Abstract
Background
Current cartilage transplantation techniques achieve suboptimal restoration and rely on patient donor cells or living grafts of chondrocytes.
Purpose
We sought to enhance allogeneic grafts by testing mosaics of genetically engineered and naïve juvenile human chondrocytes (jCh).
Methods
We obtained specimens from three humans and performed three experiments (two in vitro, one in vivo). We compared neocartilage with and without (1) supplemented serum-free medium (chondrocyte differentiation medium [CDM]), (2) adenoviral BMP-2 (AdBMP-2) transduction, and (3) varying ratios (0.1–1) of transduced and naïve jCh. We compared (4) healing with mosaic grafts with naïve neocartilage or marrow stimulation in immunosuppressed rats. For each of 10 in vitro treatment groups, we had six replicates for each human, and for each of three in vivo treatment groups, we had four replicates for one human. We scored the histology with the semiquantitative Bern score.
Results
AdBMP-2 and naïve neocartilage growth in CDM were histologically superior (Bern score, 5.2 versus 3.7; 8.0 versus 1.8) and size (8.0 versus 6.1; 7.9 versus 2.2 mg) to standard medium. In CDM, AdBMP-2 decreased viability (76% versus 90%), but increased BMP-2 production (619 ng/mL versus 43 pg/mL). Ten percent and 25% AdBMP-2 transduction had Bern scores of 6.8 and 6.5 and viability of 84% and 83%, respectively. Twenty-five percent mosaic grafts provided better healing histologically than marrow stimulation or naive neocartilage.
Conclusions
Low-level AdBMP-2 and CDM augment neocartilage parameters in vitro and vivo.
Clinical Relevance
Genetic augmentation of jCh and creation of mosaic neocartilage may improve graft viability and articular healing compared with naïve neocartilage.
Introduction
Current research in cartilage restoration has diversified in a multitude of directions [4, 10, 55]. State-of-the-art technology and numerous products are in various phases of clinical introduction (Table 1). Several major in vitro and in vivo challenges exist including preventing fibroblastic dedifferentiation of chondrocytes, producing true hyaline cartilage, and attaining adequate biomechanical strength. Four burgeoning areas of investigation are cell type, graft material, growth factors, and genetic engineering. The ideal cell source has been presumed to be autologous, but a recent study showed that allogeneic juvenile human articular chondrocytes (jCh) may be a potentially superior alternative for tissue engineering [9]. Although scaffold-based technology continues to be investigated aggressively, less attention has been directed to scaffold-independent grafts. The permissive microenvironment of micromass culture stimulates the formation of neocartilage similar in cellular distribution, extracellular matrix (ECM) composition, density, and ultrastructure to native hyaline cartilage [16, 31, 59]. BMP-2 plays an integral role in chondrocyte homeostasis and differentiation [16, 31, 51, 54], and reportedly increases type II collagen, proteoglycan, and aggrecan synthesis [53]. One method of providing bioactive factors such as BMP-2 is with genetic engineering and modification of biologic features of chondrocytes. We presume noncadaveric jCh genetically modified to amplify BMP-2 expression would form superior scaffold-independent grafts than unmodified or naive chondrocytes and would improve healing when transplanted directly into chondral lesions.
Table 1.
| Product | Description | Status |
|---|---|---|
| Carticela | First generation ACI with periosteal flap | Approved (US) |
| Matrix-induced ACIa (MACI) | ACI with seeding of chondrocytes onto surface of biodegradable type I/III collagen membrane | Phase III (Europe) |
| HYAFF 11/Hyalograft-Cb; Novocart 3Dc; BioSeed-Cd | ACI with seeding of chondrocytes by penetrating into hyaluronic acid derivative (Hyalograft C), collagen scaffold (Novocart 3D), or polyglycolic/polylactic acid polymer and fibrin glue (BioSeed-C) | Phase III (Europe/Asia; Hyalograft-C), I/II (Europe; BioSeed-C) |
| Chondrocelecte | Characterized chondrocytes selected for ACI | Phase III (Europe) |
| BST-CarGelf, VeriCarti | Adjunct to microfracture to retain mesenchymal stem cells; uses gel scaffold of chitosan and β-glycerophosphate that solidifies at body temperature (BST-CarGel) or double-structured collagen scaffold (VeriCart) | Phase II/III (US/Canada; BST-CarGel), I (US; Vericart) |
| Chondro-Gideh | Collagen type I/III membrane used for ACI or as adjunct to microfracture | Approved (Europe) |
| Neocarti, CaReSj | ACI with chondrocytes cultured in bovine (Neocart) or rat tail (CaRes) collagen type I scaffold. | Phase II (US; Neocart), Approved in Europe (CaReS) |
| Denovo NTk | Minced juvenile human allograft cartilage | Postlaunch/Phase IV (US) |
| Denovo ETk | Cultured scaffold-free juvenile human allograft cartilage implant | Phase I/II (US) |
| PEGylated fibrinogen/Gelrin Cl; ChonDuxm | Adjunct to microfracture; uses photopolymerizable hydrogel of polyethylene glycol-modified fibrinogen (Gelrin C) with chondroitin sulfate bioadhesive (ChonDux) that cross-links in situ after exposure to ultraviolet light | Preclinical development (Europe; Gelrin C); Phase II/III (Europe; ChonDux) |
| CAISn | Minced autologous cartilage with polyglycolic acid/polycaprolactone polymer with polydioxanone mesh | Phase I/II (US) |
ACI – autologous chondrocyte implantation; aGenzyme Biosurgery, Cambridge, MA, USA; bFidia Advanced Biopolymers, Abano Terme, Italy; cTissue Engineering Technologies AG, Reutlingen, Germany; dBiotissue Technologies, Freiburg, Germany; eTigenix, Leuven, Belgium; fBiosyntech, Quebec, Canada; gFidia Advanced Biopolymers, Abano Terme, Italy; hGeistlich, Wolhusen, Switzerland; iHistogenetics, Waltham, MA, USA; jArthro Kinetics, Esslingen, Germany; kZimmer, Warsaw, IN, USA; lRegentis Biomaterials, Haifa, Israel; mCartilix, Foster City, CA, USA; nDePuy Mitek, Raynham, MA, USA.
In an in vitro study we compared the size, viability, and histologic matrix formation of jCh neocartilage (1) with and without supplemented culture medium, (2) with and without adenoviral BMP-2 (AdBMP-2) transduction, and (3) with varying ratios (.1, .25, .5, .75, and 1) of transduced to naïve jCh in a mosaic graft. In an in vivo study we (4) compared healing of full-thickness articular cartilage defects using mosaic neocartilage (.25 ratio of transduced to naïve chondrocytes), naïve neocartilage, and ordinary marrow stimulation in athymic nude (immunosuppressed) rats.
Materials and Methods
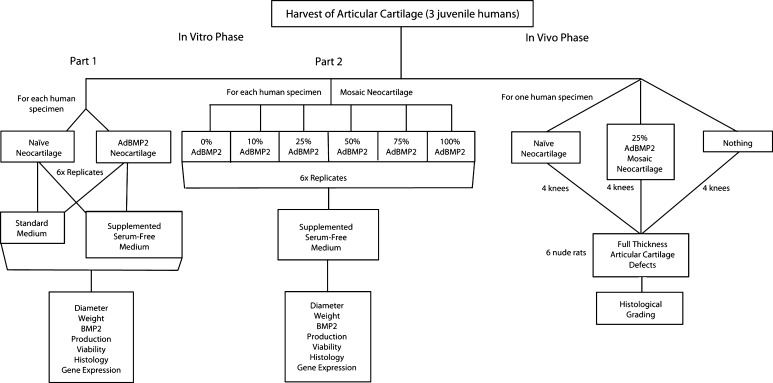
This study was designed as a pilot project to show proof of concept. We obtained specimens from three humans and performed three experiments (two in vitro, one in vivo). For each of the 10 in vitro treatment groups (four in Part 1, six in Part 2), we had six replicates for each human, and for each of the three in vivo treatment groups, we had four replicates for one human (Fig. 1).
Fig. 1.
The study design is shown in this flowchart.
In Part 1 of the in vitro phase, the effects on physical parameters of size and wet weight, soluble BMP-2 production, histologic appearance, and cellular viability were examined based on growth in supplemented serum-free medium compared with standard medium and with full AdBMP-2 transduction. In Part 2 of the in vitro phase, the effects of mosaic neocartilage (ratios of BMP-2 transduced chondrocytes to naïve chondrocytes in the graft of 0.1 to 0.9, 0.25 to 0.75, 0.5 to 0.5, 0.75 to .25, 0.9 to 0.1) on the same dependent variables were examined. For the in vivo phase, the histologic appearance of articular defect healing with one group of mosaic neocartilage was compared with traditional marrow stimulation and naïve neocartilage.
Informed, written parental consent was obtained in each case and all proceedings were in accordance with the Institutional Review Board (IRB) protocol #2009H0256, the Institutional Animal Care and Use Committees (IACUC) protocol #2009A0119, and the University Animal Care and Use Committee guidelines.
To isolate and expand articular chondrocytes we harvested articular cartilage from three patients whose digits or feet had been amputated for polydactyly or fibular hemimelia. All patients who underwent surgery were younger than 10 years, an important threshold for neocartilage potential as reported by Adkisson et al. [1]. All specimens were taken immediately from the surgical field and placed in standard media consisting of Dulbecco’s Modified Eagle’s Medium (DMEM), (Gibco, Grand Island, NY, USA), 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin. They were transported to the laboratory in a chilled container for same-day processing to optimize cellular yield [49].
Using a sterile sharp scalpel blade, all of the articular cartilage from the amputated appendage of each patient was minced into thin pieces, and washed three times with Gey’s balanced salt solution (GBSS). The minced cartilage was stirred in collagenase type I (2 mg/mL standard media) (Worthington Biochemical Corp, Lakewood, NJ, USA) and incubated at 37°C in a humidified atmosphere of 5% CO2 for 16 hours [22] to digest the cartilage matrix. The cell suspension was strained with a 100-μm pore cell strainer and centrifuged (600 g for 5 minutes) to obtain a cell pellet. After the pellet was washed twice with standard medium, the cells were resuspended and plated on T-75 flasks at a density of 1.3 × 104 cells/cm2.
When the chondrocytes became confluent, they were detached with 0.25% trypsin-EDTA (Gibco), reseeded on new T-75 flasks, and passaged no more than three times to attain adequate cell numbers. Of the chondrocyte batches gathered from different patients, three (19-month, 17-month, and 8 year-old males) were chosen for subsequent in vitro study based on greatest chondrocyte morphologic features when grown in high-density monolayer. Chondrocytes were counted with a hemocytometer and assessed with trypan blue dye exclusion for viability.
We constructed adenoviral (Ad) vectors for BMP-2 and green fluorescent protein (GFP) as previously described [27]. Briefly, Ad293 cells (a derivative of HEK293, human embryonic kidney cells, with improved cell adherence properties, modified to allow viral replication) (Agilent Technologies, Palo Alto, CA, USA) were expanded on T-75 flasks according to instructions. To minimize the risk of cell line contamination, the Ad293 cells were purchased directly from the manufacturer and used immediately on receipt. When the cell monolayer reached approximately 70% confluency, they were infected with the intended adenoviral stock at a multiplicity of infection (MOI) of 10 to 20. After approximately 3 to 4 days when the cells began to show cytopathic effects of the virus, they were collected for purification.
Purification of AdGFP and AdBMP-2 was accomplished using the Sartobind® filter unit from the AdEasy™ Virus Purification Kit (Agilent) according to manufacturer’s instructions. Infectious unit (IFU) titer was estimated using a 1:1000 viral particle: IFU ratio for the optical density at 260 nm with a spectrophotometer (Spectronic Biomate 5, Thermo Electron Corporation, Waltham, MA, USA). It then was verified using the Adeno-X™ Rapid Titer Kit (Clontech Laboratories Inc, San Francisco, CA, USA) as directed.
Chondrocytes were seeded at 4.3 × 104 cells/cm2 with standard media and grown in triplicate monolayer cultures for 24 hours to achieve approximately 80% confluency. AdBMP-2 or AdGFP was added directly to the culture with 65 μL/cm2 of warmed GBSS and swirled. After 4 hours of incubation at 37°C, the excess virus and GBSS were replaced with standard medium. A dose-response curve with 1 week of monolayer growth was created using AdBMP-2 and AdGFP to separately confirm an optimal MOI of 100 [11, 26, 27]. The transduction efficiency and gene expression of GFP was evaluated with a fluorescence microscope under blue light (488 nm). The soluble concentrations of BMP-2 were assessed using commercial competitive enzyme-linked immunosorbant assays (ELISA) (Quantikine1, R&D Systems, Minneapolis, MN, USA). The optical density of each BMP-2 sample was read by an Ultra Microplate Reader EL808 (Bio-Tek Instruments, Winooski, VT, USA) and expressed as picograms per milliliter.
Two chondrocyte treatment groups (naive versus AdBMP-2 transduction) and two growth medium groups (standard DMEM-based medium versus chondrocyte differentiation medium [CDM]) provided four groups of neocartilage cultures with six replicates each: naive-DMEM [ND]; naive-CDM [NC]; AdBMP-2-DMEM [BD]; AdBMP-2-CDM [BC]. The CDM (Lonza, Walkersville, MD, USA) was a serum-free basal medium supplemented with dexamethasone, ascorbate, insulin, transferrin, selenium, pyruvate, proline, gentamicin, amphotericin, L-glutamine, and 10 μg/mL transforming growth factor (TGF)-β.
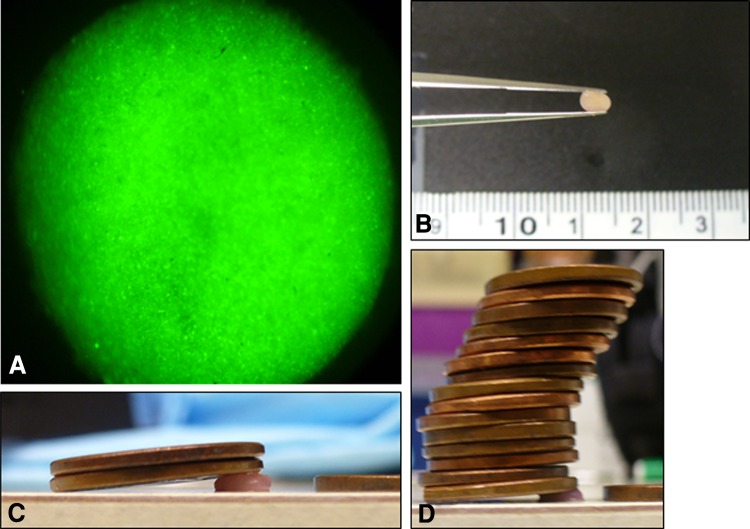
Twenty-four hours after AdBMP-2 transduction, the chondrocyte monolayer was treated with warmed trypsin for 5 minutes at 37°C. Naive chondrocytes were prepared in a similar fashion. The resultant cell suspension was pelleted and washed twice with standard medium. One million chondrocytes were placed in each of six 15-mL polypropylene conical tubes (BD Biosciences, Franklin Lakes, NJ, USA) with 2.5 mL of standard medium and centrifuged at 1500 rpm for 5 minutes. For the CDM groups, the standard medium was carefully replaced with CDM, and all were recentrifuged. The pellets were left undisturbed at 37°C in a humidifed atmosphere of 5% CO2. At 7 days, the medium was fully replaced and the pellets were elevated from the tip. At 14 days, the pellets were harvested. In addition, AdGFP pellets were created and grown for at least 8 weeks to confirm equal distribution of cells, persistent gene expression, and adequate mechanical strength (Fig. 2).
Fig. 2A–D.
Neocartilage pellets transduced with AdGFP show (A) excellent gene expression, (B) mechanical stability, and (C) compressive strength (D) at 8 weeks.
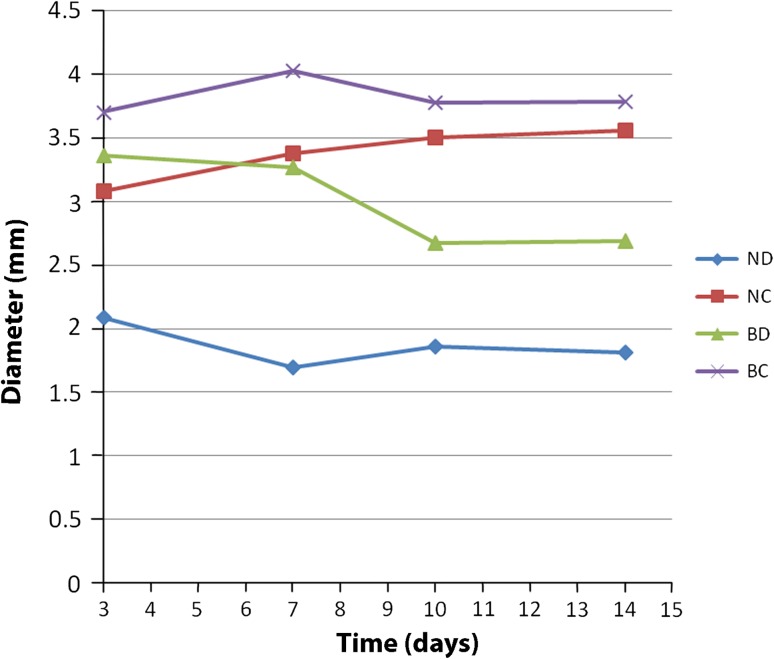
The diameters of the pellets were measured along their longest axis, by a treatment-blinded observer using a microscope, at 3, 7, 10, and 14 days [47]. Dry weight was obtained at the time of harvest. Absolute and viable cell counts were assessed by digesting the pellet with collagenase for 6 hours [22] and counting the cells with a hemocytometer and trypan blue dye exclusion. The soluble BMP-2 concentrations were measured at 7 and 14 days (Fig. 3) using an ELISA. For histologic evaluation with safranin-O and toluidine blue, each of the three pellets was fixed in 10% neutral buffered formalin (NBF) for 24 hours and embedded in paraffin. Four cross-sections (5 μm) were made from each pellet. Two of us (AB, KS) viewed each section under a light microscope. Chondrogenesis was scored according to the Bern score [21]. Briefly, the Bern score (minimum, 0; maximum, 9) was designed specifically for cartilaginous pellet cultures or engineered tissue sections, and evaluates three categories: uniformity of staining, amount of matrix formation, and type of cell morphology. It has been validated and has low intraobserver and interobserver variabilities (−0.06 ± 1.05 and −0.06 ± 0.17, respectively) [21, 48].
Fig. 3.
The growth of neocartilage pellets with time is shown. ND = naive chondrocytes, standard medium. NC = naive chondrocytes, chondrocyte differentiation medium. BD = AdBMP-2 transduced chondrocytes, standard medium. BC = AdBMP-2 transduced chondrocytes, chondrocyte differentiation medium.
Gene expression was analyzed quantitatively by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assays in triplicate. Each of three pellets was digested with collagenase for 6 hours [22], and the NucleoSpin® RNA XS kit (Clontech) was used according to the manufacturer’s instructions to isolate RNA. Complementary DNA was generated by reverse transcription using the TaqMan® (Applied Biosystems, Foster City, CA, USA) Reverse Transcription Reagents and thermal cycling parameters provided by the supplier. For PCR, primers and probes for type I collagen [Hs00164004_m1] and type II collagen [Hs00264051_m1] were purchased as TaqMan Gene Expression Assays (Assay ID in brackets). Relative expression levels for each gene were normalized to a eukaryotic 18S ribosomal RNA (rRNA) endogenous control [Hs99999901_s1]. Reagents from the TaqMan Universal Master Mix were used. Gene amplification was performed using an ABI Prism® 7000 Sequence Detection System (Applied Biosystems) at thermal cycling parameters specified by the supplier.
For the second part of the in vitro phase, six groups of six pellets from each patient with differing proportions of naive and AdBMP-2 chondrocytes were evaluated for the effect of mosaicism on viability, physical parameters, and gene expression. Similar to before, naive and AdBMP-2 chondrocytes were resuspended from monolayer. By centrifuging together a proportion from each suspension, mosaic pellets were created representing the following percentages of naive and AdBMP-2 chondrocytes: 25/75, 50/50, 75/25, 90/10. For comparison, two control groups of pellets, 0/100 and 100/0, were created. All pellets were grown for 2 weeks in CDM with medium replacement at 7 days. As in Part 1, diameter, weight, histology, cell count, BMP-2 production, and gene expression were assessed.
Six female athymic nude rats (10–12 weeks of age, Charles River Laboratories, Wilmington, MA, USA) were used to assess healing of three treatment groups: marrow stimulation alone (control), naive neocartilage graft, and 75/25 mosaic neocartilage graft.
As above, neocartilage pellets were cultivated in vitro for 2 weeks. Treatment groups were randomized to different knees and rats. Each rat had bilateral surgery. After 1 week of acclimation, the rats were induced under anesthesia with 5% isoflurane, then maintained with 1% isoflurane delivered via nose cone. The surgical site was prepared and draped in the usual sterile fashion. A medial parapatellar approach was performed and the trochlear groove exposed by subluxating the patella. A full-thickness articular cartilage defect of 2.5 mm diameter and 1.5 mm depth was created in the trochlear groove using a motorized burr (Dremel, Mount Prospect, IL, USA). In the control group (four knees), the defect received no treatment. Because of the thin nature of articular cartilage in rats, the subchondral bone invariably was penetrated, representing a marrow-stimulating technique. In the neocartilage treatment groups (four knees each), the assigned graft was press-fitted into the defect. The knee was put through a ROM with the patella reduced to assure stability of the graft. The arthrotomy was closed with absorbable suture and the skin with nylon.
Buprenorphine 0.05 mg/kg was administered intramuscularly for pain. Prophylactic Augmentin 0.35 mg/mL (GlaxoSmithKline, Research Triangle Park, NC, USA) was given 1 day preoperatively and 2 weeks postoperatively in the animals’ drinking water. The rats were allowed to roam freely in their cage. All rats tolerated the procedure well and there were no signs of infection or immunologic rejection at any time. None of the rats appeared to have lameness or loss of limb function.
At 8 weeks, the rats were euthanized with CO2. The articular surface of the knees was examined macroscopically and photographed. The distal femur was resected en bloc, fixed in NBF for 48 hours, harshly decalcified (Decalcifier-S, U.S. Biotex, Webbville, KY, USA) for 4 hours, sectioned in the sagittal plane, then further decalcified with 50% formic acid/20% sodium citrate for 48 hours. The specimen was paraffin-embedded, sectioned, and stained with toluidine blue. The histology was blindly reviewed by one trained pathologist (SB) and a specialist in cartilage research (ALB), and graded according to a modification of the scheme described by Cook et al. [14]. Prior studies have shown high intraobserver (Pearson r = 0.92–0.99) and interobserver (intraclass correlation, 0.94–0.99) reliabilities of elementary and complex histologic scoring systems for articular cartilage repair [38, 44].
Differences in quality of neocartilage formation between neocartilage grown in supplemented and ordinary medium, naïve and transduced neocartilage, neocartilage with varying ratios of transduced jCh, and articular cartilage healing in rats treated with a naïve neocartilage graft, a mosaic neocartilage graft, or no treatment were analyzed using a repeated measures ANOVA according to a fixed-effects model. An equal variance assumption was addressed by using the grouping option in Proc Mixed (Statistical Analysis System, Cary, NC, USA) model for estimation of each group variance separately to adjust the degrees of freedom for potential heteroscedasticity. A normality assumption was checked by using Anderson-Darling, Shapiro-Wilk, and Kolmogorov-Smirnov tests, where a p < 0.10 level was considered as the lack of normal distribution. To quantify the relative changes in gene expression, the comparative CT (ΔΔCT) method [34] was used, which first normalizes to a housekeeping gene, then generates the fold change between groups. All analyses were performed using the SAS 9.2 software package (Statistical Analysis System).
Results
AdBMP-2 neocartilage had larger (p < 0.001) diameter than naive neocartilage at Day 3, but by Day 10, pellets grown in CDM, regardless of transduction, were larger (p < 0.001) than those grown in standard medium (Fig. 4). At the time of harvest, NC and BC were heavier (p < 0.001) than ND and BD (Table 2). Viability was greater (p < 0.001) in naive than in AdBMP-2 transduced neocartilage regardless of growth medium. AdBMP-2 neocartilage produced more soluble BMP-2 than naïve neocartilage when grown in CDM (p = 0.0045). Based on both individual categories and summation of the Bern score, there was a histologic difference (p ≤ 0.047) between each group. Neocartilage grown in standard medium did not produce substantial cartilage-specific extracellular matrix, had predominantly fibroblast-like morphologic features, and showed limited improvement of staining intensity with AdBMP2-transduction. The mean ratio of collagen type II to I gene expression was similar between NC and BC, and higher (p ≤ 0.0043) than ND and BD.
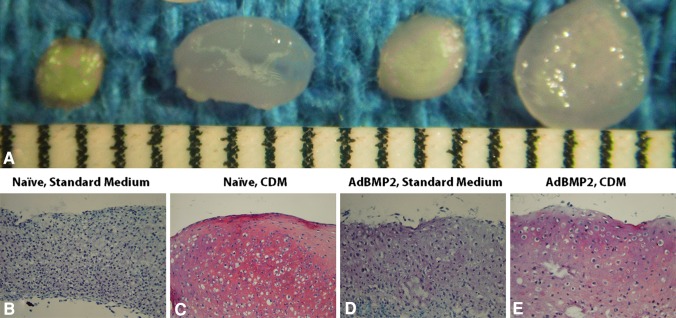
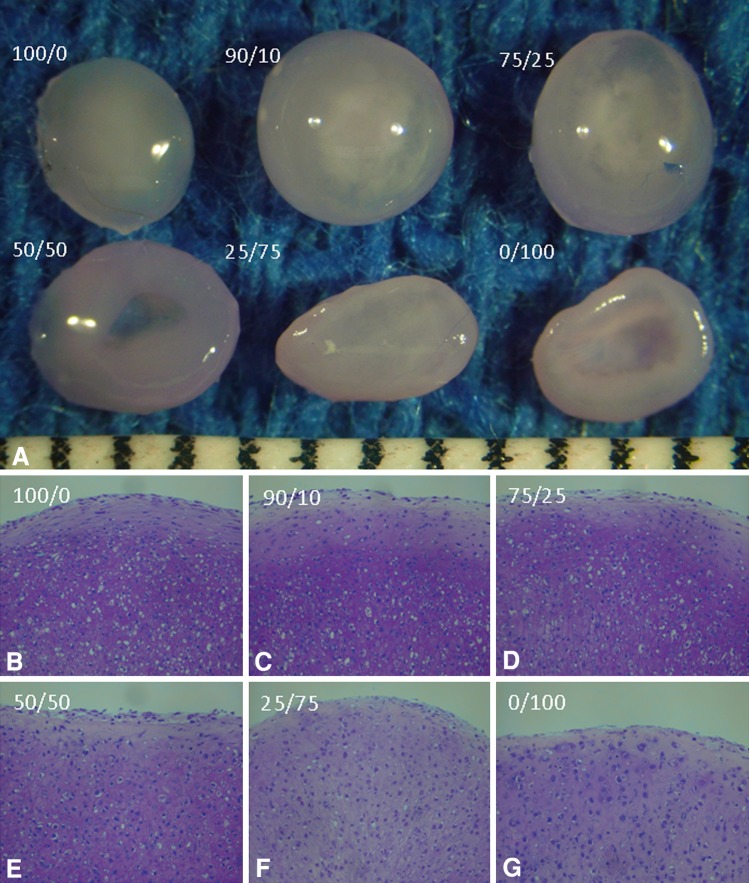
Fig. 4A–E.
(A) Macroscopic and histologic comparisons of the (B) naïve, standard medium, (C) naïve, CDM, (D) AdBMP2, standard medium, and (E) AdBMP2, CDM treatment groups for Part 1 of the in vitro phase are shown (Stain, safranin O; original magnification, ×20). CDM = chondrocyte differentiation medium. Scale = 1 mm.
Table 2.
Summary of juvenile human chondrocyte neocartilage comparing supplemented medium and adenoviral BMP-2 transduction
| Measurement | Growth condition | |||
|---|---|---|---|---|
| Naïve-DMEM | Naïve-CDM | AdBMP-2-DMEM | AdBMP-2-CDM | |
| Weight | 2.2 ± 1.3 mg | 7.9 ± 1.6 mg | 6.1 ± 0.6 mg | 8.0 ± 1.4 mg |
| Viability | 90% ± 6% | 90% ± 7% | 70% ± 12% | 76% ± 8% |
| BMP-2 production (Day 14) | 175 ± 191 pg/mL | 43 ± 38 pg/mL | 6.2 ± 6 ng/mL | 619 ± 345 ng/mL |
| Bern score total | 1.8 ± 1.5 | 8.0 ± 1.0 | 3.7 ± 1.8 | 5.2 ± 2.8 |
| Collagen type II:I ratio | 0.56 ± 0.07 | 2.4 ± 0.05 | 0.70 ± 0.42 | 2.3 ± 0.09 |
Mean ± SD; DMEM = standard medium, consisting of Dulbecco’s Modified Eagle’s Medium, 10% fetal bovine serum, 1% penicillin-streptomycin; CDM = supplemented medium, consisting of a serum-free basal medium with dexamethasone, ascorbate, insulin, transferrin, selenium, pyruvate, proline, gentamicin, amphotericin, L-glutamine, and 10 μg/ml TGF-β; Naïve = untransduced chondrocytes; AdBMP-2 = adenoviral BMP-2 transduced juvenile human articular chondrocytes.
All percentages of AdBMP-2 transduction increased the diameter (p < 0.001 to p = 0.0521) of neocartilage over the naive pellet (Fig. 5). The mean weights of the 90/10, 75/25, and 50/50 mosaics were greater (p < 0.001 to p = 0.0099) than those of the 25/75 mosaic and 100/0 or 0/100 pellets (Table 3). The viability decreased with greater than 50% AdBMP-2 transduced chondrocytes. The mean percent viability of 100/0, 90/10, and 75/25 mosaics were greater (p = .0018 to 0.0478) compared with 25/75 and 0/100. Naive neocartilage produced a negligible concentration of soluble BMP-2. Soluble BMP-2 concentrations were similar (p = 0.0311 to 0.5237). Histologically, the 90/10, 75/25, and 50/50 mosaics had higher mean Bern scores (p = 0.0038 to 0.0443) than those of the 100/0, 25/75, and 0/100 groups. Substantial extracellular matrix, rounded chondrocyte morphologic features, and intense cartilage staining were seen in the superior groups. The collagen type II: I ratio was greatest (p < 0.001 to p = 0.0169) in the 90/10 mosaic.
Fig. 5A–G.
(A) Macroscopic and (B) histologic comparisons of 100, (C) 90/10, (D) 75/25, (E) 50/50, (F) 25/75, and (G) 0/100 naïve/AdBMP-2 chondrocytes for Part 2 of the in vitro phase are shown (Stain, toluidine blue; original magnification, ×20). % of naive chondrocytes/% of AdBMP-2 transduced chondrocytes. Scale = 1 mm.
Table 3.
Summary of juvenile human chondrocyte neocartilage comparing varying ratios of adenoviral BMP-2 transduction
| Measurement | Percent ratio of naïve: AdBMP-2 neocartilage | |||||
|---|---|---|---|---|---|---|
| 100/0 | 90/10 | 75/25 | 50/50 | 25/75 | 0/100 | |
| Diameter | 3.2 ± 0.5 mm | 3.5 ± 0.5 mm | 3.6 ± 0.5 mm | 3.5 ± 0.4 | 3.3 ± 0.5 | 3.4 ± 0.2 |
| Weight | 7.4 ± 1.1 mg | 12.7 ± 3.9 mg | 11.0 ± 2.7 mg | 10.2 ± 3.7 mg | 7.5 ± 2.7 mg | 7.4 ± 4.0 mg |
| Viability | 85% ± 7% | 84% ± 8% | 83% ± 8% | 78% ± 9% | 71% ± 8% | 75% ± 10% |
| BMP-2 production | 99 ± 54 pg/mL | 66 ng/mL | 170 ng/mL | 319 ng/mL | 490 ng/mL | 587 ng/mL |
| Bern score total | 5.5 ± 1.1 | 6.8 ± 1.5 | 6.5 ± 0.6 | 6.7 ± 1.2 | 5.5 ± 1.3 | 5.3 ± 1.0 |
| Collagen type II:I ratio* | 1.0 ± 0 | 1.8 ± 0.5 | 1.2 ± 0.5 | 0.78 ± 0.5 | 0.3 ± 0.1 | 0.4 ± 0.2 |
Mean ± SD; * relative to control, 100/0; Naïve = untransduced chondrocytes; AdBMP-2 – adenoviral BMP-2 transduced juvenile human articular chondrocytes.
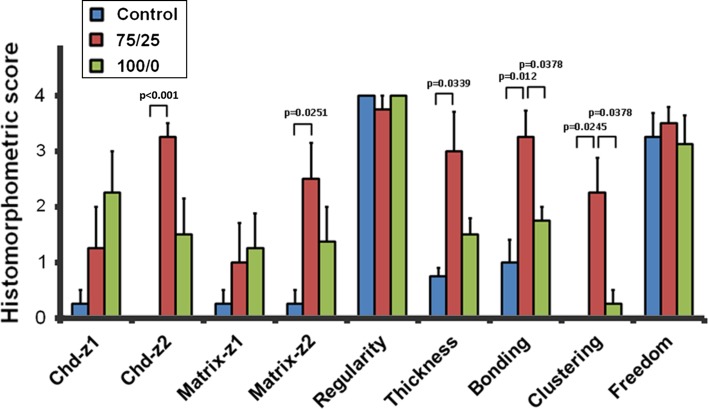
Macroscopically, the locations of the defects were still clearly visible after the 8-week duration to the control (Fig. 6), naïve neocartilage (Fig. 7), and mosaic neocartilage (Fig. 8). Histologically, all groups produced a repair tissue in the defect with excellent surface regularity and the immediately adjacent cartilage was generally free from degenerative change (Fig. 9). In the control group representing marrow stimulation, only a thin layer of fibrous tissue without any chondrocyte morphology lay superficial to the subchondral bone. There were areas where the medullary vasculature appeared to flow into the fibrous repair tissue. Staining of the early matrix was inferior to mature adjacent cartilage, but in the 75/25 mosaic group, a calcified cartilage layer and tidemark could be seen. The thickness of repair tissue, morphologic features of the chondrocytes in the central zone, and bonding to adjacent cartilage were superior in the 75/25 mosaic neocartilage group.
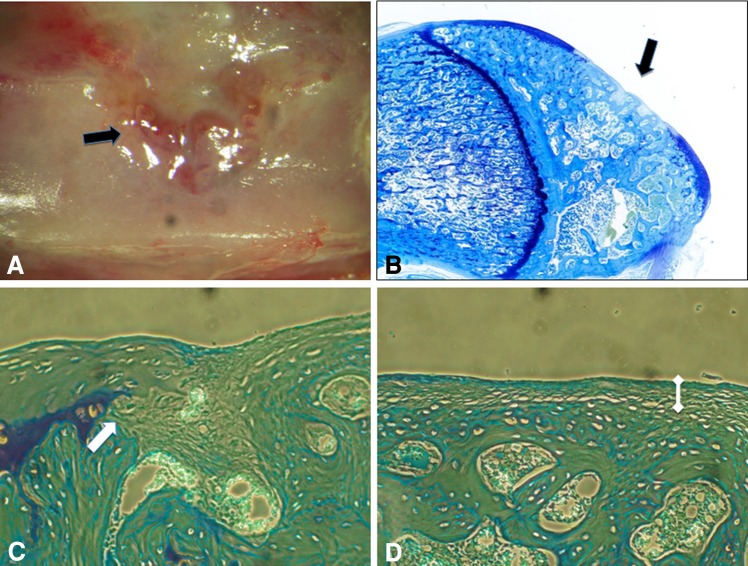
Fig. 6A–D.
(A) Macroscopic and (B) toluidine blue (Original magnification, ×4) staining appearances of the defect (black arrows) in the control group are shown. (C) The edge of the defect (white arrow) and peripheral zone of the repair tissue are shown (Stain, toluidine blue; original magnification, ×20). (D) The central zone of the defect can be seen in this illustration (Stain, toluidine blue; original magnification, ×20). There is thin, fibrous repair tissue (white dumbbell) directly overlying the subchondral bone in the central zone.
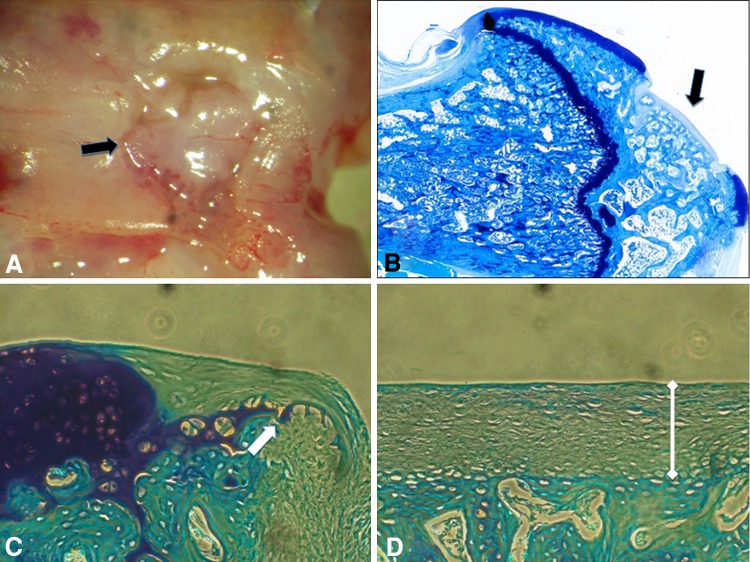
Fig. 7A–D.
(A) The macroscopic appearance of the defect (black arrows) is shown in the group treated with the 100/0 neocartilage (original magnification, ×4). (B) The toluidine blue staining appearance of the defect (black arrow) is shown in the group treated with the 100/0 neocartilage (Original magnification, ×4). (C) The edge of the defect (white arrow) and peripheral zone of the repair tissue are shown (Stain, toluidine blue; original magnification, ×20). (D) The central zone of the defect is seen in this illustration (Stain, toluidine blue; original magnification, ×20). There is a thicker layer of repair tissue (white dumbbell) and difference in its early cartilaginous appearance compared with that seen in Figure 5D.
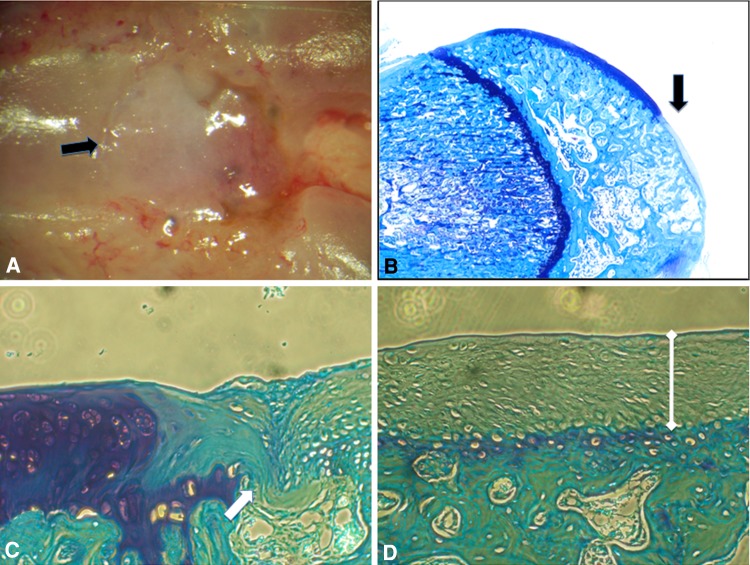
Fig. 8A–D.
(A) The macroscopic appearance of the defect (black arrow) in the group treated with the 75/25 neocartilage is shown (original magnification, ×4). (B) The toluidine blue staining appearance of the defect (black arrow) in the group treated with the 75/25 neocartilage are shown (Original magnification, ×4). (C) The edge of the defect (white arrow) and peripheral zone of the repair tissue are shown (Stain, toluidine blue; original magnification, ×20). (D) The central zone of the defect is shown (Stain, toluidine blue; original magnification, ×20). There is a thicker layer of repair tissue (white dumbbell) and difference in early cartilaginous appearance compared with that seen in Figure 5D. Early formation of a calcified cartilage zone (white bracket) and tidemark demarcating the separation from the more superficial zones can be seen.
Fig. 9.
A graphic representation of the histologic findings for in vivo healing of chondral defects is shown. Chd-z = chondrocyte morphology zone [14].
Discussion
Current research has explored numerous avenues for improving first-generation ACI including type of scaffolding material, source of cell for implantation, method of culture, delivery of beneficial factors, and alteration of genetic behavior [15, 23, 32, 33]. However, no technique has shown full hyaline restoration of cartilage lesions. The rationale for this study was to examine whether genetically augmenting jCh could improve neocartilage formation and healing of full-thickness articular defects.
The specific purposes were to compare in vitro the size, viability, and quality of matrix formation of jCh neocartilage (1) with and without supplemented culture medium, (2) with and without adenoviral BMP-2 (AdBMP-2) transduction, and (3) with varying ratios of transduced and naïve jCh in a mosaic graft. In vivo, the purpose was to compare (4) healing of full-thickness articular cartilage defects using mosaic neocartilage, naïve neocartilage, and ordinary marrow stimulation in immunosuppressed rats.
Limitations of this study include the following. First, we had a relatively short duration of in vivo followup. The course of cartilage restoration can require more than a year in large animals and humans [20, 29], but in a small animal model, reparative processes are accelerated [2, 43]. Marrow stimulating studies in rats [3, 43] have shown attainment of full histologic improvement as early as 4 weeks and stabilization of scores for as long as 24 weeks. In our study with neocartilage, although the repair tissue at 8 weeks lacks the same proteoglycan staining properties as normal adjacent tissue, there is evidence that attainment of full hyaline cartilage in ACI is an ongoing and lengthy process in which the quality of regeneration improves with time after grafting [20]. Second, we lacked a null adenoviral (AdNull) control. Although AdNull vectors have been described in some studies to control for the effect of adenoviral infection alone [19, 60], there is not a strong precedent for their use in cartilage research. Preliminary findings with AdGFP transduction and GFP production revealed its inadequacy as a neutral control for AdBMP-2 and expression of cartilage markers. Third, this study represents a pilot project to show proof of principle. The sample size of human donors is relatively small and the neocartilage potential of chondrocytes may vary among individuals. However, prior work [1] has shown an important threshold in juvenile chondrocytes that distinguishes their chondrocytes from adults. There are clear translational limitations with the rat model, but it is ideal for a cost-effective experimental design and immunosuppressed environment [13]. Additional studies are necessary to reveal hurdles necessary to apply this concept to humans.
In the in vitro phase of this study, jCh growth in serum-free supplemented medium and AdBMP-2 transduction enhanced neocartilage formation over standard medium and naive jCh, respectively. AdBMP-2 transduction reduced cellular viability in neocartilage. Numerous studies have shown an effect of age on chondrocyte proliferation and postexpansion chondrogenic capacity [6], quality of cartilage formation [1, 3], production of cell-associated matrix [30], and senescence markers [37]. Recently, one group reported 100-fold higher proteoglycan production and collagen type II mRNA expression in jCh [3], and a positive interaction on adult chondral fragments when cocultured with juvenile cartilage [9]. Viral transduction cytotoxicity has been cited as a concern [39]. Although equine chondrocytes grown in monolayer are relatively hardy at one week [26], human jCh in neocartilage may be more susceptible as shown here.
The cytotoxic effect of adenoviral transduction can be minimized by creating mosaic grafts and reducing apoptosis to levels similar to those of traditional ACI [59]. Varying proportions of AdBMP-2 transduced and naive jCh were trialed to grow neocartilage with the goal of maximizing cellular viability, maintaining soluble BMP-2 production, and optimizing histologic scores. Superior outcomes were observed in mosaic neocartilage of 75% naive jCh – 25% AdBMP-2 transduced jCh and 90% naive jCh – 10% AdBMP-2 transduced jCh. The concept of “instructor” cells in a micromass coculture has been described [17]. In an embryonic stem cell study, only 25% of the constituent cells were required to express chondroinductive protein to induce paracrine conversion to a chondrocyte phenotype [12]. The possibility of an off-the-shelf allogeneic graft is improved not only by the immunoprivileged nature of the articular environment, but also by passive and active mechanisms of juvenile neocartilage. Sequestration of the chondrocytes in their self-produced matrix before implantation can decrease immune response and host resorption compared with isolated allogeneic chondrocytes [40]. Juvenile chondrocytes do not stimulate, and may even suppress, host lymphocyte proliferation [2, 3]. De novo tissue, or neocartilage, is not only intuitively appealing as the next step in orthopaedic bioengineering beyond scaffold-dependent technology, but may prove to be clinically superior [7]. In vitro, neocartilage differs from native hyaline cartilage only in fibril orientation secondary to the lack of physiologic mechanical stimulation [16, 31, 58]. There is evidence that a neocartilage allograft may be able to adapt, mature, and remodel after implantation [35]. Although formation of extraordinary shapes and sizes of neocartilage has been proven using rabbit chondrocytes [41, 42], it is considerably more difficult with human jCh [7].
For the in vivo phase of this study, 75/25 mosaic neocartilage grafts placed into full-thickness articular defects resulted in better histologic findings at 8 weeks compared with naive neocartilage grafts and to marrow stimulation alone. In the neocartilage treatment groups, chondrons with multiple chondrocytes each could be seen in the adjacent intact cartilage, suggesting division and early clonal proliferation [46]. A member of the TGF superfamily, BMP-2 reportedly correlates with clinical improvement [50], plays a role in intrinsic and surgically induced cartilage repair [8, 50], and reduces gaps between reparative and host cartilage [52]. Because pellet growth is primarily attributable to matrix formation and not chondrocyte proliferation [36], chondroactive factors may be helpful in augmenting size of neocartilage formation. Direct supplementation of recombinant protein is limited by a short in vivo half-life, and in vivo transduction with viral vectors to sites of cartilage damage is notoriously difficult [5, 45, 53]. Ex vivo therapeutic gene transfer is a safe, controlled process and is useful for transducing multiple proteins including BMP-2, BMP-7, and insulin-like growth factor-1 [5, 11, 12, 18, 24, 25, 53]. It is more targeted and readily achievable for cartilage applications than in vivo gene transfer [5, 53]. The adenoviral vector remains episomal and does not incorporate into the host chromosome, is approved for use in clinical trials and has been explored in other areas of medicine [56], infects dividing and nondividing cells, is replication-defective, has only the potential to cause the common cold in humans, and has elevated, prolonged levels of transgene expression [54]. Although recombinant adeno-associated virus has been used to a lesser extent in cartilage research [57] and is less immunogenic [28], it is difficult to manufacture and has lower transduction efficiency [54]. As with any new technique, additional animal studies are necessary to detect potential side effects associated with the viral vector or with elevated production of recombinant growth factor.
Human jCh grown in serum-free supplemented medium and transduced with AdBMP-2 were superior in vitro and in vivo when combined in low proportions with naïve jCh to form mosaic neocartilage grafts. In light of our findings, future goals include longer in vivo followup and larger animal models.
Acknowledgments
We thank Joel L. Mayerson MD, and David C. Flanigan MD, for clinical support; Akikazu Ishihara PhD, for statistical support; Alan D. Fletchner BS, HTL (ASCP), for histology support; Eric Skinner BS, and Samantha Dyer for technical assistance; and M.M. Manring PhD, for editorial support.
Footnotes
The Dallas B. Phemister Award of the Mid-American Orthopaedic Association.
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This study was performed at the Comparative Orthopaedics Research Laboratory, The Ohio State University, Columbus, OH, USA
References
- 1.Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001;391(suppl):S280–S294. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 2.Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR. Immune evasion by neocartilage-derived chondrocytes: implications for biologic repair of joint articular cartilage. Stem Cell Res. 2010;4:57–68. doi: 10.1016/j.scr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Adkisson HD, 4th, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, Seyedin M, Amendola A, Streeter PR, Buckwalter JA. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38:1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16:305–329. doi: 10.1089/ten.teb.2009.0590. [DOI] [PubMed] [Google Scholar]
- 5.Arai M, Anderson D, Kurdi Y, Annis-Freeman B, Shields K, Collins-Racie LA, Corcoran C, DiBlasio-Smith E, Pittman DD, Dorner AJ, Morris E, LaVallie ER. Effect of adenovirus-mediated overexpression of bovine ADAMTS-4 and human ADAMTS-5 in primary bovine articular chondrocyte pellet culture system. Osteoarthritis Cartilage. 2004;12:599–613. doi: 10.1016/j.joca.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Barbero A, Grogan S, Schafer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrocyte capacity. Osteoarthritis Cartilage. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein P, Dong M, Corbeil D, Gelinsky M, Gunther KP, Fickert S. Pellet culture elicits superior chondrogenic redifferentiation than alginate-based systems. Biotechnol Prog. 2009;25:1146–1152. doi: 10.1002/btpr.186. [DOI] [PubMed] [Google Scholar]
- 8.Blaney Davidson EN, Vitters EL, van Lent PL, van de Loo FA, van den Berg WB, van der Kraan PM. Elevated extracellular matrix production and degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair and remodeling. Arthritis Res Ther. 2007;9:R102. doi: 10.1186/ar2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonasia DE, Martin JA, Marmotti A, Amendola RL, Buckwalter JA, Rossi R, Blonna D, Adkisson HD, 4th, Amendola A. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. Am J Sports Med. 2011;39:2355–2361. doi: 10.1177/0363546511417172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- 11.Brower-Toland BD, Saxer RA, Goodrich LR, Mi Z, Robbins PD, Evans CH, Nixon AJ. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001;12:117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- 12.Carlberg AL, Pucci B, Rallapalli R, Tuan RS, Hall DJ. Efficient chondrogenic differentiation of mesenchymal cells in micromass culture by retroviral gene transfer of BMP-2. Differentiation. 2001;67:128–138. doi: 10.1046/j.1432-0436.2001.670405.x. [DOI] [PubMed] [Google Scholar]
- 13.Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16:105–115. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook SD, Patron LP, Salkeld SL, Rueger DC. Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg Am. 2003;85(suppl 3):116–123. doi: 10.2106/00004623-200300003-00018. [DOI] [PubMed] [Google Scholar]
- 15.Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469:2696–2705. doi: 10.1007/s11999-010-1764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannoni P, Cancedda R. Articular chondrocyte culturing for cell-based cartilage repair: needs and perspectives. Cells Tissues Organs. 2006;184:1–15. doi: 10.1159/000096946. [DOI] [PubMed] [Google Scholar]
- 17.Giovannini S, Diaz-Romero J, Aigner T, Heini P, Mainil-Varlet P, Nesic D. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater. 2010;20:245–259. doi: 10.22203/ecm.v020a20. [DOI] [PubMed] [Google Scholar]
- 18.Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007;89:672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- 19.Gordon JW. Adenovirus gene transfer vector toxicity to mouse embryos: implications for human IVF. Hum Reprod. 2002;17:2380–2387. doi: 10.1093/humrep/17.9.2380. [DOI] [PubMed] [Google Scholar]
- 20.Grigolo B, Roseti L, De Franceschi L, Piacentini A, Cattini L, Manfredini M, Faccini R, Facchini A. Molecular and immunohistological characterization of human cartilage two years following autologous cell transplantation. J Bone Joint Surg Am. 2005;87:46–57. doi: 10.2106/JBJS.C.01685. [DOI] [PubMed] [Google Scholar]
- 21.Grogan SP, Barbero A, Winkelmann V, Rieser F, Fitzsimmons JS, O’Driscoll S, Martin I, Mainil-Varlet P. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng. 2006;12:2141–2149. doi: 10.1089/ten.2006.12.2141. [DOI] [PubMed] [Google Scholar]
- 22.Hayman DM, Blumberg TJ, Scott CC, Athanasiou KA. The effects of isolation on chondrocyte gene expression. Tissue Eng. 2006;12:2573–2581. doi: 10.1089/ten.2006.12.2573. [DOI] [PubMed] [Google Scholar]
- 23.Hettrich CM, Crawford D, Rodeo SA. Cartilage repair: third-generation cell-based technologies: basic science, surgical techniques, clinical outcomes. Sports Med Arthrosc. 2008;16:230–235. doi: 10.1097/JSA.0b013e31818cdc98. [DOI] [PubMed] [Google Scholar]
- 24.Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. 2003;21:573–583. doi: 10.1016/S0736-0266(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda T, Kubo T, Nakanishi T, Arai Y, Kobayashi K, Mazda O, Ohashi S, Takahashi K, Imanishi J, Takigawa M, Hirasawa Y. Ex vivo gene delivery using an adenovirus vector in treatment for cartilage defects. J Rheumatol. 2000;27:990–996. [PubMed] [Google Scholar]
- 26.Ishihara A, Zachos TA, Bartlett JS, Bertone AL. Evaulation of permissiveness and cytotoxic effects in equine chondrocytes, synovial cells, and stem cells in response to infection with adenovirus 5 vectors for gene delivery. Am J Vet Res. 2006;67:1145–1155. doi: 10.2460/ajvr.67.7.1145. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara A, Zekas LJ, Litsky AS, Weisbrode SE, Bertone AL. Dermal fibroblast-mediated BMP2 therapy to accelerate bone healing in an equine osteotomy model. J Orthop Res. 2010;28:403–411. doi: 10.1002/jor.20978. [DOI] [PubMed] [Google Scholar]
- 28.Ivkovic A, Pascher A, Hudetz D, Maticic D, Jelic M, Dickinson S, Loparic M, Haspl M, Windhager R, Pecina M. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther. 2010;17:779–789. doi: 10.1038/gt.2010.16. [DOI] [PubMed] [Google Scholar]
- 29.Jubel A, Andermahr J, Schiffer G, Fischer J, Rehm KE, Stoddart MJ, Hauselmann HJ. Transplantation of de novo scaffold-free cartilage implants into sheep knee chondral defects. Am J Sports Med. 2008;36:1555–1564. doi: 10.1177/0363546508321474. [DOI] [PubMed] [Google Scholar]
- 30.Kamada H, Masuda K, D’Souza AL, Lenz ME, Pietryla D, Otten L, Thonar EJ. Age-related differences in the accumulation and size of hyaluronan in alginate culture. Arch Biochem Biophys. 2002;408:192–199. doi: 10.1016/S0003-9861(02)00543-X. [DOI] [PubMed] [Google Scholar]
- 31.Kerker JT, Leo AJ, Sgaglione NA. Cartilage repair: synthetics and scaffolds: basic science, surgical techniques, and clinical outcomes. Sports Med Arthrosc. 2008;16:208–216. doi: 10.1097/JSA.0b013e31818cdbaa. [DOI] [PubMed] [Google Scholar]
- 32.Kessler MW, Ackerman G, Dines JS, Grande D. Emerging technologies and fourth generation issues in cartilage repair. Sports Med Arthrosc. 2008;16:246–254. doi: 10.1097/JSA.0b013e31818d56b3. [DOI] [PubMed] [Google Scholar]
- 33.Kon E, Delcogliano M, Filardo G, Montaperto C, Marcacci M. Second generation issues in cartilage repair. Sports Med Arthrosc. 2008;16:221–229. doi: 10.1097/JSA.0b013e31818cdbc5. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Lu Y, Adkisson HD, Bogdanske J, Kalscheur V, Maloney W, Cheung R, Grodzinsky AJ, Hruska KA, Markel MD. In vivo transplantation of neonatal ovine neocartilage allografts: determining the effectiveness of tissue transglutaminase. J Knee Surg. 2005;18:31–42. doi: 10.1055/s-0030-1248155. [DOI] [PubMed] [Google Scholar]
- 36.Lubke C, Ringe J, Krenn V, Fernahl G, Pelz S, Kreusch-Brinker R, Sittinger M, Paulitschke M. Growth characterization of neo porcine cartilage pellets and their use in an interactive culture model. Osteoarthritis Cartilage. 2005;13:478–487. doi: 10.1016/j.joca.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85:106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- 38.Moojen DJ, Saris DB, Auw Yang KG, Dhert WJ, Verbout AJ. The correlation and reproducibility of histological scoring systems in cartilage repair. Tissue Eng. 2002;8:627–634. doi: 10.1089/107632702760240544. [DOI] [PubMed] [Google Scholar]
- 39.Morrey ME, Anderson PA, Chambers G, Paul R. Optimizing nonviral-mediated transfection of human intervertebral disc chondrocytes. Spine J. 2008;8:796–803. doi: 10.1016/j.spinee.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Moskalewski S, Hyc A, Osiecka-Iwan A. Immune response by host after allogeneic chondrocyte transplant to the cartilage. Microsc Res Tech. 2002;58:3–13. doi: 10.1002/jemt.10110. [DOI] [PubMed] [Google Scholar]
- 41.Nagai T, Furukawa KS, Sato M, Ushida T, Mochida J. Characteristics of a scaffold-free articular chondrocyte plate grown in rotational culture. Tissue Eng Part A. 2008;14:1183–1193. doi: 10.1089/ten.tea.2007.0114. [DOI] [PubMed] [Google Scholar]
- 42.Nagai T, Sato M, Furukawa KS, Kutsuna T, Ohta N, Ushida T, Mochida J. Optimization of allograft implantation using scaffold-free chondrocyte plates. Tissue Eng Part A. 2008;14:1225–1235. doi: 10.1089/ten.tea.2007.0225. [DOI] [PubMed] [Google Scholar]
- 43.Nishimori M, Deie M, Kanaya A, Exham H, Adachi N, Ochi M. Repair of chronic osteochondral defects in the rat: a bone marrow-stimulating procedure enhanced by cultured allogenic bone marrow mesenchymal stromal cells. J Bone Joint Surg Br. 2006;88:1236–1244. doi: 10.1302/0301-620X.88B9.17810. [DOI] [PubMed] [Google Scholar]
- 44.Orth P, Zurakowski D, Wincheringer D, Madry H. Reliability, reproducibility, and validation of five major histological scoring systems for experimental articular cartilage repair in the rabbit model. Tissue Eng Part C Methods. 2012;18:329–339. doi: 10.1089/ten.tec.2011.0462. [DOI] [PubMed] [Google Scholar]
- 45.Palmer GD, Gouze E, Gouze JN, Betz OB, Evans CH, Ghivizzani SC. Gene transfer to articular chondrocytes with recombinant adenovirus. Methods Mol Biol. 2003;215:235–246. doi: 10.1385/1-59259-345-3:235. [DOI] [PubMed] [Google Scholar]
- 46.Poole CA. Articular cartilage chondrons: form, function and failure. J Anat. 1997;191:1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rui YF, Du L, Wang Y, Lui PP, Tang TT, Chan KM, Dai KR. Bone morphogenetic protein 2 promotes transforming growth factor β3-induced chondrogenesis of human osteoarthritic synovium-derived stem cells. Chin Med J (Engl). 2010;123:3040–3048. [PubMed] [Google Scholar]
- 48.Rutgers M, van Pelt MJ, Dhert WJ, Creemers LB, Saris DB. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18:12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Schachar NS, Cucheran DJ, McGann LE, Novak KA, Frank CB. Metabolic activity of bovine articular cartilage during refrigerated storage. J Orthop Res. 1994;12:15–20. doi: 10.1002/jor.1100120103. [DOI] [PubMed] [Google Scholar]
- 50.Schmal H, Niemeyer P, Zwingmann J, Stoffel F, Sudkamp NP, Mehlhorn AT. Association between expression of the bone morphogenetic proteins 2 and 7 in the repair of circumscribed cartilage lesions with clinical outcome. BMC Musculoskeletal Disord. 2010;11:170. doi: 10.1186/1471-2474-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitt B, Ringe J, Haupl T, Notter M, Manz R, Burmester GR, Sittinger M, Kaps C. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation. 2003;71:567–577. doi: 10.1111/j.1432-0436.2003.07109003.x. [DOI] [PubMed] [Google Scholar]
- 52.Sellers RS, Zhang R, Glasson SS, Kim HD, Peluso D, D’Augusta DA, Beckwith K, Morris EA. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2) J Bone Joint Surg Am. 2000;82:151–160. doi: 10.1302/0301-620X.82B1.10733. [DOI] [PubMed] [Google Scholar]
- 53.Smith P, Shuler FD, Georgescu HI, Ghivizzani SC, Johnstone B, Niyibizi C, Robbins PD, Evans CH. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43:1156–1164. doi: 10.1002/1529-0131(200005)43:5<1156::AID-ANR26>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 54.Steinert AF, Noth U, Tuan RS. Concepts in gene therapy for cartilage repair. Injury. 2008;39(suppl 1):S97–S113. doi: 10.1016/j.injury.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stroh DA, Johnson AJ, Mont MA. Surgical implants and technologies for cartilage repair and preservation of the knee. Expert Rev Med Devices. 2011;8:339–356. doi: 10.1586/erd.11.13. [DOI] [PubMed] [Google Scholar]
- 56.Vorburger SA, Hunt KK. Adenoviral gene therapy. Oncologist. 2002;7:46–59. doi: 10.1634/theoncologist.7-1-46. [DOI] [PubMed] [Google Scholar]
- 57.Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, Mizukami H, Ozawa K, Koshino T. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52:164–170. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, McCaffery JM, Spencer RG, Francomano CA. Hyaline cartilage engineered by chondrocytes in pellet culture: histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants. J Anat. 2004;205:229–237. doi: 10.1111/j.0021-8782.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng MH, King E, Kirilak Y, Huang L, Papadimitriou JM, Wood DJ, Xu J. Molecular characterisation of chondrocytes in autologous chondrocyte implantation. Int J Mol Med. 2004;13:623–628. doi: 10.3892/ijmm.13.5.623. [DOI] [PubMed] [Google Scholar]
- 60.Zhou SY, Xie ZL, Xiao O, Yang XR, Heng BC, Sato Y. Inhibition of mouse alkali burn induced-corneal neovascularization by recombinant adenovirus encoding human vasohibin-1. Mol Vis. 2010;16:1389–1398. [PMC free article] [PubMed] [Google Scholar]