Abstract
In neuronal systems, excitation and inhibition must be well balanced to ensure reliable information transfer. The cone/horizontal cell (HC) interaction in the retina is an example of this. Because natural scenes encompass an enormous intensity range both in temporal and spatial domains, the balance between excitation and inhibition in the outer retina needs to be adaptable. How this is achieved is unknown. Using electrophysiological techniques in the isolated retina of the goldfish, it was found that opening Ca2+-dependent Cl− channels in recorded cones reduced the size of feedback responses measured in both cones and HCs. Furthermore, we show that cones express Cl− channels that are gated by GABA released from HCs. Similar to activation of ICl(Ca), opening of these GABA-gated Cl− channels reduced the size of light-induced feedback responses both in cones and HCs. Conversely, application of picrotoxin, a blocker of GABAA and GABAC receptors, had the opposite effect. In addition, reducing GABA release from HCs by blocking GABA transporters also led to an increase in the size of feedback. Because the independent manipulation of Ca2+-dependent Cl− currents in individual cones yielded results comparable to bath-applied GABA, it was concluded that activation of either Cl− current by itself is sufficient to reduce the size of HC feedback. However, additional effects of GABA on outer retinal processing cannot be excluded. These results can be accounted for by an ephaptic feedback model in which a cone Cl− current shunts the current flow in the synaptic cleft. The Ca2+-dependent Cl− current might be essential to set the initial balance between the feedforward and the feedback signals active in the cone HC synapse. It prevents that strong feedback from HCs to cones flood the cone with Ca2+. Modulation of the feedback strength by GABA might play a role during light/dark adaptation, adjusting the amount of negative feedback to the signal to noise ratio of the cone output.
Key points
The GABAergic pathway modulates feedback between retinal horizontal cells (HCs) and cone photoreceptors, but is not mediating negative feedback, as previously hypothesized.
Opening of GABA-gated chloride channels in cone photoreceptors reduces the amplitude of feedback responses generated by HCs.
Activation of a different presynaptic chloride current, the calcium-dependent chloride current, in individual cones has a similar effect on feedback as application of GABA.
Modulation of the strength of feedback from HCs seems to be a general consequence of activation of presynaptic chloride currents in cones.
This puts the functional role of these currents in a new perspective; GABA acts as a slow and global neuromodulator enhancing feedback in the light- and attenuating feedback in the dark-adapted retina, whereas the calcium-dependent chloride current modulates feedback fast and locally to tune the size of feedback to local light conditions.
Introduction
Reliable information transfer in neuronal systems depends on well-balanced excitatory and inhibitory inputs. Cone photoreceptors are excited by light and receive inhibitory feedback from horizontal cells (HCs). For these neurons, the balance between excitation and inhibition seems particular important as they respond with graded potential changes, and their synaptic output can both increase and decrease depending on the stimulus configuration. If inhibition would be too large or too small, modulation of the cone membrane potential would not be effectively transformed into changes in glutamate release (Fahrenfort et al. 1999). How the strength of the feedback signal from HCs to cones can be tuned to maintain the balance between excitation and inhibition is unknown.
Feedback from HCs modulates the cone Ca2+ current (ICa), which regulates cone glutamate release (Verweij et al. 1996). Although GABA has been suggested as a feedback neurotransmitter (Wu & Dowling, 1980; Tachibana & Kaneko, 1984; Tatsukawa et al. 2005), surround-induced feedback responses in cones do not seem to be mediated by GABA (Verweij et al. 1996, 2003; Kamermans et al. 2001; Crook et al. 2011) The negative feedback mechanism from HCs to cones is, at least in fish, mediated by connexin hemichannels most likely via an ephaptic interaction (Kamermans et al. 2001; Kamermans & Fahrenfort, 2004; Fahrenfort et al. 2009; Klaassen et al. 2011). Feedback is strongly pH dependent (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Fahrenfort et al. 2009). The pH dependence might be due to the strong pH sensitivity of connexin hemichannels (Malchow et al. 1993; Trexler et al. 1999; Gonzalez-Nieto et al. 2008; Huckstepp et al. 2010), or it might indicate that protons are mediating feedback (Hirasawa & Kaneko, 2003; Vessey et al. 2005; Fahrenfort et al. 2009). This leaves the role of GABA-gated Cl− currents (ICl(GABA)) in cones unexplained (Tachibana & Kaneko, 1984) and thus a possible candidate for the modulation of feedback.
Apart from a ICl(GABA) (Tachibana & Kaneko, 1984; Picaud et al. 1998; Klooster et al. 2004), cones have a large Ca2+-dependent Cl− current (ICl(Ca)) (Bader et al. 1982; Corey et al. 1984; Barnes & Bui, 1991; Barnes & Deschenes, 1992; Kraaij et al. 2000a), which is located in the synaptic terminal of photoreceptors (Stohr et al. 2009; Mercer et al. 2011). The activation of ICa(Cl) depends on the intracellular Ca2+ concentration ([Ca2+]i), which is mainly determined by the influx of Ca2+ through voltage-gated Ca2+ channels (Barnes & Bui, 1991; Kraaij et al. 2000a; Lalonde et al. 2008), uptake of Ca2+ in Ca2+ stores, and the extrusion of Ca2+ via the Ca2+ pumps (Krizaj et al. 2004). The activation and inactivation time constants of this current range from a few 100 ms to seconds (Kraaij et al. 2000a) due to the slow kinetics of the Ca2+-dependent Cl− channel (Scudieri et al. 2012) and the slow extrusion of Ca2+ by Ca2+ pumps (Krizaj et al. 2004). It has been suggested that this current plays a role in controlling the intracellular chloride concentration ([Cl−]i), which might modulate the amplitude of ICa in photoreceptors (Rabl & Thoreson, 2002; Thoreson et al. 2003). Therefore, this current is a potential candidate for the modulation of the communication between cones and HCs.
In this paper, the effect of these two cone Cl− currents (ICl) on the strength of feedback from HCs to cones was studied in the goldfish retina. Activation of either ICl led to a reduction of the amplitude of the feedback signal from HCs to cones. Although both currents are carried by Cl−, they function on different time scales. The GABAergic system functions on a time scale of seconds, whereas the ICl(Ca) functions on a time scale of hundreds of milliseconds. Simulations with an extended version of an ephaptic feedback model, originally formulated by Fahrenfort et al. (2009), showed that a cone ICl can inhibit feedback from HCs to cones by interfering with the current flow in the synaptic terminal. Together these data suggest that the GABAergic pathway from HCs to cones modulates the strength of feedback globally on a slow time scale, possibly during light/dark adaptation. By contrast, ICl(Ca) modulates feedback strength locally on a much faster time scale depending on cone polarization.
Methods
Experimental animals
Goldfish, Carassius auratus (12–16 cm standard body length), were kept at 16°C under a 12 h dark, 12 h light cycle. Experiments were performed with fish that were between 6 and 9 h into their light phase. All recordings from cones and HCs were made in flat mounted isolated retinal preparations.
All experimental procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and conformed to the guidelines for the Care and Use of Laboratory Animals of The Netherlands Institute for Neuroscience acting in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Experimental procedures
Isolated retina preparation for cone and HC recordings
Fish were dark-adapted for at least 3 min, and all further preparation steps were performed under dim deep-red light illumination. After decapitation, an eye was enucleated and hemisected, and most of the vitreous was removed with filter paper. The retina was isolated, placed receptor side up in a superfusion chamber (volume 0.75 ml) mounted on a Nikon Eclipse 600FN microscope (Nikon, Tokyo, Japan) or an Olympus IMT2 inverted microscope (Olympus, Tokyo, Japan), and superfused continuously (1.5 ml min−1) with a Ringer solution of which the pH was continuously measured. The Ringer solution contained (in mm): NaCl, 102.0; KCl, 2.6; MgCl2, 1.0; CaCl2, 1.0; NaHCO3, 28.0; glucose, 5.0; and was continuously gassed with approximately 2.5% CO2 and 97.5% O2. Minor adjustments to the amount of CO2 were made such that the pH was 7.8. All chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA), except for SKF89976-A (a kind gift from SmithKline Beecham Pharmaceuticals, London, UK).
Voltage clamp measurements of cone responses
Optical stimulator
Two optical stimulators were used for the cone measurements. The first consisted of a 450 W xenon lamp that supplied two beams of light that were directed to the preparation after passing through Uniblitz VS14 shutters (Vincent Associates, Rochester, NY, USA), neutral density filters (Schott, Mainz, Germany), and a series of lenses and apertures. The second consisted of two homemade LED stimulators based on a three-wavelength high-intensity LED (Atlas, Lamina Ceramics Inc., Westhampton, NJ, USA). The peak wavelengths of the LEDs were 635, 520 and 460 nm, respectively, with a bandwidth smaller than 25 nm. An optical feedback loop ensured linearity. The output of the LEDs was coupled to the microscope via light guides.
Feedback-induced responses to 500 ms, 3000 μm spot stimulation were measured in cones at different potentials while the cone was continuously saturated with a 20 μm spot. The 20 μm spots were projected through the 60× water immersion objective (NA = 1.00) of the microscope, and the 3000 μm spots were projected through the microscope condenser (NA = 1.25). For experiments with cones, only white light stimuli were used. Light intensities are expressed in log units of attenuation relative to a luminance of 4 × 103 cd m−2.
Electrodes and recording equipment
Pipettes were pulled from borosilicate glass (GC150TF-10 Harvard Apparatus Ltd, Edenbridge, UK) with a Sutter P-87 micropipette puller (Sutter Instrument Co., Novato, CA, USA); the impedances ranged from 3 to 6 MΩ when filled with pipette medium and measured in Ringer solution. The standard patch pipette medium contained (in mm): KCl, 10.0; potassium d-gluconate, 96.0; MgCl2, 1.0; CaCl2, 0.1; EGTA, 5.0; Hepes, 5.0; ATP-K, 5.0; GTP-Na3, 1.0; 3′,5′-cGMP-Na, 0.2; phosphocreatine-Na2, 20; creatine phosphokinase, 50 units ml−1. In experiments with the standard patch pipette medium, the calculated ECl was −55 mV. Where appropriate, ECl was shifted by interchanging concentrations of KCl and potassium d-gluconate. The pH of the pipette medium was adjusted to 7.25 with KOH. The electrodes were mounted on a MP-85 Huxley/Wall-type micromanipulator (Sutter Instrument Co.) and connected to a Dagan 3900A Integrating Patch Clamp (Dagan Corporation, Minneapolis, MN, USA). The liquid junction potential was measured with a patch pipette filled with the pipette medium, and positioned in a bath filled with pipette medium. The reference electrode was filled with 3 m KCl. After the potential was adjusted to zero, the bath solution was replaced with Ringer solution. The resulting potential change was considered the junction potential, and all data were corrected accordingly. The preparation was illuminated with infrared light (λ > 850 nm; Wratten filter 87c, Kodak, Rochester, NY, USA), magnified with a Nikon 60× water immersion objective (NA = 1.00), differential interference contrast, and viewed using a video camera (Philips, Eindhoven, The Netherlands). Data acquisition, and control of the patch clamp and optical stimulator were done with a CED 1401 AD/DA converter and Signal 3.07 (both from Cambridge Electronic Design Ltd, Cambridge, UK).
Intracellular measurements of HC responses
Optical stimulator
Light stimuli were generated using two beams from a 450 W xenon light source, and a pair of circular neutral density filters (Barr & Strout, Glasgow, UK). Full-field chromatic light stimuli were projected onto the retina through a 2× objective lens (NA = 0.08) of the microscope. To spectrally classify the HC, a monochromator (Ebert, Waltman, USA) and interference filters with a bandwidth of 8 ± 3 nm (Ealing Electro-Optics Inc., South Natick, MA, USA) were used. Light intensities are expressed in log units relative to 4 × 104 photons μm−2 s−1.
Electrodes and recording equipment
Microelectrodes were pulled on a horizontal puller (P-80/PC, Sutter Instrument Co.) using aluminosilicate glass (SM100F-10, Harvard Apparatus), and had impedances ranging from 300 to 400 MΩ when filled with 3 m KCl. Intracellular recordings were made with a WPI S7000A microelectrode amplifier system (World Precision Instruments Inc., Sarasota, FL, USA), recorded on paper (Linearcorder F WR3701, Graphtec, Yokohama, Japan), and sampled using an AD/DA converter (CED 1401, Cambridge Electronic Design) coupled to a Windows-based computer system.
Model
The model describing negative feedback from HCs to cones is an extended version of a model for the goldfish retina (Fahrenfort et al. 2009; Klaassen et al. 2011). Briefly, a simple conductive network was used to evaluate whether the physiology and morphology of the cone/HC synapse allows for physiologically relevant ephaptic interaction. The HC is modelled as three conductances with their associated reversal potentials; the hemichannel conductance (ghemi), the glutamate-gated conductance (gGlu) and a non-linear potassium conductance (gK) taken from Dong & Werblin (1995). ghemi is located on the dendrites of HCs, while gGlu is located on both the dendrites and the soma of the HCs. The reversal potentials for ghemi and gGlu are 0 mV, and that for gK is −82.7 mV (EK). In the dark, the HC membrane potential (VHC) is more positive than EK, and current will flow from gK into ghemi and gGlu via an extracellular resistive pathway in the synaptic complex whose conductance is gext. This current will generate a voltage drop over gext, making the potential deep in the synaptic cleft (Vext) slightly negative. The light-induced closure of gGlu causes the HC to hyperpolarize, resulting in an increase in current through gext, and a greater negativity in Vext. The fall in Vext causes the cone membrane to depolarize locally, which modulates the cone Ca2+ current (ICa), and increases the release of neurotransmitter. All parameters and equations of the model were kept equal to parameter set 2 in Fahrenfort et al. (2009). The cone model consisted only of a voltage-gated Ca2+ current and glutamate release. ICa was modelled according to eqn (1) with parameter values: KCa=−5.4 mV, ECa= 44.6 mV, nCa= 12 mV and  (normalized units; Klaassen et al. 2011). The relation between ICa in the cone and the glutamate-dependent conductance gGlu in the HC is described by eqn (2). This relation is based upon the finding that the glutamate release depends linearly on ICa (Schmitz & Witkovsky, 1997), and that the dependence of gGlu on the glutamate concentration can be described by a Hill function with coefficient 2 (O’Dell & Christensen, 1989; Schmitz & Witkovsky, 1997). Similar to Klaassen et al. (2011),
(normalized units; Klaassen et al. 2011). The relation between ICa in the cone and the glutamate-dependent conductance gGlu in the HC is described by eqn (2). This relation is based upon the finding that the glutamate release depends linearly on ICa (Schmitz & Witkovsky, 1997), and that the dependence of gGlu on the glutamate concentration can be described by a Hill function with coefficient 2 (O’Dell & Christensen, 1989; Schmitz & Witkovsky, 1997). Similar to Klaassen et al. (2011),  and KGlu= 0.1 (normalized units). The relation between ICl and Vcone is given by eqn (3).
and KGlu= 0.1 (normalized units). The relation between ICl and Vcone is given by eqn (3).
 |
(1) |
| (2) |
| (3) |
The present extension of the model consists of only one free parameter: gCl. To determine how critical this value is for the behaviour of the model, ECl and gCl were varied.
Statistics
Data are presented as means ± standard error of the mean (SEM). Significance was determined using the Student's t test (paired if appropriate) or the Mann–Whitney test. P≤ 0.05 was considered as significant.
Results
Activation of the Ca2+-dependent Cl− current reduces feedback responses in cones
Cones possess a large ICl(Ca) with highly specific characteristics. Figure 1 illustrates these features by showing the current traces of a cone at −80 mV and stepped for 2000 ms to various potentials with ECl at −55 mV. Stepping the cone membrane potential to the depolarized potentials yields a slowly developing outward current (Fig. 1A, left). Small tail currents are visible when the membrane potential is hyperpolarized back to −80 mV. This slowly activating current can be blocked by niflumic acid, one of the most potent pharmacological blockers of ICl(Ca) (Barnes & Deschenes, 1992; Kraaij et al. 2000a; Mercer et al. 2011; Fig. 1A, right). However, some of the ability of niflumic acid to block ICl(Ca) might lie in its ability to block Ca2+ influx (Thoreson et al. 2003). This makes this drug less suitable to study the functional role of ICl(Ca) in cones. We therefore chose to manipulate the size of ICl(Ca) without the use of pharmacology, by simply manipulating the holding potential of the cone. It exploits the slow kinetics of ICl(Ca). When keeping a cone hyperpolarized for a prolonged period, [Ca2+]i will decrease and Ca2+-dependent Cl− channels will be closed. Subsequent short-term depolarization will only minimally activate ICl(Ca). On the other hand, keeping the cell depolarized for a prolonged time will lead to high [Ca2+]i and strong activation of ICl(Ca). Subsequent brief hyperpolarization will minimally inactivate ICl(Ca). Thus, after stepping to a potential at which feedback can be measured (−45 mV to −25 mV), there will be a short time window in which ICl(Ca) will be relatively stable activated or inactivated depending on the pre-pulse. To verify the above, a number of control experiments were performed.
Figure 1. Activation of the Ca2+-dependent Cl− current in individual cones decreases the size of light-induced feedback responses.

A, the cone membrane potential was clamped at −60 mV and stepped to a range of voltages from −80 mV to +30 mV in 10 mV steps. Left panel, whole-cell current traces in control conditions show that ICl(Ca) is a slowly activating current. Right panel, whole-cell current traces of a cone when ICl(Ca) is blocked by 100 μm niflumic acid. The slowly activating current is absent. B, average whole-cell I–V relations of cones after a pre-pulse of 1 min to either −20 mV (black trace, n= 5) or −80 mV (red trace, n= 5). From these curves both the peak amplitude and the half-activation potential of the ICa were determined. The peak amplitude did not differ significantly between the two VClamp values, but the half-activation potential of ICa was significantly shifted to positive potentials for a depolarized VClamp (−20 mV: −21.5 ± 1.6 mV; −80 mV: −26.6 ± 1.1 mV; n= 5; P= 0.01). C, average I–V relations of the pre-pulse-induced current (the difference between the current after the pre-pulse of −20 mV and −80 mV) in the presence or absence of niflumic acid. The pre-pulse-induced current (black trace) reverses at about the calculated value (−35 mV) for ECl (−33.9 ± 5.8 mV; n= 6), and can be partially blocked by 100 μm niflumic acid (red trace). D, activation if ICl(Ca) leads to changes in the communication between HCs and cones. Left panel, activation of ICl(Ca) leads to a sustained shift of ICa to positive potentials. Middle panel, the peak amplitude of ICa does not depend on the activation of ICl(Ca). Right panel, feedback responses in cones are significantly reduced when ICl(Ca) is activated. E, example of feedback responses to 100 ms full-field flash in a cone clamped at −40 mV, following either a pre-pulse of −20 mV (black trace) or −80 mV (red trace). A depolarizing pre-pulse significantly reduces the feedback responses compared with a hyperpolarizing pre-pulse (3.3 ± 0.8 pA vs. 16.6 ± 4.8 pA, respectively; n= 6; P= 0.021). F, the amplitude of light-induced feedback responses in cones at different clamping potentials and pre-pulse potentials. The amplitude of feedback is dependent on both the potential at which it is measured and the pre-pulse potential. Feedback is the largest for the most hyperpolarized pre-pulse.
Cones in the isolated retina were voltage-clamped for at least 1 min at either −20 mV, to activate ICl(Ca), or −80 mV, to inactivate ICl(Ca). ECl was set to −35 mV. Whole-cell I–V relations of the sustained current were constructed in conditions with ICl(Ca) activated (Fig. 1B, black line) or inactivated (Fig. 1B, red line) and subtracted from each other. The resulting difference curve, plotted in Fig. 1C (black line), is a linear current with a reversal potential at about ECl (−33.9 ± 5.8 mV; n= 6), suggesting it is carried by Cl−. This current could be blocked by the application of 100 μm niflumic acid (n= 6), shown in Fig. 1C (red line). Application of niflumic acid reduced the difference current at −95 mV by 69.8 ± 0.072% (n= 6; P= 0.017), indicating that this current is mediated through Ca2+-dependent Cl− channels. These results confirm that the designed protocol indeed modulates the activity of ICl(Ca). Moreover, ICl(Ca) remains stably activated or inactivated for a period long enough to determine the size of feedback from HCs to cones in cones.
The activation of the ICl(Ca) led to a number of changes in the cone–HC communication. First of all, the half-activation potential of ICa was significantly shifted to positive potentials after holding the cell at −20 mV (−20 mV: −21.5 ± 1.6 mV; −80 mV: −26.6 ± 1.1 mV; n= 5; P= 0.01; Fig. 1D, left), without a change in the peak amplitude of the ICa (−20 mV: 120 ± 13 pA; −80 mV: 124 ± 26 pA; n= 5; P= 0.80; Fig. 1D, middle). What would be the effect of the activation of ICl(Ca) on the communication between HCs and cones? Cones were voltage-clamped at −40 mV and saturated with a 20 μm spot of intense white light, and a full-field stimulus (0 log) was flashed on for 500 ms in addition. Full-field light stimulation leads to hyperpolarization of HCs, which induces an inward current in cones. This current is the light-induced feedback-mediated response in cones (Verweij et al. 1996; Kamermans et al. 2001; Klaassen et al. 2011).
Activating ICl(Ca) affected the light-induced feedback responses measured in cones (Fig. 1E). The black trace shows a feedback-induced response in a cone clamped for 250 ms at −40 mV after a 1 min depolarization to −20 mV (light intensity = 0 log; ECl=−55 mV). The red trace shows a similar response, but now clamped for 250 ms at −40 mV after a 1 min hyperpolarization to −80 mV. The feedback response after prolonged hyperpolarization is significantly larger than after prolonged depolarization (16.6 ± 4.8 pA and 3.3 ± 0.8 mV, respectively; n= 6; P= 0.021; Fig. 1D, right). This reduction was voltage dependent. Figure 1F shows the amplitude of feedback measured at different potentials after 1 min depolarization (−20 mV; black line) and after 1 min of hyperpolarization (−60 mV, blue line; and −80 mV, red line). After prolonged hyperpolarization the feedback amplitude was maximal at −40 mV, whereas after prolonged depolarization the feedback amplitude was at −30 mV and reduced in amplitude. This reduction was significant at −40 mV (n= 4; P= 0.04), which is the middle of the activation range of the cones Ca2+ current. These results show that activation of a ICl in an individual cone is sufficient to reduce feedback-induced response in the physiological membrane potential range of the cones.
Cones have a GABA-gated current
ICl(Ca) is not the only ICl reported in cones. In many vertebrates, cones express GABA receptors (Kamermans & Werblin, 1992; Verweij et al. 1998; Paik et al. 2003; Klooster et al. 2004) and have a ICl(GABA) (Tachibana & Kaneko, 1984; Picaud et al. 1998). HCs contain GABA and release GABA via a GABA transporter working in the reverse direction, at least in non-mammalian species (Schwartz, 1982; Yazulla & Kleinschmidt, 1983). These two findings led to the hypothesis that HCs feedback to cones via a GABAergic pathway. However, direct measurements of the light-induced feedback signal in cones in goldfish (Verweij et al. 1996; Kamermans et al. 2001) and macaque (Verweij et al. 2003) showed that the feedback responses remained present when GABAergic transmission was blocked, leaving the function of ICl(GABA) unexplained.
Cones in the isolated retina of goldfish were voltage-clamped, and I–V relations were constructed in the presence (red trace, Fig. 2A) or absence of 200 μm GABA (black and blue traces, Fig. 2A). Application of GABA led to an increase in conductance. GABA-induced currents were isolated by subtraction of the control I–V relation from that determined in the experimental condition. GABA application induced a linear current (Fig. 2B, red line) with a reversal potential of −48.8 ± 6.0 mV (n= 5). This is close to the calculated reversal potential for Cl− (ECl=−55 mV), implying that this current is mainly carried by Cl−. If this current is indeed carried by Cl−, then its reversal potential should depend on ECl. This was tested next by performing the same experiments with a different intracellular chloride concentration. The red line in Fig. 2C is the I–V relation of the GABA-induced current with ECl at −55 mV, and the black line is the I–V relation of the GABA-induced current with ECl at −20 mV (−29.1 ± 3.5 mV; n= 8). The reversal potential of the GABA-induced current shifts with ECl (Fig. 2C), corroborating that it is carried by Cl−. Application of 200 μm of the GABA-gated Cl− channel blocker picrotoxin (PTX) closed a conductance (Fig. 2B, green line) with a reversal potential close to ECl (−50.5 ± 3.0 mV; n= 4). This indicates the presence of an endogenous ICl(GABA) in cones in control conditions.
Figure 2. Goldfish cones have GABA receptor-mediated Cl− currents.
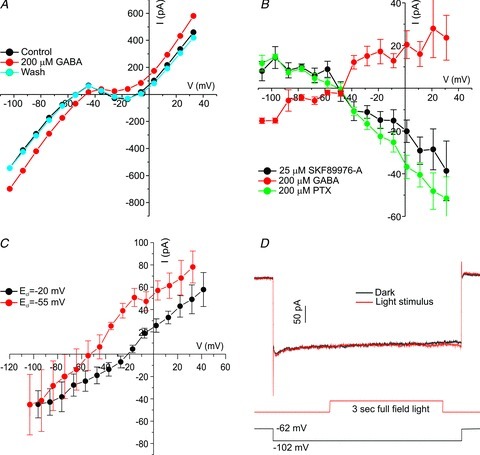
A, example of a whole-cell I–V relation of a cone in the presence or absence of GABA: Control (black trace), 200 μm GABA (red trace), Wash (blue trace). B, I–V relations of drug-induced (drug minus control) currents; 200 μm GABA induces a current reversing close to the calculated value for ECl−55 mV (red trace, n= 5). Application of 25 μm SKF89976-A, a GABA transporter blocker, leads to the closure of a current (black trace, n= 5), similar to the effect of 200 μm picrotoxin (PTX; green trace, n= 4). All currents reverse close to the estimated value of ECl. C, the GABA-induced (GABA minus control) I–V relations in conditions with a calculated ECl of −20 mV (black trace, n= 8) or −55 mV (red trace, n= 5). Changing ECl shifts the reversal potential of the currents near the values of the calculated ECl (−20 mV: −29.1 ± 3.5 mV; −55 mV: −48.8 ± 6.0 mV). D, example of cone current traces with or without light stimulation. To maximize the size of the Cl− current, ECl was set to an estimated –20 mV and the cone was stepped to −102 mV. Next, HCs were hyperpolarized by a 3 s full-field light flash (red trace). This sequence did not elicit any current changes compared with cones kept in the dark following the same potential step (black trace) in all six cones tested.
HCs are the only GABAergic neurons synapsing in the outer plexiform layer (Marc et al. 1978; Lam et al. 1980; Yazulla, 1986). HCs release GABA via GABA transporters (Schwartz, 1982, 1987), which can be blocked by SKF89976-A (Verweij et al. 1998). The black line in Fig. 2B shows that application of 25 μm SKF89976-A leads to the closure of a conductance with a reversal potential of −47.6 ± 4.4 mV (n= 5), just as the application of 200 μm PTX. Thus, blocking GABA transporters leads to a reduction of ICl(GABA) in cones. This is consistent with the hypothesis that HCs release GABA via a GABA transporter working in the reverse direction (Schwartz, 1982). These experiments show that GABA, released by HCs, opens GABA-gated Cl− conductances in cones, illustrating that HCs project to cones via a GABAergic pathway. Moreover, a significant fraction of the GABA receptors on cones are activated under control conditions.
If HCs would modulate their GABA release in a voltage-dependent manner (Schwartz, 1987), then hyperpolarizing HCs should change the GABA-gated conductance in cones. However, we were unable to modulate the GABA-gated conductance in cones by a light-induced hyperpolarization of HCs. HCs were hyperpolarized by full-field light stimulation (0 log) for 3 s (Fig. 2D). Hyperpolarization of HCs should lead to a reduction of the GABA release by HCs (Schwartz, 1987), and thus close GABA-gated conductance in cones and lead to a reduction of the current at −102 mV. Fig. 2D shows the whole-cell current of a cone when stepped from −62 mV to −102 mV without light stimulation (black trace) and with a 3 s full-field light stimulus (0 log, red trace), which hyperpolarizes HCs. The two traces are identical. This was found in all six cells tested this way. These results indicate that light-induced hyperpolarization of HCs does not modulate GABA-gated conductance in cones on a time scale of seconds (see Discussion).
GABA-gated current inhibits feedback responses in cones and HCs
What is the effect of the activation of this GABAergic mechanism on the communication between HCs and cones? Light-induced feedback-mediated responses in cones were reduced in the presence of 200 μm GABA (Fig. 3A, middle trace) compared with the control conditions (Fig. 3A, left trace) that recovered after washing out GABA (Fig. 3A, right trace). On average, feedback-induced responses were reduced by 42.2 ± 6.5% (n= 15; P= 0.00014). Blocking GABA-gated channels with 200 μm PTX had the opposite effect (Fig. 3B). Application of PTX increased the feedback-induced current by 74.9 ± 20.2% (n= 8; P= 0.018) relative to control conditions, revealing the presence of an endogenous GABA-gated current in control conditions as previously shown in Fig. 2B. Blocking GABA release from HCs by application of SKF89976-A led, in two of the four cells tested, to a similar increase in feedback as PTX (Fig. 3C). The two other cells did not respond to the application of SKF89976-A.
Figure 3. GABA reduces the size of feedback responses in cones and HCs.

A, example of light-induced feedback responses in a cone in the presence and absence of GABA. The cone was clamped at −40 mV and saturated with a 20 μm spot of intense white light to saturate the direct light response. A full-field stimulus was flashed on for 500 ms, which induces an inward current. Application of 200 μm GABA reduces the amplitude of this current by 42.2 ± 6.5% (n= 15; P= 0.00014). The feedback responses recover after washout of the drug. B, example of light-induced feedback responses in a cone in the presence and absence of picrotoxin (PTX). In contrast to GABA, application of 200 μm PTX results in an increase in the amplitude of the feedback response in cones by 74.9 ± 20.2% (n= 8; P= 0.018). The feedback response returned to pre-drug value after washing out. C, example of a light-induced feedback response in a cone with and without SKF89976-A. Blocking the GABA transporter activity leads to an enhancement of feedback responses in cones. D, example of responses in a HC to a 500 ms full-field flash in the presence of 25 μm SKF89976-A while applying GABA and PTX. The rollback in the light-induced response of the HCs (arrow, left trace) is an indirect measure for negative feedback from HCs to cones. Application of 200 μm GABA (middle trace) reduced the rollback on average by 14.9 ± 3.3% (n= 10; P= 0.0084). Conversely, co-application of 200 μm PTX (right trace) had the opposite effect and increased the rollback by 14.5 ± 4.1% (n= 6; P= 0.027) relative to the value of rollback in the presence of GABA. E, the voltage response of a BHC to diffuse light stimulation of 550 nm and 650 nm in control (left trace) and in the presence of 200 μm GABA (right trace). Feedback induces a depolarizing response to 650 nm light stimulation (arrow). This response is suppressed by GABA. F, the voltage response of a BHC to diffuse light stimulation of 550 nm and 650 nm in control (left trace) and in the presence of 25 μm SKF89976-A (right trace). The feedback-induced depolarizing response to 650 nm light (arrow) is enhanced by SKF89976-A.
The rollback, defined as the ratio between the peak and sustained light response of monophasic horizontal cells (MHCs), is an indirect measure for negative feedback from HCs to cones (Kamermans et al. 2001). To test the effect of GABA on this measure of HC feedback, we recorded full-field light (0 log) responses of MHCs in the presence of 200 μm GABA alone or in combination with 200 μm PTX (Fig. 3D). During these experiments, 25 μm SKF89976-A was present in control conditions to exclude interference of the GABA transporters on the HCs. Application of GABA alone led to a marked change in response shape (Fig. 3D, middle trace). The rollback response clearly present in control conditions (Fig. 3D, left trace, arrow) was reduced by 14.9 ± 3.3% (n= 10; P= 0.0084) after GABA application. Subsequent co-application of PTX increased the rollback response by 14.5 ± 4.1% (n= 6; P= 0.027; Fig. 3D, right trace) similar to control values. In the absence of both SKF89976-A and GABA, application of PTX increased rollback by 6.17 ± 1.60% (n= 12; P= 0.003), presumably owing to the closure of previously mentioned endogenous GABA-gated Cl− channels on cones.
A second measure for feedback in HCs is the depolarizing response of biphasic horizontal cells (BHCs) to red light. Figure 3E and F shows the responses of a BHC due to green (550 nm, 0 log) and red (650 nm, 0 log) light stimulation in control conditions, and after application of 200 μm GABA or 25 μm SKF89976-A. GABA reduced the ratio between the hyperpolarizing and depolarizing response of the BHCs by 21.0 ± 6.6% (n= 8; P= 0.03). Application of SKF89976-A led to an increase of the depolarizing response to red light in three out of three BHCs (Fig. 3F). These results illustrate that feedback-induced responses in both cones and HCs are inhibited by GABA and enhanced when the GABAergic system is blocked.
Apart from affecting feedback measures in HCs, application of GABA slightly depolarized MHC membrane potential by 3.9 ± 1.1 mV (n= 10; P= 0.008), but did not affect sustained light response amplitudes. The response amplitude in control and GABA differed by 6.7 ± 0.05%, which is not significantly different from zero (n= 10; P= 0.19). Also, PTX application did not affect the MHC membrane potential. The membrane potential in control and PTX differed by 0.04 ± 1.26% (n= 12), which is not significantly different from zero. However, the sustained light response amplitude was reduced by 18.0 ± 0.06% (n= 12; P= 0.004). The latter reduction might be fully explained by the increase in rollback response.
Feedback modulating mechanism
The results so far indicate that opening Cl− channels in cones leads to a reduction of the feedback from HCs to cones. What is the mechanism? Because the reduction can be obtained by opening Cl− channels in a single cone, the mechanism must be local in the cones. Recently, it was shown that feedback from HCs to cones is mediated by a connexin hemichannel, most likely via an ephaptic interaction (Kamermans et al. 2001; Fahrenfort et al. 2009; Klaassen et al. 2011). Next, it will be evaluated whether such an ephaptic feedback mechanism could be modulated by a ICl in the cone synaptic complex. Therefore, Cl− channels were incorporated in the ephaptic feedback model, described previously (Fahrenfort et al. 2009; Klaassen et al. 2011; Fig. 4), and its behaviour as function of the Cl− conductance was analysed. Details of the modification of the model can be found in Methods.
Figure 4. Description of the ephaptic feedback model.
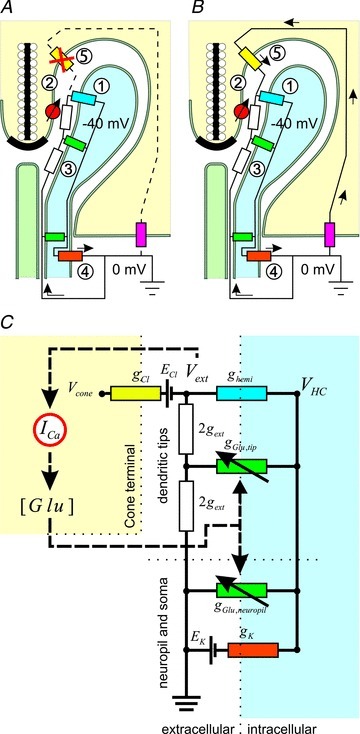
A, schematic representation of the ephaptic feedback model. The model, described by Fahrenfort et al. (2009), consists of a simple resistive network. The HC is modelled as three conductances with their associated reversal potentials: the hemichannel conductance (ghemi, blue resistor); the glutamate-gated conductance (gGlu, green resistors); and a non-linear potassium conductance (gK, orange resistor). In the dark, the HC membrane potential (VHC) is more positive than reversal potential for potassium (EK), and current will flow from gK in the HC membrane into ghemi and gGlu via an extracellular resistive pathway in the synaptic complex with conductance gext (white resistors). This current will generate a voltage drop over gext, making the potential deep in the synaptic cleft (Vext) slightly negative. Light stimulation leads to a reduction of glutamate release by cones, which ultimately leads to the closure of gGlu and hyperpolarization of the HC, and an increase in the current through ghemi and an increase of the voltage drop over gext, making Vext more negative. Vext is sensed by voltage-gated Ca2+ channels (red voltage sensor), which adjust the cone Ca2+ current (ICa) accordingly. The pink resistor is gK in the cone. B, schematic representation of the ephaptic feedback model including a Cl− current in the cone pedicle. The original feedback model was extended by incorporation of a Cl− conductance (gCl, yellow resistor) with its associated equilibrium potential (ECl) to evaluate the consequences of this current on the feedback and compare it with experimental results. C, the equivalent electrical circuit of the ephaptic model of feedback from HCs to cones. All components as described above. In addition, gGlu,tip is the glutamate-gated conductance at the tips of HC dendrites, gGlu,neuropil is the glutamate-gated conductance in the HC neuropil, [Glu] is the glutamate concentration in the synaptic cleft and Vcone is the cone membrane potential relative to outside.
In short, this mechanism functions as follows. Connexin hemichannels are located at the tips of the HC dendrites close to the synaptic ribbon (Fig. 4A). Current will flow into HCs via these hemichannels. This current has to come from outside the synaptic terminal. Because the extracellular space in the synaptic terminal has a finite resistance, a voltage drop over this intersynaptic resistance will occur. This makes the potential deep in the synaptic cleft slightly negative. The voltage-dependent Ca2+ currents in the cones will be influenced by this voltage drop. They will experience a slightly more depolarized membrane potential. Hyperpolarizing HCs will lead to an increase of the hemichannel current, which will cause an increase of the voltage drop over the intersynaptic resistance. This makes the potential deep in the synaptic cleft even more depolarized. So far, this model correctly predicted the specific way feedback is affected when the number of hemichannels in HC dendrites was reduced (Klaassen et al. 2011) and how ephaptic feedback depends on glutamate receptors (Fahrenfort et al. 2005, 2009). Now the model was extended with a ICl in the cone synaptic complex and used to evaluate the behaviour of feedback as function of the Cl− conductance (Fig. 4B). Its equivalent circuit is given in Fig. 4C.
Figure 5A shows the steady state ICa of the cone with HCs resting at their dark membrane potential of −34.7 mV (red) or when hyperpolarized to −54.7 mV (blue). HC hyperpolarization leads to a shift of ICa to negative potentials. ECl was set to the value where the ICl reverses when ECl was set to −55 mV (−50.3 mV). Increasing gCl from 0 pS (left) to 5 pS (middle) or 10 pS (right) shifted ICa to positive potentials. Next, the feedback-induced currents (i.e. ICa when HCs are depolarized, subtracted from ICa when HCs are hyperpolarized) were determined. Figure 5B shows that the maximal feedback-induced response shifts to positive potentials and reduces in amplitude with increasing gCl. This result is qualitatively similar to those experimentally obtained (Fig. 3D), although the curves do deviate somewhat from the experimental results at positive potentials. This is most likely due to the fact that gCl in the model is static, whereas ICl(Ca) changes dynamically during a voltage-clamp experiment. Changing ECl from −55 mV to −29.4 mV leads to shifts of ICa in the opposite direction with increasing gCl, but the reduction of the feedback-induced shift of ICa remained (Fig. 5C).
Figure 5. Inclusion of a Cl− conductance in the ephaptic model of HC feedback shifts ICa and reduces the size of feedback.

A, the normalized steady state ICa of the cone with the HC membrane potential either depolarized at −34.7 mV (red traces) or hyperpolarized at −54.7 mV (black traces). ECl was set to −50.3 mV. For the left panel gCl was set to 0 pS. In this condition, hyperpolarization of HCs leads to a shift of ICa to negative potentials. Increasing gCl to 5 pS (middle panel) or 10 pS (right panel) leads to a shift of ICa to positive potentials and a reduction of the feedback-induced shift of ICa. B, plot of the feedback-induced current as a function of Vcone for different values of gCl. The feedback-induced currents were calculated by subtracting ICa, when HCs were depolarized, from ICa, when HCs were hyperpolarized. Increasing gCl (0 pS, black trace; 5 pS, red trace; 10 pS, green trace) reduces the maximal feedback-induced response and shifts it to positive potentials. C, plot of the feedback-induced current as a function of Vcone for different values of gCl with ECl set to −29.4 mV. The reduction of the feedback-induced shift of ICa remains (0 pS, black trace; 5 pS, red trace; 10 pS, green trace).
Discussion
Excitatory output of photoreceptors is counteracted by negative feedback from HCs. These two elements create a balance, which ensures optimal information transfer to bipolar cells (BCs). To maintain high sensitivity throughout the broad range of stimuli encountered under natural conditions, it is essential that the contribution of both these elements can adapt. Although much is known about mechanisms facilitating photoreceptor adaptation, how the strength of inhibitory feedback is modulated has received less attention. In the present study, the role of ICa(Cl) and GABA-gated currents in cones in modulating the size of feedback from HCs was examined in the goldfish retina. It was shown that these two ICl are involved in modulating the balance between cone excitation and HC inhibition by altering the efficacy of HC feedback to cones.
Could the change in feedback strength described in this paper be due to changes in [Cl]i? Thoreson and co-workers showed that in photoreceptors of the tiger salamander, ICa is reduced when [Cl−]i is reduced (Thoreson et al. 2000, 2003). The relevance of this pathway strongly depends on ECl. The physiological value of ECl in photoreceptors seems to differ between rods and cones. Rods seem to have an ECl of −25 mV, which is more positive than the dark membrane potential (Rabl & Thoreson, 2002). In cones, ECl seems to be close or slightly negative to the dark resting membrane potential (Kraaij et al. 2000a; Thoreson & Bryson, 2004). Therefore, opening of Cl− channels in goldfish cones in the dark will not alter either membrane potential or [Cl−]i substantially. Accordingly, the peak amplitude of ICa in cones was found to be independent of activation of either ICl(GABA) or ICl(Ca). The modulation of feedback by opening a Cl− channel in cones cannot be accounted for by such modulation of ICa by changes in [Cl]i, as feedback reduced when a Cl− conductance was activated while ECl was set at −50 mV. In such conditions Cl− ions will flow into the cell instead of out, which should make ICa larger instead of smaller.
Cl− channel activity modulates ephaptic feedback
How could the activation of a cone ICl modulate feedback? One option would be that opening Cl− channels in cones would limit the change in surround-induced depolarization due to shunting inhibition, which reduces membrane potential changes in cones. Although we cannot exclude some contribution of shunting inhibition, it is highly unlikely that this would be the mechanism for the modulation of feedback, because feedback hardly alters the membrane potential of the cone, but strongly modulates the Ca2+ channels by changing the extracellular potential (Verweij et al. 1996; Kraaij et al. 2000a). Therefore, we explored how the activation of Cl− channels would affect the ephaptic feedback mechanism.
Negative feedback from HCs to cones strongly depends on the morphology of the cone synaptic terminal. The specific relative localization of Ca2+ channels, hemichannels and glutamate receptors in the invaginating cone synapse seems to be crucial for the function of the feedback mechanism. We developed a quantitative model of this ephaptic feedback mechanism based on goldfish and zebrafish data (Fahrenfort et al. 2009; Klaassen et al. 2011). The model extended with ICl in cones reproduced the inhibition of feedback by the Cl− conductance correctly (Fig. 5). The mechanism by which this happens is as follows. Activation of a Cl− conductance in the presynaptic membrane will influence the current flow in the synaptic terminal. When ECl is more negative than Vm, a ICl will flow from the inside of the cone into the synaptic cleft. This current will compensate part of the current flow over the intersynaptic resistance. The result is that the potential deep in the synaptic cleft will be slightly less negative. This leads to a shift of ICa to positive potentials as was found experimentally (Fig. 1D, left).
Apart from these sustained shifts of ICa, adding ICl in the synaptic terminal leads to modulation of the feedback-induced shift of ICa in cones. Hyperpolarization of HCs leads to an increase in current flow through the hemichannels. Without a Cl− conductance in cones all current has to come from outside the synaptic terminal and would flow through the intersynaptic space. However, with a Cl− conductance in the cone synaptic terminal, part of this current will flow via this pathway making the current through the intersynaptic space smaller. Because the potential deep in the synaptic cleft strongly depends on the modulation of the current flow through the intersynaptic space, the feedback-induced shift of ICa is reduced (Fig. 1E). Note that this reduction is independent of ECl. The reason being that in the dark ICl, the hemichannel current and the current through the intersynaptic space are balanced. In this condition ICl can either be inward or outward depending on ECl. Increasing the hemichannel current through HC hyperpolarization will lead to an increase in the current through the intersynaptic space, and to an increase in outward ICl or a decrease of inward ICl depending on ECl. However, the change in current will be the same in both conditions.
The functional role of the calcium-dependent Cl− current
It was found that activation of ICl(Ca) leads to a decrease of the amplitude of the feedback response. This implies that in cones around their dark resting membrane potential, in which ICl(Ca) is activated, feedback is suppressed. This generates an intriguing mechanism. As soon as feedback becomes too large, and too much Ca2+ is flowing into the cone synaptic terminal, ICl(Ca) gets activated and suppresses feedback. ICl(Ca) tunes the amount of feedback a cone receives at its dark resting membrane potential and in that way ensures a proper balance between the feedforward and feedback signals flowing across this synapse. Note that this balance is mainly set by the properties of ICl(Ca); i.e. its Ca2+ dependence. In other words, ICl(Ca) prevents a cone from getting a Ca2+ overload due to excessive HC feedback. This mechanism functions on a time scale from a few 100 ms to seconds.
Role of GABA in the outer retina
Both the activation of ICl(Ca) and ICl(GABA) reduce the feedback-induced responses. They might influence feedback via the mechanism outlined above. However, we cannot exclude that GABA exerts its effect via multiple pathways. These alternative pathways will be discussed next.
It has been suggested that the feedback pathway from HCs to cones is GABAergic. This suggestion was based on the evidence that at least one type of HC in goldfish (H1-HCs) contains and releases GABA (Marc et al. 1978; Yazulla & Kleinschmidt, 1983) in a Ca2+-independent manner (Schwartz, 1982; Yazulla & Kleinschmidt, 1983; Cammack & Schwartz, 1993), and that cones express GABAA receptors located close to the glutamate release sites (Yazulla & Brecha, 1980; Yazulla et al. 1989; Klooster et al. 2004) that can be activated by GABA (Murakami et al. 1982; Tachibana & Kaneko, 1984). However, direct measurements of feedback from HCs to cones in goldfish shows that HC feedback is not GABA mediated (Verweij et al. 1996). Similar results were obtained in turtle (Pottek et al. 2001) and monkey (Verweij et al. 2003).
In the present study it was confirmed that cones have an ICl(GABA) and that GABA released by HCs, via a GABA transporter working in the reverse direction, activates these receptors. Furthermore, it was shown that GABA inhibits negative feedback from HCs to cones. However, blocking this GABAergic pathway leads to an enhancement of feedback responses. These results are inconsistent with GABA as the feedback neurotransmitter, but indicate that GABA modulates feedback.
How does GABA inhibit feedback? GABA can act at many locations in the retina. First of all, GABA could hyperpolarize the cones. This would lead to a reduction in the synaptic gain of the cone and thus to a smaller HC response (VanLeeuwen et al. 2009). Secondly, GABA could act on HCs. Goldfish and salamander HCs have been shown to possess GABA receptors (Kamermans & Werblin, 1992; Verweij et al. 1998; Paik et al. 2003; Klooster et al. 2004). Opening these GABA-gated channels will polarize the HC membrane potential depending on the position of ECl and shunt the HC light response. In principle, the reduction of the feedback responses in cones could be due to a reduction of the HC response amplitude as described in the previous section. Smaller HC response amplitudes will result in smaller feedback responses in cones (Kraaij et al. 2000b). However, in this study we showed that the HC response amplitude did not significantly reduce, suggesting that the opening of GABA-gated channels on cones is the main mechanism responsible for the GABA-induced reduction of feedback responses. Secondly, GABA could act on dopaminergic interplexiform cells, which project to HCs (Dowling & Ehinger, 1978) and receive GABAergic input from the inner retina (Yazulla & Zucker, 1988). Dopamine is known to modulate the gap-junctional coupling and sensitivity of the glutamate receptors of HCs (Knapp & Dowling, 1987; Tornqvist et al. 1988; Yang et al. 1988a,b; McMahon et al. 1989). Both of these changes could result in modulation of negative feedback from HCs to cones. It is unlikely that the modulation of the gap-junctions is the underlying mechanism for the modulation of feedback as the modulation of feedback remains present for full-field stimuli. In such conditions, no current will flow through the gap-junctions, and thus modulation of the gap-junctions will not affect the system.
We were not able to activate this modulatory pathway by light stimuli. One of the reasons for this might be that this pathway functions on a time scale of seconds to minutes. The time constant of this GABAergic pathway is most likely determined by the time constant of the GABA transporter, the time constant of the GABA receptors and the volume into which GABA is released. Because both the GABA transporter and the GABA receptor time constants are fast (Cammack et al. 1994; Lukasiewicz & Shields, 1998), it seems likely that the volume of the compartment wherein GABA is released is the rate-limiting factor. Immunocytochemical evidence shows that GABA transporters are not focally localized in the synaptic complex but are also present on the somatic membrane of HCs (Klooster et al. 2004). This distribution of the GABA transporters indicates that HCs release GABA in the extracellular space around them, which is a relatively large volume. Inducing significant changes in the GABA concentration in such a large compartment will take time (Kamermans & Werblin, 1992). How much time will depend on the precise organization of the extracellular space around HCs and might vary strongly between species.
Alternatively, one could hypothesize that the GABA release by HCs is only partly voltage dependent, but that modulation of GABA transporters by, for instance, phosphorylation is a much more prominent way of regulating GABA release. In the dark-adapted retina, GABA release from HCs is increased (Yazulla, 1985). Interestingly, the efficiency of the feedback pathway changes strongly during light/dark adaptation, with feedback almost absent in the dark-adapted state (Weiler & Wagner, 1984). Choi et al. (2011) showed that the localization of GABA receptors in cones is modulated by light/dark adaptation, with most GABA receptors located in the membrane in the dark-adapted state. These observations lead to the suggestion that GABA is involved in adaptation-induced changes in feedback strength (Gilbertson et al. 1991; Yang & Wu, 1993; Yang et al. 1998).
It has been suggested that HCs feedforward to BCs via a GABAergic pathway (Yang & Wu, 1991). The results of the present paper do not exclude the existence of such a feedforward pathway. It might be that in the dark-adapted retina, the global, transporter-mediated GABAergic pathway from HCs to cones dominates. On the other hand, in the light-adapted retina, GABA may mediate a local feedforward signal from HCs to BCs (Thoreson & Mangel, 2012). This feedforward pathway might be a Ca2+-dependent, vesicular release pathway (Lee & Brecha, 2010; Hirano et al. 2011).
Functional role of GABAergic modulation of negative feedback
In this paper we have shown that activation of ICl(GABA) in cones inhibits negative feedback from HCs to cones. What is the functional consequence of this modulation? In the light-adapted retina, GABA release is low (Yazulla & Kleinschmidt, 1982; O’Brien & Dowling, 1985; Yazulla, 1985) and feedback is consequently barely inhibited by ICl(GABA). Therefore, the feedback pathway from HCs to the cones will be maximally active in this adaptational condition. This leads to a well-developed centre/surround organization in BCs and spectral coding of HCs, which are instrumental to reduce redundancies.
In the dark-adapted retina, the signal-to-noise ratio drops and redundancy reduction has to be limited in order to collect enough reliable information about the stimulus. In this condition, GABA release is high in the dark-adapted retina (Yazulla & Kleinschmidt, 1982; O’Brien & Dowling, 1985; Yazulla, 1985), resulting in the opening of GABA-gated Cl− channels and consequently a reduction of the efficiency of feedback from HCs to cones. This will result in a reduction of the centre/surround organization of BCs (Thoreson & Mangel, 2012) and a loss of spectral coding of HCs (Weiler & Wagner, 1984). In the meantime, the cone Ca2+ current has to remain in its operating range. ICl(Ca) might be responsible for this. If sustained feedback is reduced, ICl(Ca) will be activated less and sustained feedback might increase again, keeping the cone ICa in its working range. This delicate interplay between feedback, ICl(Ca) and ICa ensures that ICa will remain in its operating range independent of the adaptation state of the retina.
Acknowledgments
This research was supported by a grant ALW-FOM and ZonMW from the Netherlands Organization for Scientific Research (NWO), and by European Commission FP7 Grant RETICIRC HEALTH-F2-2009-223156 (coordinator: MK).
Glossary
- BC
bipolar cell
- BHC
biphasic horizontal cell
- HC
horizontal cell
- MHC
monophasic horizontal cell
- PTX
picrotoxin
Author contributions
DE, IF and MK designed the experiments and analysis. DE, IF and TS performed the experiments. DE, IF and MK performed the analysis. MS, HE and MK constructed and analyzed the model. DE, IF, MS and MK wrote the manuscript. All authors approved the final version of the manuscript.
References
- Bader CR, Bertrand D, Schwartz EA. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Bui Q. Modulation of calcium-activated chloride current via pH-induced changes of calcium channel properties in cone photoreceptors. J Neurosci. 1991;11:4015–4023. doi: 10.1523/JNEUROSCI.11-12-04015.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Deschenes MC. Contribution of Ca and Ca-activated Cl channels to regenerative depolarization and membrane bistability of cone photoreceptors. J Neurophysiol. 1992;68:745–755. doi: 10.1152/jn.1992.68.3.745. [DOI] [PubMed] [Google Scholar]
- Cammack JN, Rakhilin SV, Schwartz EA. A GABA transporter operates asymmetrically and with variable stochiometry. Neuron. 1994;13:949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Cammack JN, Schwartz EA. Ions required for the electrogenic transport of GABA by horizontal cells of the catfish retina. J Physiol. 1993;472:81–102. doi: 10.1113/jphysiol.1993.sp019938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Cao Y, Adelaja A, Adams L, Ribelayga C, Mangel S. GABA-A receptors are expressed and active on cones to a greater extent at night, than in the day. ARVO Meeting Abstracts. 2011;52:4109. [Google Scholar]
- Corey DP, Dubinsky JM, Schwartz EA. The calcium current in the inner segment of rods from the salamander (Ambystoma tigrinum) retina. J Physiol. 1984;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Manookin MB, Packer OS, Dacey DM. Horizontal cell feedback without cone type-selective inhibition mediates “red-green” colour opponency in midget ganglion cells of the primate retina. J Neurosci. 2011;31:1762–1772. doi: 10.1523/JNEUROSCI.4385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. Inward rectifying potassium conductance can accelerate the hyperpolarizing response in retinal horizontal cells. J Neurophysiol. 1995;74:2258–2265. doi: 10.1152/jn.1995.74.6.2258. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc R Soc Lond B Biol Sci. 1978;201:7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- Fahrenfort I, Habets RL, Spekreijse H, Kamermans M. Intrinsic cone adaptation modulates feedback efficiency from horizontal cells to cones. J Gen Physiol. 1999;114:511–524. doi: 10.1085/jgp.114.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenfort I, Klooster J, Sjoerdsma T, Kamermans M. The involvement of glutamate-gated channels in negative feedback from horizontal cells to cones. Prog Brain Res. 2005;147:219–229. doi: 10.1016/S0079-6123(04)47017-4. [DOI] [PubMed] [Google Scholar]
- Fahrenfort I, Steijaert M, Sjoerdsma T, Vickers E, Ripps H, van Asselt J, Endeman D, Klooster J, Numan R, ten Eikelder H, Von Gersdorff H, Kamermans M. Hemichannel-mediated and pH-based feedback from horizontal cells to cones in the vertebrate retina. PLoS One. 2009;4:e6090. doi: 10.1371/journal.pone.0006090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson TA, Borges S, Wilson M. The effects of glycine and GABA on isolated horizontal cells from the salamander retina. J Neurophysiol. 1991;66:2002–2013. doi: 10.1152/jn.1991.66.6.2002. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nieto D, Gomez-Hernandez JM, Larrosa B, Gutierrez C, Munoz MD, Fasciani I, O’Brien J, Zappala A, Cicirata F, Barrio LC. Regulation of neuronal connexin-36 channels by pH. Proc Natl Acad Sci U S A. 2008;105:17,169–17,174. doi: 10.1073/pnas.0804189105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Brandstatter JH, Morgans CW, Brecha NC. SNAP25 expression in mammalian retinal horizontal cells. J Comp Neurol. 2011;519:972–988. doi: 10.1002/cne.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Eason R, Sachdev A, Dale N. CO2-dependent opening of connexin 26 and related beta connexins. J Physiol. 2010;588:3921–3931. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I. Ephaptic interactions within a chemical synapse: hemichannel-mediated ephaptic inhibition in the retina. Curr Opin Neurobiol. 2004;14:1–11. doi: 10.1016/j.conb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Werblin FS. GABA-mediated positive autofeedback loop controls horizontal cell kinetics in tiger salamander retina. J Neurosci. 1992;12:2451–2463. doi: 10.1523/JNEUROSCI.12-07-02451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen LJ, Sun Z, Steijaert MN, Bolte P, Fahrenfort I, Sjoerdsma T, Klooster J, Claassen Y, Shields CR, Ten Eikelder HM, Janssen-Bienhold U, Zoidl G, McMahon DG, Kamermans M. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biol. 2011;9:e1001107. doi: 10.1371/journal.pbio.1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster J, Nunes CB, Yazulla S, Kamermans M. Postsynaptic localization of gamma-aminobutyric acid transporters and receptors in the outer plexiform layer of the goldfish retina: an ultrastructural study. J Comp Neurol. 2004;474:58–74. doi: 10.1002/cne.20114. [DOI] [PubMed] [Google Scholar]
- Knapp AG, Dowling JE. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature. 1987;325:437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Kraaij DA, Spekreijse H, Kamermans M. The nature of surround induced depolarizing responses in goldfish cones. J Gen Physiol. 2000a;115:1–14. doi: 10.1085/jgp.115.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaij DA, Spekreijse H, Kamermans M. The open- and closed-loop gain-characteristics of the cone/horizontal cell synapse in goldfish retina. J Neurophysiol. 2000b;84:1256–1265. doi: 10.1152/jn.2000.84.3.1256. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Liu X, Copenhagen DR. Expression of calcium transporters in the retina of the tiger salamander (Ambystoma tigrinum) J Comp Neurol. 2004;475:463–480. doi: 10.1002/cne.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde MR, Kelly ME, Barnes S. Calcium-activated chloride channels in the retina. Channels (Austin) 2008;2:252–260. doi: 10.4161/chan.2.4.6704. [DOI] [PubMed] [Google Scholar]
- Lam DMK, Su YYT, Chin CA, Brandon C, Wu J-Y, Marc RE, Lasater EM. GABA-ergic horizontal cells in the teleost retina. Brain Res Bull. 1980;5:137–140. [Google Scholar]
- Lee H, Brecha NC. Immunocytochemical evidence for SNARE protein-dependent transmitter release from guinea pig horizontal cells. Eur J Neurosci. 2010;31:1388–1401. doi: 10.1111/j.1460-9568.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz PD, Shields CR. A diversity of GABA receptors in the retina. Semin Cell Dev Biol. 1998;9:293–299. doi: 10.1006/scdb.1998.0238. [DOI] [PubMed] [Google Scholar]
- Malchow RP, Qiian H, Ripps H. Evidence for hemi-gap junctional channels in isolated horizontal cells of the skate retina. J Neurosci Res. 1993;35:237–245. doi: 10.1002/jnr.490350303. [DOI] [PubMed] [Google Scholar]
- Marc RE, Stell WK, Bok D, Lam DMK. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978;182:221–246. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Knapp AG, Dowling JE. Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci U S A. 1989;86:7639–7643. doi: 10.1073/pnas.86.19.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AJ, Rabl K, Riccardi GE, Brecha NC, Stella SL, Jr, Thoreson WB. Location of release sites and calcium-activated chloride channels relative to calcium channels at the photoreceptor ribbon synapse. J Neurophysiol. 2011;105:321–335. doi: 10.1152/jn.00332.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Shimoda Y, Nakatani K, Miyachi EI, Watanabe SI. GABA-mediated negative feedback from horizontal cells to cones in carp retina. Jpn J Physiol. 1982;32:911–926. doi: 10.2170/jjphysiol.32.911. [DOI] [PubMed] [Google Scholar]
- O’Brien DR, Dowling JE. Dopaminergic regulation of GABA release from the intact goldfish retina. Brain Res. 1985;360:41–50. doi: 10.1016/0006-8993(85)91218-1. [DOI] [PubMed] [Google Scholar]
- O’Dell TJ, Christensen BN. A Voltage-clamp study of isolated stingray horizontal cell non-NMDA excitatory amino acid receptors. J Neurophysiol. 1989;61:162–172. doi: 10.1152/jn.1989.61.1.162. [DOI] [PubMed] [Google Scholar]
- Paik SS, Park NG, Lee SJ, Han HK, Jung CS, Bai SH, Chun MH. GABA receptors on horizontal cells in the goldfish retina. Vision Res. 2003;43:2101–2106. doi: 10.1016/s0042-6989(03)00335-3. [DOI] [PubMed] [Google Scholar]
- Picaud S, Pattnaik B, Hicks D, Forster V, Fontaine V, Sahel J, Dreyfus H. GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor-glia co-culture model. J Physiol. 1998;513:33–42. doi: 10.1111/j.1469-7793.1998.033by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottek M, Schultz K, Janssen-Bienhold U, Weiler R. Physiological and anatomical evidence for an involvement of connexin26 in the negative feedback loop between horizontal cells and cones in turtle. Invest Ophthalmol Vis Sci. 2001;42:s671. [Google Scholar]
- Rabl K, Thoreson WB. Calcium-dependent inactivation and depletion of synaptic cleft calcium ions combine to regulate rod calcium currents under physiological conditions. Eur J Neurosci. 2002;16:2070–2077. doi: 10.1046/j.1460-9568.2002.02277.x. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Witkovsky P. Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. J Neurosci. 1997;78:1209–1216. doi: 10.1016/s0306-4522(96)00678-1. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol. 1982;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release gamma-aminobutyic acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Scudieri P, Sondo E, Ferrera L, Galietta LJ. The anoctamin family: TMEM16A and TMEM16B as calcium-activated chloride channels. Exp Physiol. 2012;97:177–183. doi: 10.1113/expphysiol.2011.058198. [DOI] [PubMed] [Google Scholar]
- Stohr H, Heisig JB, Benz PM, Schoberl S, Milenkovic VM, Strauss O, Aartsen WM, Wijnholds J, Weber BH, Schulz HL. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci. 2009;29:6809–6818. doi: 10.1523/JNEUROSCI.5546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. Gamma-aminobutyric acid acts at axon terminals of turtle photoreceptors; difference in sensitivity among cell types. Proc Natl Acad Sci U S A. 1984;81:7961–7964. doi: 10.1073/pnas.81.24.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa T, Hirasawa H, Kaneko A, Kaneda M. GABA-mediated component in the feedback response of turtle retinal cones. Vis Neurosci. 2005;22:317–324. doi: 10.1017/S0952523805223076. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Bryson EJ. Chloride equilibrium potential in salamander cones. BMC Neurosci. 2004;5:53. doi: 10.1186/1471-2202-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Bryson EJ, Rabl K. Reciprocal interactions between calcium and chloride in rod photoreceptors. J Neurophysiol. 2003;90:1747–1753. doi: 10.1152/jn.00932.2002. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res. 2012;31:407–441. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Chloride efflux inhibits single calcium channel open probability in vertebrate photoreceptors: chloride imaging and cell-attached patch-clamp recordings. Vis Neurosci. 2000;17:197–206. doi: 10.1017/s0952523800172025. [DOI] [PubMed] [Google Scholar]
- Tornqvist K, Yang X-L, Dowling JE. Modulation of cone horizontal cell activity in the teleost fish retina. III. Effects of prolonged darkness and dopamine on electrical coupling between horizontal cells. J Neurosci. 1988;8:2279–2288. doi: 10.1523/JNEUROSCI.08-07-02279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Bukauskas FF, Bennett MV, Bargiello TA, Verselis VK. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J Gen Physiol. 1999;113:721–742. doi: 10.1085/jgp.113.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanLeeuwen M, Fahrenfort I, Sjoerdsma T, Numan R, Kamermans M. Lateral gain control in the outer retina leads to potentiation of centre responses of retinal neurons. J Neurosci. 2009;29:6358–6366. doi: 10.1523/JNEUROSCI.5834-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci. 2003;23:10,249–10,257. doi: 10.1523/JNEUROSCI.23-32-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Negishi K, Spekreijse H. GABA-sensitivity of spectrally classified horizontal cells in the goldfish retina. Vis Neurosci. 1998;15:77–86. doi: 10.1017/s0952523898151039. [DOI] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R, Wagner H-J. Light-dependent change of cone-horizontal cell interactions in carp retina. Brain Res. 1984;298:1–9. doi: 10.1016/0006-8993(84)91141-7. [DOI] [PubMed] [Google Scholar]
- Wu SM, Dowling JE. Effects of GABA and Glycine on the distal cells of the cyprinid retina. Brain Res. 1980;199:401–414. doi: 10.1016/0006-8993(80)90697-6. [DOI] [PubMed] [Google Scholar]
- Yang JH, Maple B, Gao F, Maguire G, Wu SM. Postsynaptic responses of horizontal cells in the tiger salamander retina are mediated by AMPA-preferring receptors. Brain Res. 1998;797:125–134. doi: 10.1016/s0006-8993(98)00373-4. [DOI] [PubMed] [Google Scholar]
- Yang X-L, Tornqvist K, Dowling JE. Modulation of cone horizontal cell activity in the teleost fish retina. I. Effects of prolonged darkness and background illumination on light responsiveness. J Neurosci. 1988a;8:2259–2268. doi: 10.1523/JNEUROSCI.08-07-02259.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-L, Tornqvist K, Dowling JE. Modulation of cone horizontal cell activity in the teleost fish retina. II. Role of interplexiform cells and dopamine in regulating light responsiveness. J Neurosci. 1988b;8:2269–2278. doi: 10.1523/JNEUROSCI.08-07-02269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-L, Wu SM. Feedforward lateral inhibition in retinal bipolar cells: input- output relation of the horizontal cell-depolarizing bipolar cell synapse. Proc Natl Acad Sci U S A. 1991;88:3310–3313. doi: 10.1073/pnas.88.8.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-L, Wu SM. Effect of GABA on horizontal cells in the tiger salamander retina. Vision Res. 1993;10:1339–1344. doi: 10.1016/0042-6989(93)90041-t. [DOI] [PubMed] [Google Scholar]
- Yazulla S. Evoked efflux of [3H]GABA from goldfish retina in the dark. Brain Res. 1985;325:171–180. doi: 10.1016/0006-8993(85)90313-0. [DOI] [PubMed] [Google Scholar]
- Yazulla S. GABAergic mechanisms in the retina. Prog Retinal Res. 1986;5:1–52. [Google Scholar]
- Yazulla S, Brecha N. Binding and uptake of the GABA analogue, 3H-muscimol, in the retinas of goldfish and chicken. Invest Ophthalmol Vis Sci. 1980;19:1415–1426. [PubMed] [Google Scholar]
- Yazulla S, Kleinschmidt J. Dopamine blocks carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1982;233:211–215. doi: 10.1016/0006-8993(82)90944-1. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Kleinschmidt J. Carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1983;263:63–75. doi: 10.1016/0006-8993(83)91201-5. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM, Vitorica J, De Blas AL. Immunocytochemical localization of GABAa receptors in goldfish and chicken retinas. J Comp Neurol. 1989;280:15–26. doi: 10.1002/cne.902800103. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Zucker CL. Synaptic organization of dopaminergic interplexiform cells in the goldfish retina. Vis Neurosci. 1988;1:13–29. doi: 10.1017/s0952523800000997. [DOI] [PubMed] [Google Scholar]


