Abstract
Background
Prophylactic antibiotics reduce the risk of deep infection after primary TKA. However, conventional systemic dosing may not provide adequate tissue concentrations against more resistant organisms such as coagulase-negative staphylococci. Regional intravenous administration of antibiotics after tourniquet inflation achieves far higher tissue concentrations but requires foot vein cannulation. The intraosseous route may offer a rapid and reliable method of regional administration.
Questions/Purposes
We compared tissue concentrations of cefazolin achieved with systemic versus regional intraosseous administration.
Methods
Twenty-two patients undergoing primary TKA were randomized into two groups. Group 1 received 1 g cefazolin systemically 10 minutes before tourniquet inflation. Group 2 received 1 g cefazolin intraosseously in 200 mL of normal saline through a tibial cannula after tourniquet inflation and before skin incision. Subcutaneous fat and femoral bone samples were taken at set intervals during the procedure and antibiotic concentrations measured using a validated technique involving high-performance liquid chromatography.
Results
The overall mean tissue concentration of cefazolin in subcutaneous fat was 186 ug/g in the intraosseous group and 11 ug/g in the systemic group. The mean tissue concentration in bone was 130 ug/g in the intraosseous group and 11 ug/g in the systemic group. These differences were consistent across all sample time points throughout the procedure.
Conclusions
Intraosseous regional administration can achieve concentrations of antibiotic in tissue an order of magnitude higher than systemic administration. Further work is required to determine if this translates into increased efficacy in preventing infection, particularly against coagulase-negative staphylococci.
Introduction
Deep surgical site infection is one of the most devastating complications of TKA. Despite concerted efforts to reduce infection rates, the reported incidence after primary TKA persists between 0.86% and 2.5% [1, 2, 20, 21]. Coagulase-negative staphylococci cause up to 49% of deep infections with evidence that the role of these organisms is increasing [1, 2, 20, 21].
The majority of early postoperative infections results from intraoperative contamination of the surgical site [10]. Even with a strict aseptic technique, bacterial contamination occurs in most if not all arthroplasty procedures [7]. Prophylactic antibiotics reduce the risk that contamination will progress to overt clinical infection, and in orthopaedic surgery, their efficacy is well established [4, 12, 14].
For antibiotic prophylaxis to be effective, the concentration of antibiotic in the tissues must exceed the minimum inhibitory concentration (MIC) of organisms that commonly cause infection for the period between skin incision and wound closure [3]. Coagulase-negative staphylococci (CoNS), one of the most common causes of infection post-TKA, have relatively high MICs against cephalosporins [28]. Conventional systemic dosing of prophylactic cephalosporins may therefore lead to inadequate tissue concentrations against these organisms [8, 28].
Regional administration of medication using a tourniquet achieves higher tissue concentrations than systemic administration by limiting distribution of the drug to the targeted limb. Some authors have used a foot vein cannula for administering prophylactic antibiotics in TKA. With this approach, substantially higher tissue concentrations of antibiotic can be achieved at the surgical site without systemic side effects [8, 9, 15, 17] (Table 1). However, cannulation of a foot vein is difficult, time-consuming, and may compromise sterility. An alternative means of regional administration is offered by intraosseous cannulation. Since its first reported use over 70 years ago [16], the intraosseous route has gained popularity as a rapid and reliable method of accessing the circulation [26].
Table 1.
Articles investigating regional administration of prophylactic antibiotics in TKA
| Authors | Comparison | Outcomes |
|---|---|---|
| Hoddinott et al. [15] | Compared 1000 mg IV cefamandole versus 750 mg regional cefuroxime through the foot vein in the same 5 patients | Mean cefuroxime bone (133 mg/L) and fat (88 mg/L) higher than cefamandole bone (9 mg/L) and fat (10 mg/L); p < 0.001 |
| de Lalla et al. [8] | RCT in 24 patients comparing 800 mg IV teicoplanin 2.5 hours preoperatively versus 400 mg teicoplanin through the foot vein | Tissue samples (skin, subcutaneous tissue, bone, synovium) 2–10 times higher through regional route |
| de Lalla et al. [9] | Clinical study 160 patients (205 knees) undergoing TKA, 400 mg teicoplanin through the foot vein | One superficial infection; no deep infections at 2-year followup |
| Lazzarini [17] | Five patients with 800 g IV teicoplanin 2.5 hours preoperatively versus 15 patients 200 mg teicoplanin through the foot vein | Tissue samples (skin, subcutaneous tissue, bone, synovium) 2 times higher through regional route |
IV = intravenous; RCT = randomized controlled trial.
We therefore aimed to compare tissue concentrations of cefazolin achieved with systemic versus regional intraosseous administration.
Patients and Methods
Patients undergoing primary TKA at a single institution were eligible for enrollment into this prospective, randomized controlled trial. Inclusion criteria were age between 55 and 85 years and a primary diagnosis of osteoarthritis. We excluded patients with previous compartment syndrome, allergy to an antibiotic used in the study, abnormal renal or liver function, recent (< 1 week) antibiotic treatment, or a body mass index (BMI) > 35 kg/m2. From March to August 2010, we assessed 32 patients undergoing primary TKA for osteoarthritis for enrollment. Ten patients were excluded (eight patients BMI > 35 kg/m2, one refused consent, one patient on oral antibiotics for recent nasal infection) leaving 22 patients who were randomized into systemic and intraosseous groups using computer-generated random allocations placed in numbered, opaque, sealed envelopes (Table 2). We randomized patients in the preoperative area to allow appropriate setup in the operative room.
Table 2.
Patient demographics
| Demographic | Intraosseous group (n = 11) | Systemic group (n = 11) |
|---|---|---|
| Males | 6 | 4 |
| Females | 5 | 7 |
| Age (years) | 71.8 (56–87) | 65.3 (48–83) |
| Body mass index (kg/m2) | 27.7 (22.1–35) | 29.1 (23.1–35) |
| Tourniquet time (minutes) | 84 (44–135) | 82 (43–113) |
| Procedure length (minutes skin to skin) | 74 (37–122) | 76 (39–110) |
| American Society of Anesthesiologists score | 2.2 | 2.1 |
Values given as mean with range in parentheses.
Based on the published data of Hoddinott et al. [15] showing an average mean fat tissue cephalosporin concentration across five time points of 88 ug/mL with SD 88 (regional administration) versus 11 ug/mL with SD 9 (systemic), a priori power analysis calculated that 11 patients in each arm would provide > 80% statistical power to detect the expected difference of 77 ug/mL in subcutaneous fat concentrations between two groups at the 5% significance level. Similarly, this sample size also provided adequate statistical power (> 90%) to detect a difference in mean bone concentrations between the two groups (assuming the average mean bone concentration is 133 ug/mL [SD 101] versus 11 ug/mL [SD 9] in regional and systemic administration, respectively). As discussed later, it is difficult to quantify what clinical effect such a difference would have on infection rates. However, antibiotic concentrations below an organism’s MIC are unlikely to provide effective prophylaxis against that organism [3]. CoNS causes up to 49% of TKA infections [20], and at our institution, over 68% of CoNS isolates have an MIC > 32 ug/mL against cefazolin. Assuming distribution similar to the cephalosporins used in the study of Hoddinott et al. [15], such isolates would therefore not be covered by the tissue concentrations seen with systemic dosing (11 ug/mL); tissue concentrations with regional dosing (88–133 ug/mL) would however provide effective prophylaxis against such isolates, suggesting the differences used in our power analysis are clinically relevant.
Patients in both groups received 1 g systemic cefuroxime between 10 and 30 minutes before tourniquet inflation. All patients underwent limb exsanguination and tourniquet inflation to 300 mmHg before routine preparation and draping. The tourniquet remained inflated for the entire procedure. Patients in the systemic group were given 1 g cefazolin systemically through a forearm vein between 10 and 30 minutes before tourniquet inflation. Intraosseous group patients received 1 g cefazolin through an EZ-IO (Vidacare, San Antonio, TX, USA; FDA-approved) intraosseous cannula, placed into the medial aspect of the proximal tibia, after draping and before skin incision. The cefazolin was administered as a bolus in 200 mL of normal saline following the recommendations of Waisman et al. [27]. In the intraosseous group, incision occurred immediately (< 1 minute) after antibiotic injection.
During the procedure, we took samples of subcutaneous fat and femoral cancellous bone at the following four steps of the procedure and the times were recorded. The first subcutaneous fat sample was taken immediately after skin incision, then both bone and fat samples at the time of the distal femoral cut, trialing of components, and immediately before closure. Times were recorded for each sample (Table 3), which were approximately 0.5 to 1 cm2.
Table 3.
Mean tissue concentrations of cefazolin at each sample point
| Intraosseous | Systemic | |||||
|---|---|---|---|---|---|---|
| Time (minutes; ± SD) | Mean concentration (μg/g; ± SD) | 95% CI (μg/g) | Time (minutes; ± SD) | Mean concentration (μg/g; ± SD) | 95% CI (μg/g) | |
| Sample point | ||||||
| Subcutaneous fat 1 | 1.2 | 175.3 | 102–250 | 1.3 | 7.2 | 4.2–10.3 |
| (0.6) | (110) | (0.4) | (4.3) | |||
| Subcutaneous fat 2 | 11 | 193.0 | 140–247 | 14 | 12.8 | 8.4–17.2 |
| (5.1) | (79.8) | (6.6) | (6.6) | |||
| Subcutaneous fat 3 | 30 | 206.3 | 121–292 | 35 | 11.2 | 8.4–14.0 |
| (11.1) | (127) | (12.3) | (4.1) | |||
| Subcutaneous fat 4 | 56 | 169.1 | 88–250 | 54 | 11.3 | 7.1–15.4 |
| (23.2) | (120) | (17.3) | (6.2) | |||
| Bone sample 1 | 11 | 75.4 | 26–125 | 14 | 9.2 | 7.4–10.9 |
| (5.1) | (74.2) | (6.6) | (2.6) | |||
| Bone sample 2 | 30 | 165.6 | 21–311 | 35 | 14.1 | 8.6–19.6 |
| (11.1) | (216) | (12.3) | (8.2) | |||
| Bone sample 3 | 56 | 148.8 | 79–219 | 54 | 10.8 | 7.7–13.8 |
| (23.2) | (105) | (17.3) | (4.6) | |||
Times are given as minutes postsurgical incision. Differences in mean tissue concentrations between the two groups were statistically significant (p < 0.001) for all comparison points.
We rinsed samples in normal saline to remove excess blood and stored them at −90°C before undergoing analysis. Bone samples were crushed with pliers, finely cut further with a scalpel, then weighed, and immersed in phosphate-buffered saline pH 7.3 for 15 hour at 4°C. The fat samples were finely cut with a scalpel and then treated in the same way as the bone samples. The immersed bone or fat tissue suspension was vortexed for 30 seconds and centrifuged at 15,000 g for 10 minutes. We transferred the supernatant to a clean tube and perchloric acid was added to precipitate the proteins. After centrifugation at 15,000 g for 5 minutes, 50 μL of clear supernatant was injected into the high-performance liquid chromatography (HPLC) system. A validation study was carried out of the extraction and HPLC technique using bone and tissue samples spiked with known concentrations of cefazolin. We analyzed all samples in duplicate.
Means, SDs, and the 95% confidence limits were calculated for the cefazolin concentrations in the different samples. Different tissue samples were pooled according to the surgical steps at which they were taken. Coefficients of variation (CVs) of concentration levels were also summarized at each surgical step for the comparison between two drug administration routes. We used repeated-measures analysis of variance to compare the average level of cefazolin across time between groups adjusted by BMI, age, and length of the surgical procedure; the Shapiro-Wilk test was applied to assess the normality of the residuals.
Results
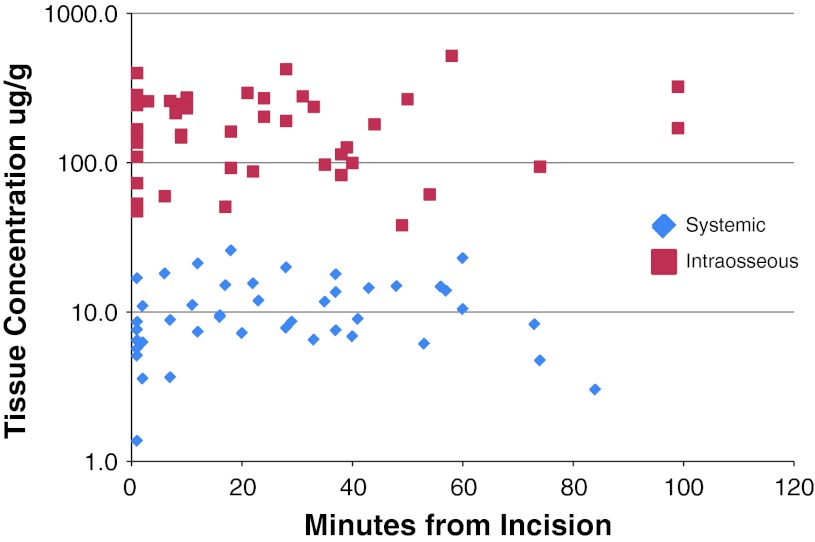
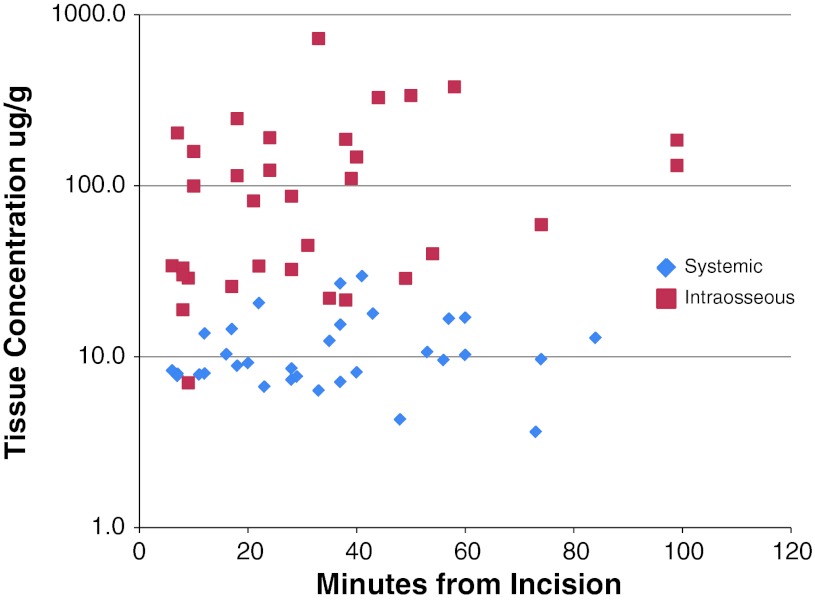
The mean tissue concentrations of cefazolin in subcutaneous fat at different collection intervals ranged from 175 (± 110) to 206 (± 127) ug/g in the intraosseous group and from 7.2 (± 4.3) to 12.8 (± 6.6) ug/g in the systemic group (Fig. 1; Table 3). The mean tissue concentration in bone ranged from 75 (± 74) to 165.6 (± 216) g/g in the intraosseous group and ranged from 9.2 (± 2.6) to 14.1 (± 8.2) μg/g in the systemic group (Fig. 2). The overall mean tissue concentration of cefazolin in subcutaneous fat was 186 ug/g in the intraosseous group and 10.6 ug/g in the systemic group (p < 0.01). The mean tissue concentration in bone was 130 ug/g in the intraosseous group and 11.4 ug/g in the systemic group (p < 0.01). The concentration was noted to be more variable with the intraosseous route for both subcutaneous fat (CV range 0.62–0.71 versus 0.37–0.56) and the bone samples (CV range 0.7–1.0 versus 0.3–0.6). The repeated-measures analysis of covariance showed no association between concentration level and age, BMI, sex, and length of procedure.
Fig. 1.
Tissue concentrations of cefazolin in subcutaneous fat for each sample.
Fig. 2.
Tissue concentrations of cefazolin in femoral bone for each sample.
No complications were seen in either group in the early postoperative period nor by 1-year followup.
Discussion
Early studies on systemic cefazolin for surgical prophylaxis reported adequate bone and soft tissue concentrations to prevent infection [6, 11, 24] and assumed MIC90 levels for CoNS of 0.5 to 1.0 ug/mL. Over recent decades, however, resistance to cephalosporins among CoNS has increased markedly, and current MIC90 is as high as 100 ug/mL in half of reported species [28]. This increase in resistance coincides with clinical data reporting a rise in the number of CoNS deep prosthetic infections [20]. Regional delivery of antibiotics may offer better protection against CoNS by achieving higher tissue concentrations (Table 1), but cannulation of a foot vein cannulation is difficult, time-consuming, and may compromise sterility. We aimed to investigate whether the more convenient intraosseous route could be used in regional delivery of prophylaxis.
There are a number of limitations to our study. First, although the intraosseous route is reported to have pharmacokinetics for both fluids and medications similar to intravenous administration [26], its use for regional administration is not as well studied. The tissue concentrations of cephalosporins in our study are comparable to those seen with intravenous regional administration [15], suggesting the two routes are similar. Second, we excluded patients with high BMIs to minimize the effect of this variable and because many authors recommend a higher systemic cefazolin dose in heavier patients [28]. Because we used a relatively high cutoff of BMI > 35 kg/m2, some of our systemic patients may have been underdosed. However, in patients undergoing TKA (mean BMI 25 kg/m2) given 2 g cefazolin systemically, Yamada [28] found a mean bone concentration of only 16 ug/g suggesting a higher systemic dose would be unlikely to alter our findings. A higher intraosseous dose could be considered for obese patients, but given the smaller volume of distribution in regional administration, 1 g is still likely to provide extremely high tissue levels. Finally, although we saw no complications with this technique in our study, the number of participants was small. Potential complications with intraosseous infusions include fluid extravasation with compartment syndrome related to incorrect needle placement in emergency situations [26]. Needle site infection has been reported rarely [26] and correlates with the length of time the needle is left in situ. Subclinical fat emboli have been seen histologically in animal studies [13], but no cases of fat embolism after intraosseous infusion have been reported in humans.
We found intraosseous regional administration of prophylactic antibiotics in TKA provides tissue concentrations 10 to 15 times higher than systemic administration. Our findings are similar to previous studies of TKA that used intravenous regional administration of other cephalosporins (Table 1). Hoddinott [15] compared 1 g intravenous regional cefuroxime to 1 g systemic cefamandole and found tissue concentrations five to 30 times higher with regional administration. During elbow surgery, Miller et al. [19] reported bone cefazolin concentrations 41 times higher and fat concentrations 133 times higher than systemic dosing, reflecting the smaller regional volume of distribution in the upper limb.
Do such high levels lead to increased efficacy? Nickinson et al. [20] reported 49% of TKA infections were the result of CoNS, and over 55% of CoNS strains were methicillin-resistant. In 1990 Friedman et al. [11] reported an MIC90 of 64 ug/mL against cefazolin for resistant strains of CoNS, and in 2011, Yamada et al. [28] reported an MIC90 of 100 ug/mL. Similar to previous studies [6, 11, 24], we found systemic dosing gave tissue concentrations of cefazolin (mean 10.6 ug/g fat and 11.4 ug/g in bone) well below these levels. In contrast, the regional intraosseous route provided mean cefazolin concentrations of 185.9 ug/g in fat and 129.9 ug/g in bone. Such levels have a plausible theoretical advantage by providing greater activity against organisms with typically high cefazolin MICs such as CoNS.
Whether such high cefazolin levels improve efficacy against more sensitive (lower MIC) bacterial strains is less clear. Although antibiotics such as aminoglycosides and fluoroquinolones exhibit concentration-dependent killing, for β-lactam antibiotics such as cefazolin, time above MIC is the most important factor. Cephalosporin treatment in animal models of infection suggests saturation of the killing rate occurs at concentrations four to five times the MIC [5]. By definition, the highest MIC90 for cefazolin-sensitive CoNS is 8 ug/g [28]; so although the intraosseous regional route will ensure tissue concentrations are at least five times this level, any clinical advantage may well be small. However, higher β-lactam concentrations (64 times MIC) are known to lead to an earlier initiation of bacterial killing [5], which may be more important for prophylaxis against infection where the goal is to prevent initial bacterial adherence and colonization.
Regional intraosseous antibiotic administration is used in the treatment of equine limb infection [18, 22, 23]; however, only one study has investigated the use of regional intraosseous medications in humans. Waisman et al. [27] reported on 109 patients given local anesthetic in 140 mL of saline through the regional intraosseous route before both upper and lower limb surgery. Two patients had inadequate anesthesia that the authors attributed to an insufficient volume (80 mL) infused in these patients. In our study, we chose a higher volume of 200 mL, because during regional administration, the circulation has effectively stopped and distribution relies on the volume of fluid to push the medication through the vasculature of the limb. We believed this volume would ensure the antibiotic is present in the tissues at incision, which occurs immediately after intraosseous injection. Our data showed very high antibiotic levels in first tissue sample, and it is possible a smaller volume may be adequate.
For regional delivery of antibiotics in TKA, the main advantages of intraosseous over foot vein cannulation are reliability and speed. The proximal tibia is already exposed during TKA, and modern intraosseous cannulation system kits offer rapid access [25]. The average time for cannulation and injection in this study was under 2 minutes, and thus a minimal difference in overall tourniquet time was observed between the two groups.
In summary, we describe a technique for administering intraosseous, regional antimicrobial prophylaxis before TKA that can achieve tissue levels an order of magnitude higher than with systemic administration. Further work is required to confirm whether this translates into increased efficacy in preventing infection, particularly against CoNS.
Acknowledgments
We thank Irene Zeng, MSc (Hons), for her assistance with statistical analysis, Grant Moore, BSc (Hons), for his assistance with laboratory analysis, and the charitable trust Clinical Research and effective practice (CCRep) for their funding support. We also thank Vidicare (San Antonio, TX, USA) for supplying the intraosseous needles without charge.
Footnotes
The institution of one of the authors (MZ) received funding from the Centre for Clinical Research and effective practice (CCRep), a charitable trust with no relationship to the subject of this article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Procedures and sample collection were performed at Middlemore Hospital, Auckland, New Zealand. Sample analysis was performed at Canterbury Health Laboratories, Christchurch, New Zealand.
References
- 1.Bengtson S, Knutson K. The infected knee arthroplasty. A 6-year follow-up of 357 cases. Acta Orthop Scand. 1991;62:301–311. doi: 10.3109/17453679108994458. [DOI] [PubMed] [Google Scholar]
- 2.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688–691. doi: 10.1302/0301-620X.86B5.14887. [DOI] [PubMed] [Google Scholar]
- 3.Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168. [PubMed] [Google Scholar]
- 4.Carlsson AK, Lidgren L, Lindberg L. Prophylactic antibiotics against early and late deep infections after total hip replacements. Acta Orthop Scand. 1977;48:405–410. doi: 10.3109/17453677708992017. [DOI] [PubMed] [Google Scholar]
- 5.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 6.Cunha BA, Gossling HR, Pasternak HS, Nightingale CH, Quintiliani R. The penetration characteristics of cefazolin, cephalothin, and cephradine into bone in patients undergoing total hip replacement. J Bone Joint Surg Am. 1977;59:856–859. [PubMed] [Google Scholar]
- 7.Davis N, Curry A, Gambhir AK, Panigrahi H, Walker CR, Wilkins EG, Worsley MA, Kay PR. Intraoperative bacterial contamination in operations for joint replacement. J Bone Joint Surg Br. 1999;81:886–889. doi: 10.1302/0301-620X.81B5.9545. [DOI] [PubMed] [Google Scholar]
- 8.de Lalla F, Novelli A, Pellizzer G, Milocchi F, Viola R, Rigon A, Stecca C. Dal Pizzol V, Fallani S, Periti P. Regional and systemic prophylaxis with teicoplanin in monolateral and bilateral total knee replacement procedures: study of pharmacokinetics and tissue penetration. Antimicrob Agents Chemother. 1993;37:2693–2698. doi: 10.1128/AAC.37.12.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lalla F, Viola R, Pellizzer G, Lazzarini L, Tramarin A, Fabris P. Regional prophylaxis with teicoplanin in monolateral or bilateral total knee replacement: an open study. Antimicrob Agents Chemother. 2000;44:316–319. doi: 10.1128/AAC.44.2.316-319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher N, Sofianos D, Berkes MB, Obremskey WT. Prevention of perioperative infection. J Bone Joint Surg Am. 2007;89:1605–1618. doi: 10.2106/JBJS.F.00901. [DOI] [PubMed] [Google Scholar]
- 11.Friedman RJ, Friedrich LV, White RL, Kays MB, Brundage DM, Graham J. Antibiotic prophylaxis and tourniquet inflation in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:17–23. [PubMed] [Google Scholar]
- 12.Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2001;1:CD000244. [DOI] [PubMed]
- 13.Hasan M, Kissoon N, Khan T, Saldajeno V, Goldstein J, Murphy S. Intraosseous infusion and pulmonary fat embolism. Pediatr Crit Care Med. 2001;2:133–138. doi: 10.1097/00130478-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Hill C, Flamant R, Mazas F, Evrard J. Prophylactic cefazolin versus placebo in total hip replacement. Report of a multicentre double-blind randomised trial. Lancet. 1981;1:795–796. doi: 10.1016/S0140-6736(81)92678-7. [DOI] [PubMed] [Google Scholar]
- 15.Hoddinott C, Lovering AM, Fernando HC, Dixon JH, Reeves DS. Determination of bone and fat concentrations following systemic cefamandole and regional cefuroxime administration in patients undergoing knee arthroplasty. J Antimicrob Chemother. 1990;26:823–829. doi: 10.1093/jac/26.6.823. [DOI] [PubMed] [Google Scholar]
- 16.Josefson A. A new method of treatment—intraosseous injection. Acta Med Scand. 1934;81:550–554. doi: 10.1111/j.0954-6820.1934.tb19683.x. [DOI] [Google Scholar]
- 17.Lazzarini L. Regional and systemic prophylaxis with teicoplanin in total knee arthroplasty. J Arthroplasty. 2003;18:342–346. doi: 10.1054/arth.2003.50053. [DOI] [PubMed] [Google Scholar]
- 18.Mattson S, Bourve L, Pearce S, Hurtig M, Burger J, Black W. Intraosseous gentamicin perfusion of the distal metacarpus in standing horses. Vet Surg. 2004;33:180–186. doi: 10.1111/j.1532-950x.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller BS, Harper WP, Hughes JS, Sonnabend DH, Walsh WR. Regional antibiotic prophylaxis in elbow surgery. J Shoulder Elbow Surg. 2004;13:57–59. doi: 10.1016/S1058-2746(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 20.Nickinson R, Board T, Gambhir A, Porter M, Kay P. The microbiology of the infected knee arthroplasty. Int Orthop. 2010;34:505–510. doi: 10.1007/s00264-009-0797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips JE, Crane TP, Noy M, Elliott TSJ, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 22.Rubio-Martínez L, López-Sanromán J, Cruz AM, Santos M. San Román F. Medullary plasma pharmacokinetics of vancomycin after intravenous and intraosseous perfusion of the proximal phalanx in horses. Vet Surg. 2005;34:618–624. doi: 10.1111/j.1532-950X.2005.00096.x. [DOI] [PubMed] [Google Scholar]
- 23.Scheuch BC, Van Hoogmoed LM, Wilson WD, Snyder JR, MacDonald MH, Watson ZE, Steffey EP. Comparison of intraosseous or intravenous infusion for delivery of amikacin sulfate to the tibiotarsal joint of horses. Am J Vet Res. 2002;63:374–380. doi: 10.2460/ajvr.2002.63.374. [DOI] [PubMed] [Google Scholar]
- 24.Schurman DJ, Hirshman HP, Kajiyama G, Moser K, Burton DS. Cefazolin concentrations in bone and synovial fluid. J Bone Joint Surg Am. 1978;60:359–362. [PubMed] [Google Scholar]
- 25.Shavit I, Hoffmann Y, Galbraith R, Waisman Y. Comparison of two mechanical intraosseous infusion devices: a pilot, randomized crossover trial. Resuscitation. 2009;80:1029–1033. doi: 10.1016/j.resuscitation.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Tobias JD, Ross AK. Intraosseous infusions: a review for the anesthesiologist with a focus on pediatric use. Anesth Analg. 2010;110:391–401. doi: 10.1213/ANE.0b013e3181c03c7f. [DOI] [PubMed] [Google Scholar]
- 27.Waisman M, Roffman M, Bursztein S, Heifetz M. Intraosseous regional anesthesia as an alternative to intravenous regional anesthesia. J Trauma. 1995;39:1153–1156. doi: 10.1097/00005373-199512000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Yamada K, Matsumoto K, Tokimura F, Okazaki H, Tanaka S. Are bone and serum cefazolin concentrations adequate for antimicrobial prophylaxis? Clin Orthop Relat Res. 2011;469:3486–3494. doi: 10.1007/s11999-011-2111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]