Abstract
Background
Local delivery of antifungals is an important modality in managing orthopaedic fungal infection. Voriconazole is a powder antifungal suitable for addition to bone cement that is released from bone cement but the mechanical properties of antimicrobial-loaded bone cement (ALBC) made with voriconazole are unknown.
Questions/Purposes
(1) Is voriconazole release dose-dependent? (2) Is released voriconazole active? (3) Is the loss of ALBC’s compressive strength caused by voriconazole dose- and elution-dependent?
Methods
Sixty standard test cylinders were fabricated with ALBC: 300 or 600 mg voriconazole per batch eluted for 30 days in deionized water. Voriconizole concentration in the eluate was measured using high-performance liquid chromatography. Cumulative-released voriconizole was calculated. Biologic activity was tested. Compressive strength was measured before and after elution. The effect of dose and time on release and compressive strength were analyzed using repeated-measure analysis of variance.
Results
Fifty-seven percent and 63% of the loaded voriconazole were released by Day 30 for the 300-mg and 600-mg formulations, respectively. The released voriconazole was active on bioassay. Compressive strength was reduced from 79 MPa to 53 MPa and 69 MPa to 31 MPa by 30 days for the 300-mg and 600-mg formulations, respectively.
Conclusions
Voriconazole release from ALBC increases with dose and is bioactive. Loss in compressive strength is greater after elution and with higher dose.
Clinical Relevance
Three hundred milligrams of voriconazole in ALBC would be expected to deliver meaningful amounts of active drug in vivo. The compressive strength of ALBC with 600 mg voriconazole is less than expected compared to commonly used antibacterials.
Introduction
Fungal musculoskeletal infections are rare. Much like bacterial infections, fungi that cause musculoskeletal infections form biofilms [1]. Fungi in biofilms are resistant to antifungals, requiring 100 to 1000× minimum inhibitory concentration [3]. Treatment of established fungal infections therefore requires high local concentrations of antifungal drugs. Local delivery has the potential to deliver high antifungal concentrations to the wound with reduced risk from systemic exposure [11]. Bone cement is an established vehicle for local delivery of antibacterials [7, 11]. Various studies describe surgeon-directed antifungal formulations of antimicrobial-loaded bone cement (ALBC) [2, 6, 9, 10, 15, 16]. Published data from amphotericin B studies establish limited release occurs, increased release using the liposome formulation, higher than expected compressive strength, possibly related to crosslinking with PMMA, and cytotoxicity at low concentrations [2, 5, 8, 9]. However, clinical experience in a case report by Marra et al. [10] suggests that sufficient amphotericin B is released in vivo to be measurable and clinically efficacious. In vitro studies on other antifungals including fluconazole, flucytosine, anidulafungin, micafungin, and turbinafine [15, 16] also suggest these agents are also released from ALBC, some in the active form. These investigations do not provide standardized release data to allow comparisons with other studies.
Voriconazole is a sterile antifungal powder suitable for use in ALBC with release data reported in two studies. It is effective against many fungal pathogens. Grimsrud et al. [6] report that voriconazole was released in an active form from bone cement. They used 800 mg/batch (approximately 30 vol%) in their ALBC formulation, but cumulative mass of voriconazole recovered in infinite sink conditions was not performed. Rousse et al. [14] reported release from ALBC made with 7.5 wt% anidulafungin or voriconazole. The voriconazole used in the study by Rousse et al. was reported as 98% pure. We are unable to obtain pure voriconazole. Clinically available formulations contain 3200 mg of sulfobutyl ether beta-cyclodextrin for every 200 mg of voriconazole (6% pure). Although both studies reporting voriconazole release from ALBC confirm release of active drug, neither provide data that can be used to compare release rates with other doses, other vehicles, or other animicrobials. It is also unknown how voriconazole dose in ALBC affects drug release or how the the voriconazole dose affects mechanical properties of the ALBC.
The purpose of this study was to answer the following study questions: (1) Is voriconazole release dose-dependant? (2) Is released voriconazole active? (3) Is the loss of ALBC’s compressive strength caused by voriconazole dose- and elution-dependent?
Materials and Methods
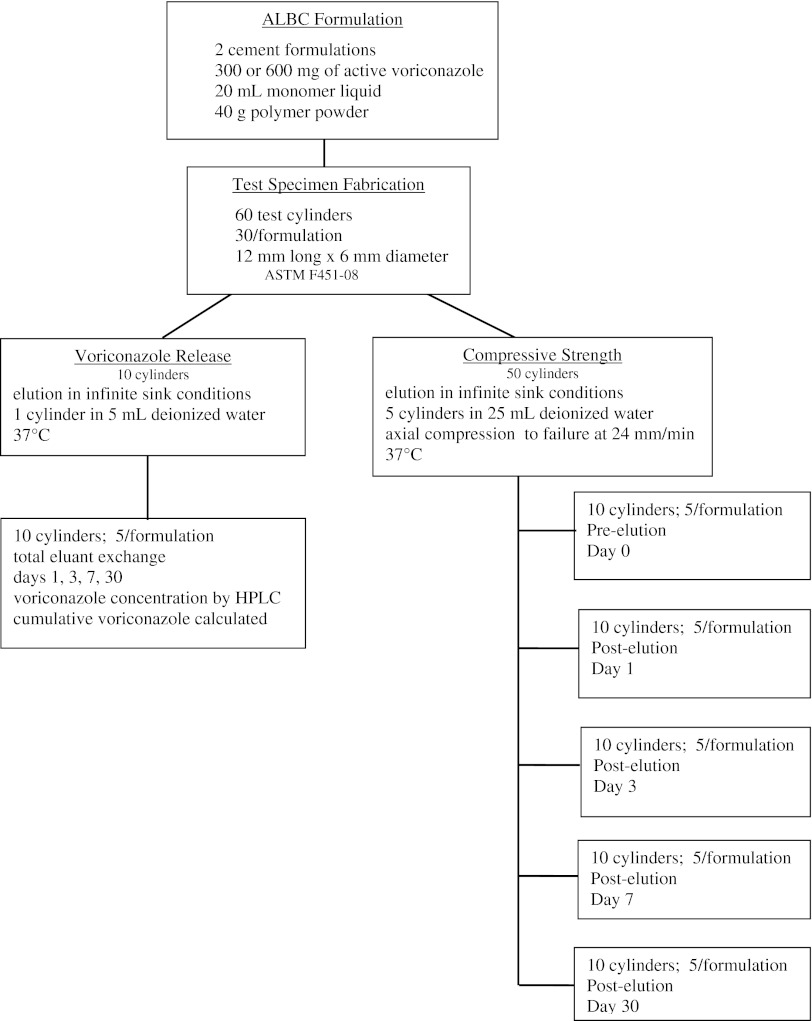
This study was designed to determine the release of voriconazole from ALBC (Fig. 1). ALBC was made with Simplex P bone cement with either 300 mg (14 vol%) or 600 mg (24 vol%) of voriconazole per batch. Voriconazole release was measured by elution using five cylinders of each formulation eluted for 30 days in deionized water. Bioassays were performed on eluent. Compressive strength was determined using 25 cylinders for each formulation in five groups of five, eluted for 0, 1, 3, 7, or 30 days, and then loaded to failure in axial compression.
Fig. 1.
Experimental layout for voriconazole study is shown.
Two formulations of ALBC were prepared by mixing either 300 mg or 600 mg of voriconazole (Hospira Inc, Lake Forest, IL, USA) per batch of Simplex P acrylic cement (Stryker, Mahwah, NJ, USA). Voriconazole powder was mixed uniformly into PMMA powder with a spatula. Monomer was subsequently added to the powder. Thirty test cylinders measuring 12 mm long × 6 mm in diameter (ASTM F451-08) were prepared in a polytetrafluoroethylene (Teflon) mold for each formulation. The ends were machined flat and square for mechanical testing and to ensure accurate length.
Five cylinders of each ALBC formulation were individually eluted in 5 mL deionized water at 37°C maintaining infinite sink conditions. Total eluent exchange was performed at 1, 3, 7, 15, and 30 days. Voriconazole concentration in the eluate was measured using isocratic high-performance liquid chromatography (HPLC) on a Beckman Gold® system (Beckman Coulter, Brea, CA, USA). A 250 mm × 4.0-mm LiChrisopher 100 RP-18E column, with 5-μm particle size, was used at a flow rate of 1 mL/min. Mobile phase was 60:40 vol:vol acetonitrile to water. Detection was performed at 220 nm [4]. A standard curve was produced using standards of known concentration. Voriconazole concentration was determined by interpolation on the standard curve using an Excel spreadsheet (Microsoft, Redmond, WA, USA). Cumulative recovered voriconazole, Mt, was calculated at 1, 3, 7, 15, and 30 days. As a pilot study, this study was performed with five cylinders per group; an a priori power analysis was not performed.
Activity of eluted voriconazole was determined using Kirby-Bauer bioassay against Candida albicans (ATCC 24433) [12] of eluent samples from each time point.
The compressive strength of both formulations was tested before elution and after 1, 3, 7, and 30 days of elution. One group of five cylinders for each formulation was eluted in 25 mL deionized water at 37°C maintaining infinite sink conditions for each of the five time periods. Total eluent exchange was carried out on Days 1, 3, 7, and 30. All test cylinders were loaded to failure in axial compression at 24.0 mm/min (ASTM F451-08) using a Test Resources 830 AT mechanical testing machine (Test Resources, Shakopee, MN, USA). Load-displacement data were analyzed using Testbuilder® (Test Resources, Shakopee, MN, USA) to determine compressive strength in accordance with ASTM Standard F451-08.
Differences in the release of voriconazole and compressive strength between ALBC formulations were determined with repeated-measures analysis of variance (ANOVA) using time in elution and voriconazole load as the factors. Appropriateness of the ANOVA model results was confirmed through the use of standard normal plots of residuals. All statistical analyses were performed using Minitab (Minitab Inc, State College, PA, USA).
Results
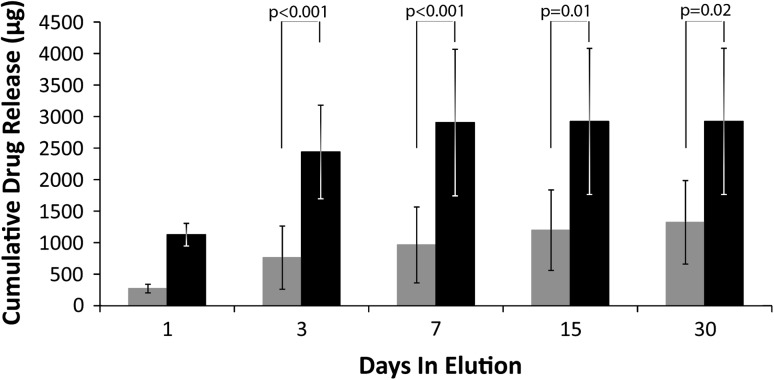
Voriconazole was released from both formulations with the 600-mg formulation releasing more drug (p < 0.001) than the 300-mg formulation. Recovered voriconazole, Mt, at 30 days was 1322 μg/cylinder (57% of the contained voriconazole) for the 300-mg formulation and 2923 μg/cylinder (63% of the contained voriconazole) for the 600-mg formulation (Fig. 2).
Fig. 2.
Cumulative elution ALBC formulated with 300 mg or 600 mg of voriconazole. Error bars show SD. Gray indicates the 300-mg formulation, whereas black indicates the 600-mg formulation. Both deliver a considerable amount of drug but the 600-mg dose cement delivers more antifungal than the 300-mg dose cement.
Voriconazole released from ALBC showed activity on Kirby-Bauer bioassay. The radii of the fungicidal zones of inhibition were consistent with concentrations measured by HPLC.
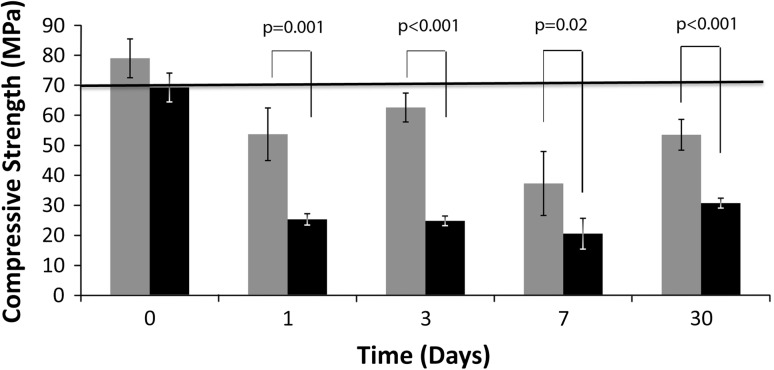
The compressive strength of ALBC with 600 mg voriconazole was less than (p < 0.001) the compressive strength of ALBC with 300 mg voriconazole (Fig. 3). The 300-mg ALBC formulation had an initial compressive strength of 79 MPa and the 600-mg formulation had an initial strength of 69 MPa. By Day 1, the 300-mg formulation had decreased to 53 MPa, and the 600-mg formulation had decreased to 25 MPa.
Fig. 3.
Compressive strength of ALBC formulated with 300 mg or 600 mg of voriconazole. Error bars show SD. Gray indicates the strength of the 300-mg batch. Black indicates the strength of the 600-mg batch. The 300-mg batch is stronger than the 600-mg batch at all time points during elution.
Discussion
Local delivery of antifungal drugs is an important component of treating fungal orthopaedic infections. Of the published studies that report release data on antifungals [6, 9, 14–16], two report voriconazole release data [6, 14] (Table 1). Both studies report release of voriconazole but had important methodological and formulation differences from our study. Our study provides release, bioactivity, and compressive strength data for high-dose voriconazole ALBC that are consistent with the conclusions reached in those previous reports. The study questions were: (1) Is voriconazole release dose-dependent? (2) Is released voriconazole active? (3) Is the loss of ALBC’s compressive strength caused by voriconazole dose- and elution-dependent?
Table 1.
Relevant literature
| Study | Antifungal | Volume fraction | Antifungal release (percent of loaded drug) | Bioactivity | Compressive strength (MPa) |
|---|---|---|---|---|---|
| Rouse et al. [14] | Anidulafungin, voriconazole | Not reported | Reported concentration only approximately 0.6% release by 24 hours | Not tested | Not tested |
| Sealy et al. [15] | Flucytosine, fluconazole, micafungin, terbinafine, anidulafungin | Not reported | Flucytosine: 0.54% Fluconazole: 0.67% Micafungin: 0.04% Terbinafine: 0.07% Anidulafungin: 9.68% |
Drug activity reported variable | Not tested |
| Kweon et al. [9] | Amphotericin B deoxycholate | < 3 vol% | 1% of amphotericin B recovered by Day 30 with poragen; 0.04% without poragen | Not tested | Compressive strength decreases with poragen; compressive strength slightly increased without poragen |
| Grimsrud et al. [6] | Voriconazole | 30 vol% | Neither percent release or concentration measured; release present after 2 weeks | Active | Not tested |
| Silverberg et al. [16] | Amphotericin B, fluconazole, 5-flucytosine | Not reported | Not measured | Amphotericin B, fluconazole active | Not tested |
Our present study has several limitations. First, these in vitro data are appropriate for comparisons with in vitro studies comparing delivery vehicles but do not represent actual tissue concentration or mechanical durability that may occur in vivo. Second, we studied only one manufacturer’s cement preparation. Further study is necessary to evaluate any differences that may exist with other cements or other antifungals. Third, we did not measure the fatigue properties of the ALBC formulations that were studied; however, high-dose ALBC is not used for implant fixation because its compressive strength falls below 70 MPa, the ISO 5833 standard for implant fixation, making fatigue studies less important. Finally, we did not study whether high concentrations of locally released voriconazole cause local host toxicity that may be important for in vivo use.
At the antifungal doses studied, voriconazole elutes effectively from ALBC (57% for 300 mg per batch of cement and 63% for 600 mg per batch of cement). Both of the voriconazole preparations studied were high-dose preparations (greater than 10 vol%) resulting from the volume of β-cyclodextran carrier required for the parenteral formulation of voriconazole (14 vol% and 24 vol%), making the resultant ALBC very porous. Additional studies are necessary to quantify release from low-dose (less than 3 vol%) cements. The release we report is in contrast to data on other antifungals. Sealy et al. [15] report elution data on ALBC made with five antifungals; however, technical details in their methods prevent comparison with our data. Amphotericin B deoxycholate with the addition of 10 g poragen [9] elutes less than 1% of the antifungal dose. In recent data reported by our laboratory, 800 mg amphotericin B deoxycholate also had low release but liposomal amphotericin B had higher release (11% of dose at 200 mg, 18% of dose at 800 mg). Release of voriconazole is five times that of liposomal amphotericin B and the amount of poragen is less by 30% (4% for AmBisome and 6% for voriconazole). Possible explanations are that some of the amphotericin B is binding to the PMMA or that cyclodextrin is a more effective poragen. It is not known if this difference in delivery efficiency will have a meaningful difference in clinical efficacy. Rouse et al. [14] report that small amounts of voriconazole are released from ALBC made with 98% pure voriconazole, presumably containing no β-cyclodextrin. This is a low-dose formulation that has low porosity and would not be expected to have the high release (63%) seen in our study. Rouse et al. use a continuous flow chamber that would be expected to achieve infinite sink conditions making comparison of drug delivery reasonable with data from our protocol; however, they report only concentrations, not the amount of drug that was released. Using their reported data to calculate accumulated voriconazole release, 0.6% of the voriconazole load was released in the first 24 hours, after which they see near zero release. The release was of much shorter duration than typical for ALBC with higher drug/poragen load. The voriconazole release observed in this study can be expected to deliver sufficient voriconazole in vivo to achieve the high concentrations necessary to treat orthopaedic fungal infections. More studies are needed to determine voriconazole toxicity to local tissues at the high levels delivered by local depot.
Rouse et al. [14] did not determine bioactivity of the delivered voriconazole; Grimsrud et al. [6] tested bioactivity at 1 and 2 weeks. This report confirms the activity of delivered voriconazole at all collection time points, verifying that active voriconazole is delivered from the cement. Recent work in our laboratory has confirmed the activity of both liposomal and deoxycholate formulations of amphotericin B eluted from bone cement [2].
Compressive strength of voriconazole ALBC has not been measured by other investigators. The initial compressive strength for the 300-mg formulation in our study was 79 MPa, above the acceptable strength for implant fixation (70 MPa, ISO 5833). Both formulations decreased rapidly in elution. The 600-mg formulation was less than half of the required strength by the first day in elution. This large decrease in compressive strength is likely related to the very large volume fraction of 24 vol%. The loss of compressive strength caused by the 300-mg formulation was consistent with previous studies of high-dose ALBA with similar volume fractions after 1 day in elution (78–52 MPa for 300 mg voriconazole, 95–59 MPa for 10 g vancomycin) [13]. The average compressive strength of Simplex P without antimicrobial is approximately 85 MPa under identical experimental conditions. The compressive strength of both of the 300-mg and the 600-mg formulations is lower than recommended for implant fixation. Compressive strength is not a limiting factor for ALBC beads in dead space.
In conclusion, biologically active voriconazole is delivered from voriconazole ALBC with increased release for higher load. The compressive strength of high-dose voriconazole ALBC is consistent with high-dose ALBC made with similar volume fractions of antibacterials. Further study is needed to address cytotoxicity from concentration achievable by local delivery and whether local delivery from these formulations is clinically effective to control orthopaedic fungal infections.
Acknowledgments
We acknowledge the help of Mary Martin PharmD at Banner Good Samaritan Medical Center and Francis Calara, BSE, at ASU. We further acknowledge assistance from John Lopez and Zachary Laughrey from the ASU Proteomics Laboratory.
Footnotes
One or more of the authors received funding from the Herbert Louis Fund at the OREF (ACM) and the donation of voriconazole by Banner Good Samaritan Medical Center (RBM). Banner Good Samaritan Medical Center provided financial support for one of the authors (RM).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham B, McLaren A, Pauken C, McLemore R. Liposomal formulation increases local delivery of amphotericin from bone cement. Clin Orthop Relat Res. 2012 Mar 31 [Epub ahead of print]. DOI: 10.1007/s11999-012-2317-4. [DOI] [PMC free article] [PubMed]
- 3.Diefenbeck M, Mückley T, Hofmann GO. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury. 2006;37(Suppl 1):S95–S104. doi: 10.1016/j.injury.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Eiden C, Mathieu O, Peyrière H, Hillaire-Buys D, Cociglio M. Simultaneous quantification of voriconazole and its N-oxide metabolite in human plasma by an easy and rapid isocratic LC nethod with UV detection. Chroma. 2008;67:275–280. doi: 10.1365/s10337-007-0508-z. [DOI] [Google Scholar]
- 5.Goss B, Lutton C, Weinrauch P, Jabur M, Gillett G, Crawford R. Elution and mechanical properties of antifungal bone cement. J Arthroplasty. 2007;22:902–908. doi: 10.1016/j.arth.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Grimsrud C, Raven R, Fothergill AW, Kim HT. The in vitro elution characteristics of antifungal-loaded PMMA bone cement and calcium sulfate bone substitute. Orthopedics. 2011;34:e378–e381. doi: 10.3928/01477447-20110627-05. [DOI] [PubMed] [Google Scholar]
- 7.Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004;427:79–85. doi: 10.1097/01.blo.0000143806.72379.7d. [DOI] [PubMed] [Google Scholar]
- 8.Harmsen S, McLaren AC, Pauken C, McLemore R. Amphotericin B is cytotoxic at locally delivered concentrations. Clin Orthop Relat Res. 2011;469:3016–3021. doi: 10.1007/s11999-011-1890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kweon C, McLaren AC, Leon C, McLemore R. Amphotericin B delivery from bone cement increases with porosity but strength decreases. Clin Orthop Relat Res. 2011;469:3002–3007. doi: 10.1007/s11999-011-1928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marra F, Robbins GM, Masri BA, Duncan C, Wasan KM, Kwong EH, Jewesson PJ. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg. 2001;44:383–386. [PMC free article] [PubMed] [Google Scholar]
- 11.McLaren A, McLemore R, Gutierrez F, Martin M. Local antimicrobial treatment. In: Cierney G III, McLaren A, Wongworowat M, editors. Orthopaedic Knowledge Update: Musculoskeletal Infection. Rosemont, IL, USA: American Academy of Orthopaedic Surgeons; 2009. pp. 95–117. [Google Scholar]
- 12.McLaren A, Nugent M, Economopoulos K, Kaul H, Vernon B, McLemore R. Hand-mixed and premixed antibiotic-loaded bone cement have similar homogeneity. Clin Orthop Relat Res. 2009;467:1693–1698. doi: 10.1007/s11999-009-0847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller R, McLaren A, Leon C, McLemore R. Mixing method affects elution and strength of high dose ALBC. Clin Orthop Relat Res. 2012 May 3 [Epub ahead of print]. DOI: 10.1007/s11999-012-2351-2. [DOI] [PMC free article] [PubMed]
- 14.Rouse MS, Heijink A, Steckelberg JM, Patel R. Are anidulafungin or voriconazole released from polymethylmethacrylate in vitro? Clin Orthop Relat Res. 2011;469:1466–1469. doi: 10.1007/s11999-010-1643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sealy PI, Nguyen C, Tucci M, Benghuzzi H, Cleary JD. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann Pharmacother. 2009;43:1606–1615. doi: 10.1345/aph.1M143. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg D, Kodali P, Dipersio J, Acus R, Askew M. In vitro analysis of antifungal impregnated polymethylmethacrylate bone cement. Clin Orthop Relat Res. 2002;403:228–231. doi: 10.1097/00003086-200210000-00033. [DOI] [PubMed] [Google Scholar]