Abstract
Background
Patient-specific instrumentation potentially improves surgical precision and decreases operative time in total knee arthroplasty (TKA) but there is little supporting data to confirm this presumption.
Questions/purposes
We asked whether patient-specific instrumentation would require infrequent intraoperative changes to replicate a single surgeon’s preferences during TKA and whether patient-specific instrumentation guides would fit securely.
Methods
We prospectively evaluated the plan and surgery in 60 patients treated with 66 TKAs performed with patient-specific instrumentation and recorded any changes. A subset of six postoperative radiographic changes to the femoral and tibial components (implant size, coronal and sagittal alignment) was analyzed to determine if surgeon intervention was beneficial. Each guide was evaluated to determine fit. We compared patient demographics and implant sizing in the patient-specific instrumentation group with a control group in which traditional instrumentation was used.
Results
We recorded 161 intraoperative changes in 66 knee arthroplasties (2.4 changes/knee) performed with patient-specific instrumentation. The predetermined implant size was changed intraoperatively in 77% of femurs and 53% of tibias. We identified a subset of 95 intraoperative changes that could be radiographically evaluated to determine if our changes were an improvement or detriment to reaching goal alignment. Eighty-two of the 95 changes (86%) made by the surgeon were an improvement to the recommended alignment or size of patient-specific instrumentation. The guide did not fit securely on eight femurs (12%) and three tibias (5%). Tourniquet time and blood loss were not improved with patient-specific instrumentation.
Conclusions
We caution surgeons against blind acceptance of patient-specific instrumentation technology without supportive data.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
TKA is a successful surgery with the majority of patients gaining rapid improvement in pain, function, and quality of life [11]. The procedure has a survival rate ranging from 91% to 95% from 15 to 23 years [1, 8, 10, 12–14]. The success of the procedure in combination with an aging population has led to an increased demand for TKA [6]. There were an estimated 402,100 TKAs performed in 2003 [7, 8] with a continued rapid increase in demand for a projected 3.48 million TKAs in 2030 [7]. An increasing need for TKA with an expected shortage of surgeons [2, 3] will likely require changes in multiple areas to include healthcare policy, reimbursement, and training programs [2]. One option to help meet this demand is to reduce operative time for the individual surgeon so a higher surgical volume can be performed.
Patient-specific instrumentation is a novel technology in TKA with the potential for decreased operative time and invasiveness, decreased blood loss, increased accuracy, and less intraoperative surgeon decision-making compared with traditional arthroplasty [4]. Computer software facilitates preoperative planning and predicts intraoperative bone resections, component sizes, and alignment. The custom guides manufactured from these data alleviate the need for multiple trays of trial implants, further increasing operative efficiency. Although available from nearly all major manufacturers and despite widespread use, the advantages to patient-specific instrumentation remain theoretical; there are no long-term implant survival data to support its use and the results of postoperative alignment accuracy are conflicting [4, 5, 15]. Multiple preoperative steps must be performed with precision for the resultant guides to be accurate. This presurgical process adds complexity, time, expense, and multiple steps to the TKA process. An error made in the initial steps of the process will lead to continued reproduction of that error.
We asked whether (1) the preoperative plan and resultant custom guides would accurately replicate surgeon preferences with infrequent intraoperative changes; (2) all femoral and tibial guides would fit securely in TKAs performed with patient-specific instrumentation; (3) surgeon-directed changes would improve postoperative alignment or component size; and (4) patient-specific instrumentation would decrease operative time and estimated blood loss compared with traditional instrumentation.
Patients and Methods
We retrospectively reviewed data on 60 prospectively followed patients who had 66 TKAs using patient-specific instrumentation from September 2010 to April 2011. The inclusion criteria were diagnosis of primary knee osteoarthritis and the ability to undergo MRI at our facility. We excluded three patients who had metal in proximity to the knee or received CT for guide production. We compared age, body mass index, and sex in these knees with those of a historical control group of 62 primary TKAs performed immediately before the use of patient-specific instrumentation from March 2010 to September 2010 with similar demographic data between groups (Table 1).
Table 1.
The average demographics of the control and PSI groups
| Demographic | Control (95% CI) | PSI (95% CI) | p value |
|---|---|---|---|
| Age (years) | 62.0 (59.4–64.6) | 61.8 (59.0–64.6) | N/A |
| Body mass index (kg/m2) | 31.5 (29.8–33.2) | 31.1 (29.5–32.7) | N/A |
| Sex | Control | PSI | |
| Male | 18 | 25 | 0.29 |
| Female | 44 | 41 |
PSI = patient-specific instrumentation; CI = confidence interval; N/A = not available.
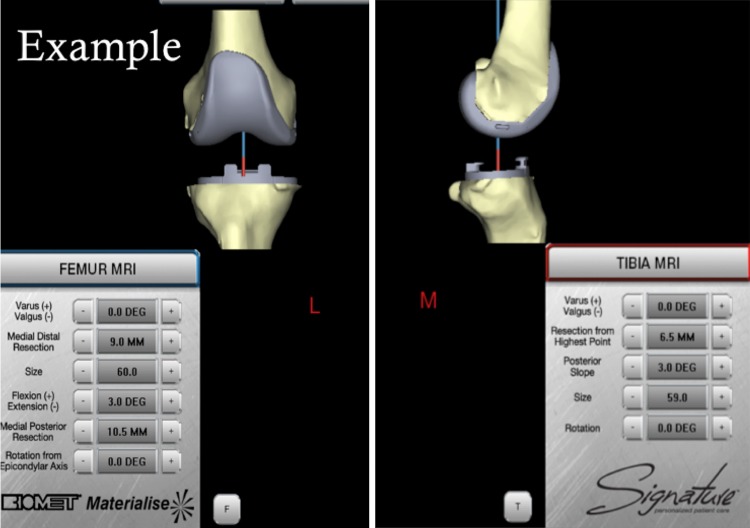
All patients received Biomet Vanguard (Warsaw, IN, USA) components. Study patients received TKAs performed with Biomet Signature patient-specific instrumentation. This process began with a preoperative MRI scanogram of the operative hip, knee, and ankle obtained at our facility per the manufacturer protocol. MRI cost was $1513 to include facility fee and radiologist interpretation. Imaging data were then provided to Materialise (Leuven, Belgium) and uploaded into proprietary software, generating a three-dimensional model of the arthritic knee. A computer-generated preoperative plan was created according to the following surgeon preferences: default alignment for femoral component rotation was parallel to the epicondylar axis, femoral component coronal alignment 90° to the mechanical axis, and femoral component sagittal alignment 3° of flexion with 9-mm distal medial resection. The tibial default alignment was 0° rotation to the AP axis, coronal alignment was 90° to the mechanical axis, and sagittal alignment was 3° of posterior slope with 8-mm resection below the highest point of the lateral plateau. The surgeon assessed each preoperative plan with the option to change multiple variables including implant size, alignment, and resection level (Fig. 1). We retained the default plan when it appeared appropriate and recorded all changes when made.
Fig. 1.
This screenshot shows an example of the preoperative templating software allowing changes to the implants and resections in a virtual format before guide manufacturing.
Once the plan was approved, femoral and tibial guides were manufactured (Materialise, Leuven, Belgium) at a cost of $900 to fit each patient’s unique anatomy and to guide surgical bone resections. The time from submission of MRI to receipt of guides required 2 to 3 weeks. Each guide was intraoperatively evaluated for fit with failure of fit defined as the inability to obtain guide stability after multiple placement attempts. On the femur, anterior holes in the custom guides directed pin placement, whereas another set of holes was drilled to dictate the position of the traditional four-in-one femoral cutting guide and eventual implant rotational alignment. In a similar manner to the femur, the patient-specific instrumentation guide was placed on the tibia and assessed for adequacy of fit. Two pins were placed anteromedially to set the tibial resection and two holes drilled superiorly to set tibial component position and rotation. Traditional cutting blocks were placed over the pins to make bone resections using the patient-specific instrumentation guidance. The knee arthroplasty was then performed in the standard fashion with soft tissue balancing as necessary.
One experienced surgeon (CLP) evaluated each step and intraoperative changes were recorded to include resection level, component size, coronal/sagittal alignment, and axial rotation. The custom guide was abandoned or modified when the proposed resection appeared malaligned to surgeon preference. Each resection that appeared reasonable with patient-specific instrumentation guidance was made and evaluated for a revision resection if deemed necessary.
We recorded tourniquet time, estimated blood loss, and implant sizes. The tourniquet was inflated directly before skin incision and deflated before closure at a consistent time point (12 minutes after cementation). Blood loss was estimated by the amount of blood present on sponges, drapes, and the suction canister at the completion of closure and verified by the anesthesiologist and surgeon.
We obtained radiographs at the 6-week postoperative visit consisting of Merchant, lateral, and standing AP knee and standing hip-knee-ankle radiographs with 100% followup obtained in study and control groups. We evaluated a subset of radiographically measurable changes to include implant size and the sagittal and coronal alignment of the femur and tibia. Femoral component downsizing was considered an improvement if there was no femoral notching on the lateral radiograph. Tibial component upsizing was considered an improvement if it resulted in no tibial component overhang on AP and lateral radiographs.
Statistical analysis was required for data involving the comparison of control and study group factors. Descriptive statistics to include mean, standard deviation, range, and confidence intervals were used to compare all continuous variables. The chi-square test was used to compare all binary variables if the expected frequencies were greater than five.
Results
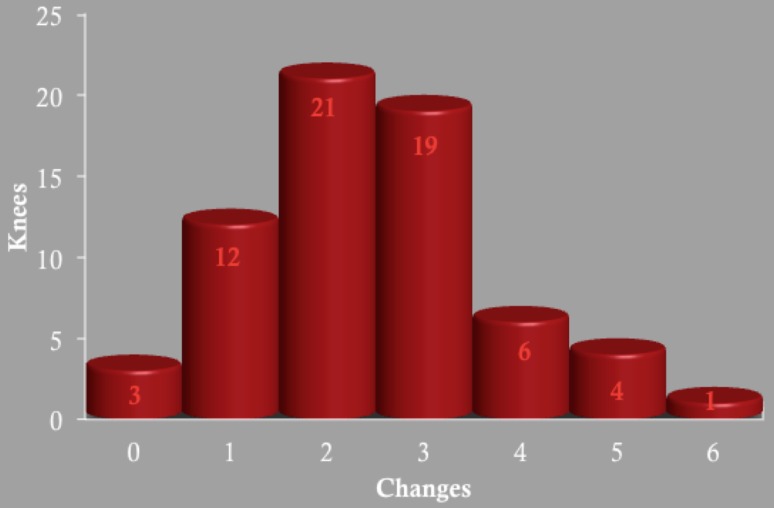
We made frequent intraoperative changes to the alignment and implant sizing proposed by patient-specific instrumentation. A total of 161 intraoperative changes were made in 66 TKAs (2.4 changes per knee) with the use of patient-specific instrumentation (Table 2). The majority of knees had one, two, or three intraoperative changes made (Fig. 2). Patient-specific instrumentation predicted the implanted component size in only 23% of femurs and 47% of tibias. The implant sizes between the study and control groups were similar for the femur and tibia (Table 3). Radiographic notching of the femur occurred with similar frequency (p = 0.30) in both groups: 10 of 66 knees with the patient-specific guides compared with 14 of 62 knees in the control group. There was no radiographic overhang of the tibial component on the AP or lateral radiographs in either group despite the frequent upsizing of the tibial component in the study group.
Table 2.
Intraoperative changes made to the femoral and tibial components in 66 patient-specific instrumentation TKAs
| Femur | Change made | Number | Percentage |
|---|---|---|---|
| Size | Up | 1 | 1.5 |
| Down | 50 | 76 | |
| Resection | Varus | 1 | 1.5 |
| Valgus | 1 | 1.5 | |
| Flexion | 0 | 0 | |
| Extension | 1 | 1.5 | |
| Proximal | 20 | 30 | |
| Distal | 0 | 0 | |
| 4:1 block | Anterior | 1 | 1.5 |
| Posterior | 2 | 3 | |
| Internal rotation | 12 | 18 | |
| External rotation | 1 | 1.5 |
| Tibia | Change made | Number | Percentage |
|---|---|---|---|
| Size | Up | 24 | 36 |
| Down | 10 | 15 | |
| Resection | Increased slope | 2 | 3 |
| Decreased slope | 2 | 3 | |
| Varus | 1 | 1.5 | |
| Valgus | 10 | 15 | |
| Increased resection | 16 | 24 | |
| Decreased resection | 0 | 0 | |
| Position | Anterior | 0 | 0 |
| Posterior | 2 | 3 | |
| Medial | 1 | 1.5 | |
| Lateral | 1 | 1.5 | |
| Internal rotation | 1 | 1.5 | |
| External rotation | 1 | 1.5 |
Fig. 2.
The total number of patient-specific instrumentation knees is listed with the number of knees requiring changes provided.
Table 3.
The average and component sizes and operative variables between the control and PSI groups
| Component sizes and operative variables | Control (95% CI) | PSI (95% CI) |
|---|---|---|
| Femur size | 63.1 (62.1–64.1) | 63.8 (62.7–64.9) |
| Tibial size | 70.1 (68.8–71.4) | 71.2 (69.9–72.5) |
| Polyethylene thickness (mm) | 12 (11.6–12.4) | 12 (11.6–12.4) |
| Tourniquet time (minutes) | 59.1 (56.2–62.0) | 59.2 (56.8–61.6) |
| Estimated blood loss (mL) | 114.2 (101.2–127.2) | 107.9 (90.9–124.9) |
PSI = patient-specific instrumentation; CI = confidence interval.
The patient-specific guides did not always obtain a secure intraoperative fit. The femoral guide did not fit securely in eight cases (12%) requiring traditional intramedullary instrumentation in three of these cases. The tibial guide did not fit securely in three cases (5%) and was abandoned for traditional instrumentation in five cases; this was the result of poor fit in one case and obviously inaccurate proposed resections in four cases.
There were 95 radiographically measurable changes with 21 involving alignment and 74 in component sizing. We improved 17 of the 21 alignment changes (81%). Forty-one of the 50 downsized femurs did not have radiographic notching and were considered an improvement (82%). All of the 24 tibias that were upsized had no radiographic overhang and were considered an improvement (100%). The surgeon improved the sizing or alignment proposed by patient-specific instrumentation in 82 of these 95 measurable changes (86%).
There was no difference in tourniquet time or estimated blood loss between the two groups (Table 3). There was also no difference in tibial polyethylene insert sizes between the groups despite frequently increasing the resections of the distal femur and proximal tibia in the study group.
Discussion
Patient-specific instrumentation technology is currently available and being used for TKA despite a lack of supporting data [9]. The rationale of this study was to determine if this technology could consistently reproduce the alignment and component sizing preferences for a single surgeon. We evaluated (1) the accuracy of the patient guides to replicate surgeon preferences based on the amount of intraoperative changes made by the surgeon; (2) the ability to obtain a stable fit with the femoral and tibial guides; (3) the quality of the surgeon-directed changes to determine if these were beneficial or detrimental to goal alignment; and (4) operative variables to include tourniquet time, estimated blood loss, and average polyethylene thickness in comparison to a control group.
There were limitations to our study. First was the subjective nature of our decision to make changes, which was based on one surgeon’s experience. The proposed resections were at times obviously incorrect, but the amount or degree to which it was deemed incorrect was not quantified. A future study that combines the use of patient-specific instrumentation and computer navigation would allow the depth and alignment of all proposed resections to be objectively evaluated. Second, we radiographically evaluated only a subset of surgeon-directed changes, including sagittal and coronal femoral and tibial alignment along with sizing. The use of postoperative CT could have been used to evaluate component rotation and provide a more comprehensive understanding of our surgeon-directed changes. As a result of time, expense, and radiation exposure concerns, we elected to not include CT in this analysis. Third, we only evaluated patient-specific instrumentation from one manufacturer. There are multiple manufacturers of this technology for TKA with variations in the computer algorithms and the functionality of the cutting guides. Our results may represent specific issues with one manufacturer and may not be representative of the overall technology. Fourth, this was not a randomized control trial but was a retrospective study performed on data collected in a prospective fashion. Several potential confounding factors to include time to treatment, medical comorbidities, and preoperative deformity were not included. We found similarities between the two groups based on the data available and this allowed us to evaluate our routine implantation practices in comparison to a novel technology. Fifth, a single experienced surgeon made all intraoperative decisions concerning changes to alignment and implant sizing. This is not representative of a low volume or inexperienced knee surgeon using this technology. A future study with multiple surgeons would provide a more comprehensive representation of this technology based on surgeon experience. A single surgeon was used to remove confounding factors that would result with multiple surgeons.
Frequent surgeon-directed changes (161 changes, 2.4 changes per knee) were made to the proposed resections, sizing, and alignment of patient-specific instrumentation. We found several changes that were made routinely (increased femoral and tibial resections, internal rotation of femur, increased tibial valgus), whereas other potential changes were never made. This is a concerning trend that the errors in guide production may not be random. Patient-specific instrumentation was unable to predictably reproduce surgeon preferences for alignment and implant sizing in our experience.
There were multiple instances of imperfect guide fit intraoperatively in which traditional instrumentation was used rather than accepting the potential risk of an undesirable resection. This raises a concern that the guide may not be an accurate reflection of patient anatomy and may be a limitation of any one of the multiple steps involved in the production of the custom guides ranging from the initial imaging acquisition to the final guide fabrication. A similar issue with guide fit has been reported with a previous patient-specific guide (OtisKnee system; OtisMed, Hayward, CA, USA), which is no longer available for use [4]. The guides must attain a stable and secure fit to ensure appropriate pin placement and to verify the guides were manufactured correctly. This was not achieved routinely in our experience.
We evaluated a subset of radiographically measurable variables including sagittal and coronal femoral and tibial alignment and found that surgeon-directed changes frequently improved (81%) the implant alignment toward the surgeon’s goal orientation. Previous concerns about component alignment with the use of patient-specific instrumentation have been reported [5] with another system (OtisMed) requiring the guides to be abandoned. In our study, the surgeon frequently changed the implanted component size to be more in line with our routine practice as evidenced by the similarity in femoral and tibial sizing between the groups. Our similar rate of femoral notching between the two groups further supports this. If changes had not been made, we have concern that many of the femoral components would have been too large in the sagittal plane, which could have repercussions for patellar tracking, soft tissue irritation, and component stability. The planned tibial component was frequently undersized, which could lead to potential subsidence and decreased component stability. Our findings indicate that the preoperatively proposed implant size and alignment from patient-specific instrumentation may be unreliable, necessitating surgeon-directed changes to ensure accurate component placement.
The use of patient-specific instrumentation required more intraoperative decision-making and was not associated with reduced operative time as measured by tourniquet time. There are initial data supporting the potential cost-effectiveness of this technology as a result of decreased operative time [16]. This benefit was not realized in our study, because we found no difference in tourniquet times between the groups. This is likely because we frequently repeated resections and took additional time to evaluate each step. We likely could have decreased our surgical times in the study group if we placed the surgical guides and immediately made the proposed cuts. However, our concern remains that the improved surgical time may come at the expense of inaccurate component position and sizing. We also found no improvement in estimated blood loss with the use of patient-specific instrumentation.
In our experience, patient-specific instrumentation did not reproducibly determine the patient’s anatomy and alignment as evidenced by the lack of an accurate preoperative plan, the need for many intraoperative adjustments, and the lack of repeatable guide fit. This complex production process is vulnerable at any step, and an error early in the process may be perpetuated throughout until ultimate guide production. In our experience, this technology does not replace the surgeon’s clinical acumen and we recommend against blindly trusting patient-specific instrumentation.
Footnotes
One of the authors (CLP) has or may receive payments or benefits, in any one year, an amount in excess of $100,000 from Biomet Orthopaedics (Warsaw, IN, USA) for royalties and as a consultant.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the University of Utah, Salt Lake City, UT, USA.
References
- 1.Ecker ML, Lotke PA, Windsor RE, Cella JP. Long-term results after total condylar knee arthroplasty. Significance of radiolucent lines. Clin Orthop Relat Res. 1987;216:151–158. [PubMed] [Google Scholar]
- 2.Fehring TK, Odum SM, Troyer JL, Iorio R, Kurtz SM, Lau EC. Joint replacement access in 2016: a supply side crisis. J Arthroplasty. 2010;25:1175–1181. doi: 10.1016/j.arth.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Hariri S, York SC, O’Connor MI, Parsley BS, McCarthy JC. A resident survey study of orthopedic fellowship specialty decision making and views on arthroplasty as a career. J Arthroplasty. 2011;26:961–968. doi: 10.1016/j.arth.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Howell SM, Kuznik K, Hull ML, Siston RA. Results of an initial experience with custom-fit positioning total knee arthroplasty in a series of 48 patients. Orthopedics. 2008;31:857–863. doi: 10.3928/01477447-20080901-15. [DOI] [PubMed] [Google Scholar]
- 5.Klatt BA, Goyal N, Austin MS, Hozack WJ. Custom-fit total knee arthroplasty (OtisKnee) results in malalignment. J Arthroplasty. 2008;23:26–29. doi: 10.1016/j.arth.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 8.Ma HM, Lu YC, Ho FY, Huang CH. Long-term results of total condylar knee arthroplasty. J Arthroplasty. 2005;20:580–584. doi: 10.1016/j.arth.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Mont MA, Johnson AJ, Zywiel MG, Bonutti PM. Surgeon perceptions regarding custom-fit positioning technology for total knee arthroplasty. Surg Technol Int. 2010;20:348–351. [PubMed] [Google Scholar]
- 10.Nafei A, Kristensen O, Knudsen HM, Hvid I, Jensen J. Survivorship analysis of cemented total condylar knee arthroplasty. A long-term follow-up report on 348 cases. J Arthroplasty. 1996;11:7–10. doi: 10.1016/S0883-5403(96)80155-0. [DOI] [PubMed] [Google Scholar]
- 11.NIH Consensus Statement on total knee replacement. NIH Consens State Sci Statements. 2003;20:1–34. [PubMed]
- 12.Pavone V, Boettner F, Fickert S, Sculco TP. Total condylar knee arthroplasty: a long-term followup. Clin Orthop Relat Res. 2001;388:18–25. doi: 10.1097/00003086-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ranawat CS, Flynn WF, Jr, Saddler S, Hansraj KK, Maynard MJ. Long-term results of the total condylar knee arthroplasty. A 15-year survivorship study. Clin Orthop Relat Res. 1993;286:94–102. [PubMed] [Google Scholar]
- 14.Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10–17. doi: 10.1097/00003086-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Spencer BA, Mont MA, McGrath MS, Boyd B. Mitrick MF Initial experience with custom-fit total knee replacement: intra-operative events and long-leg coronal alignment. Int Orthop. 2008;33:1571–1575. doi: 10.1007/s00264-008-0693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watters TS, Mather RC, 3rd, Browne JA, Berend KR, Lombardi AV, Bolognesi MP. Analysis of procedure-related costs and proposed benefits of using patient-specific approach in total knee arthroplasty. J Surg Orthop Adv. 2011;20:112–116. [PubMed] [Google Scholar]