Abstract
Background
The saddle prosthesis originally was developed to reconstruct large acetabular defects in revision hip arthroplasty and was used primarily for hip reconstruction after periacetabular tumor resections. The long-term survival of these reconstructions is unclear.
Questions/purpose
We therefore examined the long-term function, complications, and survival in patients treated with saddle prostheses after periacetabular tumor resection.
Patients and Methods
Between 1987 and 2003 we treated 17 patients with a saddle prosthesis after periacetabular tumor resection (12 chondrosarcomas, three osteosarcomas, one malignant fibrous histiocytoma, one metastasis). During followup, 11 patients died, resulting in a median overall survival of 49 months (95% CI, 30–68 months). The remaining six patients were alive without disease (mean followup, 12.1 years; range, 8.3–16.8 years). In one patient the saddle prosthesis was removed after 3 months owing to dislocation and infection. We obtained SF-36 questionnaires, Toronto Extremity Salvage Scores (TESS), and Musculoskeletal Tumor Society (MSTS) scores.
Results
Thirteen of 17 patients used walking assists for mobilization at last followup: eight patients required two crutches, five needed one crutch, and one did not use any walking aids. The other three patients were not able to mobilize independently and only made bed to chair transfers. The mean hip flexion in the six surviving patients was 60° (range, 40°–100°) at last followup. Local complications were seen in 14 of the 17 patients: nine wound infections, seven dislocations, and two leg-length discrepancies requiring additional surgery. In the five surviving patients with their index prosthesis still in situ, the mean MSTS score at long-term followup was 47% (range, 20%–77%), the mean TESS score was 53% (range, 41%–67%), and the mean composite SF-36 physical and mental component summaries were 43.9 and 50.6, respectively.
Conclusion
Reconstruction with saddle prostheses after periacetabular tumor surgery has a high risk of complications and poor long-term function with limited hip flexion; therefore, we no longer use the saddle prosthesis for reconstruction after periacetabular tumor resections.
Level of Evidence
Level IV, retrospective case series. See the Guideline for Authors for a complete description of levels of evidence.
Introduction
The pelvis accounts for 5% to 15% of primary sarcomas and is the third most frequent site for occurrence of bone metastasis. As tumor surgery evolved in the first half of the 20th century, the standard surgical treatment for primary pelvic sarcomas was hemipelvectomy, which is a mutilating procedure to a vital extremity to achieve local tumor control at the cranial margin. Furthermore this disfiguring procedure has a reported range of major morbidity in more than 53% of patients [3, 4] and is disabling. With the development of better imaging, chemotherapy, and surgical techniques there has been increased interest in internal hemipelvectomy with partial resection of the innominate bone [11, 21, 27]. With this procedure local tumor control can be obtained in patients with pelvic sarcomas without the necessity for amputation. Ideally a periacetabular reconstruction achieves tumor resection with adequate margins and minimal complications and results in the ability to mobilize without the use of walking aids [3, 4, 10, 17, 18].
Pelvic resections were classified by Enneking et al. [12–14] into three types by the portion of bone removed. Type 1 involves resection of the ilium, Type 2 involves the acetabulum, and Type 3 involves the pubic rami. The reconstructive procedure is challenging with a reported incidence of mechanical, infectious, and neurovascular complications of 33% to 56% after Type 2 internal hemipelvectomy of a periacetabular tumor [1, 8, 16, 20, 21, 23].
The saddle prosthesis (Link, Hamburg, Germany) initially was designed by Nieder et al. [23] in 1979 for large acetabular defects in revision hip arthroplasty. Since the 1980s the saddle prosthesis also was used for reconstruction after periacetabular tumor resection [5, 8]. Partly owing to the design of the saddle prosthesis, several long-term complications have been described [16, 20, 23, 26] in which iliac wing destruction and proximalization of the prosthesis resulting in leg-length discrepancy and dislocations were most prominent. Other major complications are wound-healing problems, deep infection, nerve deficits, fractures, and heterotopic ossification [1, 2, 5, 8, 29].
Advancements in radiographic imaging, adjuvant chemotherapy and radiotherapy, and surgical techniques have facilitated the resectability of periacetabular tumors with a subsequent increase in the number of pelvic reconstructions [10]. Owing to increased patient survival after internal hemipelvectomy, improvement of long-term hip function after pelvic reconstruction is required to make mobilization without walking aids possible. Although several other reconstructive procedures have been reported including pelvic prosthesis arthroplasty [1, 2, 8, 16, 20, 23, 28], allograft reconstruction (with or without a total hip prosthesis) [19, 27], arthrodesis [16, 24], and pseudarthrosis [16], a gold standard has yet to be established owing to poor postoperative function and major complication rates ranging from 33% to 56% [1, 2, 7, 8, 16, 19, 20, 23, 24, 28]
We therefore evaluated the long-term function and complications in patients with a saddle prosthesis after reconstruction of a periacetabular tumor resection.
Patients and Methods
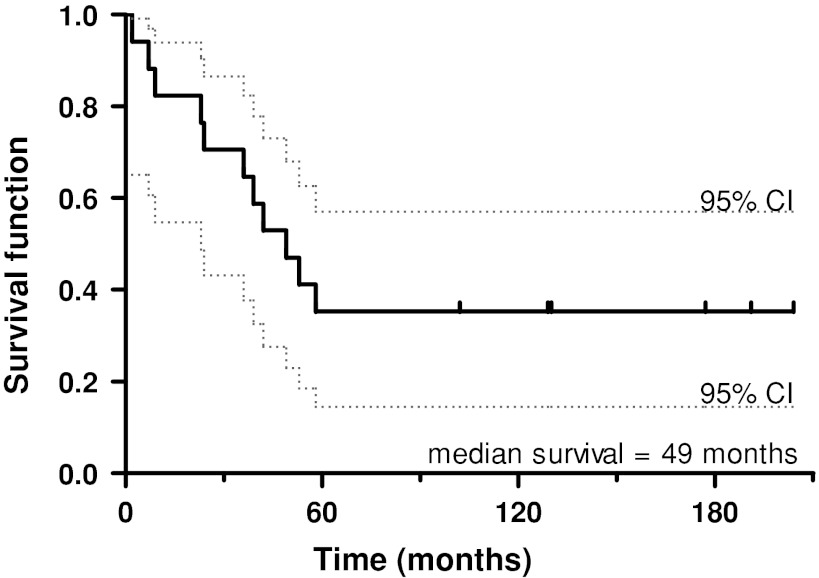
From 1987 to 2003, a total of 17 patients were treated with a saddle prostheses after a Type 2 resection of periacetabular tumors. The indications for a saddle prosthesis were: (1) pelvic tumors localized in the periacetabular region with clear surgical margins, (2) no signs of metastatic disease, (3) sufficient residual bone stock of the ilium after planned resection for creation of a stable notch for the saddle, and (4) otherwise good physical status and life expectancy. The contraindications were: (1) tumor extension across the sacroiliac joint and iliacus muscle or extensive soft tissue infiltration into the pelvis or thigh, (2) involvement of sacral nerves or sciatic nerve, (3) metastatic disease from the primary periacetabular tumor, (4) lack of residual bone stock of the ilium after planned resection, and (5) poor life expectancy and physical status. There were 10 men and seven women with a mean age of 48 years at diagnosis (range, 24–65 years). Twelve patients had chondrosarcomas, three had osteosarcomas, one had a malignant fibrous histiocytoma, and one had periacetabular metastasis of a Grawitz tumor (Table 1). No patients were lost to followup. At last followup, 11 patients had died (median survival, 36 months; range, 2–58 months), and six were still alive (median followup, 94 months; range, 2–204 months). Of this last group one patient had the saddle prosthesis removed 3 months postoperatively owing to chronic infection. No evidence of disease was found in the six patients who were still alive (Fig. 1). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs, and functional scores were taken of the surviving patients at latest followup.
Table 1.
Baseline data for 17 patients treated with a saddle prothesis
| Patient | Sex and age (years) | Type of tumor and grade | Enneking, side | Year of surgery | Graft type | Graft technique | Cerclage fixation | Surgery time (minutes) | Post-operative plaster | Adjuvant therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M 44 | Chondrosarcoma grade 2 | Zones 2/3, L | 1987 | None | None | No | 450 | Yes | RT |
| 2 | M 49 | Chondrosarcoma grade 2 | Zones 1/2/3, R | 1987 | Auto + allograft femur | Standard | Not documented | Not documented | Yes | RT |
| 3 | F 63 | Chondrosarcoma grade 2 | Zone 2, R | 1988 | Auto + allograft femur | Standard | Yes | 540 | Yes | RT |
| 4 | M 61 | Chondrosarcoma grades 2–3 | Zone s1/2, L | 1988 | Not documented | Not documented | Not documented | 240 | Not documented | RT |
| 5 | M 51 | Chondrosarcoma grade 2 (dedifferentiated) | Zone 2, R | 1989 | Auto + massive allograft | Standard + osteo-synthesis iliac wing | No | 480 | Yes | ChT |
| 6 | F 43 | Osteosarcoma | Zones 1/2, R | 1990 | Auto + massive allograft | Standard + osteo-synthesis iliac wing | No | Not documented | No | RT + ChT |
| 7 | M 50 | Metastatic renal cell carcinoma | Zone 2, R | 1992 | Not documented | Not documented | Not documented | Not documented | Not documented | RT + ChT + embolization |
| 8 | M 65 | Chondrosarcoma grade 2 | Zone 2, L | 1992 | Auto + allograft femur | Standard | Yes | 370 | Yes | None |
| 9 | M 24 | Chondrosarcoma grades 2–3 | Zones 1/2, R | 1994 | Not documented | Not documented | Not documented | 540 | Yes | Not documented |
| 10 | F 26 | Chondrosarcoma grade 1 | Zone 2, L | 1995 | Auto + massive allograft | Standard + osteo-synthesis iliac wing | No | 360 | Yes | None |
| 11 | F 53 | Chondrosarcoma grades 2–3 (dedifferentiated) | Zone 2, L | 1995 | Auto + massive allograft | Standard + osteo-synthesis iliac wing | No | 660 | Yes | ChT |
| 12 | M 34 | Chondrosarcoma grades 2–3 (dedifferentiated) | Zone 2, L | 1998 | Autograft femur | Standard | Yes | 370 | Yes | RT |
| 13 | M 27 | Malignant fibrous histiocytoma | Zones 1/2, R | 1999 | Auto + allograft femur | Standard | Yes | 300 | Yes | ChT |
| 14 | F 50 | Osteosarcoma | Zone 2, R | 1999 | Auto + allograft femur | Standard | Yes | 390 | Yes | RT + ChT |
| 15 | F 54 | Chondrosarcoma grade 2 (dedifferentiated) | Zone 2, L | 2001 | Auto + allograft femur | Standard | Yes | 420 | Yes | None |
| 16 | M 57 | Chondrosarcoma grades 2–3 (myxoid) | Zone 2, R | 2001 | Auto + allograft femur | Standard | No | 300 | Yes | ChT |
| 17 | F 59 | Osteosarcoma | Zones 2/3, R | 2003 | Autograft femur | Standard | Yes | 330 | Yes | RT + ChT |
RT = radiotherapy; ChT = chemotherapy.
Fig. 1.
The Kaplan-Meier curve of implant survivorship shows a major decrease in survival of the saddle prosthesis for the first 5 years owing to removal of the prostheses because of infection and death. The patients who survived after pelvic reconstruction require good long-term hip function from the saddle prosthesis.
During surgery the patient was positioned supine and access to the acetabulum and sciatic notch was gained by an extended ilioinguinal approach. The gluteal and abdominal muscles were spared as much as possible to restore abductor strength, hip stability, and soft tissue coverage postoperatively. The periacetabular tumors were resected en bloc and usually some iliac muscle was sacrificed to achieve a wide tumor margin. After preparation of the femoral stem a notch was engraved in the medial part of the iliac wing remnant. In the majority of patients an iliac wing augmentation was performed in standard fashion to create more stable bone stock for the saddle to articulate with. In these patients the remnant iliac wing was split between the internal and external tables, augmented with autograft femoral bone from the patients’ own resected femoral head, and if necessary with an allograft donor femoral head (Table 1, Patients 2–3, 8, 13–17). In case of insufficient bone stock for creation of a stable notch a massive fibular allograft also was used for iliac wing augmentation, which was fixated on the iliac wing by plate and screws (Table 1, Patients 5, 6, 10, 11). In seven patients a cerclage with Dacron tape also was used to further reinforce the bond between the saddle and the notch in the augmented iliac wing (Table 1, Patients 3, 8, 12–15, 17). After selection of the required saddle prosthesis length, hip stability and achieved leg length were tested perioperatively and adjusted when necessary. At the end of the procedure sufficient soft tissue coverage was achieved in all patients primarily.
The postoperative rehabilitation protocol included nonweightbearing for wound healing the first 2 weeks followed by 6 weeks of gradually increasing mobilization to partial weightbearing with two crutches and a pelvic plaster cast. Full weightbearing was allowed after 8 weeks. No braces or casts were used routinely for mobilization after the first 3 months [22]. Postoperative radiotherapy and/or chemotherapy were administered on indication guided by the specific tumor characteristics after histologic examination (Table 1).
During the first 2 years after surgery the patients were seen for clinical followup in the outpatient department three to four times a year, and on an annual basis afterwards. All patients were screened annually at our outpatient clinic with pelvic radiographs and thoracic CT for pulmonary metastatic disease. CT of the pelvis was done only if there was suspicion of local recurrence. All postoperative complications, maximal hip flexion, and use of walking aids were retrieved from the medical records. Of the remaining six surviving patients, function also was assessed by maximal hip flexion at followup, the SF-36 questionnaire [6], Toronto Extremity Salvage Score (TESS) [9], and Musculoskeletal Tumor Society score (MSTS) [13]. The TESS is a questionnaire that is being used for functional assessment in patients who have had limb surgery for bone and soft tissue tumors. The questionnaire consists of 30 activities and the result is converted to a percentage. The MSTS limb salvage scoring system consists of six factors (pain, function, emotional acceptance, support, walking, and gait). The highest possible MSTS score is 30, and the points are converted to a percentage.
Results
Eight of the 17 patients required two crutches or a walker for general mobilization. At last followup one patient was able to ambulate without walking aids, while five used one elbow crutch or cane. Three other patients were not able to mobilize even with support and only made bed to chair transfers. The mean maximal postoperative hip flexion for the five surviving patients was 60°, with a range of 40° to 100°. For the surviving patients the MSTS score at a mean followup of 12.1 years (range, 8.3–16.8 years) was 47% (range, 20%–77%), the mean TESS score was 53% (range, 41%–67%), and the mean composite SF-36 physical and mental scores were 43.9 and 50.6 respectively. Infection and delayed wound healing formed the majority of all documented postoperative complications. Nine early postoperative wound infections were diagnosed within 3 months postoperatively; all were treated with antibiotics. Three patients eventually had their saddle prosthesis removed because of ongoing infections. Two of the nine patients required débridement of necrotic wounds, one patient had a fistula excised, and in one patient the saddle prosthesis could be retained only after multiple débridements and treatment with gentamicin beads. Seven of the 17 patients had dislocations of their saddle prostheses, all of which initially were treated with reduction and casting. Three of these patients required subsequent revision surgery because of recurrent dislocations (Table 2, Patients 8, 9, 16). The mechanism of dislocation was usually a combined flexion and rotational force when getting out of bed or a chair during the early mobilization period (within 3 months after surgery). Two patients had developed leg length differences greater than 5 cm and both had revision of the modular components of the saddle prosthesis with restoration of leg length and stability (Table 2, Patients 1, 15). During surgery proximalization of the saddle prosthesis at the iliac wing was noted with decreased soft tissue tension. Seven patients had revision surgery owing to infection, dislocation, or leg length difference. One patient had a femoral artery thrombosis and one had an iatrogenic lumbosacral plexus lesion at the level of the L2-S1 nerve root that was permanent and probably attributable to extensive soft tissue excision necessary to achieve safe surgical margins. Fourteen of all the 17 patients had a postoperative complication that was documented (Table 2). Length of the surgical procedure was documented in 14 patients. Median time of surgery was 380 minutes (range, 240–660 minutes). Owing to death from disease, followup was limited in three patients to 2, 7, and 9 months, respectively (Table 2, Patients 4, 5, 17).
Table 2.
Clinical outcome data for 17 patients
| Patient number | Complications | Recurrent disease | Died of disease | Patient survival (months) | Revision, removal | Survival of prosthesis (months) | Hip flexion (degrees) | Mobilization |
|---|---|---|---|---|---|---|---|---|
| 1 | Plexus lesion, length difference | Yes | Yes | 36 | Yes, no | 36 | 60 | Two crutches |
| 2 | None | Yes | Yes | 58 | No | 58 | Not documented | Two crutches |
| 3 | Dislocation | Yes | Yes | 53 | No | 53 | 60 | Two crutches |
| 4 | Infection, wound necrosis | Yes | Yes | 9 | No | 9 | 80 | Bedridden |
| 5 | Infection, dislocation | Yes | Yes | 2 | No | 2 | Not documented | Bedridden |
| 6 | Infection | Yes | Yes | 42 | No | 42 | Not documented | One crutch |
| 7 | Infection | Yes | Yes | 49 | Yes, yes | 40 | 100 | Two crutches |
| 8 | Dislocation, length difference | No | No | 204 | Yes, no | 204 | 40 | Two crutches |
| 9 | Infection, thrombosis, dislocation | No | No | 191 | Yes, yes | 2 | Not documented | Bedridden |
| 10 | None | Yes | No | 177 | No | 177 | 40 | One crutch |
| 11 | Wound necrosis | Yes | Yes | 24 | No | 24 | 90 | One crutch |
| 12 | Infection | Yes | Yes | 23 | Yes, yes | 3 | Not documented | Two crutches |
| 13 | Dislocation | No | No | 130 | No | 130 | 80 | No walking aids |
| 14 | None | No | No | 129 | No | 129 | 50 | One crutch |
| 15 | Infection, length difference | No | No | 102 | Yes, no | 102 | 90 | One crutch |
| 16 | Infection, dislocation | Yes | Yes | 39 | Yes, yes | 12 | 80 | Two crutches |
| 17 | Infection, dislocation, wound fistula | Yes | Yes | 7 | No | 7 | 90 | Two crutches |
Discussion
The saddle prosthesis initially was designed for reconstruction of large acetabular defects in revision hip arthroplasty, but it has been used primarily for hip reconstruction after periacetabular tumor resections. For periacetabular reconstruction with the saddle prosthesis a notch is created in the iliac remnant [23], and in the event of large resections the remaining iliac wing can be augmented additionally with a cortical allograft to create a more stable notch. The saddle articulates with the iliac notch and does not require an exact anatomic fit. The saddle design has no formal constraint, but does have four modular interpositional components to build an optimal offset and length for soft tissue tensioning. The goals of surgical treatment of periacetabular tumors are wide resection providing local control and optimal chance for survival, preservation of limb function, and quality of life. Previous reports have been published on the surgical and functional outcomes of saddle prostheses [1, 2, 5, 8, 20, 23, 25, 28], but functional outcome in long-term survivors with these reconstructions remains unclear. Therefore we examined the long-term functional results and complications in patients treated with a saddle prosthesis after periacetabular tumor resection.
We recognize some limitations to our study. First, it is a retrospective case series, which makes comparison with a concurrent control group impossible. Therefore the exact influence of the extensive exposure needed for excision of periacetabular tumors with safe margins remains unclear. Second, the group of long-term survivors is relatively small and functional scoring has not been documented on an annual basis for all patients. Third, the operative time was not documented for all patients, therefore, it is not clear whether surgical technique or a learning curve could have influenced the high rate of complications. Fourth, only 17 patients have undergone surgery during a 16-year period. Although the operation rate was approximately one saddle prosthesis per year, the senior surgeons had extensive experience in this surgical field with different types of orthopaedic oncology surgery and arthroplasty during this period. The Leiden University Medical Centre has been a national referral center for orthopaedic oncology for more than 25 years. In the same 5-year period as in this study 24 pelvic reconstructions of other types were performed after periacetabular Stages P1 through 3 tumor resections. Compared with other studies the mean length of surgery also was shorter (Table 3). For these reasons we believe the limited number of procedures did not influence the complication rate.
Table 3.
Literature comparison of long-term functional outcome of saddle protheses
| Study | Year published | Patients with long-term followup | Mean length of long-term followup (months) | MSTS score | TESS score | Other outcome data |
|---|---|---|---|---|---|---|
| Kitagawa et al. [20] | 2006 | 7 | 21 | 45% | 61% | Operation time mean, 391 minutes |
| Aljassir et al. [2] | 2005 | 16 | 45 | 51% | 64% | Operation time mean, 600 minutes |
| Cottias et al. [8] | 2001 | 9 | 42 | 57% | 58% | Operation time range, 300–480 minutes |
| Renard et al. [25] | 2000 | 11 | 12 | 53% | – | Operation time median, 420 minutes |
| 6 | 24 | 51% | ||||
| Alboulafia et al. [1] | 1995 | 9 | 33 | – | – | Operation time mean, 466 minutes 7 patients “excellent” 2 patients “good” |
| Current study | 2012 | 6 | 146 | 47% | 53% | Operation time mean, 380 minutes 1 patient no crutches 3 patients one crutch 1 patient two crutches 1 patient only transfers |
MSTS = Musculoskeletal Tumor Society; TESS = Toronto Extremity Salvage Score.
In comparison to previous short-term followup series, the mean followup of 12.1 years in our study (with minimal followup of 8.3 years) of the surviving patients for whom functional scores with TESS and MSTS were measured, is substantially longer. Cottias et al. [8] reported a series of patients with a mean followup of 42 months. Functional scoring for nine patients showed a mean MSTS score of 57% and a mean TESS score of 58%. Kitagawa et al. [20] reported functional scores for a group of seven patients with a mean followup of 21 months. They reported a mean MSTS score of 45% for the seven patients, and six patients had a mean TESS score of 61%. Aljassir et al. [2] had a group of 16 patients with mean followup of 45 months, a mean MSTS score of 51%, and mean TESS score of 64%. Renard et al. [25] measured functional outcome at 1 and 2 years after a saddle prosthesis in 11 and six surviving patients respectively, and reported mean MSTS scores of 53% and 51%. Aboulafia et al. [1] described the results of patients treated with saddle prostheses by a grading system depending on oncologic outcome, ambulatory function, and use of pain medication. Of the nine patients still alive after an average of 33 months, the overall results were reported as “excellent” in seven patients and “good” in two patients. The functional outcome scores for our patients with long-term followup are slightly less (mean MSTS score, 47%; mean TESS score, 53%) than reported by other authors with substantially shorter followups. The functional results after pelvic reconstruction with the saddle prosthesis, and especially the poor hip flexion, are related to the eccentric position of the new rotational center of the hip that allows only limited ROM [29]. However, the extended resection has a negative effect on patient function [3–5].
Major complications after periacetabular reconstructions are common (ranging from 33%–65%) and can be related to the surgical procedure, tumor extension, the implant, and comorbidities of the patient [1, 8, 16, 20]. The type and incidence of complications after saddle prosthesis reconstruction in our patients are comparable to those reported by others. Wound complications, occurring in 18% to 37% of patients [1, 2, 8, 23, 25], are wound dehiscence, skin necrosis, and superficial and deep infections. Risk factors for the high incidence of wound problems are the long operating time, large surgical exposure, high volume of blood loss, lack of muscular and soft tissue coverage, large dead space after resection, and the patients’ immune system compromised by chemotherapy and radiotherapy. Reported neurologic complications are transient peroneal nerve paresis and neurapraxia of the sciatic and femoral nerves attributable to manipulation of the femur. The bony complications that frequently are described are fractures of the remaining iliac wing and proximal migration of the saddle component (range, 0%–7%), which causes leg length difference and dislocations (range, 0%–18%) [1, 8, 16, 20]. When large resection of the iliac wing is required, more proximal migration has been reported [1, 25]. In cases where continuous cranial migration of the saddle prosthesis is observed (Fig. 2), the patient is likely to have a deep wound infection [23]. We used nonresorbable sutures around the iliac wing and the saddle to prevent dislocation, and bone grafting of the iliac notch was used in case of a narrow remnant iliac wing, as described by others [8, 20]. Heterotopic ossifications can be seen growing from the iliac remnant several months postoperatively, which can have a negative effect on functional outcome. Oncologic complications are local recurrence and systemic progressive disease [16, 25, 26, 28].
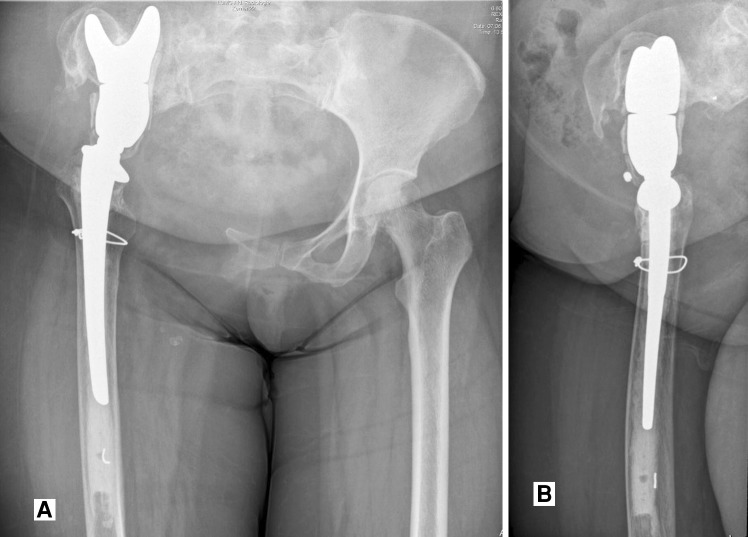
Fig. 2A–B.
This patient had a periacetabular osteosarcoma induced by radiotherapy, for which a Type 2 internal hemipelvectomy was performed, with pelvic reconstruction using the saddle prosthesis. (A) AP and (B) lateral view radiographs obtained 6 years after the initial reconstruction show signs of cranial migration of the saddle prosthesis attributable to osteolysis at the narrow iliac wing remnant proximally.
The Mark II saddle prosthesis (Link, Hamburg, Germany) used in our series is a second-generation design that offers better mobility and stability because of its modular design compared with the first-generation nonmodular Mark I design [23]. It still requires additional bone resection to create an iliac notch and provides an unstable articulation causing a high risk of mechanical failure at the ilium-to-saddle interface. Furthermore, the postoperative function based on the MSTS and TESS scores of the patients after saddle prosthesis reconstruction was not superior to other reconstructions with pelvic prostheses, allograft reconstruction, arthrodesis, or pseudarthrosis [20]. To address the mechanical complications of the saddle prosthesis, the periacetabular reconstruction (PAR) endoprosthesis was developed, which is a third-generation modular design consisting of an iliac wing component fixed to the ilium with screws and cement [15]. The modular femoral stem articulates with a constrained socket joint, which is embedded in the iliac wing component. Menendez et al. [21] reported results for 25 patients treated with the PAR endoprosthesis with a mean followup of 29 months and an average MSTS score of 67%. Although this third-generation saddle design did provide some functional improvement, major complications still occurred in 56% of the patients and implant survivorship after 5 years was reported at 60% [21]. In the study by Menendez et al. [21], 14 of 25 patients had at least one major complication: there were eight infections, five reoccurrences, three dislocations, two fractures, one malposition, one necrosis, and one heterotopic ossification.
Some authors prefer pelvic reconstruction by pseudarthrosis [16] instead of reconstruction with allografts [24] or an endoprosthesis [30], because of difficulty in providing a firm long-lasting reconstruction and high complication rates. However, in addition to limited motion and inconvenient leg length discrepancy, failure of fusion often occurs, resulting in a painful pseudarthrosis with unsatisfactory functional outcome [7, 9, 29].
To improve outcome after reconstruction of large defects after periacetabular resection new custom implants and ball and socket-type implants with pedestal-based cups have been developed. These newer types of tumor prostheses consist of a socket with a cone-shaped pedestal attached, which is inserted into the remaining iliac body toward the superior border of the sacroiliac joint for stable fixation. Some designs offer a modular cup for restoration of anatomic inclination and anteversion, which articulates with a large ceramic or tripolar head potentially offering more stability. Because no iliac notch has to be created less bone resection and soft tissue exposure is required, but complications otherwise related to the challenges of periacetabular tumor resections probably will remain unchanged. Although these new pedestal-based designs theoretically may offer improved function, no long-term followup data are available yet.
Reconstruction with saddle prostheses after periacetabular tumor surgery has a high risk of complications and poor long-term functional outcome with limited hip flexion. Based on our study with limited patient numbers but long-term followup the saddle prosthesis cannot be recommended for pelvic reconstruction after internal hemipelvectomy. Therefore in our center we no longer use the saddle prosthesis for reconstruction after periacetabular tumor resections. New more anatomic modular designs for pelvic reconstruction may offer better stability and mobility with cone-shaped, pedestal-based designs, but long-term followup is required to assess survivorship and functional outcome.
Acknowledgments
We thank Vivian M. Spaans MD, for her contribution in computing implant survivorship and generating the Kaplan-Meier curve.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
References
- 1.Aboulafia AJ, Buch R, Mathews J, Li W, Malawer MM. Reconstruction using saddle prosthesis following excision of primary and metastatic periacetabular tumors. Clin Orthop Relat Res. 1995;314:203–213. [PubMed] [Google Scholar]
- 2.Aljassir F, Beadel GP, Turcotte RE, Griffin AM, Bell RS, Wunder JS, Isler MH. Outcome after pelvic sarcoma resection reconstructed with saddle prosthesis. Clin Orthop Relat Res. 2005;438:36–41. doi: 10.1097/00003086-200509000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Apffelstaedt JP, Driscoll DL, Spellmann JE, Velez AF, Gibbs JF, Karakousis CP. Complications and outcome of external hemipelvectomy in the management of pelvic tumors. Ann Surg Oncol. 1996;3:304–309. doi: 10.1007/BF02306287. [DOI] [PubMed] [Google Scholar]
- 4.Beck LA, Einertson MJ, Winemiller MH, DePompolo RW, Hoppe KM, Sim FF. Functional outcomes and quality of life after tumor-related hemipelvectomy. Phys Ther. 2008;88:916–927. doi: 10.2522/ptj.20070184. [DOI] [PubMed] [Google Scholar]
- 5.Benevenia J, Cyran FP, Biermann JS, Patterson FR, Leeson MC. Treatment of advanced metastatic lesions of the acetabulum using the saddle prosthesis. Clin Orthop Relat Res. 2004;426:23–31. doi: 10.1097/01.blo.0000141387.03035.3e. [DOI] [PubMed] [Google Scholar]
- 6.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campanna R, van Horn JR, Guernelli N, Briccoli A, Ruggieri P, Biagini R, Bettelli G, Campanacci M. Complications of pelvic resections. Arch Orthop Trauma Surg. 1987;106:71–77. doi: 10.1007/BF00435417. [DOI] [PubMed] [Google Scholar]
- 8.Cottias P, Jeanrot C, Vinh TS, Tomeno B, Anract P. Complications and functional evaluation of 17 saddle prostheses for resection of periacetabular tumors. J Surg Oncol. 2001;78:90–100. doi: 10.1002/jso.1127. [DOI] [PubMed] [Google Scholar]
- 9.Davis AM, Bell RS, Badley EM, Yoshida K, Williams JI. Evaluating functional outcome in patients with lower extremity sarcoma. Clin Orthop Relat Res. 1999;358:90–100. doi: 10.1097/00003086-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Deloin X, Dumaine V, Biau D, Karoubi M, Babinet A, Tomeno B, Anract P. Pelvic chondrosarcomas: surgical treatment options. Orthop Traumatol Surg Res. 2009;95:393–401. doi: 10.1016/j.otsr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Eilber FR, Grant TT, Sakai D, Morton DL. Internal hemipelvectomy: excision of the hemipelvis with limb preservation. An alternative to hemipelvectomy. Cancer. 1979;43:806–809. doi: 10.1002/1097-0142(197903)43:3<806::aid-cncr2820430305>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60:731–746. [PubMed] [Google Scholar]
- 13.Enneking WF, Dunham WK, Gebhart MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 14.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 15.Falkinstein Y, Ahlmann ER, Menendez LR. Reconstruction of type II pelvic resection with a new peri-acetabular reconstruction endoprosthesis. J Bone Joint Surg Br. 2008;90:371–376. doi: 10.1302/0301-620X.90B3.20144. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs B, O’Connor MI, Kaufman KR, Padgett DJ, Sim FH. Iliofemoral arthrodesis and pseudarthrosis: a long-term functional outcome evaluation. Clin Orthop Relat Res. 2002;397:29–35. doi: 10.1097/00003086-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Griesser MJ, Gillette B, Crist M, Pan X, Muscarella P, Scharschmidt T, Mayerson J. Internal and external hemipelvectomy or flail hip in patients with sarcomas: quality-of-life and functional outcomes. Am J Phys Med Rehabil. 2012;91:24–32. doi: 10.1097/PHM.0b013e318232885a. [DOI] [PubMed] [Google Scholar]
- 18.Ham SJ, Schraffordt Koops H, Veth RP, van Horn JR, Eisma WH, Hoekstra HJ. External and internal hemipelvectomy for sarcomas of the pelvic girdle: consequences of limb salvage treatment. Eur J Surg Oncol. 1997;23:540–546. doi: 10.1016/S0748-7983(97)93173-5. [DOI] [PubMed] [Google Scholar]
- 19.Harrington KD. The use of hemipelvic allografts or autoclaved grafts for reconstruction after wide resections of malignant tumors of the pelvis. J Bone Joint Surg Am. 1992;74:331–341. [PubMed] [Google Scholar]
- 20.Kitagawa Y, Ek ET, Choong PF. Pelvic reconstruction using saddle prosthesis following limb salvage operation for periacetabular tumour. J Orthop Surg (Hong Kong). 2006;14:155–162. doi: 10.1177/230949900601400210. [DOI] [PubMed] [Google Scholar]
- 21.Menendez LR, Ahlmann ER, Falkinstein Y, Allison DC. Periacetabular reconstruction with a new endoprosthesis. Clin Orthop Relat Res. 2009;467:2831–2837. doi: 10.1007/s11999-009-1043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray TG, Wetters NG, Moric M, Sporer SM, Paprosky WG, Della Valle CJ. The use of abduction bracing for the prevention of early postoperative dislocation after revision total hip arthroplasty. J Arthroplasty. 2012:27(8 suppl);126–129. [DOI] [PubMed]
- 23.Nieder E, Elson RA, Engelbrecht E, Kasselt MR, Kellar A, Steinbrink K. The saddle prosthesis for salvage of the destroyed acetabulum. J Bone Joint Surg Br. 1990;72:1014–1022. doi: 10.1302/0301-620X.72B6.2246283. [DOI] [PubMed] [Google Scholar]
- 24.Ozaki T, Hillmann A, Bettin D, Wuisman P, Winkelmann W. High complication rates with pelvic allografts: experience of 22 sarcoma resections. Acta Orthop Scand. 1996;67:333–338. doi: 10.3109/17453679609002326. [DOI] [PubMed] [Google Scholar]
- 25.Renard AJ, Veth RP, Schreuder HW, Pruszczynski M, Keller A, van Hoesel Q, Bökkerink JP. The saddle prosthesis in pelvic primary and secondary musculoskeletal tumors: functional results at several postoperative intervals. Arch Orthop Trauma Surg. 2000;120:188–194. doi: 10.1007/s004020050041. [DOI] [PubMed] [Google Scholar]
- 26.Renard AJ, Veth RP, Schreuder HW, van Loon CJ, Koops HS, van Horn JR. Function and complications after ablative and limb salvage therapy in lower extremity sarcoma of bone. J Surg Oncol. 2000;73:198–205. doi: 10.1002/(SICI)1096-9098(200004)73:4<198::AID-JSO3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz AJ, Kiatisevi P, Eilber FC, Eilber FR, Eckardt JJ. The Freidman-Eilber resection arthroplasty of the pelvis. Clin Orthop Relat Res. 2009;467:2825–2830. doi: 10.1007/s11999-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Lei B, Hoekstra HJ, Veth RP, Ham SJ, Oldhoff J, Schraffordt Koops H. The use of the saddle prosthesis for reconstruction of the hip joint after tumor resection of the pelvis. J Surg Oncol. 1992;50:216–219. doi: 10.1002/jso.2930500403. [DOI] [PubMed] [Google Scholar]
- 29.Windhager R, Karner J, Kutschera HP, Polterauer P, Salzer-Kuntschik M, Kotz R. Limb salvage in periacetabular sarcomas: review of 21 consecutive cases. Clin Orthop Relat Res. 1996;331:265–276. doi: 10.1097/00003086-199610000-00038. [DOI] [PubMed] [Google Scholar]
- 30.Witte D, Bernd L, Bruns J, Gosheger G, Hardes J, Hartwig E, Lehner B, Melcher I, Mutschler W, Schulte M, Tunn PU, Wozniak W, Zahlten-Hinguranage A, Zeifang F. Limb-salvage reconstruction with MUTARS hemipelvic endoprosthesis: a prospective multicenter study. Eur J Surg Oncol. 2009;35:1318–1325. doi: 10.1016/j.ejso.2009.04.011. [DOI] [PubMed] [Google Scholar]