Abstract
Background
Postoperative pain after total knee arthroplasty remains one of the most important challenges facing patients undergoing this surgery. Providing a balance of adequate analgesia while limiting the functional impact of regional anesthesia and minimizing opioid side effects is critical to minimize adverse events and improve patient satisfaction.
Questions/Purposes
We asked whether bupivacaine delivered through an elastomeric device decreases the (1) patients’ perception of pain after TKA; (2) narcotic consumption; and (3) narcotic-related side effects as compared with a placebo.
Methods
In this prospective, double-blind, placebo-controlled study, all patients received standardized regional anesthesia, a preemptive and multimodal analgesic protocol, and a continuous intraarticular infusion at 5 mL/hour through an elastomeric infusion pump. The patients were randomized to receive either an infusion pump filled with (1) 300 mL of 0.5% bupivacaine, the experimental group (n = 75); or (2) 300 mL of 0.9% normal saline solution, the control group (n = 75). Data concerning postoperative pain levels through a visual analog scale, postoperative opioid consumption, opioid-related side effects, and complications were collected and analyzed.
Results
Patients in the experimental group receiving the bupivacaine reported a reduction in pain levels in highest, lowest, and current visual analog scale scores compared with the placebo group on the first postoperative day and highest visual analog scale score on postoperative Day 2 along with a 33% reduction in opioid consumption on postoperative Day 2 and a 54% reduction on postoperative Day 3.
Conclusion
In patients undergoing TKA, continuous intraarticular analgesia provided an effective adjunct for pain relief in the immediate postoperative period without the disadvantages encountered with other analgesic methods.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
TKA is one of the most successful orthopaedic procedures in improving quality of life and restoring patients to a higher level of function with less pain. The management of pain in the immediate postoperative period is one of the most critical aspects in allowing speedier rehabilitation and reducing the risk of postoperative complications. The level of pain control is the most important element of patient satisfaction [4, 9, 31] and influences patient choice of physician, willingness to return to the same physician, and likelihood of recommending a physician [5, 9, 16]. Additionally, improved analgesic methods may be responsible for much of the reported success of so-called minimally invasive surgery for TKA [23].
Uncontrolled postoperative pain results in a multitude of consequences including cardiovascular complications related to increased pain levels, delayed mobilization secondary to the inability to actively take part in physical therapy and rehabilitation, increased incidence of opioid-related side effects, and prolonged hospitalization resulting in above-average use of healthcare funds [2, 6–8, 15, 18, 20, 21, 27, 31]. Narcotic-related side effects from intravenous opioid analgesia include gastrointestinal effects in 37% of patients, cognitive effects in 34%, urinary retention in 16%, pruritus in 15%, and respiratory depression in 2% of patients [32]. These effects can delay mobilization, prolong the hospital stay, and have the potential to result in substantial morbidity. An approach that focuses on multimodal perioperative regimens or comprehensive anesthesia protocols that incorporate both regional analgesia with systemic medications and preemptive analgesic techniques has recently been emphasized in the orthopaedic and anesthesia literature [13, 14, 24].
Elastomeric pain pumps delivering local anesthesia have been used by orthopaedic surgeons for many years for postoperative pain control after arthroscopic procedures of the knee and shoulder, but also after total joint arthroplasty [1, 3, 10, 12, 26]. Recent concerns of chondrolysis with continuous local anesthetic have limited the use in recent years in native joints [28, 29, 33]. The effectiveness of the elastomeric pain pump and continuous infiltration of local anesthetic after TKA has been evaluated briefly by three studies in particular [11, 19, 22]. Mauerhan and colleagues [19] randomized patients to receive intraarticular saline, morphine, bupivacaine, or bupivacaine and morphine. They reported a reduction in pain in the group with the combination of morphine and bupivacaine, but only for the first 4 hours after surgery with no effect on overall opioid consumption. Nechleba et al. [22] randomized 30 patients to receive either 0.25% bupivacaine or saline and found higher narcotic consumption in the group receiving bupivacaine. Finally, Gomez-Cardero and Rodriguez-Merchan [11] randomized patients to either ropivacaine or saline and reported decreased pain, opioid use, reduced hospital stay, and no increase in complication rate in patients randomized to the ropivacaine infusion.
We asked whether bupivacaine delivered through an elastomeric device decreases the (1) patients’ perception of pain after TKA; (2) narcotic consumption; and (3) narcotic-related side effects as compared with a placebo.
Patients and Methods
We enrolled 160 patients (70 men; 90 women) in the study from June 2010 to May 2011. Inclusion criteria for the study were patients older than age 18 years undergoing primary, unilateral TKA for degenerative arthritis. We excluded patients if their medical history included peripheral inflammatory disease, hypersensitivity to opiates, fibromyalgia, Paget’s disease, allergy or intolerance to local anesthetic medications, sleep apnea (contraindication for the intrathecal opioid), and chronic opioid use possibly leading to opioid tolerance or opioid-induced hyperalgesia. Patients with a body mass index (BMI) greater than 40 kg/m2, American Society of Anesthesiologists score of 4 or higher, or any major renal (potential contraindication to nonsteroidal antiinflammatory drugs) or liver (potential contraindication to acetaminophen) impairment were excluded as well. During the study period we performed unilateral primary TKA on a total of 1017 patients. Eligible and willing patients gave written informed consent before participation in the study. The study was approved by the institutional review board at Thomas Jefferson University Hospital.
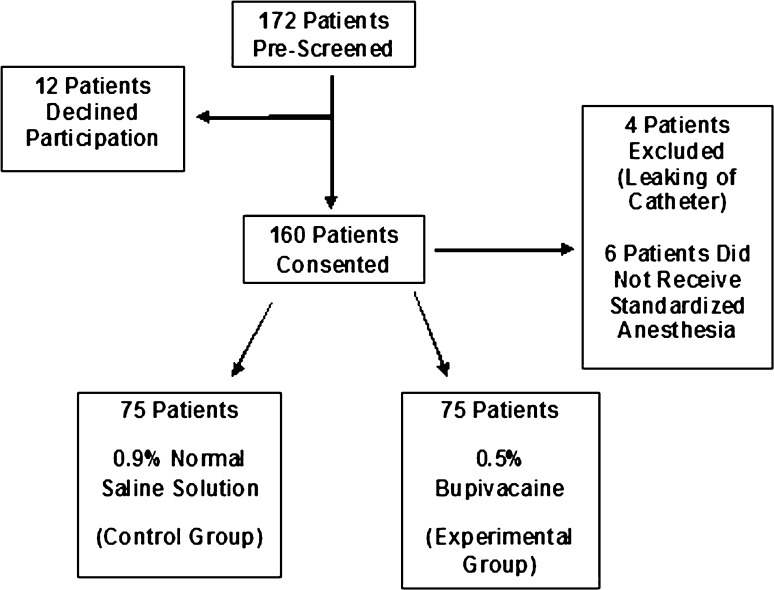
We randomly assigned participating patients to one of the two treatment groups: local anesthetic (bupivacaine) (n = 80) or control (normal saline solution) (n = 80) using a random number generator (1 or 2). The experimental group received an elastomeric pump, the On-Q PainBuster® (I-Flow Corp, Lake Forest, CA, USA) (Fig. 1), filled with 300 mL of 0.5% bupivacaine, and the control group received an identical pump filled with 300 mL of 0.9% normal saline solution. The pump released the fluid at a rate of 5 mL/h−1, staying with the patient for 2 days postoperatively. The Investigational Drug Service prepared the pumps under sterile conditions and patients, study personnel, and attending surgical and floor staff were blinded to the contents of the pumps. One hundred sixty patients had consented with 150 patients (65 male; 85 female) completing the study. Four patients were excluded postoperatively because of leaking from the catheter site during physical therapy requiring early removal, and six patients were excluded for having comorbidities that contraindicated spinal anesthesia and/or Duramorph as part of their anesthesia (not recognized at the time of enrollment) (Fig. 2). Seventy-five patients completed the study from each group. The overall study population (control and experimental groups) had a mean age of 63.9 years (range, 35–81 years), a mean BMI of 30.2 kg/m2 (range, 16.4–39.6 kg/m2), and a mean length of stay of 3.38 days (range, 1–7 days). There was no difference in age (p = 0.25), BMI (p = 0.62), or sex (p = 0.74) between the two groups (Table 1).
Fig. 1.
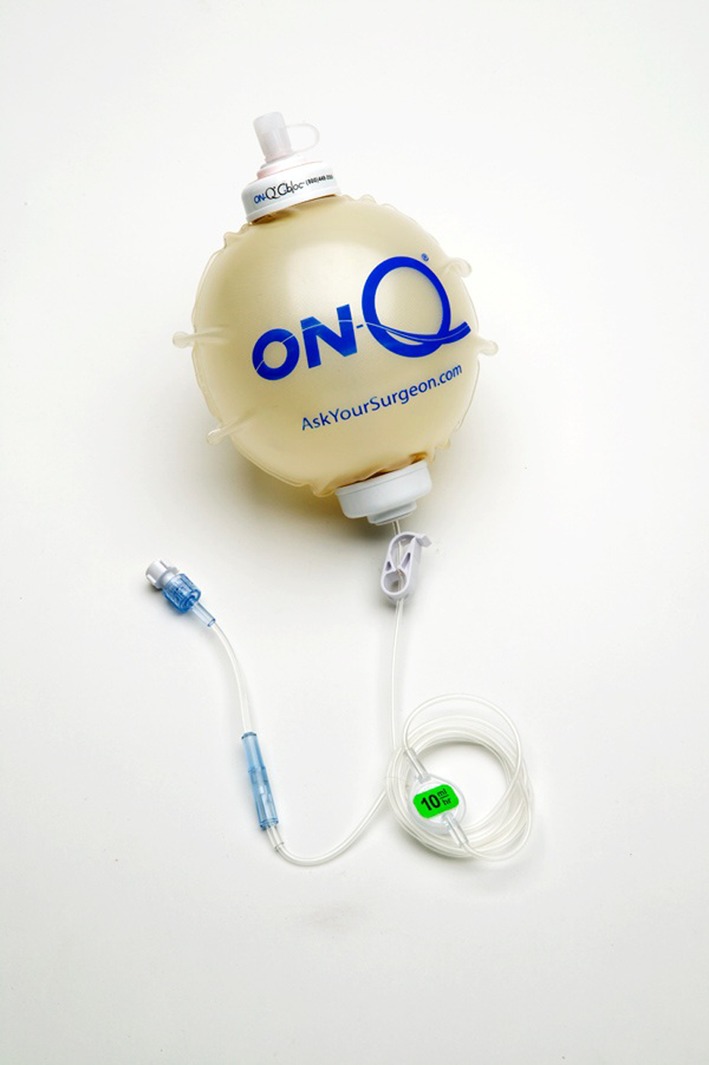
The On-Q PainBuster® (I-Flow Corp, Lake Forest, CA, USA) elastomeric infusion pump was used in this study.
Fig. 2.
The study screening, consent, and enrollment flowchart demonstrates the number of patients who completed the study at each step.
Table 1.
Study population demographic and length of stay data
| Demographic | Control | Experimental | p value |
|---|---|---|---|
| Number | 75 | 75 | Not applicable |
| Mean age (years) | 63.09 | 64.80 | 0.257 |
| Sex | 34 male, 41 female | 31 male, 44 female | 0.742 |
| Mean body mass index (kg/m2) | 30.43 | 30.05 | 0.621 |
| Mean length of stay (days) | 3.35 | 3.43 | 0.514 |
In the power analysis, based on a previous study by Kelly [17], a minimal clinically important difference (MCSD) using visual analog scale (VAS) scores was 1.2 cm. With these data we establish 1.2 cm as the MCSD for our study with a 95% CI of 0.9 to 1.5 cm and a SD of 1.9 cm. The effect size assumed was then 0.63 (1.2/1.9), and with a p < 0.05 for significance and power level of 0.80, the resulting sample size was 41 patients per group for a total of 82. Assuming a nearly 50% subject attrition rate considering the randomized, prospective nature of the study, we recruited a study population of 160 patients, of whom 150 completed the study.
All patients in the study received uniform spinal anesthesia with bupivacaine (12.5 mg) and Duramorph (0.2 mg) to standardize the perioperative analgesia. Patients underwent implantation with a cemented, posterior-stabilized TKA, including patellar resurfacing. Knee arthroplasty procedures were conducted by five distinct attending adult reconstruction surgeons. After the total knee components were cemented, the intraarticular catheter was placed in the joint space before application of the surgical dressings. The insertion site of the catheter was bandaged securely and covered with a sterile dressing. No drain was inserted into the knee as per our standard TKA protocol. The tourniquet was deflated after closure of the entire wound and application of a sterile dressing.
Patients were mobilized on the day of surgery as tolerated. Continuous passive motion machines were used at a minimum twice daily (2 hours per use) with progressive ROM increases as tolerated by the patient. Patients received warfarin with a goal international normalized ratio between 1.5 and 2.0 for deep vein thrombosis prophylaxis.
The infusion pump was activated immediately postoperatively and was removed on postoperative Day 2 unless the patient was discharged at an earlier time. During their hospital stay, patients were placed into our standardized analgesia protocol that is used for all patients undergoing TKA. Preoperative oral doses of 975 mg acetaminophen, 75 mg pregabalin, and 400 mg celecoxib were administered. Postoperatively, oral doses of 650 mg acetaminophen every 6 hours, 75 mg pregabalin every 12 hours, and intravenous doses of 30 mg ketorolac every 6 hours were administered (Fig. 3). If patients remained in the hospital after postoperative Day 3, the ketorolac was discontinued. Patients were offered narcotic medication as necessary to alleviate breakthrough pain not managed through the scheduled drug administration and the On-Q PainBuster®.
Fig. 3.
This figure delineates the preemptive and multimodal analgesic regimen and breakthrough pain medication dosing protocol that was used in the perioperative period for the study. POD = postoperative day; PCA = patient-controlled analgesia; NSAID = nonsteroidal antiinflammatory drug.
We first measured pain values for overall pain value using the VAS at approximately 5 pm on the day of the operation. Starting on the day of surgery, patients filled out a pain management questionnaire including VAS scales for current pain, least pain over the last 12 hours, most pain over the last 12 hours, and pain during various daily activities (physical therapy, walking, sitting out of bed) and other potential opioid-related symptoms experienced by the patient (nausea, vomiting, pruritus, constipation, urinary retention, dizziness, confusion, difficulty concentrating, drowsiness, headache, dry mouth) (Appendix 1). For the completion of the VAS, patients were advised that the left-most end of the 10-cm line represented no pain and the right-most end represented the worst pain they had ever felt. We asked the patient to put an X on the line at the location corresponding to pain level they were experiencing. These questionnaires were filled out every morning at approximately 8 am and every evening at approximately 5 pm until discharge. Additionally, the patients filled out a short followup questionnaire at 4 weeks postoperatively (Appendix 2). Narcotic consumption was recorded by the study personnel through electronic medical records and converted into morphine equivalents for subsequent analysis.
A Student’s t-test and chi-square test with an alpha level of 0.05 and a power level of 0.80 were used to compare the continuous and categorical variables, respectively. The null hypothesis was that continuous intraarticular infiltration of bupivacaine through an intraarticular catheter does not decrease the patients’ perception of pain, opioid consumption, and frequency of opioid-related side effects postoperatively after TKA. Secondary outcomes included for clinical significance and data analysis involved the comparison of narcotic consumption and postoperative adverse opioid effects. All data were analyzed using SPSS Version 16.0 (SPSS Inc, Chicago, IL, USA).
Results
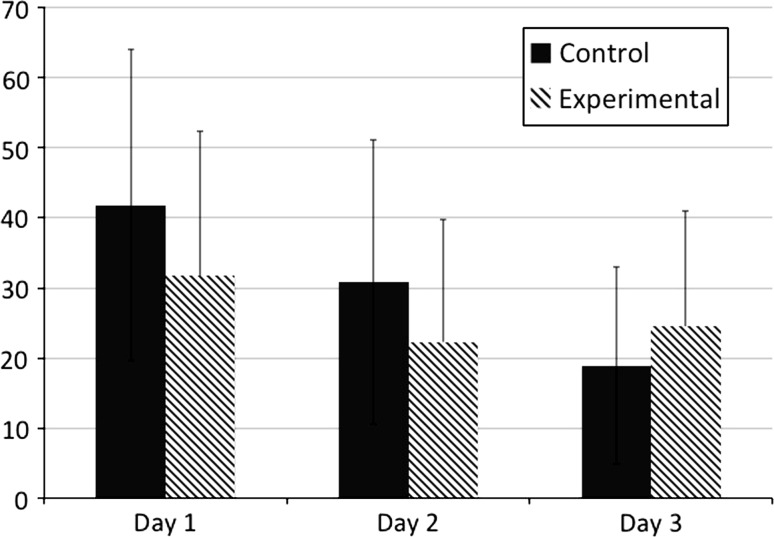
There was a difference in average highest (p = 0.01), lowest (p = 0.01), and current (p = 0.03) VAS scores between the experimental and placebo groups on postoperative Day 1 and highest (p = 0.04) VAS score on postoperative Day 2 with a universal trending decrease in VAS scores throughout postoperative Day 2 (Fig. 4). Mean VAS scores for highest pain level over the last 12 hours were less in the experimental group at postoperative Day 1 am (p = 0.02), postoperative Day 1 pm (p = 0.02), and postoperative Day 2 am (p = 0.01) and pm (p = 0.03). Mean VAS scores for lowest pain level over the last 12 hours were less in the experimental group at postoperative Day 1 am (p = 0.02). After postoperative Day 2, the patients remaining in the hospital in the experimental group tended to have higher (p = 0.54) VAS scores for maximum pain during the last 12 hours (Table 2).
Fig. 4.
The mean VASs on postoperative Days 1 to 3 are shown here.
Table 2.
Postoperative outcome data: overall daily visual analog score
| Postoperative day | Control | Experimental | p value |
|---|---|---|---|
| POD 1 now | 39.59 | 30.30 | 0.015 |
| POD 1 most | 61.13 | 49.55 | 0.013 |
| POD 1 least | 21.57 | 15.28 | 0.032 |
| POD 2 now | 24.19 (N = 70) | 19.30 (N = 72) | 0.147 |
| POD 2 most | 49.87 (N = 70) | 40.85 (N = 72) | 0.048 |
| POD 2 least | 15.39 (N = 70) | 12.34 (N = 72) | 0.256 |
| POD 3 now | 13.27 (N = 22) | 20.94 (N = 27) | 0.141 |
| POD 3 most | 35.57 (N = 22) | 39.94 (N = 27) | 0.536 |
| POD 3 least | 7.68 (N = 22) | 12.76 (N = 27) | 0.176 |
POD = postoperative day.
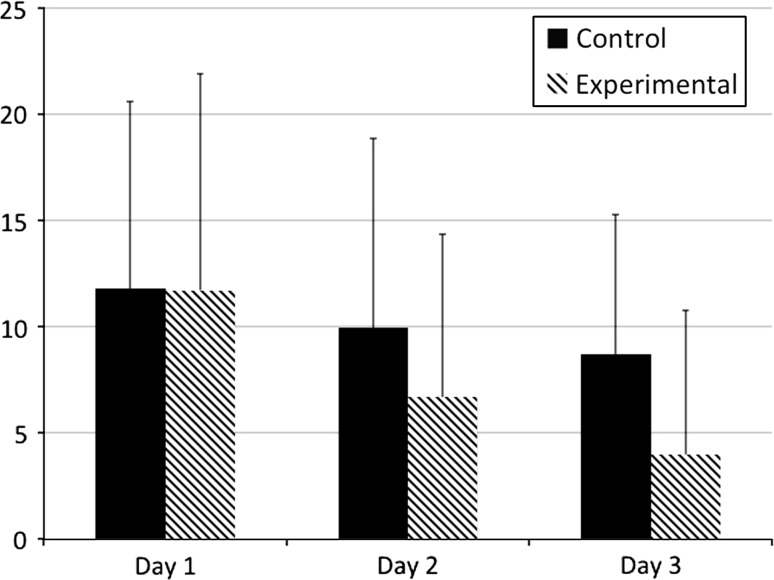
Narcotic consumption was decreased in the experimental group throughout the hospital stay with a difference on Day 2 (p = 0.02) and Day 3 (p = 0.03) (Fig. 5). We observed a decrease in average opioid consumption of 33% on Day 2 and nearly 54% for patients remaining on Day 3 (Table 3). One of the patients in the experimental group was an outlier in narcotic consumption, receiving 12 times the average amount of narcotics, and was excluded in the narcotic analysis. We found no differences in postoperative complications or VAS scores getting out of bed, walking, and during physical therapy, although there was a trend toward improved pain control in the experimental group. At the 4-week postoperative followup, patient perception of highest pain level while in the hospital was less (p = 0.02) in the experimental group.
Fig. 5.
The mean postoperative narcotic consumption on Days 1 to 3 is shown here.
Table 3.
Postoperative outcome data: narcotic consumption (morphine equivalents, mg)
| Postoperative day | Control | Experimental | p value |
|---|---|---|---|
| POD 1 | 11.84 | 11.73 | 0.957 |
| POD 2 | 9.96 (N = 70) | 6.69 (N = 72) | 0.021 |
| POD 3 | 8.7 (N = 22) | 4.0 (N = 27) | 0.038 |
POD = postoperative day.
Although narcotic consumption was different between the two groups, there was no difference with regard to narcotic-related side effects (Table 4). Additionally, there was no difference (p = 0.51) with regard to length of stay between the two groups. On postoperative Day 1, five patients in the control group were discharged and three patients in the experimental group were discharged. On postoperative Day 2, 48 patients in the control group were discharged and 45 patients in the experimental group were discharged.
Table 4.
Postoperative outcome data narcotic-related side effects (visual analog scale score)
| Side effect | Control | Experimental | p value |
|---|---|---|---|
| Nausea | 6.8 | 5.1 | 0.562 |
| Vomiting | 3.7 | 5.4 | 0.078 |
| Constipation | 4.0 | 5.2 | 0.409 |
| Passing urine | 1.2 | 2.1 | 0.122 |
| Difficulty concentrating | 2.3 | 3.6 | 0.100 |
| Drowsiness | 5.9 | 7.8 | 0.144 |
| Dizziness | 5.1 | 6.1 | 0.386 |
| Confusion | 1.6 | 2.6 | 0.302 |
| Fatigue | 5.3 | 6.4 | 0.363 |
| Itchiness | 8.1 | 8.3 | 0.835 |
| Dry mouth | 11.9 | 12.9 | 0.614 |
| Headache | 2.46 | 2.48 | 0.980 |
During the study, there were six complications in the experimental (bupivacaine) group (four reoperations in three patients) and nine complications (seven reoperations in five patients) in the control group. In the experimental group, three patients underwent manipulation under anesthesia for postoperative stiffness (at an average of 6 weeks postoperatively). One of these patients also had persistent drainage from his incision 8 days postoperatively and underwent irrigation and débridement with polyethylene exchange and had a large hematoma. Two patients in the experimental group had complications that required readmission or an extended stay; one patient sustained a pulmonary embolism, and the other was readmitted for treatment of possible cellulitis, which was later determined to be allergic dermatitis. In the control group, three patients underwent manipulation under anesthesia for postoperative stiffness (at approximately 6 weeks postoperatively) with two of these patients undergoing repeat manipulation under anesthesia for recurrent stiffness. Also in the control group, one patient underwent irrigation and débridement and polyethylene exchange for treatment of a draining knee incision, one patient sustained a lateral condyle fracture that was treated with revision TKA during the study period, one patient was treated with oral antibiotics for postoperative cellulitis, and one patient was treated conservatively after readmission for a supratherapeutic international normalized ratio and hemarthrosis.
Discussion
Successful postoperative rehabilitation hinges on pain management, and the adverse effects of current analgesic approaches continue to limit achievement of rehabilitation goals. The balance between providing adequate analgesia and limiting the functional impact of regional anesthesia while reducing opioid side effects is critical to not only minimization of adverse events, but improving patient satisfaction. In this study we asked whether continuous intraarticular analgesia with bupivacaine delivered through an elastomeric device is beneficial in terms of decreasing (1) the patients’ perception of pain after TKA; (2) narcotic consumption; and (3) narcotic-related side effects as compared with a placebo.
This study has certain limitations that should be noted. First, the assessment of VAS scores had to be standardized for the practicality of the study. This involved the VAS scores being assessed each morning at 8 am and each afternoon at 5 pm. For each assessment, patients may have experienced either an inciting factor for pain (physical therapy or independent ambulation) or an ameliorating factor (analgesic medication dose) at varying times before or after the assessment. This could not be controlled as a result of a variety of study factors. However, this variability was present in both the control and experimental cohorts and therefore we believe the potential effect is dampened in the final results of the study. Second, all patients received the same dose of intrathecal spinal anesthetic agent with the same dose of intrathecal opioid; however, with patients having surgery at different points during the day, the spinal anesthetic effect may have continued on for certain patients at the time of the assessment and it may have dissipated for others. Again, this could not be controlled; however, we believe that because this variability was present in both the control and experimental cohorts, the potential effect is dampened in the final results. Third, because intrathecal opioid was used in all patients, the potential narcotic side effect difference between the control and experimental groups may have been diminished because of the substantial intrathecal opioid effect. Our institutional standard for patients undergoing knee arthroplasty includes the intrathecal opioid at the time of the spinal and the opioid dose given was the standard dose for our institution (0.2 mg). This may be increased from the dose administered at other institutions and it must be noted that with a higher dose, there is a potentially longer-lasting spinal narcotic effect as well as a potential for increased opioid-related side effects. We believe that if there is to be a clinically valid difference with regard to narcotic-related side effects, this difference should be demonstrated regardless of the intrathecal opioid effect, which will wear off over an approximately 12-hour period of time. The fourth limitation is that we did not measure the changes in infection rate because of the limited size of the cohort. This was not a primary outcome measure and would have potentially required an exponentially larger cohort to determine a difference.
We found the patients with the bupivacaine pump had lower VAS scores during the first 2 days and had a reduction in the opioid consumption during the second and third postoperative days. More specifically, on postoperative Day 1, the levels of current pain, most pain during the last 12 hours, and least pain during the last 12 hours were all decreased in the experimental group. On postoperative Day 2, only the most pain during the last 12 hours was lower, although the current and least pain did trend toward significance. Postoperative Day 3 scores were similar in the two groups in concordance with the catheter being removed on postoperative Day 2. Interestingly, when patients were asked about the immediate postoperative pain at the 4-week followup visit, patients in the experimental group had a lower VAS score for maximum pain during the hospitalization than the control group. These data are in contrast with much of the previous literature on the use of intraarticular analgesia with local anesthetic demonstrating mixed results. Mauerhan and colleagues [19] randomized patients to receive intraarticular saline, morphine, bupivacaine, or bupivacaine and morphine. They reported a reduction in VAS scores for pain in the group with the combination of morphine and bupivacaine, but only for the first 4 hours after surgery. On the other hand, Gomez-Cardero and Rodriguez-Merchan [11] randomized 50 patients to either ropivacaine or saline and found decreased pain scores at every time point in which VAS scores were taken (once daily).
Opioid consumption differed between the control and experimental groups on the second and third postoperative days after the procedure. Of note, the opioid consumption on the third postoperative day for patients remaining in the hospital was nearly 54% lower in the experimental group, although the catheter and bupivacaine infiltration had been removed the previous day. Based on available data on the half-life of bupivacaine and the systemic reabsorption and clearance indicating that bupivacaine is reabsorbed and metabolized by this point, this may indicate the role of local anesthetics in the pain pathway and a preemptive analgesic effect. In patients remaining 2 days postoperatively, the experimental group received less than 7 mg morphine equivalent narcotic in the 24-hour period, whereas on the third postoperative day, the experimental group received only 4 mg in the 24-hour period. We believe this remarkably small amount of opioid is a result of the combination of the multimodal and preemptive regimen [24, 25] along with the effects of local anesthesia in dampening the sensitization of the pain response. Prior studies have reported varying findings on this question. The previously described study by Mauerhan et al. [19] demonstrated no effect on opioid consumption in the group receiving the continuous local anesthetic infiltration, whereas in the study by Gomez-Cardero and Rodriguez-Merchan [11], there was a reduction in the number of patients requiring opioids for pain control in the group that received the local anesthetic infiltration. Toftdahl and colleagues [30] studied the effectiveness of a combination of ropivacaine, ketorolac, and epinephrine infiltration into the soft tissues (one-shot injection) and found lower opioid consumption than a group receiving continuous femoral nerve blockade.
In our study, opioid-related side effects (as reported by the patient), including nausea, vomiting, somnolence, pruritus, and constipation, were not reduced in the experimental group, although there was a reduction in opioid use. This is believed to be directly related to the small amount of opioid used by both groups as a whole. Additionally, it is possible that considering the overall small amount of opioid, the side effects realized from the intrathecal opioid disrupted any potential to evaluate a difference between the two groups of patients. In this matter, previous investigations have also disagreed. Nechleba et al. [22] randomized 30 patients to receive either 0.25% bupivacaine or saline and observed higher narcotic consumption in the group receiving bupivacaine, whereas the previously quoted study by Gomez-Cardero and Rodriguez-Merchan [11] reported a concomitant decrease in opioid-related side effects with the decrease in opioid consumption in the group receiving the continuous local anesthetic infiltration.
This cohort of patients undergoing TKA was not without complications. One patient in both the experimental and the control groups (1.3%) required irrigation and débridement with tibial polyethylene exchange for persistent wound drainage/hematoma evacuation; however, of the entire cohort of 150 patients, this would be within the historically typical range for a reoperation rate at our institution after TKA [34]. In addition, the incidence of manipulation under anesthesia for postoperative stiffness/arthrofibrosis was 4% (three patients of 75 total) in the experimental group and 6.7% (five patients of 75 total) in the control group. However, to truly identify a change in the incidence of infection or treatment for stiffness, we would need a much larger cohort of patients. Furthermore, one of the patients in the experimental group who underwent manipulation for stiffness also underwent irrigation and débridement for a draining incision, which limited the postoperative rehabilitation.
The advantages of the spinal anesthesia, preemptive analgesia with the multimodal pain regimen protocol along with the intraarticular analgesia include excellent pain control, low opioid consumption, and continued operating room efficiency. Operating time was not increased by placement of the catheter, providing a substantial practical advantage over use of peripheral nerve blockade, which can be somewhat time-consuming if performed in the operating room. Overall when looked at individually, the opioid consumption in our experimental group is extraordinarily low throughout the hospital stay. Based on our results, patients undergoing TKA may positively benefit from the use of a postoperative intraarticular catheter in decreasing overall pain levels and reducing the need for opioids for pain management, and intraarticular analgesia may provide an effective alternative for pain relief in the immediate postoperative time period without the disadvantages encountered with epidural anesthesia, regional nerve blockade, and patient-controlled analgesia pumps.
Acknowledgments
We thank The Knee Society research review committee for the honor of receiving the 2012 Chitranjan Ranawat Award.
Appendix 1. Pain Management Questionnaire (Day of Surgery)




Appendix 2. Pain Management Questionnaire (~6-week Postoperative Visit)

Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Alford JW, Fadale PD. Evaluation of postoperative bupivacaine infusion for pain management after anterior cruciate ligament reconstruction. Arthroscopy. 2003;8:855–861. doi: 10.1016/s0749-8063(03)00734-5. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists Task Force on Acute Pain Management Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2004;6:1573–1581. doi: 10.1097/00000542-200406000-00033. [DOI] [PubMed] [Google Scholar]
- 3.Bianconi M, Ferraro L, Traina GC, Zanoli G, Antonelli T, Guberti A, Ricci R, Massari L. Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth. 2003;6:830–835. doi: 10.1093/bja/aeg277. [DOI] [PubMed] [Google Scholar]
- 4.Brokelman RB, van Loon CJ, Rijnberg WJ. Patient versus surgeon satisfaction after total hip arthroplasty. J Bone Joint Surg Br. 2003;4:495–498. [PubMed] [Google Scholar]
- 5.Burroughs TE, Davies AR, Cira JC, Dunagan WC. Understanding patient willingness to recommend and return: a strategy for prioritizing improvement opportunities. Jt Comm J Qual Improv. 1999;6:271–287. doi: 10.1016/s1070-3241(16)30444-8. [DOI] [PubMed] [Google Scholar]
- 6.Chelly JE, Ben-David B, Williams BA, Kentor ML. Anesthesia and postoperative analgesia: outcomes following orthopedic surgery. Orthopedics. 2003;8(Suppl):s865–s871. doi: 10.3928/0147-7447-20030802-08. [DOI] [PubMed] [Google Scholar]
- 7.Cheville A, Chen A, Oster G, McGarry L, Narcessian E. A randomized trial of controlled-release oxycodone during inpatient rehabilitation following unilateral total knee arthroplasty. J Bone Joint Surg Am. 2001;4:572–576. doi: 10.2106/00004623-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Coley KC, Williams BA, DaPos SV, Chen C, Smith RB. Retrospective evaluation of unanticipated admissions and readmissions after same day surgery and associated costs. J Clin Anesth. 2002;5:349–353. doi: 10.1016/S0952-8180(02)00371-9. [DOI] [PubMed] [Google Scholar]
- 9.DeLeo JA. Basic science of pain. J Bone Joint Surg Am. 2006;Suppl 2:58–62. doi: 10.2106/JBJS.E.01286. [DOI] [PubMed] [Google Scholar]
- 10.DeWeese FT, Akbari Z, Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001;392:226–231. doi: 10.1097/00003086-200111000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Cardero P, Rodriguez-Merchan EC. Postoperative analgesia in TKA: ropivacaine continuous intraarticular infusion. Clin Orthop Relat Res. 2010;5:1242–1247. doi: 10.1007/s11999-009-1202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottschalk A, Burmeister MA, Radtke P, Krieg M, Farokhzad F, Kreissl S, Strauss M, Standl T. Continuous wound infiltration with ropivacaine reduces pain and analgesic requirement after shoulder surgery. Anesth Analg. 2003;4:1086–1091. doi: 10.1213/01.ANE.0000081733.77457.79. [DOI] [PubMed] [Google Scholar]
- 13.Hebl JR, Kopp SL, Ali MH, Horlocker TT, Dilger JA, Lennon RL, Williams BA, Hanssen AD, Pagnano MW. A comprehensive anesthesia protocol that emphasizes peripheral nerve blockade for total knee and total hip arthroplasty. J Bone Joint Surg Am. 2005;Suppl 2:63–70. doi: 10.2106/JBJS.E.00491. [DOI] [PubMed] [Google Scholar]
- 14.Horlocker TT, Kopp SL, Pagnano MW, Hebl JR. Analgesia for total hip and knee arthroplasty: a multimodal pathway featuring peripheral nerve block. J Am Acad Orthop Surg. 2006;3:126–135. doi: 10.5435/00124635-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Ilfeld BM, Morey TE, Wang RD, Enneking FK. Continuous popliteal sciatic nerve block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesthesiology. 2002;4:959–965. doi: 10.1097/00000542-200210000-00031. [DOI] [PubMed] [Google Scholar]
- 16.Kehlet H, Dahl JB. The value of ‘multimodal’ or ‘balanced analgesia’ in postoperative pain treatment. Anesth Analg. 1993;5:1048–1056. doi: 10.1213/00000539-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;3:205–207. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marino J, Russo J, Kenny M, Herenstein R, Livote E, Chelly JE. Continuous lumbar plexus block for postoperative pain control after total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;1:29–37. doi: 10.2106/JBJS.H.00079. [DOI] [PubMed] [Google Scholar]
- 19.Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM. Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. J Arthroplasty. 1997;5:546–552. doi: 10.1016/S0883-5403(97)90178-9. [DOI] [PubMed] [Google Scholar]
- 20.Modig J, Borg T, Karlstrom G, Maripuu E, Sahlstedt B. Thromboembolism after total hip replacement: role of epidural and general anesthesia. Anesth Analg. 1983;2:174–180. [PubMed] [Google Scholar]
- 21.Morrison RS, Magaziner J, McLaughlin MA, Orosz G, Silberzweig SB, Koval KJ, Siu AL. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;3:303–311. doi: 10.1016/S0304-3959(02)00458-X. [DOI] [PubMed] [Google Scholar]
- 22.Nechleba J, Rogers V, Cortina G, Cooney T. Continuous intra-articular infusion of bupivacaine for postoperative pain following total knee arthroplasty. J Knee Surg. 2005;3:197–202. doi: 10.1055/s-0030-1248181. [DOI] [PubMed] [Google Scholar]
- 23.Nuelle DG, Mann K. Minimal incision protocols for anesthesia, pain management, and physical therapy with standard incisions in hip and knee arthroplasties: the effect on early outcomes. J Arthroplasty. 2007;1:20–25. doi: 10.1016/j.arth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Miller AG, Gandhi K. Current concepts review: multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;11:1075–1084. doi: 10.2106/JBJS.J.01095. [DOI] [PubMed] [Google Scholar]
- 25.Parvizi J, Porat M, Gandhi K, Viscusi ER, Rothman RH. Postoperative pain management techniques in hip and knee arthroplasty. Instr Course Lect. 2009:769–779. [PubMed]
- 26.Rasmussen S, Kramhoft MU, Sperling KP, Pedersen JH. Increased flexion and reduced hospital stay with continuous intraarticular morphine and ropivacaine after primary total knee replacement: open intervention study of efficacy and safety in 154 patients. Acta Orthop Scand. 2004;5:606–609. doi: 10.1080/00016470410001501. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, Sage D, Futter M, Saville G, Clark T, MacMahon S. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;7275:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheffel PT, Clinton J, Lynch JR, Warme WJ, Bertelsen AL, Matsen FA., 3rd Glenohumeral chondrolysis: a systematic review of 100 cases from the English language literature. J Shoulder Elbow Surg. 2010;6:944–949. doi: 10.1016/j.jse.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Serrato JA Jr, Fleckenstein CM, Hasan SS. Glenohumeral chondrolysis associated with use of an intra-articular pain pump delivering local anesthetics following manipulation under anesthesia: a report of four cases. J Bone Joint Surg Am. 2011;17:e99.1–8. [DOI] [PubMed]
- 30.Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen EK, Soballe K. Comparison of peri- and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthop. 2007;2:172–179. doi: 10.1080/17453670710013645. [DOI] [PubMed] [Google Scholar]
- 31.Wall PD. The prevention of postoperative pain. Pain. 1988;3:289–290. doi: 10.1016/0304-3959(88)90286-2. [DOI] [PubMed] [Google Scholar]
- 32.Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3:159–180. doi: 10.1054/jpai.2002.123652. [DOI] [PubMed] [Google Scholar]
- 33.Wiater BP, Neradilek MB, Polissar NL, Matsen FA., 3rd Risk factors for chondrolysis of the glenohumeral joint: a study of three hundred and seventy-five shoulder arthroscopic procedures in the practice of an individual community surgeon. J Bone Joint Surg Am. 2011;7:615–625. doi: 10.2106/JBJS.I.01386. [DOI] [PubMed] [Google Scholar]
- 34.Zmistowski B, Restrepo C, Kahl LK, Parvizi J, Sharkey PF. Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res. 2011;1:138–145. doi: 10.1007/s11999-010-1558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]