Abstract
Propranolol is a non-selective beta-adrenergic antagonist successfully used in a case of kaposiform hemangioendothelioma associated with Kasabach-Merritt phenomenon. We report eleven patients treated with propranolol for kaposiform hemangioendothelioma and the related variant tufted angioma, six of whom also had Kasabach-Merritt phenomenon. The varied responses to treatment, with only 36% responding in our series, demonstrate the need for further study of this medication before routine use for these indications.
Keywords: propranolol, kaposiform hemangioendothlioma, tufted angioma, vascular tumor, Kasabach-Merritt, treatment
Introduction
Kaposiform hemangioendothelioma (KHE) and tufted angioma (TA) are rare vascular tumors of infancy and early childhood. They were first reported independently but now thought to be related entities on the same spectrum of disease (1-3). They often present in infancy and early childhood as solitary, firm, blue-red soft tissue tumors of the head, neck, trunk or extremities, or in the retroperitonium (1, 3-5). Histologically, the tumors are composed of infiltrative nodules of convoluted vessels with surrounding lymphatic vessels.
KHE and TA account for the majority of vascular neoplasms associated with Kasabach-Merritt Phenomenon (KMP), a consumptive coagulopathy characterized by severe thrombocytopenia and hypofibrinogenemia (6, 7). The mortality rate from hemorrhagic complications may be as high as 30% (6, 7).
Enlarging tumors and those associated with KMP often require aggressive treatment. Given the rarity of KHE and TA, controlled trials comparing treatment modalities have not yet been performed. Evaluation of treatment response is difficult as some tumors have the potential to spontaneously regress and objective response criteria have not been developed. Without guidance from prospective trials, management decisions are based on anecdotal opinion and isolated case reports and series.
One publication reports the success of propranolol when used in a single case of KHE with KMP (8). In contrast, response rates from this series are not favorable, and clinicians should proceed cautiously before treating these life-threatening conditions with propranolol.
Case Reports
Patient 1
A healthy full-term male was noted to have a right face mass at birth. The tumor progressed slowly until 5 months of age, when rapid growth resulted in involvement of most of the right head and neck. The patient was initially diagnosed with infantile hemangioma (IH) and treatment with propranolol 1 mg/kg/day was initiated. Despite 4 weeks of therapy, the tumor continued to grow. A platelet count showed severe thrombocytopenia of 19,000/μL, and biopsy confirmed the diagnosis of KHE. Therapy with propranolol was discontinued. After treatment with embolization, prednisolone 2 mg/kg/day, and vincristine 0.05 mg/kg/week, the KHE decreased significantly in size and the KMP resolved without evidence of relapse 12 months later.
Patient 2
A healthy full-term male was noted to have asymptomatic right leg swelling at birth. At 5 weeks of age, he developed progressive tumor growth, warmth, pain, and inability to extend the right leg. Laboratory values showed KMP with a platelet count of 45,000/μL. He had good response to prednisolone 2 mg/kg/day, which was gradually decreased and eventually discontinued 8 months later. Within 2 weeks, however, there was clinical worsening and prednisolone 2 mg/kg/day was restarted. Propranolol 2 mg/kg/day was added, allowing prednisolone to be discontinued over the next 2 months. The patient remained on propranolol monotherapy for the next 6 months with continued response. An attempt to discontinue the propranolol at that time resulted in rapid regrowth and leg pain within 2 weeks, and both propranolol 2 mg/kg/day and prednisolone 2 mg/kg/day were restarted. The prednisolone was weaned off over 7 months, then propranolol was subsequently tapered and eventually stopped 10 months later. In total, the patient was on propranolol monotherapy for 16 months with complete response to therapy. The patient remains without clinical evidence of tumor 9 months after completion of all therapy.
Patients 3-11
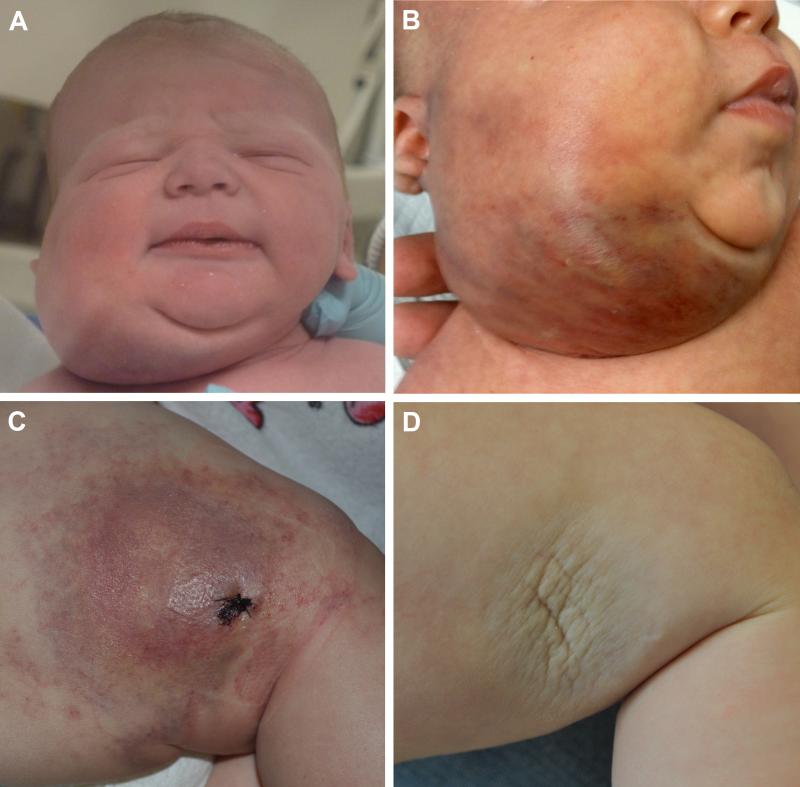
Propranolol was used in 9 additional cases (Table I). In patient 3, there was no response to propranolol, although efficacy is difficult to assess as the patient had already failed multiple other therapies. Patient 4 had partial response to steroids and vincristine but relapse with taper, and adding propranolol did not give benefit. In patient 5, propranolol and aspirin were started in combination, but the patient relapsed quickly after the aspirin was discontinued and subsequently responded to aspirin alone, suggesting that the propranolol itself was not effective. Patient 6 was treated with propranolol for only 3 days before KMP developed, prompting a switch to prednisolone and vincristine. Patients 7 (Figures 1A and B) and 8 worsened on first-line propranolol monotherapy, but both subsequently improved with prednisolone and vincristine. In contrast, propranolol was used as first-line monotherapy in patient 9 with complete response (Figures 1C and D). Additionally, patients 10 and 11 had partial response to propranolol monotherapy for 4 and 9 months, respectively.
Table I.
Summary of patient cases.
| Age at initiation of propranolol | Diagnosis | Location | Approximate size | KMP at initiation of propranolol | Previously attempted treatments | Highest dose of propranolol | Duration of propranolol treatment | Concurrent treatments with propranolol | Response to propranolol | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 5 months | KHE with KMP | Right scalp, right face, and right neck | N/A | Unknown | None | 1 mg/kg/day | 4 weeks | None | No response. Propranolol discontinued when KMP was diagnosed. |
| Patient 2 | 12 months | KHE with KMP (no biopsy) | Right inferior thigh, right knee, and right superior tibia | N/A | Present | Prednisolone for 10 months (good response until tapered off) | 2 mg/kg/day | 25 months | Prednisolone (variable doses up to 2 mg/kg/day) for 9 of the months | Complete response† to combination then alone. Off all therapy for 9 months without relapse. |
| Patient 3 | 4 years | KHE with KMP | Left scalp, left face, left neck, and bilateral chest | 8 × 11 cm | Present | Multiple courses of vincristine ± cyclophosphamide and actinomycin D for 3.5 years. Prednisolone for 2 years. Interferon-α2b for 2 months. Radiation therapy (1000 centiGray total) for 5 days. Thalidomide, celecoxib, etoposide, and cyclophosphamide regimen for 2 months. |
1.3 mg/kg/day | 8 weeks | Prednisolone up to 2 mg/kg/day for entire course Vincristine 0.05 mg/kg/week for first 2 weeks |
No response. Partial response subsequently to repeated vincristine 0.05 mg/kg/week for 4 weeks. |
| Patient 4 | 13 months | KHE with KMP | Retroperitonium | 5 × 8 cm | Present | Vincristine for 6 months. IV methylprednisolone and prednisolone for 10 months (good response but recurrent milder KMP when tapered to low doses) |
2 mg/kg/day | 3.5 months | Prednisolone <1.5 mg/kg/day for first 6 weeks. Vincristine 0.025-0.05 mg/kg q1-2 weeks for entire course. |
No response. KHE slightly larger, but no KMP recurrence. Partial response subsequently to sirolimus 0.8 mg/m2/dose twice daily with smaller tumor. Off all therapy for 7 months without worsening, |
| Patient 5 | 21 months | TA | Right thigh | 6 × 8 cm | No | None | 2 mg/kg/day | 4 weeks | Aspirin 5 mg/kg/day | No response. Relapsed within 2 days when aspirin discontinued. Aspirin restarted and propranolol discontinued with partial response to aspirin alone. |
| Patient 6 | 8 months | KHE with KMP | Left shoulder, left upper arm, and left elbow | 8 × 12 cm | No | None | 0.3 mg/kg/day | 3 days | None | No response. Discontinued after only 3 days due to KMP. Very good response subsequently to prednisolone 2 mg/kg/day and vincristine 0.05 mg/kg/week. |
| Patient 7 | 17 days | KHE with KMP (no biopsy) | Right face and neck | 6 × 6 cm | Present | None | 2 mg/kg/day | 19 days | Prednisolone 2 mg/kg/day for last 8 days | No response. Worsened on treatment. Very good response subsequently to prednisolone 2 mg/kg/day and vincristine 0.05 mg/kg/week. |
| Patient 8 | 9 days | KHE with KMP (no biopsy) | Left chest | 9 × 9 cm | Present | None | 2 mg/kg/day | 19 days | None | No response. Worsened on treatment. Very good response subsequently to prednisolone 2 mg/kg/day and vincristine 0.05 mg/kg/week. |
| Patient 9 | 2 days | TA with KMP | Right thigh | 5 × 6 cm | Present | None | 3 mg/kg/day | 11 months | None | Complete response.† Treated for 3 months, with relapse after discontinuation. Resolution of TA and KMP with second course of propranolol for 8 months. Off all therapy for 6 months without relapse. |
| Patient 10 | 3 years | KHE | Right scalp, right face, and right neck | N/A | No | Prednisolone for 2 months Aminocaproic acid for 13 months |
2 mg/kg/day | 4 months | None | Partial response.‡ KHE stable in size and KMP remained quiescent. |
| Patient 11 | 26 months | KHE | Right buttock | 5 × 8 cm | No | None | 2 mg/kg/day | 9 months | None | Partial response.‡ Softening of lesion and symptom improvement. |
Complete response defined as complete resolution of tumor, and in cases with KMP, normalization of hematologic parameters.
Partial response defined as stabilization of tumor growth, partial regression of tumor, improvement of KMP, and/or improvement of symptoms.
IV: intravenous; N/A: not available
Figure 1.
(A) Patient 7 at birth. He was misdiagnosed as an IH and started on propranolol 2 mg/kg/day at 17 days. (B) KHE after 11 days of propranolol monotherapy. His KHE and KMP worsened during the 19 days that he was on propranolol, despite the addition of prednisolone 2 mg/kg/day. (C) Patient 9 at 4 months of age. She was also misdiagnosed as an IH at birth and started on propranolol with stabilization of growth and thrombocytopenia. The propranolol was discontinued at 3 months of age as the tumor was thought to be quiescent. Within 1 month, she presented with an enlarging tumor and platelet count of 5,000/μL and was diagnosed with TA and KMP. (D) TA at 8 months of age, 4 months into second course of propranolol. Treatment with propranolol rapidly decreased tumor size within 24 hours of achieving 3 mg/kg/day and normalization of platelet count within 2 weeks. She was treated for an additional 8 months with complete resolution of her TA and KMP.
Discussion
Treatment of KHE and TA is in part limited by knowledge gaps due to their rare nature and lack of prospective trials. Complete surgical excision is the treatment of choice, although many tumors are unresectable due to location, size, or tissue infiltration (2). A variety of pharmacologic treatments have been reported in the literature including ticlopidine plus aspirin, heparin, low molecular weight heparin, corticosteroids, interferon-α, vincristine, actinomycin-D, cyclophosphamide, and sirolimus (4). While vincristine has been suggested as the first-line agent of choice with response rates of 86-100%, complete resolution is rare, relapse rates are 14-27%, and its use is limited by neurotoxicity and other side effects (9, 10). In difficult cases, multidrug or multimodal regimens are often required.
A case report describes the use of propranolol in the treatment of a 6-week-old male with KHE and KMP (8). The infant was treated with propranolol 2 mg/kg/day and vincristine 1.0 mg/m2/dose for 4 weeks. There was dramatic clinical improvement, and oral propranolol was continued alone for an additional 13 months. This report suggests that propranolol holds promise as a potential treatment option for KHE. In contrast, another case report highlights a patient with KHE and KMP who did not respond to numerous therapies, including propranolol (11).
Our results indicate that therapy with propranolol was ineffective in nearly two-thirds of patients. In our series of 11 patients, only 4 patients responded (patients 2, 9, 10, 11) and 7 others did not. In contrast to treatment of IH, the improvement was slow and gradual in 3 (patients 2, 10, 11) of the 4 patients where propranolol was efficacious; the 4th patient (patient 9) partially improved on lower doses of propranolol but had a dramatic response to 3 mg/kg/day, suggesting that higher doses may be necessary (12). In 4 (patients 1, 6, 7, 8) of the 6 patients who received it as first-line monotherapy, propranolol did not result in response even as late as 4 weeks. In comparison to monotherapy, propranolol did not appear to be more effective in those cases where it was used in combination with other agents. There were no distinguishing clinical features between those cases that responded and those that did not.
A controlled prospective trial with objective response criteria is necessary to determine the best therapies for KHE and TA with and without KMP, although accumulating sufficient patients for such a trial would be extremely difficult. In the interim, our knowledge about treating these tumors continues to rely on retrospective series and reviews. Propranolol is currently used for the treatment of vascular tumors without complete understanding of its mechanism of action. Of the 13 reported patients with KHE and TA treated with propranolol, including our series of 11, only 5 patients (38%) have responded to the medication. Given the lack of efficacy in the majority of patients and the availability of other medications with a better response rate, more experience with propranolol in the setting of KHE and TA is necessary before use of this medication as a first-line agent.
Acknowledgements
The authors would like to thank Drs. Susan Lindemulder, Sanjay Mahant, and Nameeta Richard for the excellent clinical care that they provided to these patients.
Dr. Tom is funded by a Research Career Development Award, K23AR060274, from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (NIAMS/NIH).
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
References
- 1.Jones EW, Orkin M. Tufted angioma (angioblastoma). A benign progressive angioma, not to be confused with kaposi's sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20:214–225. [PubMed] [Google Scholar]
- 2.Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. An aggressive neoplasm associated with kasabach-merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321–328. doi: 10.1097/00000478-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: A study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559–568. doi: 10.1097/00000478-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez Y, Bernabeu-Wittel M, Garcia-Morillo JS. Kaposiform hemangioendothelioma. Eur J Intern Med. 2009;20:106–113. doi: 10.1016/j.ejim.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: Variability of the clinical morphology. Pediatr Dermatol. 2002;19:394–401. doi: 10.1046/j.1525-1470.2002.00113.x. [DOI] [PubMed] [Google Scholar]
- 6.Enjolras O, Wassef M, Mazoyer E, et al. Infants with kasabach-merritt syndrome do not have “true” hemangiomas. J Pediatr. 1997;130:631–640. doi: 10.1016/s0022-3476(97)70249-x. [DOI] [PubMed] [Google Scholar]
- 7.Kelly M. Kasabach-merritt phenomenon. Pediatr Clin North Am. 2010;57:1085–1089. doi: 10.1016/j.pcl.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with kasabach-merritt syndrome: A new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:e171–173. doi: 10.1097/MPH.0b013e3182152e4e. [DOI] [PubMed] [Google Scholar]
- 9.Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-merritt phenomenon: A retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459–462. doi: 10.1097/00043426-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Fahrtash F, McCahon E, Arbuckle S. Successful treatment of kaposiform hemangioendothelioma and tufted angioma with vincristine. J Pediatr Hematol Oncol. 2010;32:506–510. doi: 10.1097/MPH.0b013e3181e001a9. [DOI] [PubMed] [Google Scholar]
- 11.Blatt J, Stavas J, Moats-Staats B, et al. Treatment of childhood kaposiform hemangioendothelioma with sirolimus. Pediatr Blood Cancer. 2010;55:1396–1398. doi: 10.1002/pbc.22766. [DOI] [PubMed] [Google Scholar]
- 12.Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]