Abstract
Background
Fresh osteochondral allograft transplantation is an increasingly common treatment option for chondral and osteochondral lesions in the knee, but the long-term outcome is unknown.
Questions/purposes
We determined (1) pain and function, (2) frequency and types of reoperations, (3) survivorship at a median of 13.5 years, and (4) predictors of osteochondral allograft failure in the distal femur.
Methods
We evaluated 122 patients (129 knees) who underwent osteochondral allograft transplantation of the femoral condyle. Mean age was 33 years and 53% were male. Clinical evaluation included the modified Merle d’Aubigné-Postel (18-point), IKDC, and Knee Society function (KS-F) scores. We defined graft failure as revision osteochondral allografting or conversion to arthroplasty. We determined whether patient characteristics or attributes of the graft influenced failure. Minimum followup was 2.4 years (median, 13.5 years); 91% had more than 10 years of followup.
Results
Mean modified Merle d’Aubigné-Postel score improved from 12.1 to 16, mean IKDC pain score from 7.0 to 3.8, mean IKDC function score from 3.4 to 7.2, and mean KS-F score from 65.6 to 82.5. Sixty-one knees (47%) underwent reoperations. Thirty-one knees (24%) failed at a mean of 7.2 years. Survivorship was 82% at 10 years, 74% at 15 years, and 66% at 20 years. Age of more than 30 years at time of surgery and having two or more previous surgeries for the operated knee were associated with allograft failure.
Conclusions
Followup of femoral condyle osteochondral allografting demonstrated durable improvement in pain and function, with graft survivorship of 82% at 10 years.
Level of Evidence
Level IV, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Chondral and osteochondral lesions of the knee share a common pathway that may lead to pain, disability, and osteoarthritis [7, 32, 36]. Treatment of chondral and osteochondral lesions has been challenging, especially in young, active patients. Arthroplasty has been a reluctant choice due to concerns over implant longevity [8, 23, 25] and functional limitations. Therefore, biologic alternatives have established themselves as an appropriate first line of treatment. These include marrow-stimulating techniques (microfracture) [28, 30, 37], cell-based cartilage repair (autologous chondrocyte implantation) [2, 34, 40], and whole tissue transfer such as osteochondral autologous transplantation and osteochondral allograft transplantation [1, 3, 4, 9]. These methods have had variable success rates in articular cartilage restoration, with clinical success rates between 50% and 90% and reoperation rates up to 49% [2, 3, 17, 24, 30, 37].
Fresh osteochondral allografting has traditionally been employed for reconstruction of large osteochondral defects or in the restoration of posttraumatic knee defects [1, 4, 15, 16, 27]. More recently, fresh osteochondral allografting has evolved into a versatile treatment option for a wide spectrum of chondral and osteochondral injuries and disorders [3, 4, 9, 27]. Osteochondral allografts emulate normal joint architecture by providing full-thickness viable hyaline cartilage tissue, along with underlying subchondral bone that acts primarily as a scaffold [33, 39]. Osteochondral allografts have resurfaced large and deep osteochondral defects, such as those seen in osteochondritis dissecans [9] or osteonecrosis [14]. Recently, LaPrade et al. [24], Williams et al. [38], McCulloch et al. [26], and Davidson et al. [6] published short-term followup studies utilizing osteochondral allografting in articular cartilage repair. At a mean followup of between 2 and 4 years, these studies demonstrated graft survival rates of between 79% and 100%. However, it is unclear whether allografts remain durable for longer time periods.
We therefore determined (1) objective and subjective measurements of pain and function, (2) frequency and types of reoperations, (3) survivorship, and (4) predictors for osteochondral allograft failure in a cohort of 122 patients who underwent fresh osteochondral allografting of the femoral condyle.
Patients and Methods
Between 1983 and 2011, an institutional review board-approved osteochondral allografting outcomes program prospectively collected data on 614 osteochondral allografting procedures of the knee performed in 536 patients. The indication for allograft surgery was presence of a painful chondral or osteochondral lesion(s) of the femoral condyle (Table 1) and failure of previous nonsurgical or surgical treatments. The contraindications for allograft surgery were advanced osteoarthritis, inflammatory joint disease, infection, and inability to follow the postoperative rehabilitation protocol. We excluded 302 patients (341 knees) who had surgery after 2001 because the tissue bank changed at that time, along with the protocols for processing the allografts (including retrieval and storage times). We also excluded 103 patients (135 knees) who had concomitant allografting of multiple anatomic sites of the index knee (trochlea, patella, tibial plateau). These exclusions left 131 patients (138 knees) who underwent isolated osteochondral allograft transplantation of the femoral condyle. Nine of these 131 patients (nine knees) were excluded: three patients (three knees) were deceased and six (six knees) were lost before the minimum 2-year followup. These nine exclusions left 122 patients (129 knees). The mean age at time of surgery was 32.8 years (range, 15–68 years); 85% were younger than 45 years. Fifty-three percent of the patients were male and 47% were female. The average BMI was 25.7 kg/m2 (range, 19–41 kg/m2). The minimum followup of patients whose grafts were not surgically removed at time of followup was 2.4 years (median, 13.5 years; range, 2.4–27.5 years); 111 of the 122 patients (91%) had more than 10 years of followup.
Table 1.
Distribution of patient diagnosis and surgical procedures before allograft surgery
| Variable | Number of knees |
|---|---|
| Diagnosis | |
| Osteochondritis dissecans | 58 (45.0%) |
| Traumatic chondral injury | 29 (22.5%) |
| Degenerative chondral lesion* | 20 (15.5%) |
| Avascular necrosis | 19 (14.7%) |
| Osteochondral fracture | 3 (2.3 %) |
| Previous surgical procedures† | |
| Chondral débridement | 86 (73.5%) |
| Drilling and microfracture | 47 (40.0%) |
| Removal of loose body | 41 (35.0%) |
| Meniscal surgery | 26 (22.0%) |
| Corrective osteotomy | 3 (2.6%) |
| Extensor mechanism realignment | 3 (2.6%) |
| Autologous chondrocyte implantation | 1 (0.9%) |
* Symptoms persisting for longer than 18 months without a discrete identifiable episode leading to the lesion; †some knees had more than one procedure.
Before allograft surgery, diagnosis of full-thickness chondral or osteochondral lesions was established after assessment of the patients’ history, physical examination, radiographic and/or MRI evaluation, and review of arthroscopic examination when available. All data were entered into our prospective institutional review board-approved database.
Fresh allograft tissue was obtained from healthy donors between the ages of 16 and 40 years who met the criteria of the American Association of Tissue Banks [18]. All the grafts passed a visual and tactile inspection of cartilage quality. The grafts were processed at the University of California, San Diego, regional tissue bank and stored at 4°C in lactated Ringer’s solution containing 1 g/L cefazolin and 10 mg/L gentamicin. Transplantation was performed within 7 days postmortem. Preoperatively, donors and recipients were matched based on the mediolateral dimension of the tibial plateau using a standard AP radiograph adjusted for magnification [12]. No blood or tissue typing was performed and no immunosuppressive therapy was employed.
Allograft surgery was performed through a midline skin incision as described previously [12]. Once the lesion was exposed, the location on the femoral condyle, size in centimeters, and depth, including any involvement of subchondral bone, were assessed, and either a shell or dowel allograft technique was performed. For the shell technique, the margins of the lesion were scored in a geometric shape and the dissected tissue removed using osteotomes and a high-speed burr (Fig. 1A). Matching grafts were removed from the donor tissue and tailored to fit the recipient site (Fig. 1B). For the dowel technique, the margins of the cartilage lesion were outlined using cylindrical templates 15 to 35 mm in diameter, and a guide wire was driven into the center of the lesion. The diseased cartilage and subchondral bone were removed with a cylindrical reamer, until normal bleeding bone was encountered (5–10 mm total depth) (Fig. 2A). Matching orthotopic grafts were harvested from identical anatomic locations on the donor allografts using a coring reamer. The grafts were copiously lavaged to remove residual bone marrow elements. Fixation of the graft was achieved either by press fit (Fig. 2B) or with the use of bioabsorbable pins (OrthoSorb®; DePuy Synthes, Warsaw, IN, USA) (Fig. 1C). The majority of knees had one or more previous surgeries before allograft surgery (Table 1). Bilateral knee surgeries were performed in seven patients. The medial femoral condyle was involved in 77 (60%) knees, the lateral femoral condyle in 45 (35%), and both condylar involvement in seven (5%). One hundred six (82%) knees received a shell allograft and 23 (18%) received a dowel allograft. The mean total graft surface area was 8.1 cm2 (range, 1–27 cm2). Ten knees had an additional procedure performed at the time of allograft surgery: hardware removal (n = 5), osteotomy (n = 2), and meniscectomy (n = 3).
Fig. 1A–C.
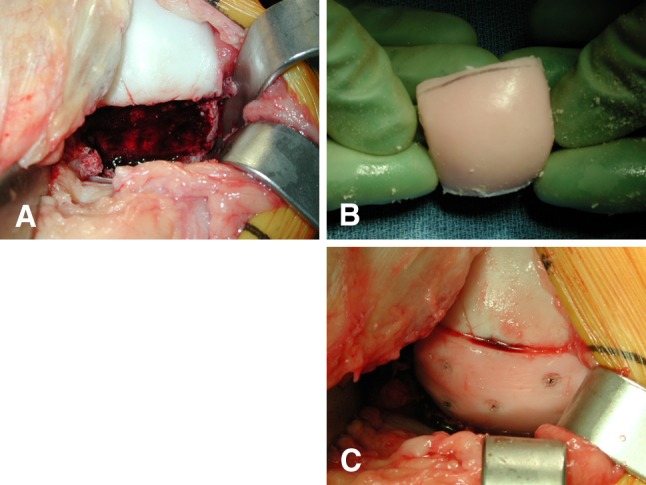
(A) An intraoperative photograph shows the femoral condyle after removal of the diseased tissue and preparation of the graft bed for shell allograft. (B) A shell allograft matched for size and thickness is obtained from the donor tissue before transplantation. (C) The shell allograft is in place, fixed with bioabsorbable pins.
Fig. 2A–B.
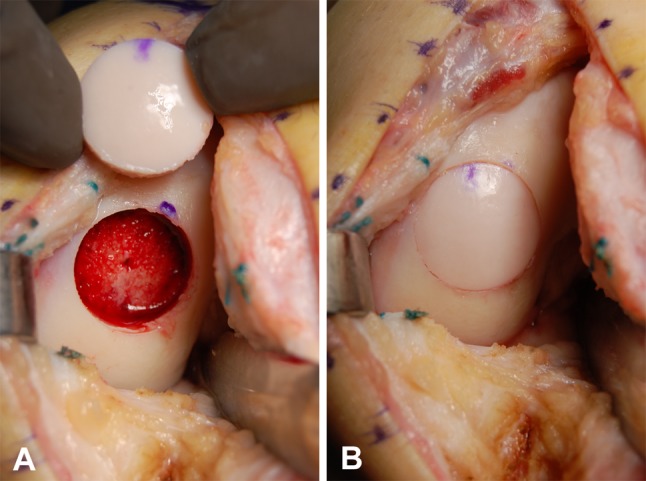
(A) An intraoperative photograph shows the femoral condyle after removal of the diseased tissue and preparation of the allograft bed with the cylindrical reamer, with the size-matched donor dowel allograft. (B) The dowel allograft is in place, secured by press-fit technique.
Postoperatively, full active ROM and protected weightbearing were applied for a period of 8 to 12 weeks. Closed-chain exercises were initiated at 4 weeks postoperatively. Progressive weightbearing was initiated at 12 weeks, depending on clinical assessment and radiographic evidence of osseous integration. Patients were allowed to return to recreational and sports activity 6 months after surgery [3].
Clinical and functional evaluation was performed postoperatively at 6 weeks, 3 months, 6 months, and annually thereafter, using the modified Merle d’Aubigné-Postel (18-point) scale [4, 5], IKDC pain and function scores [20], and Knee Society function (KS-F) scores [19]. One of the authors (WDB) performed clinical patient assessment for 34 of the 122 patients (28%) who returned for followup for this study. Eighty-eight patients (72%) did not return for followup, but all completed the modified Merle d’Aubigné-Postel, IKDC, and KS-F questionnaires by mail or telephone. We documented any additional surgeries after the osteochondral allografting procedure, including operations considered not related to the allograft. We defined osteochondral allograft failure as revision of the graft or conversion to partial or total knee arthroplasty.
We calculated means and frequencies to describe characteristics of the study population (sex, age, previous surgeries on the operative knee, diagnosis), details regarding the allograft (size, location, number of grafts), and data collected at followup (number and type of reoperations, status of the allograft). Osteochondral allograft survivorship was calculated using the Kaplan-Meier method [22] with failure of the allograft (revision of the allograft or conversion to arthroplasty) as the end point. We performed univariate analyses (chi-square tests for categorical variables and independent t-tests for continuous variables) to determine which variables were associated with allograft failure. Variables with p values less than 0.05 in the univariate analysis and variables suggested to be related to graft failure in previous studies were entered into a logistic regression analysis to evaluate the following risk factors for allograft failure: sex, age, preoperative diagnosis, number of previous surgeries on the index knee, and allograft size and location. After checking the data for normality, we used paired t-tests to assess change in patients’ pain and function scores from preoperative to followup as measured by modified Merle d’Aubigné-Postel, IKDC, and KS-F scores. SPSS® Version 13.0 (SPSS Inc, Chicago, IL, USA) was used for all analyses.
Results
The mean ± SD modified Merle d’Aubigné-Postel score improved (p < 0.001) from 12.1 ± 2.1 points to 16.0 ± 2.2 points (range, 7–18 points). At latest followup, 36% of the patients had scores of 18 points (maximum possible), 43% 15 to 17 points, 19% 12 to 14 points, and 2% less than 12 points. The mean IKDC pain score improved (p < 0.001) from 7.0 ± 1.9 points to 3.8 ± 2.9 points, the mean IKDC function score improved (p < 0.001) from 3.4 ± 1.3 points to 7.2 ± 2.0 points, and the mean KS-F score improved (p = 0.005) from 65.6 points to 82.5 points (Table 2).
Table 2.
Preoperative and followup clinical outcome measures
| Outcome measure | Preoperative | Followup | p value |
|---|---|---|---|
| Modified d’Aubigne-Postel score (points)* | 12.2 ± 2.1 | 16.0 ± 2.2 | < 0.001 |
| Excellent (18 points) | 0% | 35.5% | |
| Good (15–17 points) | 14.0% | 43.0% | |
| Fair (12–14 points) | 48.4% | 19.4% | |
| Poor (< 12 points) | 37.6% | 2.1% | |
| IKDC pain score (points)* | 7.0 ± 1.9 | 3.8 ± 2.9 | < 0.001 |
| IKDC function score (points)* | 3.4 ± 1.3 | 7.2 ± 2.0 | < 0.001 |
| Knee Society function score (points)* | 65.6 ± 15.5 | 82.5 ± 17.5 | 0.005 |
* Values are expressed as mean ± SD.
Of the 129 knees, 61 (47%) had an average of 1.5 ± 0.7 (range, 1–4) further surgeries after the osteochondral allografting procedure (Table 3). Thirty knees (23%) had one or more reoperations not necessarily related to the allograft. Thirty-one knees (24%) underwent reoperations defined as osteochondral allograft failure. The mean time to failure was 7.2 ± 5.2 years (range, 1–19.7 years). Five knees had a mean of 2.2 ± 0.8 surgeries before failure, and 26 knees failed without preceding surgeries.
Table 3.
Frequency and types of reoperations after allograft surgery
| Reoperations | Number of knees |
|---|---|
| Reoperations not necessarily related to the allograft* | |
| Arthroscopic débridement | 29 (22.5%) |
| Meniscectomy | 3 (2.3%) |
| Meniscus repair | 5 (3.9%) |
| Loose body removal | 3 (2.3%) |
| ACL reconstruction | 2 (1.6%) |
| Extensor mechanism realignment | 1 (0.8%) |
| Osteotomy | 1 (0.8%) |
| Heterotopic ossification removal | 1 (0.8%) |
| Reoperations defined as allograft failure | |
| Revision allograft | 15 (11.6%) |
| Conversion to TKA | 13 (10.1%) |
| Conversion to partial knee arthroplasty | 3 (2.3%) |
* Some knees had more than one procedure.
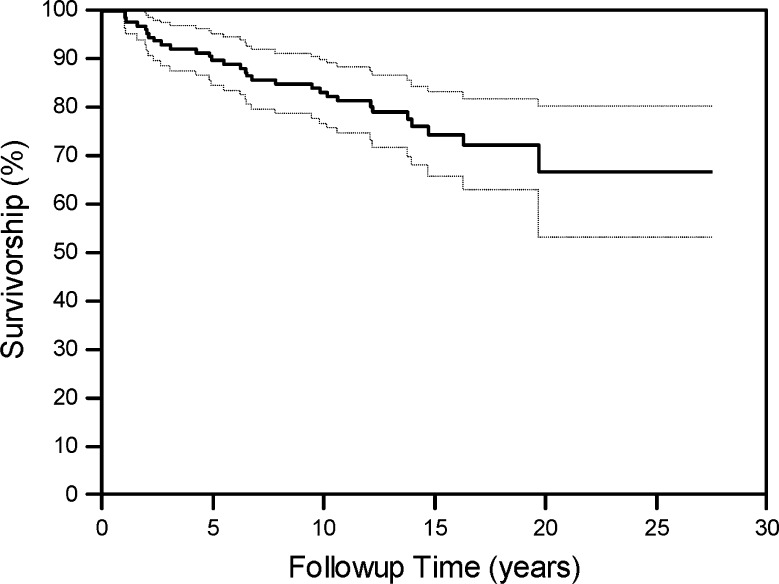
At followup, 98 of 129 (76%) knees did not have the allograft surgically removed. Survivorship rates of the osteochondral allograft were 89% (95% CI, 82%–94%) at 5 years, 82% (95% CI, 74%–88%) at 10 years, 74% (95% CI, 66%–81%) at 15 years, and 66% (95% CI, 57%–74%) at 20 years (Fig. 3).
Fig. 3.
A graph shows the overall graft survivorship and 95% CIs with revision of the allograft or arthroplasty conversion as the end point. Survivorship rates were 89% (95% CI, 82%–94%) at 5 years, 82% (95% CI, 74%–88%) at 10 years, 74% (95% CI, 66%–81%) at 15 years, and 66% (95% CI, 57%–74%) at 20 years.
Age and number of previous surgeries were associated with osteochondral allograft failure after controlling for the other variables in the model (Table 4). Patients who were 30 years or older at the time of surgery were 3.5 times more likely to fail than patients who were younger than 30 years. Patients who had two or more previous surgeries in the operative knee before undergoing osteochondral allografting were 2.8 times more likely to have a failure of the allograft compared to patients who had one or no previous surgeries.
Table 4.
Logistic regression analysis for variables predicting failure of the osteochondral allograft
| Predictor | Reference group | Odds ratio compared to reference group | p value |
|---|---|---|---|
| Age | |||
| ≥ 30 years | < 30 years | 3.54 | 0.012 |
| Diagnosis | |||
| Other | Degenerative chondral lesion | 3.73 | 0.062 |
| Number of previous surgeries | |||
| 2 or more | ≤ 1 | 2.77 | 0.030 |
| Graft location | |||
| Medial femoral condyle | Lateral femoral condyle | 2.23 | 0.099 |
Discussion
Osteochondral allografting has emerged as an important treatment for both posttraumatic reconstruction [1, 11, 16] and cartilage restoration [4, 10, 13]. With the development of commercial tissue banking programs for recovery, processing, and storage of osteochondral allografts, the availability of allograft tissue has increased, rendering this treatment modality accessible to more surgeons and patients. While a number of short-term studies [4, 6, 9, 14, 24, 26, 38] support the efficacy of osteochondral allograft transplantation, few studies describe the long-term outcomes of allografting of the femoral condyle [1, 15]. Therefore, we investigated the long-term outcomes of fresh osteochondral allografting of the femoral condyle. Specifically, we were interested in the long-term pain and function, reoperation rates, graft survivorship, and parameters determining graft failure.
Our study had several limitations. First, being a retrospective case series, no control group was available, and therefore we could not assess the success of this treatment in comparison to other potential treatment modalities or to the natural history of the diseased knee in the 91% of our patients with surviving grafts. Second, one of our outcome measures, the modified Merle d’Aubigné-Postel score, has not been validated for use in knees; however, it has been used in our allograft program since 1983 and predates the advent of other validated outcome measures. Although not validated, it was a simple method, describing objective measures of pain and function and allowing a historical, intrasample comparison within our cohort. Third, we obtained followup via telephone or mail for 72% of the patients. Patient examination in person would have been ideal, as this could have potentially uncovered more details about patient status. Being a national referral center (with patients having a wide geographic distribution) and based on our previous experience in osteochondral allograft outcome studies [9, 14, 21], we anticipated difficulties obtaining personal clinical followup. As a result, we designed the study so the important outcome measures (pain, function, reoperation) were readily obtainable by questionnaire via telephone or mail. Fourth, we lacked radiographic followup. Although all patients were followed radiographically until healing of the allograft was documented, long-term radiographic followup was available in less than 25% of knees. Radiographic evaluation may have added additional objective parameters not observed by examination or questionnaire. Nevertheless, we were not aware of any validated radiographic outcome measures useful in followup of osteochondral allograft transplantation or other cartilage repair procedures.
All clinical outcome measures improved from preoperatively to latest followup and were comparable to short-term clinical results of other cartilage repair procedures [17, 28, 31, 34]. Preoperatively, using the modified Merle d’Aubigné-Postel score, only 14% of our patients had a score of 15 points or more, while postoperatively, 79% did. Gross et al. [15, 16], using the modified Hospital for Special Surgery score, reported a mean score of 83 of 100 points in 48 of 60 surviving grafts at 10 years after osteochondral allograft transplantation of the femoral condyle. Zaslav et al. [40] reported the outcome of autologous chondrocyte implantation in 126 patients. At 48 months’ followup, the mean modified Cincinnati knee score improved from 3.3 points to 6.3 points. Although comparisons to knee arthroplasty populations may not have been valid due to differences in patient demographics, Keeney et al. [23], in a meta-analysis of TKAs in patients younger than 55 years, reported an overall KS-F score of 81.6 points, comparable to our postoperative KS-F score. This result may have reflected the lack of sensitivity of the current Knee Society scoring system in young patient with higher functioning knees.
Sixty-one of 129 knees (47%) underwent reoperation after the index osteochondral allografting procedure. This was a substantial reoperation rate and demands further analysis. We determined whether further operations were directly related to the allograft. We defined two types of operations: those resulting in removal of allograft (revision or arthroplasty) and those not directly related to allograft failure. Thirty of 61 (23% of entire study population) knees had reoperations that did not involve removal of the allograft; all had some form of arthroscopic treatment. This was not surprising for a young active population in which further knee injury (meniscus tear/ACL tear) or onset of new knee symptoms requiring further surgery was not uncommon. Zaslav et al. [40] reported on 126 patients undergoing autologous chondrocyte implantation after failed chondroplasty or microfracture. At 48 months’ followup, 49% underwent further surgery. Minas et al. [29] reported a 26% failure rate for autologous chondrocyte implantation in patients who had undergone prior marrow stimulation procedures. Thirty-one of 61 (24% of entire study population) knees had reoperation with removal of the allograft. Fifteen knees underwent revision allografting and 16 had a conversion to arthroplasty. The decision to either revise the allograft or convert to arthroplasty included clinical variables, mode of failure of the primary surgery, progression of arthritis, and the patient’s and surgeon’s desire to avoid arthroplasty. We believe the ability to revise an allograft to another allograft is an important advantage of the osteochondral allografting procedure. Further study of the outcomes of revision allografting and arthroplasty after osteochondral allograft transplantation is warranted. Although there are no comparable studies documenting reoperation rates in patients with osteochondral allograft, Gross et al. [15] reported 12 failures in 60 patients but did not report total number of reoperations. In a study of patients undergoing autologous chondrocyte implantation after failed marrow stimulation, Minas et al. [29] reported a 26% failure rate.
One of the most important questions regarding the use of osteochondral allografting in knee restoration has been durability. While many studies have shown short-term graft survival, many patients want to know how long the allograft will last. Survivorship is a recognized method of determining the durability of a procedure or implant [22]. Our survivorship rates of 82% at 10 years and 74% at 15 years are comparable to reported survivorship of osteochondral allograft transplantation for posttraumatic reconstruction of the tibia and the distal femur [1, 35]. Shasha et al. [35] reported on 65 fresh tibia osteochondral allografts for failed tibia plateau fractures. At a mean of 12 years, 21 of 65 patients had undergone conversion to TKA. Survivorship was 80% at 10 years and 65% at 15 years. Aubin et al. [1] reported survivorship in a cohort of 72 patients undergoing surgery for defects of the femoral condyle. Twelve patients were lost to followup and 12 had failed at the mean 10-year followup. Survivorship was 85% at 10 years and 74% at 15 years.
We used a logistic regression model to attempt to determine which variables were associated with clinical failure of the osteochondral allografting procedure. We chose to determine predictors of failure rather than success, as failure was more easily defined in this diverse patient population. The model showed only two variables associated with a greater risk of failure: age of greater than 30 years and two or more prior surgical procedures. No correlation was noted with patient sex or graft size. It is possible the size of the study population or number of failures was insufficient for the logistic regression model to predict other potential risk factors, such as preoperative diagnosis or graft location. Other studies [11, 27] have demonstrated a higher failure rate with osteochondral allografting in older patients, uncorrected limb malalignment, diagnosis of osteoarthritis, and bipolar allografts. Younger individuals with no more than one previous operation have appeared to be the best candidates for allografting of the femoral condyle.
Osteochondral allografting is an increasingly common restorative procedure for a wide variety of knee pathologies. This study demonstrated fresh osteochondral allografting was a successful and durable treatment for chondral and osteochondral lesions of the femoral condyle. Further investigation is warranted to better understand factors leading to clinical failure.
Footnotes
The institution of one of the authors (WBD) has received, during the study period, funding from the Joint Restoration Foundation (Centennial, CO, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Scripps Clinic and Scripps Green Hospital (La Jolla, CA, USA).
References
- 1.Aubin PP, Cheah HK, Davis AM, Gross AE. Long-term followup of fresh femoral osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2001;(391 Suppl):S318–S327. [DOI] [PubMed]
- 2.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 3.Bugbee WD. Fresh osteochondral allografts. J Knee Surg. 2002;15(3):191–195. [PubMed] [Google Scholar]
- 4.Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation. Clinical results in the knee. Clin Orthop Relat Res. 1999;(360):159–168. [PubMed]
- 5.D’Aubigne RM, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36-A(3):451–475. [PubMed] [Google Scholar]
- 6.Davidson PA, Rivenburgh DW, Dawson PE, Rozin R. Clinical, histologic, and radiographic outcomes of distal femoral resurfacing with hypothermically stored osteoarticular allografts. Am J Sports Med. 2007;35(7):1082–1090. doi: 10.1177/0363546507299529. [DOI] [PubMed] [Google Scholar]
- 7.Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G, Ding C, Cicuttini FM. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage / OARS, Osteoarthritis Res Soc. 2008;16(3):337–342. doi: 10.1016/j.joca.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Eickmann TH, Collier MB, Sukezaki F, McAuley JP, Engh GA. Survival of medial unicondylar arthroplasties placed by one surgeon 1984-1998. Clin Orthop Relat Res. 2006;452:143–149. doi: 10.1097/01.blo.0000238793.74843.dc. [DOI] [PubMed] [Google Scholar]
- 9.Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907–914. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 10.Garrett JC. Fresh osteochondral allografts for treatment of articular defects in osteochondritis dissecans of the lateral femoral condyle in adults. Clin Orthop Relat Res. 1994;(303):33–37. [PubMed]
- 11.Ghazavi MT, Pritzker KP, Davis AM, Gross AE. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg Br. 1997;79(6):1008–1013. doi: 10.1302/0301-620X.79B6.7534. [DOI] [PubMed] [Google Scholar]
- 12.Gortz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88(6):1374–1384. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Gortz S, Bugbee WD. Allografts in articular cartilage repair. Instr Course Lect. 2007;56:469–480. [PubMed] [Google Scholar]
- 14.Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468(5):1269–1278. doi: 10.1007/s11999-010-1250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;(435):79–87. [DOI] [PubMed]
- 16.Gross AE, Kim W. Las Heras F, Backstein D, Safir O, Pritzker KP. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466(8):1863–1870. doi: 10.1007/s11999-008-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(suppl 2):25–32. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 18.Hornicek FJ, Woll JE, Kasprisin D, McLean VA, editors. Standards for Tissue Banking. 10. McLean, VA: American Association of Tissue Banks; 2002. [Google Scholar]
- 19.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13–14. [PubMed]
- 20.Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29(5):600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 21.Jamali AA, Emmerson BC, Chung C, Convery FR, Bugbee WD. Fresh osteochondral allografts: results in the patellofemoral joint. Clin Orthop Relat Res. 2005;(437):176–185. [PubMed]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53(282):457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 23.Keeney JA, Eunice S, Pashos G, Wright RW, Clohisy JC. What is the evidence for total knee arthroplasty in young patients?: a systematic review of the literature. Clin Orthop Relat Res. 2011;469(2):574–583. doi: 10.1007/s11999-010-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91(4):805–811. doi: 10.2106/JBJS.H.00703. [DOI] [PubMed] [Google Scholar]
- 25.Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000;(380):85–90. [DOI] [PubMed]
- 26.McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411–420. doi: 10.1177/0363546506295178. [DOI] [PubMed] [Google Scholar]
- 27.McDermott AG, Langer F, Pritzker KP, Gross AE. Fresh small-fragment osteochondral allografts. Long-term follow-up study on first 100 cases. Clin Orthop Relat Res. 1985;(197):96–102. [PubMed]
- 28.Miller BS, Steadman JR, Briggs KK, Rodrigo JJ, Rodkey WG. Patient satisfaction and outcome after microfracture of the degenerative knee. J Knee Surg. 2004;17(1):13–17. doi: 10.1055/s-0030-1247141. [DOI] [PubMed] [Google Scholar]
- 29.Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902–908. doi: 10.1177/0363546508330137. [DOI] [PubMed] [Google Scholar]
- 30.Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911–1920. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 31.Mithofer K, Minas T, Peterson L, Yeon H, Micheli LJ. Functional outcome of knee articular cartilage repair in adolescent athletes. Am J Sports Med. 2005;33(8):1147–1153. doi: 10.1177/0363546504274146. [DOI] [PubMed] [Google Scholar]
- 32.O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80(12):1795–1812. [PubMed] [Google Scholar]
- 33.Pearsall AW, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32(1):125–131. doi: 10.1177/0095399703258614. [DOI] [PubMed] [Google Scholar]
- 34.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85-A(suppl 2):17–24. doi: 10.2106/00004623-200300002-00003. [DOI] [PubMed] [Google Scholar]
- 35.Shasha N, Krywulak S, Backstein D, Pressman A, Gross AE. Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Joint Surg Am. 2003;85-A(suppl 2):33–39. doi: 10.2106/00004623-200300002-00005. [DOI] [PubMed] [Google Scholar]
- 36.Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. J Bone Joint Surg Am. 2003;85-A(suppl 2):8–16. doi: 10.2106/00004623-200300002-00002. [DOI] [PubMed] [Google Scholar]
- 37.Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16(2):83–86. [PubMed] [Google Scholar]
- 38.Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89(4):718–726. doi: 10.2106/JBJS.F.00625. [DOI] [PubMed] [Google Scholar]
- 39.Williams SK, Amiel D, Ball ST, Allen RT, Tontz WL, Jr, Emmerson BC, Badlani NM, Emery SC, Haghighi P, Bugbee WD. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35(12):2022–2032. doi: 10.1177/0363546507305017. [DOI] [PubMed] [Google Scholar]
- 40.Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37(1):42–55. doi: 10.1177/0363546508322897. [DOI] [PubMed] [Google Scholar]