded ethanol, and air dried. Cells were stained with DAPI (2 μg/ml) and scored under fluorescent microscope (Imager- A1 Carl Zeiss, Germany). Minimum of 500 spermatozoa were scored in each slide. Spermatozoa with no halo, small halo, large halo and fragmented nucleus were counted separately under oil immersion objective (1000X magnification). The percentage of sperm with DNA damage was determined by counting the spermatozoa having fragmented nuclei and spermatozoa with no halo.
Sperm mitochondrial potential
The mitochondrial function was assessed in fresh and frozen-thawed semen samples using the method described by Johnson et al. [21] with minor modifications. Briefly, the samples were centrifuged at 1800 rpm for 8 min and resuspended in EBSS medium containing 10 μg/ml of Rhodamine 123 (Sigma Aldrich, USA, Cat. No. R8004), followed by incubation at 37 °C and 5 % CO2 for 20 min. After washing, a drop of sperm suspension was placed on a clean glass slide and observed under fluorescent microscope (Imager A1, Carl Zeiss, Germany) to assess mitochondrial integrity. Spermatozoa exhibiting bright fluorescence only at the mid piece region were considered to have functional mitochondria. From each sample a minimum of 500 spermatozoa were scored to determine functional or non-functional mitochondria.
Chlortetracycline (CTC) assay
The ability to undergo capacitation was assessed in frozen-thawed spermatozoa by CTC assay as described by Colas et al. [22] with minor modifications. The frozen-thawed semen samples were washed, pellet was resuspended in EBSS medium supplemented with HSA and incubated at 37 °C and 5 % CO2 for 1 h. The experiment was initiated by incubating 18 μl of sperm suspension with 2 μl of Ethidium Bromide (23.3 μM) and 20 μl of 750 μM CTC solution (Sigma Aldrich, USA, Cat. No. C4881) at 37 °C in the dark. After 15 s, the reaction was stopped by adding 5 μl of glutaraldehyde solution (12.2 %) and capacitation pattern was evaluated using fluorescent microscope (Imager A1, Carl Zeiss, Germany). At least 200 spermatozoa were scored from each sample and classified as non-capacitated, capacitated, and dead spermatozoa.
Acrosome reaction
The ability to undergo acrosome reaction was assessed in frozen-thawed spermatozoa by triple stain technique as described by Garde et al. [23] with minor modifications. Briefly, the washed pellet was resuspended with EBSS and the cell density was adjusted to 5–6 millions/ml. To 0.5 ml of sperm suspension, 0.5 mM of calcium ionophore A23187 (Sigma Aldrich, USA, Cat. No. C7522) was added and incubated at 37 °C and 5 % CO2. After 1 h, the suspension was mixed with equal volume of trypan blue (0.5 %) and incubated further at 37 °C for 15 min. The sperm suspension was washed with phosphate buffered saline (PBS) and pellet was fixed in 3 % paraformaldehyde (PFA) at 4 °C for 30 min followed by washing with distilled water. A thin smear was prepared on a clean glass slide and air dried at room temperature. The cells were stained with 2 % Bismark brown (SD Fine-Chem. Ltd. India, Cat. No. 440-10-0925) for 3 min at 37 °C followed by washing with Milli Q and later stained with 0.8 % Rose Bengal (Sisco Research Laboratory, India, Cat. No. 184033) for 5 min at 37 °C. The slides were serially dehydrated using graded alcohol, washed twice in xylene and mounted in DPX mounting agent. The slides were observed under light microscope and acrosome pattern was classified into acrosome intact live sperm, acrosome intact dead sperm, acrosome reacted live sperm and acrosome reacted dead sperm. Total of 200 spermatozoa were counted for each sample and results were expressed in percentage.
Statistical analysis
Data are presented as Mean±SE. The DNA damage, mitochondrial function and motility data were analyzed by one way ANOVA (Analysis of number of variance) using Bonferroni post-test method. Capacitation and acrosome reaction data were analyzed by unpaired Student’s t test. The statistical evaluation was performed using GraphPadInstat 3.0 software (San Deigo, CA, USA). Statistical significance was set at 0.05.
Results
The study included 109 infertile men (mean age 35.23±0.57 years) among which 58 were having normal semen profile and 51 were with abnormal semen profile. The abnormal group included 27 teratozoospermic, 3 oligozoospermic, 1 asthenozoospermic, 14 oligoteratozoospermic, 3 asthenoteratozoospermic and 3 oligoasthenoteratozoospermic subjects. The seminal characteristics are given in Table 2.
Table 2.
The semen profile of study subjects (n=109*)
| Parameters | Mean±SD |
|---|---|
| Age (years) | 34.69±05.29 |
| Volume (ml) | 03.46±01.59 |
| Sperm count (millions/ml) | 38.29±30.01 |
| Total motility (%) | 61.36±12.76 |
| Progressive motility (%) | 44.30±12.50 |
| Rapid progressive motility (%) | 06.80±05.27 |
| Morphology (%) | 27.30±08.12 |
| Viability (%) | 63.55±13.96 |
*Normozoospermic subjects- 58; subjects with abnormal semen parameters 51; The abnormal group included oligozoospermic (n=3), asthenozoospermic (n=1), teratozoospermic (n=27), oligoteratozoospermic (n=14), asthenoteratozoospermic (n=3) and oligoasthenoteratozoospermic subjects (n=3)
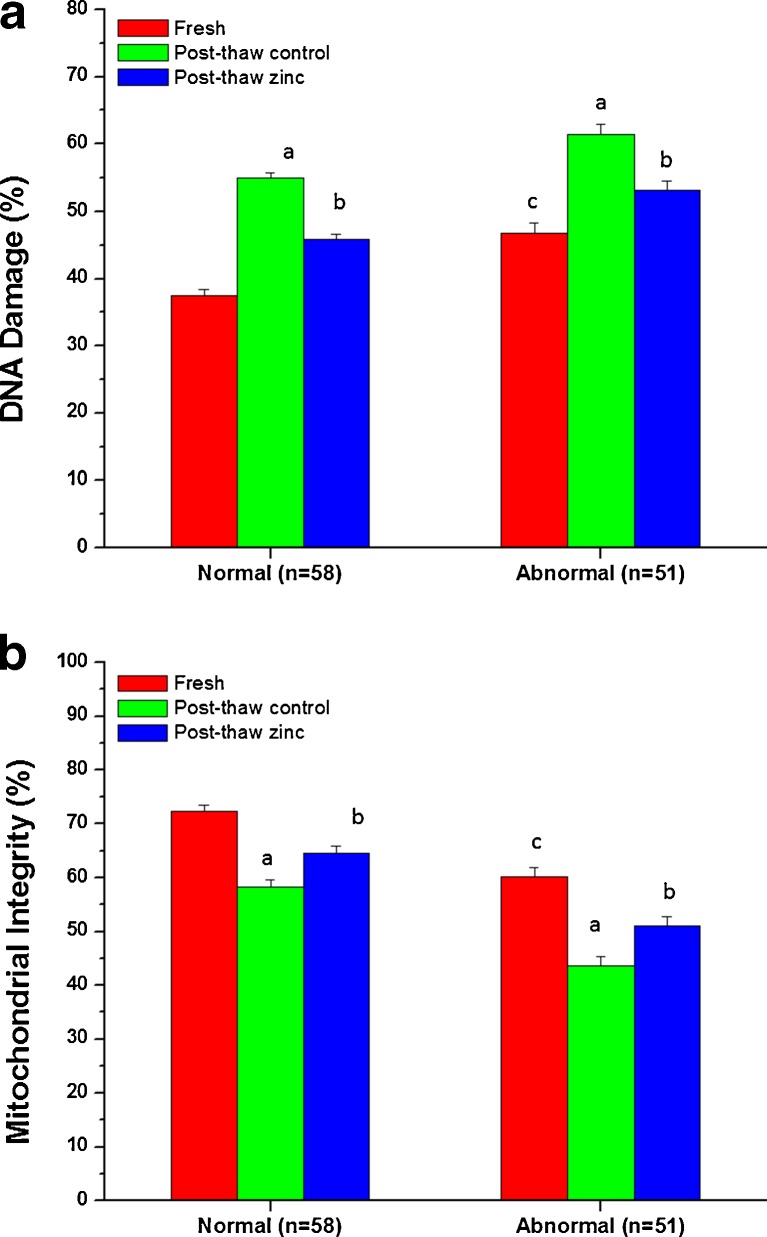
The fresh ejaculates of the abnormal group had significantly higher (p<0.001) percentage of spermatozoa with DNA damage compared to normozoospermic subjects (Fig. 1a). Freeze-thaw process resulted in a significant increase (p<0.001) in DNA damage in normozoospermic as well as abnormal group. Addition of zinc prior to cryopreservation significantly reduced the DNA damage compared to the corresponding control groups (p<0.001), irrespective of the ejaculate quality.
Fig. 1.
a. DNA damage in post-thaw semen samples cryopreserved after addition of zinc (100 μM) to semen samples. a:p<0.001 compared to fresh ejaculate of respective group; b:p<0.001 compared to post-thaw control of respective group; c:p<0.001 compared to fresh ejaculate of normozoospermic subjects; b: Mitochondrial function in post-thaw semen samples cryopreserved after addition of zinc (100 μM) to semen samples. a:p<0.001 compared to fresh ejaculate of respective group; b:p<0.05 compared to post-thaw control of respective group group; c:p<0.001 compared to fresh ejaculate of normozoospermic subjects;
The mitochondrial function was significantly higher (p<0.001) in fresh ejaculates of normozoospermic semen samples in comparison to the abnormal group (Fig. 1b). Loss of mitochondrial potential was observed in spermatozoa of both normozoospermic and abnormal semen samples subjected to freeze-thaw process (p<0.001). Zinc supplementation prior to cryopreservation was found to reduce the mitochondrial damage in both normozoospermic and abnormal semen samples (p<0.05).
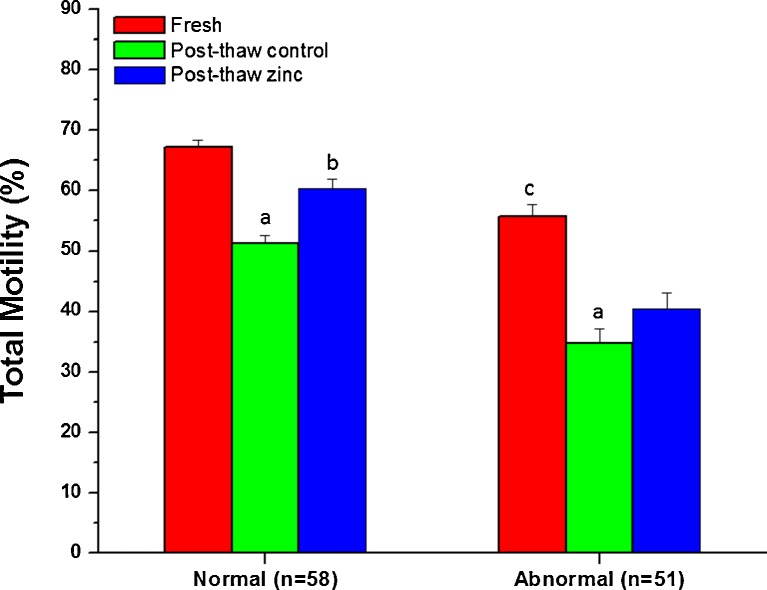
The percentage of total motile spermatozoa in fresh ejaculates of normozoospermic and abnormal group was 67.17±1.21 and 55.80±1.98 respectively (Fig. 2), and the difference was statistically significant (p<0.001). Freeze-thaw process resulted in a significant decline in total motility in both groups. Results indicate that addition of zinc prior to cryopreservation prevents the loss of motility in both normozoospermic and abnormal groups, although the protective effect was statistically significant only in normozoospermic group (p<0.05).
Fig. 2.
Total motility in frozen-thawed spermatozoa crypreserved with or without zinc supplementation to the sample prior to cryopreservation. a:p<0.001 compared to fresh ejaculate of respective group; b:p<0.01 compared to post-thaw control of normozoospermic group; c:p<0.001 compared to fresh ejaculate of normozoospermic subjects;
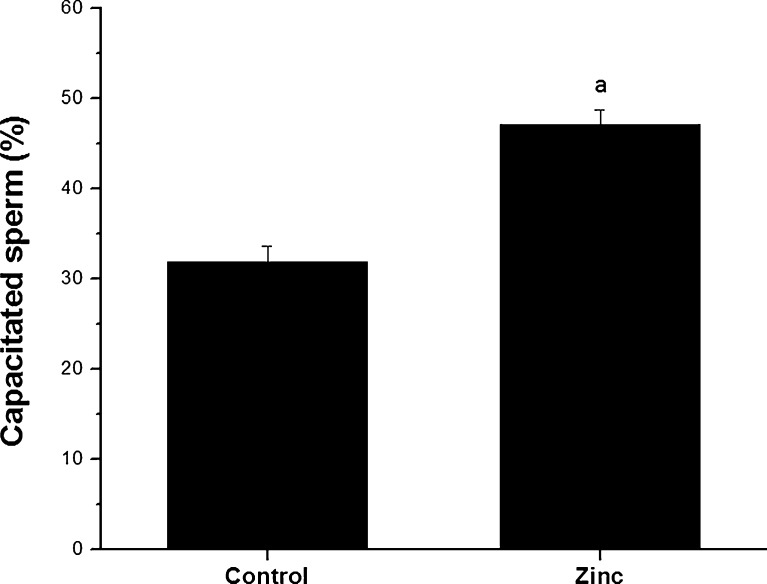
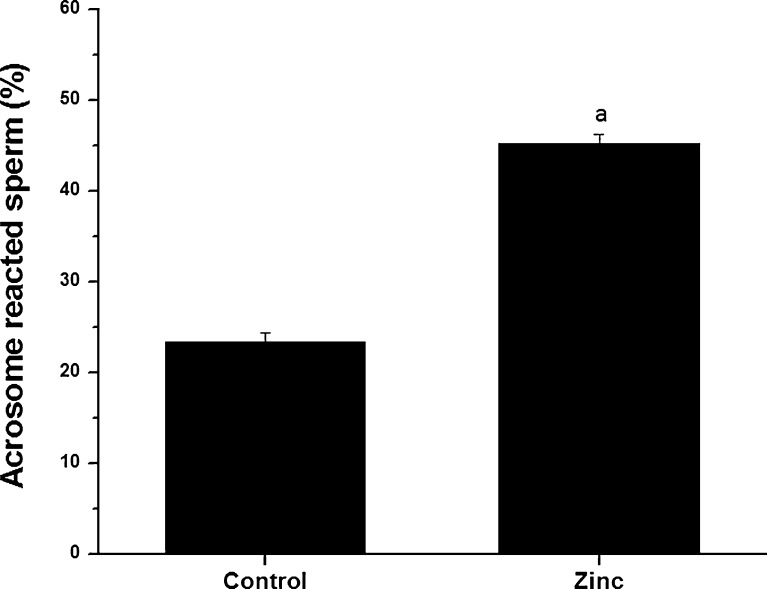
Sperm fertilizing ability was studied in normozoospermic semen samples (n=25) by assessing their potential to undergo capacitation (Fig. 3) and acrosome reaction (Fig. 4). At the end of 1 h incubation, ~32 % of spermatozoa were capacitated in control group, which was significantly higher (p<0.0001) in the zinc supplemented group (~47 %). Similarly, zinc supplemented group had almost 2 fold higher percentage of acrosome reacted spermatozoa compared to control group (45.20±0.98 and 23.32±1.03 respectively, p<0.001).
Fig. 3.
Capacitation in frozen-thawed spermatozoa of normozoospermic semen samples cryopreserved with or without addition of zinc to semen sample. a:p<0.0001 compared to control
Fig. 4.
Acrosome reaction after ionophore challenge in frozen-thawed spermatozoa of normozoospermic semen samples cryopreserved with or without addition of zinc to semen sample. a:p<0.0001 compared to the post-thaw control group
Discussion
Cryopreservation induces chromatin instability, DNA strand breaks and apoptosis in spermatozoa [4, 5, 24, 25] and thereby reduces their functional competence. Poorly organized chromatin of spermatozoa from infertile men [26] increases their susceptibility to freeze-thaw-induced DNA damage compared to fertile men [27], which poses further challenge in semen cryopreservation technology. Therefore, the recent research focus is mainly on the methods to prevent the freeze-thaw-induced DNA damage, especially in poor quality ejaculates. Previous studies have concentrated on improving the post-thaw sperm quality by freezing the semen samples with free radical scavengers such as vitamin C and Vitamin E [12, 13]. In the present study we hypothesized that increasing the chromatin stability of sperm by adding zinc to the ejaculate prior to cryopreservation can prevent the DNA damage.
Fresh ejaculates of the normozoospermic subjects had ~37 % DNA damaged spermatozoa which was significantly lower than that of abnormal semen group (~47 %). Earlier reports suggest that infertile men, especially with abnormal semen profile carry higher incidence of DNA damage in comparison to the fertile donors [4, 5, 28]. Significant increase in DNA damage (p<0.001) observed in post-thaw spermatozoa of normozoospermic and abnormal group is in agreement with earlier reports [4, 13, 19].
The result of present study indicates that adding zinc to the ejaculates prior to freezing can prevent freeze-thaw-induced DNA damage. Addition of zinc may help in stabilizing the organization of nuclear proteins and chromatin of spermatozoa [16, 17]. It is postulated that the human sperm chromatin contains a zinc ion for each protamine molecule per turn of the DNA [29] and, helps in stabilizing the chromatin by forming salt bridges with protamine thiols and with imidazole groups of histidine. Such a salt bridge is considered as strong as a covalent disulfide bridge and can serve as a reversible and temporary stabilizer of the sperm chromatin [30]. An earlier study by Kvist et al. [31] demonstrated that zinc prevents the human sperm chromatin from undergoing decondensation during its storage at 22 °C for 24 h.
Since motility is the most seriously affected parameter in spermatozoa after freeze-thaw process it is usually considered as a reliable parameter to assess the efficiency of cryopreservation protocol. In the present investigation we observed that the post-thaw motility declines by ~20 % compared to the fresh ejaculate (p<0.001). Even though the exact mechanism by which motility drops is not yet elucidated, the most probable reason appears to be the loss of mitochondrial activity [3]. Concurrent loss of mitochondrial function and reduction in progressive motility (~15 % and 22 % respectively) of frozen-thawed spermatozoa observed in the present study further supports this. The role of zinc on sperm motility has contradictory reports in the literature. Majority of the studies on human sperm suggest that zinc has inhibitory effect on motility [32, 33]. In contrary to these reports, few studies have observed that zinc enhances sperm motility [34, 35]. However, no significant change in the motility pattern was observed in the present study after supplementing zinc to the semen sample (data not shown).
The significantly higher percentage of post-thaw motility in semen samples cryopreserved after addition of zinc could be due to the stabilizing effect of zinc on the microfilaments in the outer dense fibers [36]. In addition, the protective effect of zinc on mitochondrial potential of spermatozoa observed in this study may help in providing the ATP required for progressive motility. Mitochondria are rich in zinc-dependent SOD that may help in preventing the free radical induced membrane damage, loss of mitochondrial potential and subsequently the loss of motility.
Spermatozoa subjected to freeze-thaw process are known to have reduced functional abilities. In the present study, we have observed a significantly higher percentage of capacitated and acrosome reacted sperm in normozoospermic semen samples (n=25) stored after addition of zinc. This could be due to the higher percentage of sperm surviving the freeze-thaw process which is evident from the data on motility and mitochondrial potential. In addition, zinc may help in preventing the freeze-thaw-induced destabilization of the plasma membrane [37, 38] of spermatozoa and thus help in retaining the acrosomal contents.
There are reports in the literature indicating that presence of zinc in culture medium can inhibit capacitation and acrosome reaction [33, 39–41]. On the contrary, the present study has demonstrated that the survival and ability to undergo capacitation and acrosome reaction is significantly increased in semen samples cryopreserved with zinc. It is possible that the zinc added to semen samples are lost from the frozen-thawed spermatozoa during washing process and hence may not have any negative influence on sperm function. However, we have studied these changes only in normozoospermic semen samples. Since the protective effect of zinc on motility, mitochondrial function and DNA damage is similar in both normal and abnormal group, a similar effect could be expected on the fertilizing ability of spermatozoa from abnormal semen samples. In conclusion, our data clearly suggests that adding zinc to semen samples prior to freeze-thaw process can significantly protect the spermatozoa from freeze-thaw-induced DNA damage and maintain the sperm functional characteristics.
Acknowledgments
Authors acknowledge financial assistance from Kasturba Medical College, Manipal, India, in the form of Postgraduate thesis grant sanctioned to APK (Grant No. PGR042). Technical support from Ms. Jayalaxmi Pai and Ms. Kirthi Patil (Clinical Embryology, Kasturba Medical College, Manipal) and Ms. Gimna Rathesh (Division of Nutrition, St. John’s Research Institute, St. John’s National Academy of Health Sciences, Bangalore) is thankfully acknowledged. Authors thank Dr. Prashanth Naik, Mangalore University for critical editing of this manuscript.
Footnotes
Capsule
Addition of zinc to liquefied ejaculate prior to cryopreservation using glycerol- egg yolk- citrate buffered medium protects spermatozoa from freeze-thaw-induced DNA damage and loss of sperm function.
Author contribution
APK, SK, KG and SRS performed the study (APK and SK had equal contribution)
GK designed the study and wrote the paper
PT, SKA and PK helped in analyzing the data and writing the paper
References
- 1.Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD. Cryopreservation of human spermatozoa. The effect of cryoprotectants on motility. Fertil Steril. 1988;50:314–320. [PubMed] [Google Scholar]
- 2.Zribi N, Chakroun NF, EI Euch H, Gargouri J, Bahloul A, Keskes LA. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril. 2010;93:159–166. doi: 10.1016/j.fertnstert.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell M, McClure N, Lewis SE. The effect of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod. 2002;17:704–709. doi: 10.1093/humrep/17.3.704. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly ET, McClure N, Lewis SEM. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril. 2001;76:892–900. doi: 10.1016/S0015-0282(01)02834-5. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly ET, Steele KE, McClure N, Lewis SEM. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum Reprod. 2001;16:1191–1199. doi: 10.1093/humrep/16.6.1191. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida H, Hoshiai H, Fukaya T, Yajima A. Fertilization of fresh and frozen spermatozoa. Assist Reprod Technol Androl. 1990;1:164–172. [Google Scholar]
- 7.Bakos HW, Thompson JG, Feil D, Lane M. Sperm DNA damage is associated with assisted reproductive technology pregnancy. Int J Androl. 2008;31:518–526. doi: 10.1111/j.1365-2605.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 8.Thomson LK, Fleming SD, Aitken RJ, Iuliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009;24:2061–2070. doi: 10.1093/humrep/dep214. [DOI] [PubMed] [Google Scholar]
- 9.Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod. 1998;13:896–900. doi: 10.1093/humrep/13.4.896. [DOI] [PubMed] [Google Scholar]
- 10.Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of the human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13:1429–1436. doi: 10.1093/humrep/13.6.1429. [DOI] [PubMed] [Google Scholar]
- 11.Duru NK, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000;74:1200–1207. doi: 10.1016/S0015-0282(00)01591-0. [DOI] [PubMed] [Google Scholar]
- 12.Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology. 2010;60:235–237. doi: 10.1016/j.cryobiol.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Kalthur G, Raj S, Thiyagarajan A, Kumar S, Kumar P, Adiga SK. Vitamin E supplementation in semen-freezing medium improves the motility and protects sperm from freeze-thaw-induced DNA damage. Fertil Steril. 2011;95:1149–1151. doi: 10.1016/j.fertnstert.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Kaji M. Zinc in endocrinology. Pediatr Int. 2001;16:1–7. [Google Scholar]
- 15.Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielthuis GA, Steegers-Theunissen RP. Effect of folic acid and zinc sulphate on male factor sub fertility, a double blind, randomized placed controlled trial. Fertil Steril. 2002;77:491–498. doi: 10.1016/S0015-0282(01)03229-0. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T, Nakajima K, Yamamoto A, Yamanaka H. Metallothionein binding zinc inhibits nuclear chromatin decondensation of human spermatozoa. Andrologia. 1995;27:161–164. doi: 10.1111/j.1439-0272.1995.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 17.Bjorndahl L, Kvist U. A model for the importance of zinc in the dynamics of human sperm chromatin stabilization after ejaculation in relation to sperm DNA vulnerability. Syst Biol Reprod Med. 2011;57:86–92. doi: 10.3109/19396368.2010.516306. [DOI] [PubMed] [Google Scholar]
- 18.Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 19.Kalthur G, Adiga SK, Upadhya D, Rao S, Kumar P. Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil Steril. 2008;89:1723–1727. doi: 10.1016/j.fertnstert.2007.06.087. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 21.Johnson LV, Walsh ML, Chen LB. Localization of mitochondria in living cells by rhodamine 123. Proc Natl Acad Sci USA. 1980;77:990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colas C, Grasa P, Casao A, Gallego M, Abecia JA, Forcada F. Changes in calmodulin immunocytochemical localization associated with capacitation and acrosomal exocytosis of ram spermatozoa. Theriogenology. 2009;71:789–800. doi: 10.1016/j.theriogenology.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Garde JJ, Ortiz N, García A, Gallego L. Use of a triple-stain technique to detect viability and acrosome reaction in deer spermatozoa. Arch Androl. 1997;39:1–9. doi: 10.3109/01485019708987895. [DOI] [PubMed] [Google Scholar]
- 24.Chohan KR, Griffin JT, Carrell DT. Evaluation of chromatin integrity in human sperm using acridine orange staining with different fixatives and after cryopreservation. Andrologia. 2004;36:321–326. doi: 10.1111/j.1439-0272.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 25.Adiga SK, Khan Z, Upadhya D, Kalthur G, Kumar P. Ability of deoxyribonucleic acid-damaged sperm to withstand freeze-thaw-induced damage during cryopreservation. Fertil Steril. 2009;92:959–963. doi: 10.1016/j.fertnstert.2008.07.1754. [DOI] [PubMed] [Google Scholar]
- 26.Erenpreiss J, Elzanaty S, Giwercman A. Sperm DNA damage in men from infertile couples. Asian J Androl. 2008;10:786–790. doi: 10.1111/j.1745-7262.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- 27.Hammadeh ME, Askari AS, Georg T, Rosenbaum P, Schmidt W. Effect of freeze–thawing procedure on chromatin stability, morphological alteration and membrane integrity of human spermatozoa in fertile and subfertile men. Int J Androl. 1999;22:155–162. doi: 10.1046/j.1365-2605.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- 28.Varghese AC, Bragais FM, Mukhopadhyay D, Kundu S, Pal M, Bhattacharyya AK. Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia. 2009;41:207–215. doi: 10.1111/j.1439-0272.2009.00917.x. [DOI] [PubMed] [Google Scholar]
- 29.Berg JM. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990;265:6513–6516. [PubMed] [Google Scholar]
- 30.Balhorn R. The protamine family of sperm nuclear proteins. Genome Biol. 2007;8:227. doi: 10.1186/gb-2007-8-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kvist U, Bjorndahl L. Zinc preserves an inherent capacity for human sperm chromatin decondensation. Acta Physiol Scand. 1985;24:195–200. doi: 10.1111/j.1748-1716.1985.tb07652.x. [DOI] [PubMed] [Google Scholar]
- 32.Danscher G, Hammen R, Fjerdingstad E, Rebbe H. Zinc content of human ejaculate and the motility of sperm cells. Int J Androl 1978;1:1576–81.
- 33.Riffo M, Leiva S, Astudillo J. Effect of zinc on human sperm motility and the acrosome reaction. Int J Androl. 1992;15:229–237. doi: 10.1111/j.1365-2605.1992.tb01343.x. [DOI] [PubMed] [Google Scholar]
- 34.Stankovic H, Mikac-Devic D. Zinc and copper in human semen. Clin Chem Acta. 1976;70:123–126. doi: 10.1016/0009-8981(76)90013-9. [DOI] [PubMed] [Google Scholar]
- 35.Caldamone AA, Freytag MK, Cockett AT. Seminal zinc and male infertility. Urology. 1979;13:280–281. doi: 10.1016/0090-4295(79)90421-7. [DOI] [PubMed] [Google Scholar]
- 36.Calvin HI, Hwang FH-F, Wohlrab H. Localisation of zinc in a dense fiber-connecting piece fraction of rat sperm tails analogous chemically to hair keratin. Biol Reprod. 1975;13:228–239. doi: 10.1095/biolreprod13.2.228. [DOI] [PubMed] [Google Scholar]
- 37.Bettger WJ, O’Dell BL. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981;28:1425–1438. doi: 10.1016/0024-3205(81)90374-X. [DOI] [PubMed] [Google Scholar]
- 38.Kendall NR, McMullen S, Green A, Rodway RG. The effect of a zinc, cobalt and selenium soluble glass bolus on trace element status and semen quality of ram lambs. Anim Reprod Sci. 2000;62:277–283. doi: 10.1016/S0378-4320(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 39.Andrews JC, Nolan JP, Hammerstedt RH, Bavister BD. Role of zinc during hamster sperm capacitation. Biol Reprod. 1994;51:1238–1247. doi: 10.1095/biolreprod51.6.1238. [DOI] [PubMed] [Google Scholar]
- 40.Bilaspuri GS, Babbar BK. Effect of albumin and zinc on capacitation and acrosome reaction of buffalo spermatozoa. Indian J Anim Sci. 2007;77:688–692. [Google Scholar]
- 41.Liu DY, Sie BS, Liu ML, Agresta F, Baker HW. Relationship between seminal plasma zinc concentration and spermatozoa–zona pellucida binding and the ZP-induced acrosome reaction in sub fertile men. Asian J Androl. 2009;11:499–507. doi: 10.1038/aja.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]