Abstract
Background
We observed isolated tibial component debonding from the cement in one modern primary TKA design (NexGen LPS 3° tibial tray; Zimmer, Warsaw, IN, USA). This failure mechanism is sparsely reported in the literature.
Questions/Purposes
We (1) assessed survivorship of this tibial tray with special emphasis on debonding; (2) described clinical and radiographic features associated with tibial failure; and (3) compared patient and radiographic features of the failures with a matched cohort.
Methods
A total of 1337 primary TKAs were performed with a cemented NexGen LPS 3° tibial tray over an 11-year period. Twenty-five knees (1.9%) were revised for tibial debonding. BMI and radiographic alignment in the tibial debonding group were compared with a matched control group. Implant survivorship was assessed using tibial debonding as the end point.
Results
Survival free of revision from tibial debonding was 100% at 1 year and 97.8% at 5 years. The tibial failures shared a typical radiographic pattern with debonding at the cement-implant interface and subsidence into varus and flexion. We found no link between limb alignment or individual component alignment and failure because 22 of the 25 failures occurred in well-aligned knees.
Conclusions
Our standardized followup of patients undergoing TKA at routine intervals allowed us to discover a higher rate of revision resulting from tibial debonding. We have discontinued the use of this particular tibial tray for primary TKA and surveillance for patients undergoing TKA continues to be warranted.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Primary TKA continues to be the gold standard for treating the painful arthritic knee with survival rates of over 90% with followup of 2 to 19 years [1, 2, 4, 5, 18–20, 26, 28]. Although revision rates remain low, the absolute numbers rise steadily [6, 12] and account for approximately 8.2% of all knee arthroplasties performed annually in the United States alone [17].
The most common reason for failure of TKA is infection followed by implant loosening [6], polyethylene wear, and instability [12, 30]. Although in some early TKA designs, failure of the tibial component was more prevalent [8], subsequent changes in design and surgical technique have for the most part decreased early aseptic failure rates of the tibial or femoral components [19, 28].
In 1997 the NexGen Legacy posterior-stabilized (LPS) prosthesis (Zimmer, Warsaw, IN, USA) was introduced as an evolution of the Insall-Burstein II PS prosthesis (Zimmer). Several metal-backed tibial implant options exist in this system for primary TKA with the most commonly used being the 3° and 7° fluted stem options. The 3° tibial tray differs from the 7° because it allows for the attachment of stem extensions and tibial augments during revision arthroplasty (Fig. 1). The tibial tray consists of a tivanium Ti-6Al-4 V alloy that incorporates a dovetail locking mechanism for the tibial polyethylene insert [32]. Recent literature suggests survival rates of 100% at 2 to 9 years for the NexGen LPS prosthesis with no mention of tibial component problems but it is unclear which of the several available tibial designs were used in those studies [10, 16, 23].
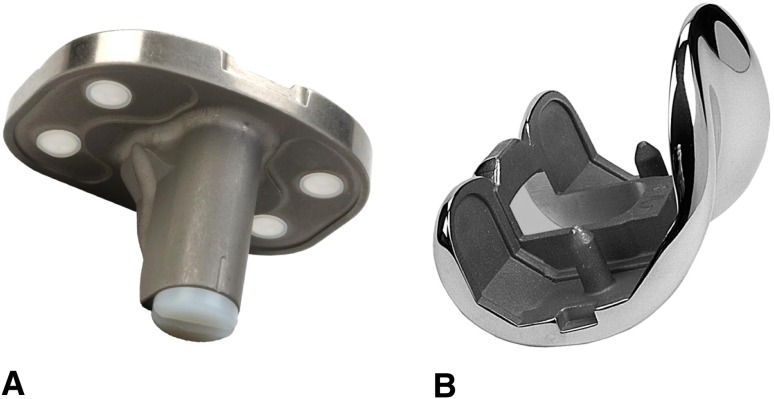
Fig. 1A–B.
(A) NexGen 3° Fluted Stem Tibial Plate (Option) and (B) NexGen LPS femoral option. This design can be used with a stem extension and augments if needed.
The NexGen LPS implant has been in use at our institution for primary TKA since 2001. Most commonly the LPS femoral component was combined with a cemented NexGen fluted 3° option tibia. In the course of routine clinical and radiographic followup, several of our surgeons noted isolated tibial component debonding from the cement mantle as a cause of failure with this design and prompted this formal review.
Our objectives were to (1) assess survivorship of the cemented LPS NexGen TKA system with a 3° fluted stem tibial tray with special emphasis on tibial component failure; (2) describe the clinical and radiographic features associated with such failure; and (3) compare patient and radiographic features in the failed group with a matched cohort whose implants had not failed.
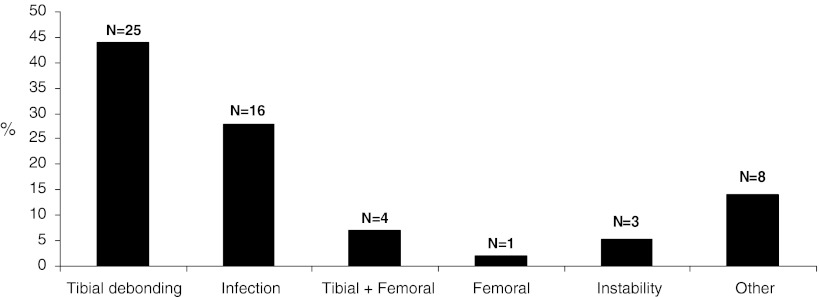
Patients and Methods
We used the Mayo Clinic total joint registry to identify 13,028 primary TKAs performed between January 1, 2000, and October 10, 2011. We then identified 2347 Zimmer NexGen Legacy posterior-stabilized primary TKAs of which 1337 were performed with a cemented NexGen fluted 3° tibial tray. The remaining 1010 TKAs were implanted with other tibial components also used with the NexGen system. Fifty-seven knees in 55 patients (4.3%) were revised at a median time of 38 months (range, 1–95 months) for various reasons. Aseptic loosening accounted for 44% of the revision cases followed by deep infection. From the group that were revised, 25 knees in 23 patients were revised because of aseptic debonding of the tibial component from the cement and comprise the study group (Fig. 2). The records of these patients were reviewed for demographic characteristics and operative reports were used to note intraoperative findings during revision surgery. In this tibial debonding group, there were 16 women (two bilateral knees) and seven men. The mean age was 58 years (range, 42–77 years). The mean BMI at the time of primary TKA was 35.6 kg/m2 (range, 24.5–45.9 kg/m2). The median time to revision was 39 months (range, 13–95 months) (Table 1). The minimum followup after revision surgery resulting from tibial component debonding was 1 month (mean, 1.4 years; range, 1 month to 5.5 years). None of the patients who underwent revision were lost to followup. The status of the remaining unrevised knees (including the control group) was followed through our joint registry with scheduled longitudinal followup at 3 months, 2 years, 5 years, and every 5 years thereafter. Followup was obtained with either a clinical visit (n = 625) or through a written or telephone questionnaire and radiographs (n = 546). The median followup for all unrevised and alive primary TKAs (n = 1171) was 51.7 months (range, 1–112 months). One hundred nine patients died during the followup period. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. We obtained institutional review board approval for this retrospective review.
Fig. 2.
Reason for failure within the 57 primary TKAs with isolated tibial debonding being the most common cause.
Table 1.
Patient demographics
| Parameter | Failed group | Control group 1 | |
|---|---|---|---|
| Sex (male/female) | 7/16 | 15/35 | |
| Age (years) | Mean | 58 | 58 |
| SD | 9.1 | 8.71 | |
| Range | 42–77 | 42–78 | |
| Body mass index (kg/m2) | Mean | 35.6 | 35.1 |
| SD | 5.9 | 7.38 | |
| Range | 24.5–45.9 | 23.7–54.4 | |
| Time to revision/followup period for control group (months) | Median | 39 | 56.5 |
| Range | 13–95 | 12–112 |
To analyze patient clinical and radiographic characteristics associated with failure, we compared a 2:1 matched control group with the tibial debonding group. The control group included 50 patients who had the NexGen LPS with the same cemented 3° fluted stem tibial tray and were matched for age (± 10 years), sex, and surgeon. All patients in the control group had well-functioning TKAs without pain, stiffness, effusion, or signs of instability on physical examination. Knee Society scores [15] at latest followup needed to be > 140 points and the patient was overall satisfied with the operative outcome. The median followup for the control group was 4 years (range, 12–112 months) (Table 1).
All surgeries were performed by one of eight surgeons specialized in adult hip and knee reconstruction. The implant choice was based on the surgeon’s preference but some patients were part of an ongoing prospective randomized trial. The surgical technique differed slightly between surgeons. Simplex cement with or without antibiotics was used in all cases and the powder was warmed before cementation of components. All but one surgeon (ADH) cemented the tibial and femoral components with the same batch of cement.
Two of us (RJS, DA) assessed pre- and postoperative AP and lateral radiographs for each study and control patient. The initial 300 radiographs were simultaneously reviewed by both reviewers and judged by consensus while subsequent radiographs were reviewed by one of the authors (DA). Immediate postoperative radiographs were defined as radiographs obtained within 6 months from the day of each subject’s index primary TKA. Standing AP long-leg films were available as hard copies only and a goniometer was used to measure the femorotibial alignment defined as the angle created by the long axes of the femur and tibia. All other radiographs (AP, lateral) of the knees were 18-inch films that were readily available by electronic image archiving software and measurements were carried out through computerized imaging software. Radiographic measurements included femorotibial (coronal) alignment, tibial component alignment, posterior tibial slope, and femoral component extension/flexion angles. Varus alignment numbers were presented as negative values; valgus measurements had positive values. All other patient radiographs underwent review for aseptic loosening.
Continuous variables are reported as mean (SD) or median (range), and discrete variables are reported as number (percentage). Conditional logistic regression, preserving the matched nature of the data, was used to assess association of the four radiographic measures and BMI with need for revision. Results are reported as odds ratio (OR) and 95% confidence interval (CI). Assessment of survival free of revision, any and for tibial debonding, used all 1337 knees implanted with this component. Survival was estimated using the Kaplan-Meier method and associations of age, sex, and BMI with revision were made using Cox proportional hazards regression; results are reported as hazard ratio and 95% CI. All analyses were performed using SAS Version 9.3 software (SAS Institute Inc, Cary NC, USA). For a risk factor with a 10% prevalence in controls, assuming low or no correlation between cases and controls, this study had 80% power to detect an OR of greater than 5.0; for a risk factor with 25% prevalence in controls, there was 80% power to detect an OR of greater than or equal to 4.0.
Results
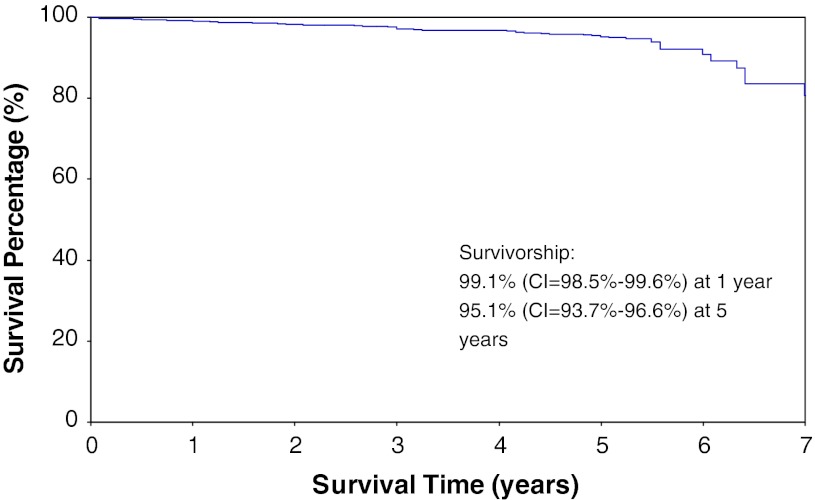
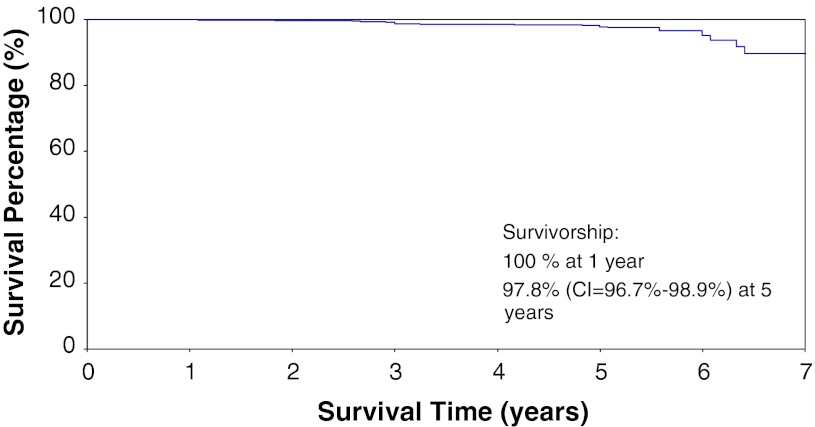
The survivorship free of revision for any reason with the cemented NexGen LPS 3° tibial tray was 99.1% (98.5%–99.6%) at 1 year and 95.1% (93.7%–96.6%) at 5 years (Fig. 3). The survivorship free from tibial debonding with the cemented NexGen LPS 3° tibial tray was 100% at 1 year and 97.8% (96.7%–98.9%) at 5 years (Fig. 4).
Fig. 3.
Kaplan-Meier survivorship with revisions resulting from any reason as the end point (CI = Confidence Interval).
Fig. 4.
Kaplan-Meier survivorship with revisions resulting from aseptic tibial debonding as the end point (CI = Confidence Interval).
Revision for tibial component debonding with the cemented NexGen LPS 3° tibial tray was performed in 1.9% of knees (25 of 1337) with a median time to revision of 39 months (range, 13–95 months). In those patients who required revision for tibial debonding, the prerevision radiographs shared characteristic findings: debonding of the tibial component at the prosthesis-cement interface and subsidence of the tibial component into varus and into flexion (Fig. 5). In some cases, radiographic documentation of debonding was present before subsequent subsidence into varus and flexion (Fig. 6). In all cases, intraoperative findings revealed a grossly loose tibial component with most of the cement mantle still attached to the bone (Fig. 7). No case exhibited signs of macroscopic polyethylene wear. Osteolysis involving the posterior femoral condyles was apparent in some cases and was attributed to cement debris.
Fig. 5A–B.
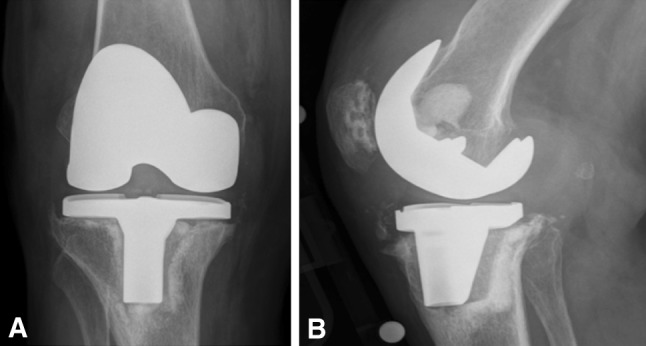
The AP view (A) reveals a pronounced varus position of the tibial tray. The lateral view (B) demonstrates flexion subsidence with debonding at the cement-prosthesis interface.
Fig. 6A–D.
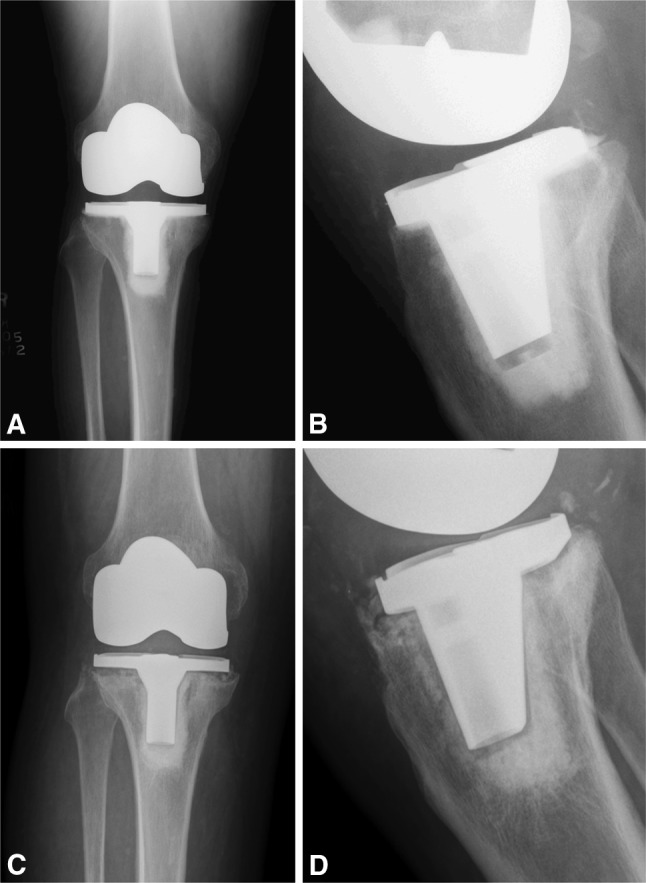
(A) Anterior and (B) posterior radiographs of a patient with an asymptomatic TKA at 24 month postoperatively. There are radiolucent lines present underneath the tibial tray. (C-D) Nine months later the patient presents with radiographs showing debonding of the tibial component.
Fig. 7.
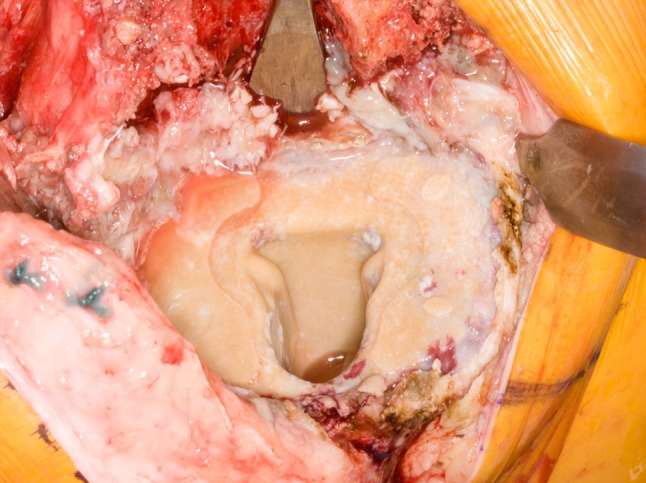
Debonding of the tibial component leaves behind a nearly intact cement mantle attached to the proximal tibia.
Using a matched control group of unrevised patients, we could not find a causative link between revision for tibial debonding and postoperative limb alignment, individual component position, or patient BMI. Although a small difference (p = 0.02) in postoperative femorotibial angle was measured in the tibial debonding group compared with the control group (+3.1º valgus versus +4.9º valgus), only three of the 25 knees in the tibial debonding group could be considered outliers (Table 2). Similarly, although a small difference (p = 0.003) in the mean postoperative tibial component alignment was measured in the tibial debonding group compared with the control group (1º varus versus +0.4º valgus), again only three of 25 tibial components in the debonding could be considered outliers beyond 3° of varus/valgus (Table 2). There was no association (p = 0.9456; OR, 1.002) of BMI and the risk of revision for tibial debonding.
Table 2.
Multivariate analysis of radiographic variables (failed versus control group 1)
| Parameters | Failed group | Control group 1 | Odds ratio | 95% CI | p value | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||||
| Coronal (º) (long films) | +3.1 ± 4.48 | −6.6 to +8.9 | +4.89 ± 2.61 | −0.7 to +8.5 | 0.8 | 0.67–0.97 | 0.02 |
| Tibial (º) | −1.1 ± 2 | −6.7 to +1.8 | +0.36 ± 1.3 | −3.1 to +2.6 | 0.5 | 0.32–0.79 | 0.003 |
| Tibial slope (º) | +5.37 ± 2.35 | +0.6 to +11.3 | +5.99 ± 2.2 | +0.8 to +10.7 | 0.85 | 0.66–1.11 | 0.21 |
| Femoral extension/flexion (º) | +4.25 ± 1.52 | +0.7 to +7.61 | +4.54 ± 2.1 | −0.91 to +7.8 | 0.92 | 0.7–1.2 | 0.52 |
Number of failures and average postoperative followup differed among surgeons who had experienced at least one failure and surgeons with longer mean followup tended to have revised more patients (Table 3). An increased awareness of this failure mechanism has led to closer surveillance and prompted earlier revision in some patients noted by less followup time to revision. An overview of the literature denotes that tibial component debonding is a rare cause of failure in primary TKA (Table 4).
Table 3.
Failure incidence (FI), mean time to revision (MTR), and overall followup time (FU)
| Surgeon | FI | MTR (months) | FU (months) |
|---|---|---|---|
| 1 | 5/183 (2.7%) | 52.7 | 52.6 |
| 2 | 3/271 (1.1%) | 34.8 | 34.0 |
| 3 | 7/154 (4.5%) | 52.3 | 52.0 |
| 4 | 2/281 (0.7%) | 45.0 | 45.9 |
| 5 | 5/202 (2.5%) | 25.4 | 35.2 |
| 6 | 3/66 (3%) | 59.1 | 25.7 |
| 7 | 1/152 (0.7%) | 76 | 56.6 |
Table 4.
Comparison of rates of aseptic tibial component failure in our study and other studies in the literature
| Study | Implant type (all cemented) | Number | Rates (%) | Mean time to revision (MTR) (years/months)/followup (FU) | Comments |
|---|---|---|---|---|---|
| Abdeen et al. [1] | IBS PS I | 0/31 | 0 | 16.2 years FU | |
| Alemparte et al. [2] | ACG | 1/94 | 1.1 | 2–8 years FU | Small tibial component |
| Berend et al. [4] | ACG | 6/3152 | 0.2 | 5 years FU | |
| Bertin [5] | NexGen CR (stemmed/pegged) | 0/225 | 0 | 5.9 years FU | |
| Foran et al. [9] | NexGen MIS Short Keel | 8/529 | 1.5% | 17 months MTR | All at the metal-cement interface |
| Fuchs et al. [10] | NexGen LPS | 0/279 | 0% | 48 months FU | |
| Indelli et al. [14] | IBS PS II | 0/92 | 0% | 7.5 years FU | |
| Ip et al. [16] | NexGen LPS | 0/60 | 0% | 21 months FU | |
| Lachiewicz and Soileau [18, 19] | IBS PS II | 0/112 | 0% | 7 years FU | |
| Mahoney and Kinsey [20] | Scorpio PS TKA | 5/1030 | 0.5% | 4.1 years MTR 7 years FU | All at bone-cement interface |
| Mikulak et al. [21] | PFC PS | 10/557 (isolated tibial debonding 4/557) | 1.8% (0.7%) | N/A MTR 56 months FU | All at metal-cement interface |
| Oh et al. [23] | IBS PS II/NexGen LPS | 0/91 | 0% | 10.3 years FU | |
| O’Rourke et al. [22] | IBS PS II | 0/126 | 0% | 6.4 years FU | |
| Rasquinha et al. [26] | PFC PS | 0/105 | 0% | 12 years FU | |
| Schwartz et al. [28] | NexGen CR four-pegged | 0/126 | 0% | 11.3 years FU | |
| Scuderi and Clarke [29] | NexGen LPS | 0/195 | 0% | 48 months FU | |
| Current study | NexGen LPS | 25/1337 | 1.9% | 39 months MTR |
PS = posterior-stabilized; CR = cruciate-retaining; IBS I = Insall-Burstein-I; IBS II = Insall-Burstein-II; LPS = Legacy Posterior Stabilized; PFC = Press-Fit Condylar Posterior Stabilized; ACG = anatomically graduated components.
Discussion
Several studies document survival rates of over 90% up to 19 years with modern TKA designs using a cemented metal-backed stemmed tibial tray [14, 19, 22, 26, 29]. In recent years, failure of tibial fixation has been a rare cause of revision [8, 11, 31]. In contrast, we found tibial component debonding from the cement was the most common cause of failure in over 1300 primary NexGen LPS TKAs implanted with a 3° fluted tray at the Mayo Clinic since 2001.
This study has several limitations. First, it is a retrospective study that used revision surgery as the end point. As such, it may underestimate the prevalence of tibial debonding if there are some patients who are asymptomatic or have not returned for routine followup radiographs. We did review the latest available radiographs for all patients and no additional cases of tibial debonding were identified but no patients were specifically contacted as part of this study [13, 24]. Second, multiple surgeons with variation in surgical technique, volume, and experience with this specific design were involved. Whether this truly represents a limitation of the study is unclear because that variation does allow us to comment that these tibial debondings are likely independent of surgical technique with failures being noted among seven of the eight surgeons who implanted this device.
The overall survivorship that we report with this TKA design certainly is satisfactory and is very similar to what has been published previously for other primary TKA systems. What is unique to this study is that tibial component debonding accounted for the majority of failures. With the same followup, other comparable series report higher failure rates as a result of infection, for example with early loosening occurring rarely. Furthermore, this particular mode of failure, tibial debonding, has not been detected with any substantial frequency in our standardized clinical and radiographic followup of other implant designs used at our institution.
Debonding of the tibial component from the cement has been reported with other designs. One case report stated isolated tibial component debonding as a cause of failure in a patient with an Insall-Burstein TKA [7]. In a study of 557 primary press-fit condylar TKAs, 10 knees were revised as a result of aseptic loosening and osteolysis around the cemented tibial component [21]. The authors found the components were easily removed by hand indicating loosening at the metal-cement interface. Retrieval analyses demonstrated impingement of the sides of the post against the box, and the authors speculated that entrapment of the post could potentially lead to transmission of increased stresses to the modular interfaces of the tibia and could be a cause of failure [21]. We did not see changes on the polyethylene post to suggest such a failure mechanism.
In a previous series of 279 primary NexGen LPS TKAs, there was no evidence of radiographic loosening or revisions of the tibial component at a mean followup of 48 months [10]. It is not clear from that article which of the several available tibial designs for this knee system was used and there may well be tibial implant design differences between these different versions that predispose to failure. For example, one series has reported a high failure rate at 17 months with a short-keeled minimally invasive version of the traditional NexGen stemmed tibial tray [9]. Both the NexGen MIS short-keeled version and the LPS 3° option stem tibial tray share the same matte surface finish and material properties (tivanium Ti-6Al-4 V alloy) [32]. However, mechanical testing has suggested that titanium alloy components such as these actually have superior bonding properties when compared with cobalt-chrome implants [25].
In this series, most patients had very few symptoms and the tibial debonding was detected at the time of routine surveillance radiographs. For a small subset of patients, their first presentation was with a catastrophic event involving marked tibial component subsidence into varus with subsequent inability to ambulate. We currently do not use this tibial implant design but continue to follow these patients through our institutional total joint arthroplasty registry-based surveillance protocol. Surveillance has recently allowed us to detect some cases of tibial debonding before subsidence of the tibial component into varus and flexion has occurred. Once debonding is demonstrated radiographically, revision surgery is recommended in an effort to minimize damage to the proximal tibial bone stock. Our detection of this clinical problem in minimally symptomatic patients underscores the importance of continued followup of all patients undergoing TKA, regardless of design, and documents the value of a total joint registry as a tool for ensuring such.
It is clear that there was a cascade of events that occurred during the debonding and failure of fixation seen in this series, but the critical inciting event and the critical design differences between this tibial tray and others remain unclear to us.
At the time of revision, the polyethylene plugs used to cover the holes in the baseplate were in some cases displaced from their seated position. It is unclear however if this occurred as a result of backside wear or after the migration of these implants. The polyethylene was also loose within the tray in some cases with evidence of burnishing on the topside baseplate. This suggests failure of the locking mechanism as a possible inciting event in some. The baseplate holes used for fixation of tibial blocks and augments on the undersurface of the tibial tray are not present on some other trays in this system and may allow egress of particulate-laden fluid into the cement-implant interface. Whether this contributes to clinical failure is also unclear.
Varus alignment of the tibial component has been implicated as a cause for accelerated tibial component failure [27]. Other factors associated with increased tibial component failure include a high BMI, postoperative varus limb alignment, and ligamentous imbalance [4]. The vast majority of the failures in this study (22 of the 25 debonding cases) however occurred in knees with good femorotibial alignment and good tibial component position. Although there were slight differences in the mean alignment values between the group with tibial debonding and the controls, that difference is largely the result of two knees in one patient with both tibial varus alignment and varus femorotibial alignment. BMI was not predictive of failure with a relatively high BMI of 35 kg/m2 noted in both the tibial debonding and the control groups. Varus limb alignment, varus tibial component position, and high BMI cannot explain more than a small fraction of these 25 cases of tibial debonding [3].
In conclusion, our standardized institutional total joint registry-based system for clinical and radiographic followup of all patients undergoing TKA at routine intervals allowed us to discover a higher than expected rate of revision resulting from tibial debonding with the cemented NexGen LPS 3° tibial tray. Although we were unable to establish the failure mechanism of this device, we have discontinued the use of this particular tibial tray and it is clear that surveillance for all patients undergoing TKA is warranted reinforcing the need of proposed comprehensive joint registries.
Footnotes
The institution of one or more of the authors (DGL, RJS, MWP, ADH) has received funding from DePuy (Warsaw, IN, USA), Zimmer (Warsaw, IN, USA), Stryker (Mahwah, NJ, USA), and Biomet (Warsaw, IN, USA). One author (DGL) certifies he has or may receive payments or benefits, in any one year, an amount in excess of $100,000 from a commercial entity (Zimmer) related to this work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abdeen AR, Collen SR, Vince KG. Fifteen-year to 19-year follow-up of the Insall-Burstein-1 total knee arthroplasty. J Arthroplasty. 2010;25:173–178. doi: 10.1016/j.arth.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Alemparte J, Cabezas A, Azocar O, Hernandez R, Acevedo M. Mid-term results of an AGC total knee arthroplasty system survival and function analysis: 2- to 8-year follow-up results. J Arthroplasty. 2003;18:420–425. doi: 10.1016/S0883-5403(03)00017-2. [DOI] [PubMed] [Google Scholar]
- 3.Berend ME, Ritter MA, Hyldahl HC, Meding JB, Redelman R. Implant migration and failure in total knee arthroplasty is related to body mass index and tibial component size. J Arthroplasty. 2008;23:104–109. doi: 10.1016/j.arth.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R, Faris GW, Davis KE. Tibial component failure mechanisms in total knee arthroplasty. Clin Orthop Relat Res. 2004;428:26–34. doi: 10.1097/01.blo.0000148578.22729.0e. [DOI] [PubMed] [Google Scholar]
- 5.Bertin KC. Tibial component fixation in total knee arthroplasty: a comparison of pegged and stemmed designs. J Arthroplasty. 2007;22:670–678. doi: 10.1016/j.arth.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng K, Pruitt L, Zaloudek C, Ries MD. Osteolysis caused by tibial component debonding in total knee arthroplasty. Clin Orthop Relat Res. 2006;443:333–336. doi: 10.1097/01.blo.0000196044.42413.c7. [DOI] [PubMed] [Google Scholar]
- 8.Ducheyne P, Kagan A, 2nd, Lacey JA. Failure of total knee arthroplasty due to loosening and deformation of the tibial component. J Bone Joint Surg Am. 1978;60:384–391. [PubMed] [Google Scholar]
- 9.Foran JR, Whited BW, Sporer SM. Early aseptic loosening with a precoated low-profile tibial component a case series. J Arthroplasty. 2011;26:1445–1450. doi: 10.1016/j.arth.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs R, Mills EL, Clarke HD, Scuderi GR, Scott WN, Insall JN. A third-generation, posterior-stabilized knee prosthesis: early results after follow-up of 2 to 6 years. J Arthroplasty. 2006;21:821–825. doi: 10.1016/j.arth.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton LR. UCI total knee replacement. A follow-up study. J Bone Joint Surg Am. 1982;64:740–744. [PubMed] [Google Scholar]
- 12.Heck DA, Melfi CA, Mamlin LA, Katz BP, Arthur DS, Dittus RS, Freund DA. Revision rates after knee replacement in the United States. Med Care. 1998;36:661–669. doi: 10.1097/00005650-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Ilahi OA, Kadakia NR, Huo MH. Inter- and intraobserver variability of radiographic measurements of knee alignment. Am J Knee Surg. 2001;14:238–242. [PubMed] [Google Scholar]
- 14.Indelli PF, Aglietti P, Buzzi R, Baldini A. The Insall-Burstein II prosthesis: a 5- to 9-year follow-up study in osteoarthritic knees. J Arthroplasty. 2002;17:544–549. doi: 10.1054/arth.2002.32186. [DOI] [PubMed] [Google Scholar]
- 15.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 16.Ip D, Wu WC, Tsang WL. Early results of posterior-stabilised NexGen Legacy total knee arthroplasty. J Orthop Surg (Hong Kong). 2003;11:38–42. doi: 10.1177/230949900301100109. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 18.Lachiewicz PF, Soileau ES. The rates of osteolysis and loosening associated with a modular posterior stabilized knee replacement. Results at five to fourteen years. J Bone Joint Surg Am. 2004;86:525–530. doi: 10.1302/0301-620X.86B8.15438. [DOI] [PubMed] [Google Scholar]
- 19.Lachiewicz PF, Soileau ES. Fifteen-year survival and osteolysis associated with a modular posterior stabilized knee replacement. A concise follow-up of a previous report. J Bone Joint Surg Am. 2009;91:1419–1423. doi: 10.2106/JBJS.H.01351. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney OM, Kinsey TL. 5- to 9-year survivorship of single-radius, posterior-stabilized TKA. Clin Orthop Relat Res. 2008;466:436–442. doi: 10.1007/s11999-007-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikulak SA, Mahoney OM. dela Rosa MA, Schmalzried TP. Loosening and osteolysis with the press-fit condylar posterior-cruciate-substituting total knee replacement. J Bone Joint Surg Am. 2001;83:398–403. doi: 10.2106/00004623-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 22.O’Rourke MR, Callaghan JJ, Goetz DD, Sullivan PM, Johnston RC. Osteolysis associated with a cemented modular posterior-cruciate-substituting total knee design: five to eight-year follow-up. J Bone Joint Surg Am. 2002;84:1362–1371. doi: 10.2106/00004623-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Oh KJ, Goodman SB, Yang JH. Prospective, randomized study between Insall-Burstein II and NexGen Legacy with a minimum 9-year follow-up. J Arthroplasty. 2011;26:1232–1238. doi: 10.1016/j.arth.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Petersen TL, Engh GA. Radiographic assessment of knee alignment after total knee arthroplasty. J Arthroplasty. 1988;3:67–72. doi: 10.1016/S0883-5403(88)80054-8. [DOI] [PubMed] [Google Scholar]
- 25.Pittman GT, Peters CL, Hines JL, Bachus KN. Mechanical bond strength of the cement-tibial component interface in total knee arthroplasty. J Arthroplasty. 2006;21:883–888. doi: 10.1016/j.arth.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Rasquinha VJ, Ranawat CS, Cervieri CL, Rodriguez JA. The press-fit condylar modular total knee system with a posterior cruciate-substituting design. A concise follow-up of a previous report. J Bone Joint Surg Am. 2006;88:1006–1010. doi: 10.2106/JBJS.C.01104. [DOI] [PubMed] [Google Scholar]
- 27.Ritter MA, Davis KE, Meding JB, Pierson JL, Berend ME, Malinzak RA. The effect of alignment and BMI on failure of total knee replacement. J Bone Joint Surg Am. 2011;93:1588–1596. doi: 10.2106/JBJS.J.00772. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz AJ, Della Valle CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468:2160–2167. doi: 10.1007/s11999-010-1360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scuderi GR, Clarke HD. Cemented posterior stabilized total knee arthroplasty. J Arthroplasty. 2004;19:17–21. doi: 10.1016/j.arth.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Skolnick MD, Coventry MB, Ilstrup DM. Geometric total knee arthroplasty. A two-year follow-up study. J Bone Joint Surg Am. 1976;58:749–753. [PubMed] [Google Scholar]
- 32.Inc Zimmer. Zimmer Tibial Component Pocket Guide, United States Version. Warsaw, IN, USA: Zimmer Inc; 2009. [Google Scholar]