Abstract
Background
Studies suggest a trend in the selection of younger and healthier individuals to undergo bilateral TKAs in an attempt to diminish the incidence of complications. It remains unclear whether this development has reduced overall perioperative morbidity and mortality.
Questions/purposes
We investigated whether changes in demographics and comorbidity patterns of patients undergoing bilateral TKAs are detectable and coincide with changes in length and cost of hospitalization, incidence of perioperative complications, morbidity, and mortality.
Methods
We accessed Nationwide Inpatient Survey data files between 1999 and 2008. One-year periods were created and changes in demographics, length of in-hospital stay, and perioperative morbidity and mortality were analyzed.
Results
An estimated 258,524 bilateral TKAs were performed between 1999 and 2008 in the United States. The number of annual procedures increased from 19,288 to 33,679 (75%). Length of hospital stay decreased from 4.98 to 4.01 days. Absolute in-hospital mortality rates decreased at an average rate of 10% per year. The unadjusted percent and adjusted incidence per 1000 inpatient days decreased from 0.42% and 0.85 to 0.16% and 0.39. Although the unadjusted incidence of pneumonia, pulmonary embolism, and nonmyocardial infarction cardiac complications did not change, an increase with time was detectible after adjustment for length of stay. No changes in adjusted incidence were seen for other complications.
Conclusions
Although a decreased incidence was seen for some major complications, others either remained unchanged or had an increased incidence when adjusted for length of stay. Future interventions should focus on reducing perioperative risk to improve patient safety.
Introduction
Approximately 6% of all knee arthroplasties performed in the United States are performed on both knees under the same anesthetic [18]. Based on data collected primarily in the 1990s, there appears to be a recent increase in the use of bilateral TKAs [13]. Despite these trends, controversy persists among physicians regarding the perioperative safety of these procedures [16, 18, 20]. Likely to address safety concerns, a trend toward selection of younger and healthier individuals was evident in nationally representative data collected between 1990 and 2004 [13]. However, it remains unclear whether this approach has reduced overall perioperative morbidity and mortality associated with bilateral TKAs.
A decrease in the cumulative incidence of in-hospital procedure-related complications was detected between 1990 and 2004 when analyzing data collected for the National Hospital Discharge Survey [13]. The available information clearly was limited; no distinction between major or minor morbidity could be made, and no information on individual complications was available. As such, minor complications such as subclinical urinary tract infections or deep venous thrombosis may be much less consequential than those that pose a potential threat to life, ie, pulmonary embolism or myocardial infarction. Furthermore, mortality rates could not be studied because of data source limitations. The effect of a substantial decrease in length of hospital stay (LOS), and thus the capture time for perioperative complications, could not be accounted for in this prior analysis. Therefore, in addition to presenting the absolute incidence of perioperative complications, the presentation of data as number of events per 1000 inpatient days has gained popularity to provide a measure of observation period-adjusted data. This strategy was used to verify whether a decrease in complications could be explained by a shortening of the observation time for these complications. Despite the limitation that such an approach assumes even distribution of complications during the length of hospitalization, the results gained from such analysis provide an additional method of analysis of this topic [5, 15], particularly because most life-threatening complications are known to occur during the early postoperative phase [12, 19].
Major improvements in perioperative care continue to take place. The cumulative impact of such changes in practice on patient safety remains dynamic, thus requiring review of recent data and trends. For example, while a shift to the selection of younger patients with reduced prevalence of cardiopulmonary disease was detected in the mid to late 1990s [13], it is unknown whether this trend has continued more recently. Detailed and accurate knowledge of perioperative risk is important to the contemporary care of patients having orthopaedic procedures, promoting the implementation of appropriate management strategies.
Studying major complications (ie, those that are an immediate threat to life) in the immediate postoperative period during which a patient is hospitalized is not only important but also relevant because (1) it represents the time during which perioperative clinicians can influence events and (2) most major complications after knee arthroplasty reportedly occur during hospitalization [6].
Using representative data collected between 1999 and 2008 for the largest all-payer database available in the United States, we determined whether (1) demographics and comorbidity patterns of patients undergoing bilateral TKAs changed with time, and if there were detectable changes in (2) length and cost of hospitalization, or (3) the in-hospital mortality rate and incidence of major complications.
Patients and Methods
For this retrospective cohort study, we obtained and analyzed Nationwide Inpatient Sample (NIS) data files. The NIS constitutes the largest all-payer inpatient discharge database in the United States. Detailed information on the NIS design is available electronically [6]. Because data used in this study are sufficiently deidentified, our institutional review board exempted it from review. We accessed and analyzed annual data files from 1999 to 2008. Admissions for bilateral TKAs were identified using the ICD-9-CM and entries with two 81.54 codes were included as described previously [13, 16, 18].
One-year periods were used for temporal analysis. We analyzed trends in patient demographics, including average age, comorbidity burden as indicated by the Deyo index [1, 2], LOS, total hospital charges, average age, and disposition status. As data for total hospital charges were not available for 1999 to 2000, only entries from 2001 to 2008 were used for this portion of the analysis.
Additionally, trends in the incidence of individual comorbidities were computed using definitions accepted for analysis of administrative databases [2, 3]. Rates of major complications were tabulated by identifying entries that listed ICD-9-CM diagnosis codes consistent with postoperative cerebral infarction, sepsis, shock/cardiorespiratory arrest, acute myocardial infarction, cardiac complications (except myocardial infarction), pneumonia, other pulmonary complications, and pulmonary embolism [7]. This approach has been described when analyzing major complication risk among recipients of bilateral knee arthroplasties [17], and definitions were chosen to represent potentially life-threatening events. In-hospital mortality rates were determined for each period. Morbidity and mortality rates were adjusted for changes in LOS by computing the number of events per 1000 inpatient days.
We calculated and plotted weighted means and percentages for continuous and categorical variables, respectively, and 95% CIs were provided [21]. Weighted estimates were calculated by uniformly multiplying stratum weights to the number of discharges per stratum, summing the multiple over all strata, and dividing by the total sum of weights. Different regression methods for trend analysis were used to characterize the association between various variables of interest and time [4]. In particular, Poisson regression, linear regression, and logistic regression were applied to person-time data (ie, in-hospital mortality per 1000 inpatient days, etc), continuous data (ie, average age, average comorbidity burden, average LOS, etc), and proportions (ie, percentage of female patients, etc), respectively. The regression coefficient associated with time, which illustrates the direction and magnitude of the trend with time, was reported along with its p value and 95% CI. For linear regression, the magnitude of the coefficient has a direct interpretation as the increase or decrease corresponding to a one-unit difference in the predictor (ie, per year in this analysis). For Poisson regression, the exponentiated value of the regression coefficient is a rate ratio per year. For the binary variables, we used the estimated logistic regression formula to back-transform the proportion and computed average percentage change per year. The trend analysis was performed using STATA® Version 11 (StataCorp LP, College Station, TX, USA), and other descriptive statistical analyses were performed using SAS® Version 9.2 (SAS Institute, Inc, Cary, NC, USA). To facilitate analysis of data and to obtain consistent estimates of mean and variance parameters taking into account the complex survey data setting, we used SAS® procedures SURVEYMEANS and SURVEYFREQ for descriptive analyses and STATA® procedures SVYSET and SVY LINEARIZED for trend analysis.
Results
An estimated 258,524 bilateral TKAs were performed between 1999 and 2008 in the United States. During this time, the total number of procedures increased annually by 75.0% (from 19,288 to 33,679) (Fig. 1). We observed a decrease in average patient age (p < 0.001; coefficient, −0.299; 95% CI, −0.328, −0.269) and a decrease in the proportion of Medicare recipients (p < 0.001; coefficient, −0.063; 95% CI, −0.070, −0.057), meaning the number of patients insured through Medicare decreased by 2.9% per year and the average age of patients undergoing bilateral TKAs decreased by 4 months per year (Table 1). More women underwent bilateral TKAs at any given time compared with men. The sex gap tended to widen (p = 0.124) with time (Table 1). An increase in the overall comorbidity burden (Deyo index) was evident (p < 0.001; coefficient, 0.013; 95% CI, 0.011, 0.016) (Table 1). An increase in all comorbidities examined was also observed (p < 0.001 for all comorbidities), except for congestive heart failure, which decreased at an average rate of 4.6% per year (p < 0.001; coefficient, −0.048; 95% CI, −0.067, −0.028) (Fig. 2).
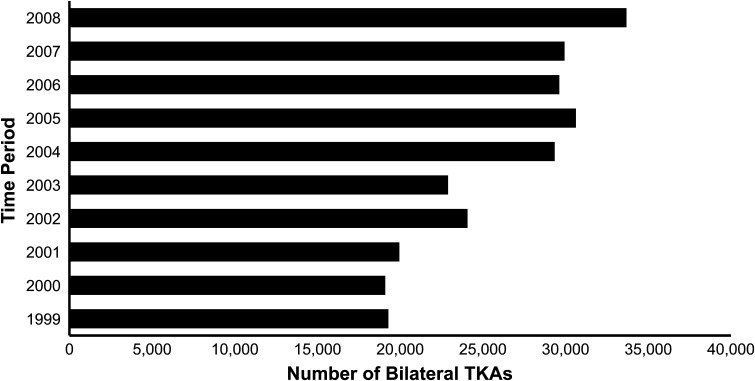
Fig. 1.
A graph shows the total number of bilateral TKAs with time in 1-year periods. The data were obtained from the NIS data files for 1999 to 2008. There was a 75% overall increase in the number of procedures performed per period from 1999 to 2008.
Table 1.
Patient demographics with time
| Variable | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | Coefficient | p value | Average % change/year |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 67.29 (66.98, 67.59) |
67.02 (66.71, 67.33) |
66.38 (66.07, 66.69) |
66.16 (65.89, 66.44) |
65.35 (65.07, 65.64) |
65.69 (65.45, 65.93) |
65.36 (65.12, 65.60) |
65.18 (64.94, 65.41) |
64.79 (64.55, 65.03) |
64.36 (64.14, 64.58) |
−0.299 (−0.328, −0.269) |
< 0.001 | NA |
| Deyo Comorbidity Index | 0.44 (0.42, 0.47) |
0.44 (0.42, 0.46) |
0.47 (0.45, 0.50) |
0.46 (0.44, 0.48) |
0.49 (0.46, 0.51) |
0.50 (0.47, 0.52) |
0.50 (0.48, 0.52) |
0.54 (0.52, 0.56) |
0.53 (0.51, 0.55) |
0.56 (0.54, 0.58) |
0.013 (0.011, 0.016) |
< 0.001 | NA |
| Women (%) | 56.23 (54.67, 57.79) |
57.23 (55.66, 58.8) |
59.47 (57.94, 61.00) |
58.01 (56.63, 59.39) |
60.09 (58.68, 61.50) |
61.29 (60.05, 62.53) |
59.07 (57.84, 60.30) |
58.19 (56.95, 59.43) |
58.72 (57.48, 59.96) |
58.35 (57.18, 59.52) |
0.005 (−0.001, 0.011) |
0.125 | 0.20 |
| Medicare patients (%) | 60.97 (59.42, 62.52) |
57.97 (56.4, 59.53) |
56.28 (54.73, 57.82) |
55.07 (53.69, 56.46) |
52.66 (51.23, 54.10) |
53.47 (52.21, 54.74) |
50.56 (49.32, 51.81) |
50.33 (49.07, 51.58) |
47.88 (46.62, 49.14) |
44.84 (43.66, 46.02) |
−0.063 (−0.07, −0.057) |
< 0.001 | −2.94 |
| Length of stay (days) | 4.98 (4.87, 5.1) |
5.12 (4.96, 5.29) |
5.05 (4.97, 5.12) |
4.75 (4.67, 4.83) |
4.59 (4.50, 4.68) |
4.52 (4.46, 4.59) |
4.42 (4.35, 4.48) |
4.37 (4.31, 4.43) |
4.29 (4.24, 4.35) |
4.01 (3.96, 4.05) |
−0.116 (−0.126, −0.106) |
< 0.001 | NA |
Values are expressed as mean, with 95% CI in parentheses; NA = not applicable; average % change per year is expressed for categorical variables only.
Fig. 2.
A graph shows the incidence of individual comorbidities among recipients of bilateral TKAs. Data are shown for each period of study. An increase in all comorbidities was found, except for congestive heart failure, which decreased in incidence. The incidence of obesity increased by 131% during the study period. CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease.
The average LOS decreased from 4.98 days (95% CI, 4.87, 5.10) in 1999 to 4.01 days (95% CI, 3.96, 4.05) in 2008 (p < 0.001; coefficient, −0.116; 95% CI, −0.126, −0.106), while the proportion of routine discharges to the patients’ customary residence without home health care decreased at an average rate of 5.5% per year (p < 0.001; coefficient, −0.067; 95% CI, −0.075, −0.058) (Fig. 3). Combined hospital charges for bilateral TKAs were approximately $1.4 billion (US dollars) between 2001 and 2008. The average charges increased from $17,357 in 2001 to $23,252 in 2008 per procedure, representing a $965 increase per year (p < 0.001; coefficient, 0.965; 95% CI, 0.928, 1.001).
Fig. 3.
A graph shows national trends in the disposition status after bilateral TKAs. The proportion of routine discharges to the patients’ customary residence without home health care decreased from 17.4% to 13.2% with time.
In-hospital mortality decreased at an average rate of 9.8% per year (p = 0.001; coefficient, −0.100; 95% CI, −0.157, −0.042) (Table 2). The unadjusted incidence decreased with time from 0.42% to 0.16% while the adjusted incidence decreased from 0.85 to 0.39 deaths per 1000 inpatient days (p = 0.021; coefficient, −0.070; 95% CI, −0.129, −0.011) (Table 3). The incidence of stroke decreased at an average rate of 6.9% per year (p = 0.012; coefficient, −0.072; 95% CI, −0.129, 0.016) (Table 2) and results remained similar when adjusted for LOS (p = 0.078; coefficient, −0.050; 95% CI, −0.107, 0.006).
Table 2.
Incidence of major complications and mortality with time
| Complication | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | Coefficient | p value | Average % change/year |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stroke | 0.31 (0.13, 0.48) |
0.44 (0.23, 0.65) |
0.45 (0.24, 0.66) |
0.29 (0.14, 0.44) |
0.35 (0.18, 0.53) |
0.16 (0.06, 0.27) |
0.28 (0.15, 0.41) |
0.28 (0.15, 0.41) |
0.19 (0.08, 0.30) |
0.23 (0.12, 0.34) |
−0.072 (−0.129, 0.016) |
0.012 | −6.90 |
| Pulmonary compromise | 0.74 (0.47, 1.01) |
1.18 (0.79, 1.57) |
0.88 (0.59, 1.17) |
0.99 (0.71, 1.27) |
0.90 (0.63, 1.17) |
0.92 (0.67, 1.16) |
1.10 (0.84, 1.36) |
0.89 (0.65, 1.13) |
0.97 (0.72, 1.21) |
0.91 (0.69, 1.14) |
0.001 (−0.03, 0.033) |
0.932 | 0.12 |
| Sepsis | 0.22 (0.07, 0.37) |
0.20 (0.06, 0.35) |
0.28 (0.11, 0.44) |
0.15 (0.05, 0.26) |
0.24 (0.1, 0.38) |
0.23 (0.11, 0.35) |
0.31 (0.17, 0.45) |
0.27 (0.14, 0.40) |
0.37 (0.22, 0.52) |
0.12 (0.04, 0.2) |
0.012 (−0.048, 0.072) |
0.695 | 0.95 |
| Shock/cardiopulmonary arrest | 0.24 (0.08, 0.40) |
0.22 (0.08, 0.37) |
0.15 (0.03, 0.28) |
0.12 (0.02, 0.22) |
0.29 (0.14, 0.44) |
0.17 (0.06, 0.27) |
0.15 (0.05, 0.24) |
0.18 (0.07, 0.29) |
0.18 (0.08, 0.29) |
0.22 (0.11, 0.33) |
−0.006 (−0.079, 0.067) |
0.872 | −0.56 |
| Acute myocardial infarction | 0.65 (0.40, 0.91) |
0.72 (0.45, 0.98) |
0.36 (0.18, 0.55) |
0.42 (0.24, 0.6) |
0.53 (0.32, 0.74) |
0.33 (0.19, 0.48) |
0.62 (0.42, 0.82) |
0.54 (0.36, 0.73) |
0.42 (0.26, 0.58) |
0.33 (0.19, 0.46) |
−0.044 (−0.088, < 0.001) |
0.049 | −4.23 |
| Cardiac (except myocardial infarction) | 6.08 (5.33, 6.83) |
6.30 (5.52, 7.09) |
6.24 (5.49, 6.99) |
6.31 (5.63, 7.00) |
6.21 (5.51, 6.90) |
6.60 (5.97, 7.23) |
6.66 (6.03, 7.28) |
6.82 (6.18, 7.46) |
6.58 (5.96, 7.21) |
6.17 (5.59, 6.74) |
0.006 (−0.006, 0.018) |
0.344 | 0.57 |
| Pneumonia | 1.03 (0.71, 1.35) |
1.02 (0.7, 1.33) |
0.81 (0.53, 1.09) |
0.75 (0.51, 0.99) |
0.99 (0.71, 1.28) |
0.79 (0.57, 1.01) |
1.13 (0.87, 1.39) |
1.00 (0.75, 1.26) |
1.10 (0.84, 1.35) |
0.86 (0.64, 1.08) |
0.007 (−0.025, 0.039) |
0.657 | 0.71 |
| Pulmonary embolism | 0.57 (0.33, 0.80) |
0.79 (0.51, 1.07) |
0.78 (0.51, 1.06) |
0.73 (0.49, 0.97) |
0.82 (0.57, 1.08) |
0.72 (0.51, 0.93) |
1.14 (0.87, 1.40) |
0.92 (0.68, 1.16) |
0.67 (0.47, 0.88) |
0.95 (0.71, 1.18) |
0.032 (−0.002, 0.065) |
0.064 | 3.21 |
| Mortality | 0.42 (0.22, 0.63) |
0.43 (0.23, 0.63) |
0.24 (0.09, 0.39) |
0.26 (0.12, 0.41) |
0.42 (0.23, 0.61) |
0.27 (0.14, 0.39) |
0.24 (0.12, 0.36) |
0.19 (0.08, 0.31) |
0.20 (0.08, 0.31) |
0.16 (0.06, 0.25) |
−0.100 (−0.157, 0.042) |
0.001 | −9.79 |
Values are expressed as incidence, with 95% CI in parentheses.
Table 3.
Incidence of major complications and mortality per 1000 inpatient days with time
| Complication | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | Coefficient | p value | Rate ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stroke | 0.62 (0.27, 0.97) |
0.85 (0.45, 1.26) |
0.89 (0.48, 1.31) |
0.61 (0.29, 0.94) |
0.77 (0.38, 1.15) |
0.36 (0.14, 0.59) |
0.63 (0.33, 0.93) |
0.64 (0.34, 0.95) |
0.44 (0.19, 0.69) |
0.57 (0.29, 0.85) |
−0.050 (−0.107, 0.006) |
0.078 | 0.95 |
| Pulmonary compromise | 1.49 (0.95, 2.04) |
2.31 (1.54, 3.07) |
1.74 (1.16, 2.32) |
2.09 (1.5, 2.68) |
1.96 (1.37, 2.55) |
2.02 (1.49, 2.56) |
2.48 (1.89, 3.08) |
2.04 (1.5, 2.59) |
2.25 (1.68, 2.82) |
2.27 (1.71, 2.84) |
0.027 (−0.003, 0.059) |
0.080 | 1.03 |
| Sepsis | 0.44 (0.13, 0.75) |
0.4 (0.12, 0.67) |
0.55 (0.22, 0.87) |
0.33 (0.1, 0.55) |
0.52 (0.21, 0.82) |
0.51 (0.24, 0.78) |
0.70 (0.38, 1.01) |
0.61 (0.31, 0.91) |
0.87 (0.51, 1.22) |
0.30 (0.09, 0.50) |
0.038 (−0.023, 0.100) |
0.222 | 1.04 |
| Shock/cardiopulmonary arrest | 0.49 (0.17, 0.8) |
0.43 (0.15, 0.71) |
0.30 (0.06, 0.55) |
0.25 (0.05, 0.46) |
0.63 (0.3, 0.96) |
0.36 (0.14, 0.59) |
0.33 (0.11, 0.55) |
0.42 (0.17, 0.66) |
0.43 (0.18, 0.67) |
0.55 (0.27, 0.83) |
0.013 (−0.062, 0.087) |
0.735 | 1.01 |
| Acute myocardial infarction | 1.31 (0.79, 1.82) |
1.40 (0.88, 1.91) |
0.72 (0.35, 1.09) |
0.89 (0.51, 1.27) |
1.15 (0.69, 1.62) |
0.73 (0.41, 1.06) |
1.41 (0.96, 1.86) |
1.24 (0.81, 1.66) |
0.98 (0.6, 1.36) |
0.82 (0.48, 1.16) |
−0.021 (−0.064, 0.023) |
0.354 | 0.98 |
| Cardiac (except myocardial infarction) | 12.21 (10.65, 13.76) |
12.30 (10.71, 13.88) |
12.37 (10.84, 13.90) |
13.30 (11.82, 14.78) |
13.53 (11.98, 15.09) |
14.59 (13.15, 16.02) |
15.06 (13.61, 16.51) |
15.59 (14.09, 17.1) |
15.34 (13.84, 16.85) |
15.39 (13.92, 16.86) |
0.031 (0.019, 0.043) |
< 0.001 | 1.03 |
| Pneumonia | 2.07 (1.43, 2.71) |
1.98 (1.36, 2.61) |
1.61 (1.05, 2.16) |
1.58 (1.06, 2.09) |
2.17 (1.55, 2.79) |
1.74 (1.25, 2.23) |
2.56 (1.96, 3.16) |
2.29 (1.71, 2.87) |
2.55 (1.94, 3.16) |
2.15 (1.6, 2.7) |
0.033 (0.001, 0.064) |
0.042 | 1.03 |
| Pulmonary embolism | 1.14 (0.66, 1.61) |
1.55 (1.00, 2.09) |
1.55 (1,20, 1.00) |
1.54 (1.03, 2.04) |
1.80 (1.23, 2.36) |
1.60 (1.13, 2.07) |
2.58 (1.97, 3.18) |
2.10 (1.55, 2.65) |
1.57 (1.09, 2.04) |
2.36 (1.78, 2.94) |
0.061 (0.028, 0.094) |
< 0.001 | 1.06 |
| Mortality | 0.85 (0.43, 1.27) |
0.84 (0.45, 1.24) |
0.47 (0.18, 0.77) |
0.54 (0.24, 0.84) |
0.89 (0.49, 1.30) |
0.59 (0.31, 0.87) |
0.55 (0.27, 0.82) |
0.44 (0.19, 0.7) |
0.46 (0.2, 0.72) |
0.39 (0.16, 0.63) |
−0.070 (−0.129, 0.011) |
0.021 | 0.93 |
Values are expressed as incidence per 1000 inpatient days, with 95% CI in parentheses.
Before adjustment for LOS, the incidence of major morbid events did not decrease (pneumonia: +0.7%, p = 0.657; coefficient, 0.007; 95% CI, −0.025, 0.039; pulmonary embolism: +3.2%; p = 0.064; coefficient, 0.032; 95% CI, −0.002, 0.065; nonmyocardial infarction cardiac: +0.6%; p = 0.344; coefficient, 0.006; 95% CI, −0.006, 0.018), except for acute myocardial infarction. The incidence for the latter event decreased by −4.2% (p = 0.049; coefficient, −0.044; 95% CI, −0.088, < 0.001). However, when LOS adjustments were considered, we found increased incidence rate ratios of 1.03 in the occurrence of pneumonia (p = 0.042; coefficient, 0.033; 95% CI, 0.001, 0.064), 1.06 in pulmonary embolism (p < 0.001; coefficient, 0.061; 95% CI, 0.028, 0.094), and 1.03 in nonmyocardial infarction cardiac complications (p < 0.001; coefficient, 0.031; 95% CI, 0.019, 0.043). The rate ratio of acute myocardial infarction, however, was insignificantly decreased at 0.98 (p = 0.354; coefficient, −0.021; 95% CI, −0.064, 0.023). Trends for other complications were not significant (Table 3).
Discussion
Attempts have been made to diminish the incidence of complications among recipients of bilateral TKAs [16, 17, 20]. It remains unclear whether the trends to performing surgery on younger and presumably healthier patients have reduced perioperative morbidity and mortality with time.
Our study has some limitations. First, owing to the nature of the database, detailed clinical information is not available and coding errors cannot be excluded. Multiple different coding options in the ICD-9-CM coding system may lead to a disparity in codes used to describe the same event. However, we used validated methods to identify and analyze these variables in this study and the NIS undergoes rigorous quality checks to assure overall accuracy of data. Second, the NIS does not capture postdischarge events. Thus, information on readmissions remains unknown. It is likely that the actual incidence of morbidity and mortality is underestimated. Further, although the incidence of events was adjusted for changes in LOS in this study, we cannot control for different distribution of complications across postoperative days. In other words, the assumption is made that the risk of complications is the same on each day of hospitalization, which likely is not the case. Unfortunately, the timing of complications is not recorded in the NIS and further analysis is not possible. Being aware that our attempt to adjust complication rates by LOS is associated with limitations, we also have provided absolute rates for major complications to provide the reader with as much information as possible. Adjusted rates should be interpreted with this limitation in mind. Regardless, the in-hospital period potentially is amenable to appropriate interventions by perioperative physicians, and thus the information reported here regarding perioperative morbidity and mortality remains highly relevant. In addition, this trend analysis provides only information on changes in the rates of events and not on potential changes in risk. Finally, no causal relationships can be established using NIS data.
We identified numerous clinically relevant trends associated with the performance of bilateral TKAs. The total number of bilateral procedures performed annually increased by approximately 75% in the United States during the study period. This increase cannot be explained by the growth of the civilian population alone but represents a proportionally comparable increase to that seen for unilateral procedures. With approximately 1.1 million TKAs performed in 2007 and 2008 (data extracted from the NIS), the proportion of bilateral procedures compared with all knee arthroplasties remained at approximately 6%, a rate similar to that previously reported [18]. However, this number represents a major increase compared with data primarily collected in the 1990s, when bilateral procedures comprised 3.7% of all knee arthroplasties [16]. A decrease in the average age of patients of approximately 2.5 years was seen with time, consistent with earlier findings [13] and findings for patients having unilateral TKAs during the same time [9]. Bilateral knee procedures reportedly are associated with increased risk for postoperative morbidity and mortality, and advanced age is a risk factor for such complications [12, 18, 20]. Although it may be argued that the trend toward decreasing age among patients having bilateral TKAs reflects a deliberate selection of younger patients for this procedure, it cannot be disregarded that the average age generally has been decreasing among patients requiring TKAs and thus the reasons for the changes among recipients have to remain speculative [8, 14].
We previously described an increase in the incidence of comorbidities among patients undergoing unilateral and bilateral TKAs [13, 14], likely representing a trend that affects the entire arthroplasty population [9, 10, 14]. The overall comorbidity burden among patients receiving bilateral procedures is lower compared with that for patients receiving unilateral procedures [9, 14, 16]. The incidence of congestive heart failure, one of the most important risk factors for perioperative morbidity and mortality after TKA [18], steadily decreased with time.
We identified a decrease in the average LOS, a decrease in discharge to the patients’ customary residence, and an increase in hospital charges with time. Although bilateral TKAs may have advantages in terms of cost-effectiveness compared with staged surgery [22] and shorter LOS may contribute to cost savings, the impact of the increase in nonroutine discharges must be considered [11]. The charges among patients admitted for unilateral TKAs increased (from $11,146 to $14,718 [extracted from the NIS]) by a similar factor between 2001 and 2008 compared with those seen for patients admitted for bilateral TKAs in this study (32% versus 34%). The impact of these trends on patient safety remains largely unstudied.
The majority of life-threatening complications occur during the first days postoperatively [12, 19]. This and the observation that most patients having these complications develop have no identifiable risk factors [19] should caution against shortening LOS without further study on the affect on perioperative safety. The safety of such a step requires rigorous study before expansion of this practice to patients having bilateral TKAs [18, 20].
The disparity in the trends of major complications and mortality may be explained by improvements in perioperative care (eg, more extensive use of telemetry) that may have an impact in preventing a fatal event, while the increased rates of many complications may represent the consequence of patients presenting with a higher comorbidity burden. However, our analysis was not able to identify causes for the trends identified. In addition, with concomitant trends in demographics, our results may lend themselves to different interpretations.
We identified numerous trends in the United States between 1999 and 2008 associated with bilateral TKAs. Reduced mortality rates represent a remarkable achievement and decreases were seen in the incidence of certain major complications. However, the unchanged incidence of many perioperative events and increased rates of some major complications when adjusted for LOS demands that future interventions focus on reducing perioperative risk to improve patient safety. Perioperative physicians should strive to maintain the appropriate balance of cost-effectiveness and patient safety for bilateral TKAs.
Acknowledgments
We thank Ya-Lin Chiu MS, for her expertise with the analysis of data.
Footnotes
The institution of one of the authors (MM) has received funding, during the study period, from the Clinical Translational Science Center, National Center for Advancing Translational Sciences (NCATS) (Grant UL1-RR024996), and the Center for Education, Research, and Therapeutics, Agency for Healthcare Research and Quality (AHRQ) (Grant U18 HSO16-75). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources NCATS and AHRQ, Rockville, MD, USA. One of the authors (SGM) certifies that he, or a member of his immediate family, has received or may receive funding supporting research related expenses, during the study period, from the Department of Anesthesiology, Hospital for Special Surgery, New York, NY, USA. The institution of one of the authors (JP) has received funding from Zimmer (Warsaw, IN, USA), Ceramtec (Laurens, SC, USA), Orthopaedic Research and Education Foundation (Rosemont, IL, USA), Stryker Orthopaedics (Mahwah, NJ, USA), Smith and Nephew (Memphis, TN, USA), Baxter (Deerfield, IL, USA), DePuy (Raynham, MA, USA), Biomimetics (Franklin, TN, USA), Medtronic (Elizabeth, NJ, USA) and 3 M (Flemington, NJ, USA). One of the authors certifies that he (JP) has or may receive payments or benefits, during the study period, an amount of $10,000–100,000 from each of the following: Ceramtec (Laurens, SC, USA); Smith and Nephew (Memphis, TN, USA); Convatec (Skillman, NJ, USA); TissueGene (Rockville, MD, USA), Zimmer (Warsaw, IN, USA); Orthopaedic Research and Education Foundation (Rosemont, IL, USA); United States Department of Defense (Washington, DC, USA); $ Musculoskeletal Transplant Foundation (Edison, NJ, USA); The Knee Society (Rosemont, IL, USA); Stryker Orthopaedics (Mahwah, NJ, USA); DePuy (Raynham, MA, USA); Baxter (Deerfield, IL, USA); Biomimetics (Franklin, TN, USA); Wyeth (New York, NY, USA); OsteoMEM (Ladera Ranch, CA, USA); SmarTech (Atlanta, GA, USA); Elsevier (Waltham, MA, USA); Wolters Kluwer (New York, NY, USA); Slack (Thorofare, NJ, USA); Hip Innovation Technology (Plantation, FL, USA), and $100,000–1,000,000 from 3 M (Flemington, NJ, USA). Two of the authors (CBM, OS) certify that they and their immediate family have no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Analysis of Nationwide Inpatient Sample data files was performed at Weill Medical College of Cornell University, New York, NY, USA.
References
- 1.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 2.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 3.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ely JW, Dawson JD, Lemke JH, Rosenberg J. An introduction to time-trend analysis. Infect Control Hosp Epidemiol. 1997;18:267–274. doi: 10.1086/647609. [DOI] [PubMed] [Google Scholar]
- 5.Galbraith JG, Butler JS, Memon AR, Dolan MA, Harty JA. Cost analysis of a falls-prevention program in an orthopaedic setting. Clin Orthop Relat Res. 2011;469:3462–3468. doi: 10.1007/s11999-011-1932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Healthcare Cost and Utilization Project. Overview of the HCUP nationwide inpatient sample (NIS). 2011. Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed August 18, 2011.
- 7.Iezzoni LI, Daley J, Heeren T, Foley SM, Fisher ES, Duncan C, Hughes JS, Coffman GA. Identifying complications of care using administrative data. Med Care. 1994;32:700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Jain NB, Higgins LD, Ozumba D, Guller U, Cronin M, Pietrobon R, Katz JN. Trends in epidemiology of knee arthroplasty in the united states, 1990–2000. Arthritis Rheum. 2005;52:3928–3933. doi: 10.1002/art.21420. [DOI] [PubMed] [Google Scholar]
- 9.Kirksey M, Lin Chiu Y, Ma Y, Gonzalez Della Valle A, Poultsides L, Gerner P, Memtsoudis SG. Trends in in-hospital major morbidity and mortality after total joint arthroplasty: United states 1998–2008. Anesth Analg. 2012;115:321–327. doi: 10.1213/ANE.0b013e31825b6824. [DOI] [PubMed] [Google Scholar]
- 10.Liu SS, Della Valle AG, Besculides MC, Gaber LK, Memtsoudis SG. Trends in mortality, complications, and demographics for primary hip arthroplasty in the united states. Int Orthop. 2009;33:643–651. doi: 10.1007/s00264-008-0549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macario A, Schilling P, Rubio R, Goodman S. Economics of one-stage versus two-stage bilateral total knee arthroplasties. Clin Orthop Relat Res. 2003;414:149–156. doi: 10.1097/01.blo.0000079265.91782.ca. [DOI] [PubMed] [Google Scholar]
- 12.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96:1140–1146. doi: 10.1097/00000542-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Memtsoudis SG, Besculides MC, Reid S, Gaber-Baylis LK, Gonzalez Della Valle A. Trends in bilateral total knee arthroplasties: 153,259 discharges between 1990 and 2004. Clin Orthop Relat Res. 2009;467:1568–1576. [DOI] [PMC free article] [PubMed]
- 14.Memtsoudis SG, Della Valle AG, Besculides MC, Gaber L, Laskin R. Trends in demographics, comorbidity profiles, in-hospital complications and mortality associated with primary knee arthroplasty. J Arthroplasty. 2009;24:518–527. doi: 10.1016/j.arth.2008.01.307. [DOI] [PubMed] [Google Scholar]
- 15.Memtsoudis SG, Dy CJ, Ma Y, Chiu YL, Della Valle AG, Mazumdar M. In-hospital patient falls after total joint arthroplasty incidence, demographics, and risk factors in the United States. J Arthroplasty. 2012;27:823.e1–828.e1. doi: 10.1016/j.arth.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Memtsoudis SG, Gonzalez Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466:2617–2627. doi: 10.1007/s11999-008-0402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Memtsoudis SG, Ma Y, Chiu YL, Poultsides L, Gonzalez Della Valle A, Mazumdar A. Bilateral total knee arthroplasty: risk factors for major morbidity and mortality. Anesth Analg. 2011;113:784–790. doi: 10.1213/ANE.0b013e3182282953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Memtsoudis SG, Ma Y, Gonzalez Della Valle A, Mazumdar M, Gaber-Baylis LK, MacKenzie CR, Sculco TP. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111:1206–1216. doi: 10.1097/ALN.0b013e3181bfab7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89:27–32. doi: 10.2106/JBJS.E.01443. [DOI] [PubMed] [Google Scholar]
- 20.Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2007;89:1220–1226. doi: 10.2106/JBJS.F.01353. [DOI] [PubMed] [Google Scholar]
- 21.Rosner B. Design and analysis techniques for epidemiologic studies. In: Rosner B, editor. Fundamental of Biostatistics. 6. Pacific Grove, CA: Duxbury; 2000. pp. 630–743. [Google Scholar]
- 22.Stubbs G, Pryke SE, Tewari S, Rogers J, Crowe B, Bridgfoot L, Smith N. Safety and cost benefits of bilateral total knee replacement in an acute hospital. ANZ J Surg. 2005;75:739–746. doi: 10.1111/j.1445-2197.2005.03516.x. [DOI] [PubMed] [Google Scholar]