Abstract
The amygdala plays an important role in the processing of emotional events. This information processing is altered by development, but little is known about the development of electrophysiological properties of neurons in the amygdala. We studied the postnatal development of electrophysiological properties of neurons in the basolateral amygdala (BLA) of the common marmoset (Callithrix jacchus). Whole-cell patch-clamp recordings were obtained from BLA pyramidal neurons in brain slices prepared from developing and adult marmosets, and electrophysiological properties known to change during development in rats were analysed. Two passive electrical properties of the neuronal membrane – the input resistance (Rin) and the membrane time constant (τ) – significantly decreased with postnatal development. In contrast, the action potential only showed a slight decrease in duration during the first month of life, whereas the amplitude did not change after birth. Passive electrical properties and action potentials in neurons of 4-week-old marmosets were similar to those in neurons of 4-year-old marmosets. The development of the action potential duration was not correlated with the development of Rin or τ, whereas the development of Rin and τ was correlated with each other. Abundant spontaneous and noradrenaline-induced GABAergic currents were present immediately after birth and did not change during postnatal development. These results suggest that newborn infant marmoset BLA pyramidal neurons possess relatively mature action potentials and receive vigorous GABAergic synaptic inputs, and that they acquire adult-like electrophysiological properties by the fourth week of life.
Key points
The amygdala plays an important role in the processing of emotional events, but little is known about the postnatal development of electrophysiological properties of amygdala neurons.
We performed slice patch-clamp recordings from basolateral amygdala (BLA) pyramidal neurons in the developing and adult common marmoset (Callithrix jacchus).
The marmoset is a non-human primate that has been increasingly used as an animal model of human disease, but no previous studies have used brain slice patch-clamp techniques in marmosets.
We found that BLA pyramidal neurons from newborn marmosets possess relatively mature action potentials and receive vigorous GABAergic synaptic inputs, and that marmoset BLA neurons acquire adult-like electrophysiological properties within the first 4 weeks of life.
Our study provides novel information on several features of postnatal development of BLA pyramidal neurons in marmosets.
Introduction
The amygdala is part of the limbic system and plays an important role in information processing of emotional events, such as fearful experiences, in both rodents and primates (reviewed in Phelpes & Ledoux, 2005). The processing of emotion is known to be altered by postnatal development. For example, an unfamiliar conspecific male poses a threat to infant but not juvenile rats (Wiedenmayer et al. 2005), and the circuit components responsible for fear extinction are altered between infancy and juvenility in rats (Kim et al. 2009). The amygdala is also known to be involved in anxiety disorders (reviewed in Holzschneider & Mulert, 2011; Mahan & Ressler, 2012). Epidemiological research has suggested that the age of onset for some anxiety disorders occurs early in life. For example, the onset of specific phobia and separation anxiety disorders occurs at about 7 years old (Kessler et al. 2005). Therefore, knowledge about the postnatal development of amygdala function, such as the development of electrophysiological properties and connectivity, is fundamental for understanding the normal development of emotion processing and its pathological alteration. So far, little is known about the postnatal development of electrophysiological properties of rodent and primate amygdala neurons.
In rat hippocampal CA1 pyramidal neurons, electrical properties such as input resistance (Rin), membrane time constant (τ), and action potential duration and amplitude, change dramatically over the first two postnatal weeks and remain largely unchanged thereafter (Spigelman et al. 1992). Similar results have been obtained in hippocampal CA3 pyramidal neurons (Tyzio et al. 2003) and layer V cortical pyramidal neurons in rats (McCormick & Prince, 1987). Postnatal changes in action potential properties have been observed in rat intracerebellar nuclei (Gardette et al. 1985) and in spinal motoneurons (Fulton & Walton, 1986). Some of these studies have raised the possibility that the different membrane properties –Rin, τ and action potential parameters – develop independently of each other (McCormick & Prince, 1987; Spigelman et al. 1992), but this suggestion has not been fully verified.
Immature rat hippocampal neurons, which have high Rin, slow τ, long action potential duration and small action potential amplitude, gradually start to receive GABAergic synaptic input around birth, prior to glutamatergic input (Tyzio et al. 1999). GABAergic synaptic responses are depolarizing in immature neurons in area CA3 of the hippocampus (Ben-Ari et al. 1989; Tyzio et al. 2007, 2008). The GABAergic system in the amygdala is influenced by maternal care during the postnatal period (Caldji et al. 1998, 2003, 2004), and is also altered by maternal separation stress (Seidel et al. 2008). In adult rats, stress such as restraint or footshock induces the release of noradrenaline into the amygdala (Tanaka et al. 1983; Galvez et al. 1996). The GABAergic system in the basolateral amygdala (BLA) is known to be activated by this catecholamine (Braga et al. 2004; Kaneko et al. 2008; Sekiguchi et al. 2009; Miyajima et al. 2010). Namely, in the adult rat and mouse BLA, noradrenaline excites unidentified GABAergic neurons via α1-adrenoceptors, resulting in increased action potential firing. Consequently, noradrenaline induces GABAergic inhibitory postsynaptic currents in BLA pyramidal neurons. The ontogeny of this system has not been examined.
The common marmoset (Callithrix jacchus) is a non-human primate that has been increasingly used as an animal model of human disease (Sasaki et al. 2009), including psychiatric disorders (reviewed in Pryce et al. 2011). Like in rodents, stimuli such as medication or environmental stress can be applied to prenatal or early postnatal marmosets, and the later emergence of behavioural or physiological abnormalities can be studied (reviewed in Pryce et al. 2011). A brain slice patch-clamp study of the development of neuronal function in the hippocampus of the cynomolgus monkey (Macaca fascicularis) reported that GABAergic synaptic currents could already be detected around midgestation (Khazipov et al. 2001). However, there have been no such patch-clamp studies in the case of marmosets.
In this study, we examined the postnatal development of the electrophysiological properties of BLA pyramidal neurons in common marmosets. We performed patch-clamp recordings from pyramidal neurons in BLA brain slices from developing and adult marmosets, and measured the following parameters: Rin, τ, action potential amplitude, action potential duration, and spontaneous, miniature and noradrenaline-induced GABAergic synaptic currents, along with other basic electrophysiological parameters.
Methods
Ethical approval
Common marmosets were bred in the Primate Center of the National Institute of Neuroscience, the National Center of Neurology and Psychiatry (Japan), and all experiments were performed in the Primate Center in strict accordance with the guidelines of the National Institute of Neuroscience. Experiments were approved by the Primate Ethics Committee of the National Institute of Neuroscience.
Subjects
A total of 18 marmosets of different ages were used: four at postnatal day (PD)0 (three male and one female), one at PD1 (female), two at 2 weeks old (male), one at 4 weeks old (female), two at 7 weeks old (male and female), one at 8 weeks old (male), one at 14 weeks old (male), one at 23 weeks old (male), one at 26 weeks old (male), two at 33 weeks old (male and female), one at 1 year old (male) and one at 4 years old (female). We co-used these individuals with other researchers who used other organs. In the case of PD0 animals, brain slices were prepared 2–3 h after birth. Because the neurons from PD0 and PD1 animals showed similar electrophysiological properties, the data from these marmosets were grouped for statistical analysis (‘PD0–1 group’). Similarly, we grouped the data from 7-, 8- and 14-week-old marmosets (‘7–14-week-old group’), and from 23-, 26- and 33-week-old marmosets (‘23–33-week-old group’). GABAergic current data were grouped from 4-, 7-, 8- and 14-week-old marmosets (‘4–14-week-old group’), and from 23-, 26-, 33-week-old and 4-year-old marmosets (‘>23-week-old group’).
Slice preparation and patch-clamp recording
Because patch-clamp recordings in marmoset brain slices have not previously been reported, we here describe our method in detail. Brain slice preparation and patch-clamp recordings were performed as reported previously (Zushida et al. 2007; Amano et al. 2008; Sekiguchi et al. 2009), with minor modifications. Marmosets were anaesthetized with ketamine and administered an overdose of pentobarbital. Ketamine was intramuscularly administered at the dose of 30–50 mg kg−1. Pentobarbital was administered intraperitoneally for PD0 to 8-week-old marmosets (50–70 mg kg−1), and intravenously for >14-week-old marmosets (35–50 mg kg−1). Following apnoea, they were decapitated. The brain was rapidly removed and immersed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mm): NaCl, 124; KCl, 3.0; MgSO4, 1.3; CaCl2, 2.0; KH2PO4, 1.2; NaHCO3, 26; glucose, 10; pH 7.4, 290–300 mosmol l−1, continuously bubbled with 95% O2−5% CO2. The brain was first cut at the midline, and a coronal hemispheric block that included the amygdala was prepared. The ventral half of the block (Fig. 1A) was used to prepare coronal slices (300 μm thick). We did not discriminate between the right and left hemispheres. The slices were prepared using a Vibratome 3000 (Vibratome Company, St Louis, MO, USA) or a Linear Slicer Pro7 (Dosaka EM, Kyoto, Japan) in ACSF at 2.5°C. Figure 1B shows a typical slice from an 8-week-old marmoset, stained with propidium iodide after recording. Slices were maintained for at least 30 min at room temperature in ACSF before being transferred, one at the time, to the recording chamber (∼1.5 ml volume). The brain slice was perfused with ACSF at 3.0 ml min−1 (gravity flow) heated to 28–32°C by an in-line heater and an automatic temperature controller (Warner Instruments, Hamden, CT, USA).
Figure 1. Preparation of marmoset brain slices for patch-clamp recordings.

A, a coronal marmoset brain slice at the level of the amygdala. The line labelled ‘cut’ indicates the position where the slice was trimmed. Inset: marmoset brain. The white line indicates the position where the slice in A was cut. The anterior aspect is shown to the left. B, slice from an 8-week-old marmoset stained post hoc with propidium iodide after recording. C, identification of brain regions for the slice in B using a marmoset brain atlas (Yuasa et al. 2010). A1, primary auditory area; acp, anterior commissure, posterior part; AH, Ammon's horn; BLA, basolateral amygdala; BMA, basomedial amygdala; CeA, central amygdala; CoA, cortical amygdala; Er, entorhinal cortex; ITG, inferotemporal gyrus; LA, lateral amygdala; MeA, medial amygdala; opt, optic tract; S, subiculum. D, a NIR-DIC image of the BLA and surrounding regions from an 8-week-old marmoset. ICM, intercalated cell masses of the amygdala. E, a NIR-DIC image from a PD0 marmoset. F, a high-magnification image of BLA pyramidal neurons and a patch pipette. The pipette-filling solution is blown onto the neuron using positive pressure. The slice was taken from an 8-week-old marmoset. G, whole-cell recordings were performed from a BLA pyramidal neuron in current-clamp mode, and a 180 pA depolarizing current step was injected into an 8-week-old neuron at RMP. In this neuron, the current threshold for action potential generation was 140 pA. H, neuron selected as a pyramidal neuron based on morphology, but that showed an interneuron-like fast-spiking firing pattern in response to a 40 pA current step.
Patch-clamp electrodes (resistance 4–7 MΩ) were pulled from borosilicate capillary tubing (World Precision Instruments, Sarasota, FL, USA) and filled with a solution containing (in mm): potassium gluconate, 105; KCl, 30; Hepes, 10; EGTA, 0.5; MgCl2, 1; sodium phosphocreatine, 12; Mg-ATP, 3; Na-GTP, 0.5; pH 7.3, 295 mosmol l−1. The BLA was identified using a marmoset brain atlas (Yuasa et al. 2010). Figure 1C shows our identification of the brain regions in the 8-week-old slice from Fig. 1B. The BLA was easily identified by its black appearance in the near-infrared differential interference contrast (NIR-DIC) video image (Fig. 1D). The BLA was easily identified using NIR-DIC video microscopy also in the case of neonates (Fig. 1E).
Pyramidal-shaped large BLA cells (Fig. 1F) were visually identified using an upright microscope and NIR-DIC imaging (Axio Scope, Carl Zeiss Microimaging, Thornwood, NY, USA). In most cases, biocytin was included in the pipette-filling solution, and the slices were stained with streptavidin-FITC for post hoc verification of pyramidal morphology and location of the recorded cell.
Whole-cell patch-clamp recordings were performed from the soma of the pyramidal cells. The electrophysiological signal was amplified and filtered at 3 kHz using a MultiClamp 700B patch-clamp amplifier (Axon Instruments, Union City, CA, USA). Data were digitized at 50 kHz and acquired with Clampex 9.2 (Molecular Devices, Sunnyvale, CA, USA). The access resistance ranged from 7 to 22 MΩ initially, and was monitored frequently to check for resealing. At the end of recordings, we confirmed that no resealing had occurred.
Analysis of membrane and action potential properties
Immediately after establishing the whole-cell configuration, recordings were performed in current-clamp mode without current injection, and resting membrane potential (RMP) was measured after stabilization for a couple of minutes. After that, a series of hyperpolarizing and depolarizing rectangular current steps was applied (500 ms; 10–40 pA increments; interpulse interval 3 s). Voltage measurements were not corrected for a calculated liquid junction potential of 12.8 mV at 29.5°C. The voltage change occurring in response to current injection was measured near the end of the step. If spontaneous activity interfered with the measurement, 0.5 μm TTX was bath-applied to block action potential firing. Rin was calculated for each cell from the slope of a regression line fit with the least-squares method to the current–voltage plot (Manko et al. 2011). The time constant τ was calculated by a single exponential fit to the hyperpolarizing phase of the voltage response elicited by a −40 pA current step. Action potential amplitude, duration and threshold were measured for the first action potential generated in response to a series of depolarizing 500 ms current steps. The half-duration (ms) was defined as the duration of the action potential at half-maximal amplitude. Action potential threshold, visually identified as the data point immediately preceding the upstroke, was used as the baseline for measurements. The number of action potentials generated in response to a 500 ms current step at an intensity corresponding to rheobase +40 pA was also counted.
Analysis of K+ currents
Immediately after establishing whole-cell configuration, recordings were performed in voltage-clamp mode (−80 mV holding potential). First, recordings were carried out at a physiological K+ concentration in Ca2+-free ACSF, containing TTX (0.5 μm), 4-aminopyridine (4-AP; 2 mm) and tetraethylammonium (TEA) chloride (10 mm; ‘4.2 mm K+-ACSF’). The Ca2+-free ACSF was prepared by replacing CaCl2 with MgCl2 to block Ca2+-dependent K+ currents. 4-AP and TEA were added to partially block voltage-dependent K+ currents. A series of rectangular voltage steps was applied (200 ms; 10 mV increments; interpulse interval 3 s). Next, the slice was perfused with high-K+, Ca2+-free ACSF, containing TTX (0.5 μm), 4-AP (2 mm) and TEA (10 mm; ‘135.2 mm K+-ACSF’). The 135.2 mm K+-ACSF contained (in mm): KCl, 134; MgSO4, 1.3; MgCl2, 2.0; KH2PO4, 1.2; NaHCO3, 26; glucose, 10; pH 7.4, 290–300 mosmol l−1. After 3–5 min, the membrane potential was held at 0 mV and a series of rectangular voltage steps was applied (200 ms; 10 mV increments; interpulse interval 3 s). Current–voltage plots were constructed from the current response near the end of the step.
Analysis of GABAergic synaptic currents
Spontaneous, miniature and noradrenaline-induced GABAergic currents were studied in voltage-clamp (−70 mV holding potential) under blockade of glutamatergic currents by 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX disodium; 20 μm) and (5R,10S)- (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (MK-801; 10 μm). Miniature GABAergic currents were recorded in the presence of TTX (0.5 μm). Gap-free 30 s current traces were obtained before, during and after application of noradrenaline (10 μm). The traces were analysed using software (Mini Analysis Program, v.6.0.7, Synaptosoft, Decatur, GA, USA). The threshold amplitude for current detection was 5 pA, and the times required for a 37% decay and a 10–90% rise in the response were taken as the decay and rise times, respectively. The Kolmogorov–Smirnov (KS) test was used for probability distribution comparisons between two data groups (Miyajima et al. 2010).
Reagents
CNQX disodium (Sigma, St Louis, IL, USA), MK-801 hydrogen maleate (Sigma), picrotoxin (Sigma), (–)-noradrenaline (Sigma), 4-AP (Tokyo Chemical Industry, Tokyo, Japan), TEA chloride (Sigma) and TTX (Wako Pure Chemicals, Tokyo, Japan) were all dissolved in ACSF and bath-applied. All other chemicals were purchased from Wako Pure Chemicals.
Statistics
Data are expressed as mean ± standard error of the mean. For statistical comparisons, the data were analysed by one-way ANOVA. If the ANOVA indicated significance, post hoc Tukey's multiple comparison tests were performed. Unpaired t tests were used to compare the data from males and females. The Gaussian distribution of the data was tested by the KS test, and the Spearman's rank correlation coefficient (ρ) was calculated for analysis of correlation. A value of P < 0.05 was considered statistically significant.
Results
Marmoset BLA pyramidal neurons
BLA pyramidal neurons were identified for recording by their shape and large size (Fig. 1F), and by the presence of spike frequency accommodation in response to a supra-threshold current injection (Fig. 1G; Sah et al. 2003). The data for the present study were collected from 100 pyramidal neurons in 78 slices prepared from 18 marmosets. Two BLA neurons that were identified as pyramidal neurons by morphology showed an interneuron-like firing pattern in response to current injection (Fig. 1H), and were therefore excluded from analysis.
Postnatal changes of membrane electrophysiological properties
The numerical data are summarized in Table 1. The RMP of BLA pyramidal neurons was between −75 and −60 mV. There were no significant differences in RMP among the six age groups tested (Table 1).
Table 1.
Summary of membrane and action potential properties in marmoset BLA pyramidal neurons
| Age (number) | PD0–1 (4) | 2 week (2) | 4 week (1) | 7–14 week (4) | 23–33 week (4) | 4 year (1) |
|---|---|---|---|---|---|---|
| RMP (mV) | −64.9 ± 0.8 (23) | −64.1 ± 0.6 (20) | −66.9 ± 1.6 (7) | −65.7 ± 1.0 (22) | −65.3 ± 1.4 (12) | −67.6 ± 2.2 (5) |
| Rin (MΩ) | 431.0 ± 30.3 (22) | 213.7 ± 15.0 (18)*** | 111.0 ± 13.3 (7)*** | 105.3 ± 10.3 (20)***,†† | 134.3 ± 16.6 (12)*** | 124.3 ± 14.8 (5)*** |
| τ (ms) | 45.6 ± 2.3 (22) | 30.8 ± 3.4 (11) | 27.6 ± 3.9 (7)* | 24.3 ± 2.9 (16)*** | 26.0 ± 3.5 (9)** | 21.6 ± 3.5 (5)** |
| Action potential | ||||||
| Amplitude (mV) | 71.6 ± 1.2 (23) | 70.8 ± 2.5 (20) | 80.0 ± 2.1 (7) | 79.6 ± 1.7 (22) | 73.9 ± 2.3 (12) | 77.8 ± 2.7 (5) |
| Half-duration (ms) | 1.8 ± 0.1 (23) | 1.9 ± 0.1 (20) | 1.4 ± 0.1 (7)*,††† | 1.2 ± 0.0 (22)***,††† | 1.3 ± 0.0 (12)***,††† | 1.2 ± 0.0 (5)**,††† |
| Threshold (mV) | −38.7 ± 0.7 (23) | −40.9 ± 1.3 (20) | −45.8 ± 1.2 (7) | −42.0 ± 1.2 (22) | −40.3 ± 1.9 (12) | −42.6 ± 1.3 (5) |
| Number/500 ms1 | 5.5 ± 0.4 (23) | 5.4 ± 0.4 (20) | 5.4 ± 1.0 (7) | 5.2 ± 0.5 (22) | 5.2 ± 0.7 (12) | 4.0 ± 0.4 (5) |
(number) after ‘Age’: the number of marmoset used; (number) after values: the number of cells tested. Abbreviations: PD, postnatal day; Rin, input resistance; RMP, resting membrane potential; τ, membrane time constant. 1The number of action potentials generated in response to a 500 ms current step at an intensity corresponding to rheobase +40 pA. *P < 0.05, **P < 0.01, ***P < 0.001 vs. corresponding PD0 & 1 values (Tukey's multiple comparison test after one-way ANOVA). ††P < 0.01, †††P < 0.001 vs. a corresponding 2 week value (Tukey's multiple comparison test after one-way ANOVA).
Rin was highest at PD0–1 and decreased with postnatal development (Table 1). Statistical analysis showed that Rin at 2 weeks old and older was significantly smaller than at PD0–1 (ANOVA, F5,78= 39.3, P < 0.0001; Tukey, P < 0.001). In neurons from 4-week-old animals, Rin decreased to 100 MΩ, and this level was stable thereafter, including at 4 years old (Table 1). The upper traces in Fig. 2A–C show examples of voltage responses to the current steps illustrated in the lower traces. As revealed by the steeper current–voltage slope near RMP, the voltage response to current steps was greater at PD1 (Fig. 2A) than at 4 weeks (Fig. 2B) and 23 weeks old (Fig. 2C), consistent with the higher Rin (Fig. 2D). Figure 2E shows the distribution of Rin values (filled circles show neurons from male marmoset; open circles correspond to females). There was considerable variability in Rin at PD0–1, and this variation was reduced with development (Fig. 2E). There were no significant differences in Rin values between males and females in age groups with subjects of both sexes (PD0–1: male, 414.3 ± 46.0 MΩ (n= 10) vs. female, 445.0 ± 39.7 MΩ (n= 12) (P= 0.63 with t test); 7–14 weeks old: male, 116.8 ± 13.7 MΩ (n= 15) vs. female, 85.5 ± 6.9 MΩ (n= 7) (P= 0.27)).
Figure 2. Voltage responses (upper traces) of three marmoset BLA pyramidal neurons to depolarizing and hyperpolarizing current steps (lower traces), and cell-to-cell variations of the analysed parameters.

A, current-clamp recording showing the response to −80, −40, 0 and +40 pA current steps in a postnatal day (PD)1 neuron. B and C, voltage traces showing the response to −80, −40, 0, +40, +80, +120 and +160 pA current steps in neurons from a 4-week-old (4W) (B) and a 23-week-old (23W) (C) marmoset. Scale bars are common to A−C. D, membrane potential plotted as a function of injected current amplitude for neurons from PD1, 4W and 23W animals. The lines indicate the regression lines obtained by the least-squares method. E–H, cell-to-cell variations of the input resistance (Rin) (E), membrane time constant (τ) (F), action potential (AP) duration (G) and AP amplitude (H). Each dot represents one cell. The bar indicates the mean value. See Table 1 for the number of neurons and marmosets used. 2W, 2-week-old; 4W, 4-week-old; 7–14W, 7-, 8- and 14-week-old; 23–33W, 23-, 26- and 33-week-old; 4Y, 4-year-old.
The τ value was also highest at PD0–1, and decreased with age (Table 1). The τ value at PD0–1 was significantly different from the values at the older ages (ANOVA, F5,64= 10.7, P < 0.0001; see Table 1 for the result of Tukey's test). Figure 2F illustrates the large variation in the value of τ at all ages analysed. There was no significant difference between males and females (PD0–1: male, 42.5 ± 3.8 ms (n= 10) vs. female, 44.4 ± 2.6 ms (n= 12) (P= 0.91 with t test); 7–14 weeks old: male, 23.6 ± 4.0 ms (n= 11) vs. female, 25.9 ± 3.4 ms (n= 5) (P= 0.47)).
These results suggest that Rin and τ decrease during postnatal development in BLA pyramidal neurons of the marmoset.
Postnatal changes of the action potential
All cells, including those in slices from PD0–1 animals, generated action potentials in response to current steps. The action potential duration was significantly longer at PD0–1 and in 2 week olds than in the four older groups (Table 1). At age 4 weeks to 4 years, the mean action potential duration was stable at 1.2–1.4 ms (Table 1). The action potential duration varied considerably at PD0–1 (range 1.2–2.3 ms) and at 2 weeks old (1.3–2.7 ms), and this variation was reduced from 4 weeks old (Fig. 2G). There was no significant difference in action potential duration between males and females (PD0–1: male, 1.6 ± 0.1 ms (n= 11) vs. female, 1.9 ± 0.1 ms (n= 12) (P= 0.09 with t test); 7–14 weeks old: male, 1.3 ± 0.0 ms (n= 15) vs. female, 1.2 ± 0.0 ms (n= 7) (P= 0.51)). These results suggest that maturation of the action potential duration occurs between the age of 2 and 4 weeks.
As shown in Table 1 and Fig. 2H, the mean action potential amplitude was between 70 and 80 mV in all groups, and did not show any changes dependent on development. There was also no significant difference in action potential amplitude between males and females (PD0–1: male, 71.4 ± 2.0 mV (n= 11) vs. female, 71.8 ± 1.3 mV (n= 12) (P= 0.87 with t test); 7–14 weeks old: male, 77.1 ± 2.0 mV (n= 15) vs. female, 76.6 ± 1.2 mV (n= 7) (P= 0.61)). The absence of amplitude changes in marmoset BLA pyramidal neurons during development is in contrast to the increase in action potential amplitude seen during postnatal development in rat hippocampal and cortical neurons (McCormick & Prince, 1987; Spigelman et al. 1992).
The mean voltage threshold for action potential generation was between −46 and −40 mV in the six groups, and did not show any changes dependent on development (Table 1), consistent with findings in rat cortical layer V neurons (McCormick & Prince, 1987). The frequency of action potential firing (expressed as the number of action potentials generated per 500 ms depolarizing current injection) also showed no developmental changes (Table 1).
K+ currents
The current–voltage plots (Fig. 2D) were linear near RMPs at all ages, suggesting that passive leak K+ channels (reviewed in Goldstein et al. 2001) contributed to the changes in Rin across development. To test this possibility, we carried out voltage–clamp recordings from BLA neurons in brain slices from PD0 and 1-year-old marmosets. Figure 3A and B shows the two voltage step protocols used for experiments using 4.2 mm K+-ACSF (Fig. 3A) and 135.2 mm K+-ACSF (Fig. 3B). The latter concentration was chosen to reduce the electrochemical gradient of K+ across the plasma membrane. Figure 3C and D shows the typical current responses of PD0 neurons to the voltage step protocols shown in Fig. 3A and B, respectively, and Fig. 3E shows the current–voltage plots prepared from four PD0 neurons for both K+ concentrations. Similarly, Fig. 3F–H shows the typical current responses and the current–voltage plots prepared from four 1-year-old neurons. When the extracellular K+ concentration was changed from 4.2 to 135.2 mm, the reversal potential shifted by 94.8 ± 2.0 mV (n= 4) at PD0 and by 97.6 ± 3.0 mV (n= 4) at 1 year. These values are close to the theoretical value of 90.6 mV calculated using the Nernst equation, suggesting that the recorded current was mainly conducted by K+ ions. Furthermore, the current–voltage relationships obtained in 135.2 mm K+-ACSF (Fig. 3E and H, filled circles) were almost linear, suggesting a major contribution of two-pore domain K+ channels, which are open at all membrane potentials (reviewed in Goldstein et al. 2001). The mean currents recorded in 135.2 mm K+ were significantly smaller at PD0 than at 1 year old (P= 0.014 at +40 mV, t test; Fig. 3E vs. H, filled circles). These results suggest that the observed changes in Rin across development were in part mediated by a change in passive K+ currents mediated by two-pore domain K+ channels.
Figure 3. Voltage-clamp analysis of K+ currents.
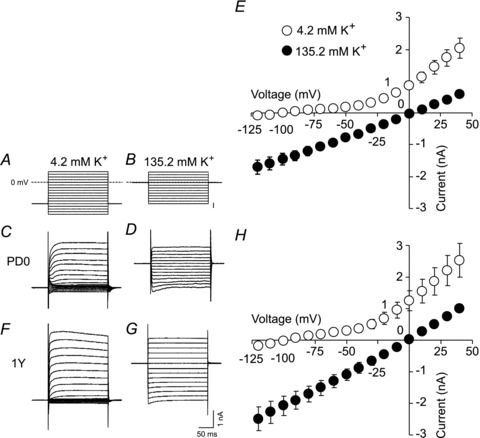
A and B, voltage step protocols used in ACSF with a physiological extracellular K+ concentration (4.2 mm; A) and in ACSF with a high extracellular K+ concentration (135.2 mm; B). C and D, typical current responses to voltage steps shown in A and B, respectively (PD0). E, current–voltage plots prepared from four PD0 neurons in 4.2 and 135.2 mm K+. F and G, typical current responses to the voltage steps shown in A and B, respectively (1 year old). H, current–voltage plots prepared from four 1-year-old neurons at 4.2 and 135.2 mm K+.
Correlation between parameters
Our results are consistent with previous research suggesting that the passive (Rin and τ) and active (action potential generation) membrane properties develop independently of each other (McCormick & Prince, 1987; Spigelman et al. 1992). Even though Rin and τ values showed great developmental changes between PD0–1 and 2 weeks old, full maturation of the action potential duration occurred considerably later – between 2 and 4 weeks old (Fig. 2E, and F vs. G). To verify this finding, we performed correlation analysis. The scattergram in Fig. 4A shows the Rin of individual neurons in PD0–1 and 2-week-old animals plotted against the corresponding τ values. Because the two data sets did not have a Gaussian distribution (KS test), the non-parametric Spearman's rank correlation coefficient (ρ) was calculated. The ρ value was 0.57 and the correlation was significant (P= 0.0005). Similar analysis was performed for Rin vs. action potential duration (Fig. 4B) and τvs. action potential duration (Fig. 4C). The ρ values were −0.16 and −0.19, respectively, and the correlations were not significant (P= 0.32 and 0.29, respectively). Thus, the maturation of Rin and τ are correlated with each other, whereas the development of the action potential duration is independent and different from Rin and τ.
Figure 4. Correlation between Rin and τ.

A–C, scattergrams showing the relationship between Rin and τ (A), between Rin and action potential (AP) duration (B), and between τ and AP duration (C). Each dot corresponds to one cell. Data from PD0–1 and 2-week-olds were used (a total of 33, 40 and 33 cells for A, B and C, respectively). Spearman's rank correlation coefficient (ρ) was calculated to assess the statistical significance of correlations.
Vigorous spontaneous GABAergic synaptic activity in neonatal marmosets
When BLA neurons were voltage-clamped at −70 mV under glutamate receptor blockade by CNQX and MK-801, spontaneous inward currents were abundant at PD0 (example in Fig. 5A, upper trace). These currents were suppressed by application of TTX (0.5 μm, n= 3; Fig. 5A, lower trace). Similar activity was also seen in neurons from animals of other ages (example from a 32-week-old marmoset in Fig. 5B, upper trace). The vigorous activity was blocked by application of picrotoxin (100 μm, n= 6; Fig. 5B, lower trace), suggesting that they were GABAergic postsynaptic currents. The 13 PD0 neurons and three PD1 neurons all generated similar activity continuously, although the frequency varied between cells (Fig. 5C; filled circles, male; open circles, female). The mean frequencies of synaptic currents were 18.0 ± 2.7 Hz at PD0–1 (n= 16 neurons from four marmosets), 11.8 ± 2.0 Hz at 2 weeks old (n= 8 neurons), 12.7 ± 1.8 Hz at 4–14 weeks olds (n= 13 neurons in five marmosets) and 14.4 ± 2.7 Hz at >23 weeks old (n= 12 neurons in five marmosets). These values were not significantly different (ANOVA, F3,45= 1.24, P= 0.31). We did not find any obvious developmental changes in amplitude, rise time or decay time of the GABAergic spontaneous synaptic currents (Fig. 5D–F). In addition, we did not find any significant differences between males and females in frequency, amplitude, rise or decay (P > 0.05 with t test for all). These results suggest that marmoset BLA pyramidal neurons already at age PD0–1 receive vigorous GABAergic input that is not different from the activity in adult neurons.
Figure 5. Vigorous spontaneous GABAergic currents in marmoset BLA pyramidal neurons.
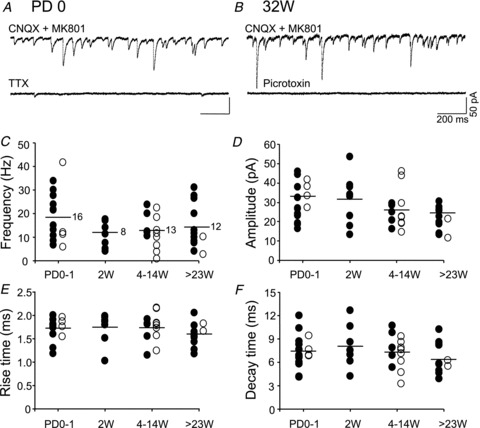
A, upper trace shows a recording from a neuron at postnatal day (PD)0 in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μm) and (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (MK-801; 10 μm). The lower trace shows a trace from the same neuron after 2 min of TTX application (0.5 μm). B, the upper trace shows a recording from a neuron of a 32-week-old (32W) marmoset in the presence of CNQX and MK-801. The lower trace shows a trace from the same neuron after 3 min of perfusion with picrotoxin (100 μm). Holding potential =−70 mV. Scale bars are common to A and B. C–F, age-related changes in frequency (C), amplitude (D), rise time (E) and decay time (F) of GABAergic events in BLA pyramidal neurons. Each dot represents one cell. The bar indicates the mean, and the number in C shows the number of neurons tested (common to D−F). Please see text for the number of marmosets used. 4–14W, 4-, 7-, 8- and 14-week-old; >23W, 23-, 26- and 33-week-old and 4-year-old.
Miniature GABAergic synaptic currents
Significant acceleration of the kinetics of GABAergic synaptic currents across development has been shown in rodents (Brickley et al. 1996; Draguhn & Heinemann, 1996; Tia et al. 1996; Hollrigel & Soltesz, 1997; Pouzat & Hestrin, 1997; Dunning et al. 1999; Okada et al. 2000; Vicini et al. 2001). As mentioned above, however, we found no significant changes in rise and decay times of spontaneous GABAergic synaptic currents in marmosets. To clarify whether or not there are developmental changes in the kinetics of GABAergic synaptic responses in marmosets, we analysed miniature GABAergic synaptic currents, which better reflect quantal release from individual synapses. Figure 6A shows typical miniature GABAergic synaptic currents from neurons at PD0 (thin line) and 1 year old (thick line) in the presence of TTX (0.5 μm), CNQX (20 μm) and MK-801 (10 μm). The peak amplitude of the response in a neuron at PD0 was scaled to that in a neuron at 1 year. The decay phase could be fitted with a single exponential function (Fig. 6A; R= 0.88 and 0.97 for PD0 and 1 year old, respectively, where R is the correlation coefficient). The mean miniature GABAergic current frequency, amplitude, rise time and decay time in neurons at PD0 and 1 year (n= 5 per group) are shown in Fig. 6B–E. The rise and decay times were significantly different between the two ages (P= 0.024 and P= 0.029 for rise and decay time, respectively; t test). In the absence of TTX, these parameters were not significantly different between the PD0 and 1-year-old marmosets (P > 0.05). These results suggest that the kinetics of miniature GABAergic currents accelerated with age between birth and 1 year old.
Figure 6. Analysis of miniature GABAergic synaptic currents.

A, typical traces showing miniature GABAergic synaptic currents from a neuron at postnatal day (PD)0 (thin line) and a neuron at 1 year old (thick line) in the presence of TTX (0.5 μm), CNQX (20 μm) and MK-801 (10 μm). The peak amplitude of the response in a neuron at PD0 was scaled to that in a neuron at 1 year. The superimposed curves are single exponential fits of the decay phase. B–E, mean frequency (B), amplitude (C), rise time (D) and decay time (E) of miniature GABAergic synaptic currents at PD0 (open bar, n= 5) and 1 year (filled bar, n= 5). *P < 0.05.
Development of noradrenaline-induced GABAergic currents
Noradrenaline application induced facilitation of the frequency of GABAergic postsynaptic currents in BLA pyramidal neurons (example from a 2-week-old marmoset in Fig. 7A). The GABAergic nature of the currents was confirmed by application of picrotoxin at the end of recordings (data not shown). Application of 10 μm noradrenaline for 2 min did not cause any statistically significant change in the leak current of BLA pyramidal neurons (P > 0.05 compared with baseline leak current, t test). Under control conditions (‘Cont’; obtained in the presence of CNQX and MK-801), the neuron in Fig. 7A received GABAergic inputs at a frequency of 11.5 Hz. When noradrenaline (10 μm) was applied, the discharge frequency increased to 17.9 Hz at 3 min after the start of noradrenaline application (trace labelled ‘+NA’ in Fig. 7A). The action of noradrenaline partially recovered after wash-out (‘Wash’ in Fig. 7A). Figure 7B shows scatter plots of the frequency in the presence of noradrenaline (10 μm) versus baseline frequency in 13 neurons from PD0–1, eight neurons from 2 week olds, 12 neurons from 4–14 week olds, and nine neurons from >23 week olds (n= 42 in total). The dotted line indicates equal frequencies with and without noradrenaline. As seen in Fig. 7B, noradrenaline facilitated GABAergic current frequency in 40 out of 42 neurons. To confirm this, we plotted inter-event intervals against the cumulative probability for each neuron (Fig. 7C; open circles, baseline; filled circles, +noradrenaline), and each dataset was statistically analysed using the KS test. The KS test indicated significant differences in the probability distribution of all 40 datasets in which noradrenaline apparently increased the current frequency, and showed no significant difference in the two remaining cells. Figure 7D shows plots of the cumulative probability versus current amplitude (open circles, baseline; filled circles, +noradrenaline), indicating that the amplitude was not affected by noradrenaline. Figure 7E shows the mean fold increases in frequency, amplitude, rise time and decay time caused by noradrenaline. As reported previously (Braga et al. 2004; Kaneko et al. 2008; Sekiguchi et al. 2009; Miyajima et al. 2010), the effect of noradrenaline on event frequency was pronounced, whereas other parameters showed little change in all the groups. One-way ANOVA revealed that there were no statistically significant differences among the four groups in frequency (F3,38= 0.84, P= 0.48), amplitude (F3,38= 1.324, P= 0.28), rise time (F3,38= 2.07, P= 0.12) and decay time (F3,38= 2.16, P= 0.11). These results suggest that the neuronal circuit responsible for facilitation of GABAergic synaptic transmission by noradrenaline is fully functional at PD0–1.
Figure 7. Facilitation of the frequency of spontaneous GABAergic postsynaptic currents by noradrenaline (NA) in marmoset BLA pyramidal neurons.
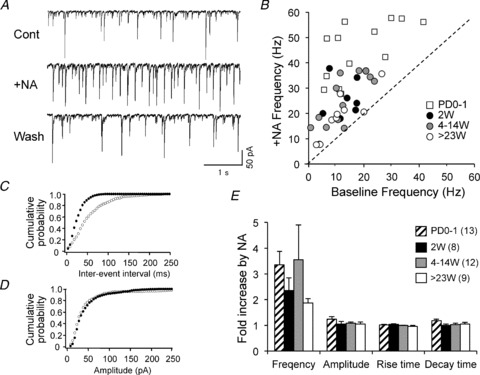
Sample traces showing activity before application of NA (‘Cont’), during application of 10 μm NA (‘+NA’) and after 10 min of wash-out (‘Wash’). Holding potential =–70 mV. Recordings were performed in the presence of CNQX and MK-801. B, scatter plots of baseline frequency versus frequency in the presence of NA, for 13 neurons at postnatal day (PD)0–1; 8 neurons from two 2-week-olds (2W); 12 neurons from one 4-week-old, two 7-week-olds, one 8-week-old and one 14-week-old; and 9 neurons from one 23-week-old, one 26-week-old and two 33-week-olds (total 42 neurons). The dotted line indicates equal frequencies with and without NA. C, inter-event interval versus cumulative probability plots in the neuron shown in A. Open circles, control; filled circles, 10 μm NA. D, amplitude versus cumulative probability plots in the neuron shown in A. Open circles, control; filled circles, 10 μm NA. E, the mean fold increases in frequency, amplitude, rise time and decay time by NA in each age group.
Discussion
Our study provides novel information on several features of postnatal development of BLA pyramidal neurons in marmosets. The passive electrical membrane properties changed significantly during the first weeks of development, whereas the action potential was relatively mature at birth but showed a slight decrease in duration over the first month of life. In addition, we found that GABAergic synaptic neurotransmission appeared functional in the marmoset amygdala at birth.
Previous morphological studies have suggested that the development of the hippocampus, another major region of the limbic system, occurs much earlier in primates than in rodents. In fact, most of the developmental process takes place in utero both in human (Humphrey, 1967; Paldino & Purpura, 1979; Kostovic et al. 1989; Arnold & Trojanowski, 1996; Hevner & Kinney, 1996) and non-human primates (Nowakowski & Rakic, 1979; Duffy & Rakic, 1983; Eckenhoff & Rakic, 1991; Seress & Ribak, 1995; Berger & Alvarez, 1996). Only one previous report has described patch-clamp recordings from primate fetuses, and it supported previous morphological results (Khazipov et al. 2001). It was found that both GABAergic and glutamatergic synaptic currents appeared around mid-gestation in hippocampal neurons of the cynomolgus monkey (Macaca fascicularis), with GABAergic currents appearing first. During the last third of gestation, numerous new glutamatergic synapses were established, and epileptiform discharges could be evoked by the GABAA receptor antagonist bicuculline already in utero. Thus, the early development of action potentials and GABAergic synaptic transmission in marmoset BLA neurons observed in the present study is consistent with these previous findings.
Membrane electrical properties and action potentials
The most remarkable postnatal change in the present study was that of Rin, which at 4 weeks old had decreased to about 1/4 of the value at birth. This degree of change nearly matches the changes reported in rat hippocampal and cortical pyramidal neurons (1/8 and 1/3, respectively; McCormick & Prince, 1987; Spigelman et al. 1992). The high Rin at birth greatly enhances neuronal excitability. The developmental decrease of Rin is thought to be caused by an increase in ion channel densities (McCormick & Prince, 1987). Our voltage-clamp analysis suggested that the decrease in Rin was in part mediated by an increase in the current mediated by passive K+ channels, mainly two-pore domain K+ leak channels. However, the possible role of other channels should be examined in the future.
Although cellular variations were large, the membrane time constant τ decreased to an extent similar to the developmental decrease in rat hippocampal and cortical pyramidal neurons (∼50% reduction). A membrane with a small time constant can be rapidly charged by a transmembrane current. Thus, the first month of life is accompanied by significant developmental changes in the basic electrical properties of marmoset BLA pyramidal neurons.
In rats, the action potential amplitude in neonatal neurons is about 50% of the mature amplitude (30 days old) in the hippocampus (Spigelman et al. 1992) and about 70% in the cortex (McCormick & Prince, 1987). This developmental change did not occur in marmoset BLA pyramidal neurons. However, the average duration of the action potential decreased to about 2/3 of neonatal levels between the second and fourth week of life (from 1.9 to 1.4 ms). This decrease is considerably milder than that seen in rat hippocampal neurons (about 1/8 of the neonatal duration) and cortical layer V neurons (about 1/4; McCormick & Prince, 1987; Spigelman et al. 1992). Therefore, the ‘immaturity’ of action potentials just after birth is low in marmoset BLA neurons compared with rats, although full maturation occurs during the first month of life. A possible explanation might be that action potential maturation starts during the fetal stage in the marmoset, so that the small postnatal changes observed in this study reflect the final step towards full maturation. As we mentioned earlier, morphological (Nowakowski & Rakic, 1979; Duffy & Rakic, 1983; Eckenhoff & Rakic, 1991; Seress & Ribak, 1995; Berger & Alvarez, 1996) and electrophysiological (Khazipov et al. 2001) studies have shown that a large part of hippocampal development takes place in utero in non-human primates. While no morphological data are available for the BLA, our results are consistent with predominantly fetal development of the action potential in the marmoset.
Correlation between parameters
The observed correlation between Rin and τ is not unexpected, because τ is a product of membrane capacitance and resistance in the equivalent circuit model of the neuronal membrane. The lack of a significant correlation between Rin/τ and the action potential duration suggests that the fairly large variations in Rin and τ seen in neurons in the first 2 weeks were not related to variations in action potential duration in the same period. Developmental changes in the spatiotemporal expression patterns of Na+ and K+ channels and changes in neuronal morphology may be important in regulating the action potential duration. Our results indicate that the developmental processes shaping the characteristics of adult neurons occur at different time scales. These processes might be targets of critically timed pathological processes that affect neuronal maturation.
GABAergic synaptic activity
Our findings suggested that the GABAergic network has acquired adult-like properties in the neonatal marmoset BLA. Such early development of GABAergic function has also been shown in the hippocampus of the cynomolgus monkey (Khazipov et al. 2001), where GABAergic synaptic currents appear around midgestation (gestation period 165 days). In addition, GABAergic interneurons that project to the dendrites of pyramidal neurons develop early (during the first half of gestation) in rhesus monkeys (Macaca mulatta; Berger & Alvarez, 1996). Our results are consistent with these previous reports. Because GABA is thought to act as a development signal (Ben-Ari et al. 2007), the strong GABAergic transmission seen in neonatal marmosets deserves notice. A high level of maternal care during infancy is associated with higher adult levels of benzodiazepine receptor binding and GABAA receptor α1/γ2 subunit expression in the BLA and central amygdala (CeA) of rats (Caldji et al. 1998, 2003). Because marmoset mothers exert close maternal care and neonates always cling to the mother, marmosets may be particularly suitable to investigate the influence of maternal separation on GABAergic synaptic transmission. For such studies, identification of the origin of GABAergic projection may be necessary. The GABAergic responses in the present study may be caused by a mixture of inputs from BLA interneurons, projections from other amygdala nuclei (Marowsky et al. 2005) and projections from other brain structures (Pitkanen, 2000). Further studies are needed to identify the origins of GABAergic inputs to neonatal BLA pyramidal neurons.
Our data suggest that the kinetics of miniature GABAergic currents changed in marmosets during development, as reported in rodents (Brickley et al. 1996; Draguhn & Heinemann, 1996; Tia et al. 1996; Hollrigel & Soltesz, 1997; Pouzat & Hestrin, 1997; Dunning et al. 1999; Okada et al. 2000; Vicini et al. 2001). One possible explanation for the absence of any significant developmental changes in the kinetics of spontaneous events is the developmental regulation of firing of presynaptic GABAergic neurons. As mentioned above, it is likely that BLA pyramidal neurons receive synaptic inputs from multiple kinds of GABAergic neurons. The firing of different GABAergic neurons might be differentially regulated across development. For example, developmentally different regulation of two distinct classes of GABAergic interneurons has been reported in area CA1 of the rat (Banks et al. 2002). In the absence of TTX, such regulation results in changes in the frequency of particular spontaneous GABAergic synaptic currents, which would disturb the statistical detection of the developmental changes in kinetics. Several mechanisms have been proposed to explain developmental changes of GABAergic synaptic currents, including changes in the expression of specific GABAA receptor subunits (Tia et al. 1996; Dunning et al. 1999; Okada et al. 2000; Vicini et al. 2001) and changes in reuptake mechanisms (Draguhn & Heinemann, 1996). The role of these mechanisms in the marmoset should be examined in future studies.
The finding that GABAergic synaptic responses could be elicited by noradrenaline in the marmoset BLA immediately after birth was particularly unexpected. The presence of a binding site for radioactive prazosin (indicating the presence of α1-adrenoceptors) has been reported in the adult marmoset (2–3 years old), but the expression of α1-adrenoceptors in the BLA of developing marmosets has not been studied (Gebhard et al. 1993). Noradrenaline is known to be released in the adult rat BLA in response to aversive stimuli such as footshock and restraint (Tanaka et al. 1983; Galvez et al. 1996), and noradrenaline-induced GABAergic currents in the BLA were greatly attenuated in stressed adult rats (Braga et al. 2004). These results imply that noradrenaline-induced GABAergic transmission is one of the systems implicated in stress-induced alteration or re-modelling of the BLA neuronal circuit. It seems logical for such a system to be active in the neonate, as their motor function is not yet fully developed and they are exposed to the menace of predators.
Other nuclei and brain regions
The BLA is one of the main nuclei of the amygdala (Pitkanen, 2000), suggesting that our findings have functional implications. Further studies on the development of the marmoset amygdala should also consider the maturation of other nuclei. For example, the development of CeA neurons is likely to be important because this nucleus forms an output centre of the amygdala. The pyramidal morphology of principal neurons in the BLA implies that these neurons are of a different cellular lineage to CeA principal neurons, which are GABAergic neurons. Indeed, principal neurons in the basolateral complex are thought to originate from the neuroepithelium of the ventral and lateral pallia, whereas those of the CeA originate from the neuroepithelium in the ganglionic eminence (Soma et al. 2009). In addition, the birth dates of neurons are different in the CeA and BLA: embryonic day 11 for CeA and embryonic day 12 for BLA (Soma et al. 2009). A comparison of neuronal development in the different amygdala nuclei will provide insights into the development of the amygdala as a whole.
The results in the present study are restricted to BLA pyramidal neurons, and cannot be applied to neurons in other brain regions. In addition, it is possible that age-related differences observed in this study were in part caused by differences in resilience between young and adult brain tissue. For instance, impaired resistance to hypoxia during slice preparation in older tissue may have introduced differences in slice health, and possibly cellular physiology, across ages. Therefore, care should be taken in interpreting comparisons between neonatal/young and adult slices. Because sexual maturity occurs at 15–18 months (Pryce et al. 2005), it seems that marmosets older than this are regarded as adults. However, this general drawback is not specific to marmosets, and brain slice patch-clamp recordings from various brain regions in marmosets may be available for acquiring further knowledge of primate development. Marmosets are particularly advantageous because of their smaller brain size compared with other frequently used monkey species.
Conclusions
In summary, we have characterized several features of the postnatal development of BLA pyramidal neurons in marmosets. The values of Rin and τ decreased significantly during the first 2 weeks of life, whereas the action potential showed relatively mature properties at birth. Action potential duration was slightly but significantly decreased during the first month, but its amplitude did not change after birth. Our findings suggest that BLA pyramidal neurons in marmosets acquire adult-like electrophysiological properties within 4 weeks of life. In addition, vigorous spontaneous and noradrenaline-induced GABAergic activity was present at all ages, indicating relative maturity of the GABA system in the marmoset at birth.
Acknowledgments
We thank Dr. Shigeki Yuasa (NCNP) for providing their marmoset brain atlas to us. This work was supported by the following grants to M.S.: KAKENHI (grant number 23500474) and Intramural Research Grant for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (grant number 22-5 & 24-2). The authors have no conflicts of interest to declare.
Glossary
- ACSF
artificial cerebrospinal fluid
- 4-AP
4-aminopyridine
- BLA
basolateral amygdala
- CeA
central amygdala
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- KS
Kolmogorov–Smirnov
- MK-801
(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate
- NIR-DIC
near-infrared differential interference contrast
- PD
postnatal day
- Rin
input resistance
- RMP
resting membrane potential
- τ
membrane time constant
- TEA
tetraethylammonium
Author contributions
M.S. planned and supervised the project. D.Y. and M.S. designed and performed the electrophysiological experiments. D.Y., M.M. and M.S. analysed and interpreted the data. M.S. drafted the article and revised it critically for important intellectual content. H.I. was responsible for anaesthesia. K.S. and K.W. provided marmosets. All authors approved of the final version of the manuscript.
References
- Amano T, Wada E, Yamada D, Zushida K, Maeno H, Noda M, Wada K, Sekiguchi M. Heightened amygdala long-term potentiation in neurotensin receptor type-1 knockout mice. Neuropsychopharmacology. 2008;33:3135–3145. doi: 10.1038/npp.2008.38. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ. Human fetal hippocampal development: I, Cytoarchitecture, myeloarchitecture, and neuronal morphologic features. J Comp Neurol. 1996;367:274–292. doi: 10.1002/(SICI)1096-9861(19960401)367:2<274::AID-CNE9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Banks MI, Hardie JB, Pearce RA. Development of GABAA receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol. 2002;88:3097–3107. doi: 10.1152/jn.00026.2002. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurons. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa J-L, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Berger B, Alvarez C. Neurochemical development of the hippocampal region in the fetal rhesus monkey. III: Calbindin-D28K, calretinin and parvalbumin with special mention of Cajal-Retzius cells and the retrosplenial cortex. J Comp Neurol. 1996;366:674–699. doi: 10.1002/(SICI)1096-9861(19960318)366:4<674::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004;29:45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Anisman H, Meaney MJ. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology. 2004;29:1344–1352. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABAA receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Heinemann U. Different mechanisms regulate IPSC kinetics in early postnatal and juvenile hippocampal granule cells. J Neurophysiol. 1996;76:3983–3993. doi: 10.1152/jn.1996.76.6.3983. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Rakic P. Differentiation of granule cell dendrites in the dentate gyrus of the rhesus monkey: a quantitative Golgi study. J Comp Neurol. 1983;214:224–237. doi: 10.1002/cne.902140210. [DOI] [PubMed] [Google Scholar]
- Dunning DD, Hoover CL, Soltesz I, Smith MA, O’Dowd DK. GABAA receptor-mediated miniature postsynaptic currents and a subunit expression in developing cortical neurons. J Neurophysiol. 1999;82:3286–3297. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- Eckenhoff MF, Rakic P. A quantitative analysis of synaptogenesis in the molecular layer of the dentate gyrus in the rhesus monkey. Brain Res Dev Brain Res. 1991;64:129–135. doi: 10.1016/0165-3806(91)90216-6. [DOI] [PubMed] [Google Scholar]
- Fulton BP, Walton K. Electrophysiological properties of neonatal rat motoneurones in vitro. J Physiol. 1986;370:651–678. doi: 10.1113/jphysiol.1986.sp015956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Mesches MN, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gardette R, Debono M, Dupont JL, Crepel F. Electrophysiological studies on the postnatal development of intracerebellar nuclei neurons in rat cerebellar slices maintained in vitro. II. Membrane conductances. Brain Res. 1985;352:97–106. doi: 10.1016/0165-3806(85)90091-4. [DOI] [PubMed] [Google Scholar]
- Gebhard R, Zilles K, Schleicher A, Everitt SB, Robbins TW, Divac I. Distribution of seven major neurotransmitter receptors in the striate cortex of the new world monkey Callithrix jacchus. Neuroscience. 1993;56:877–885. doi: 10.1016/0306-4522(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Goldstein SAN, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Kinney HC. Reciprocal entorhinal-hippocampal connections established by human fetal midgestation. J Comp Neurol. 1996;372:384–394. doi: 10.1002/(SICI)1096-9861(19960826)372:3<384::AID-CNE4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Hollrigel GS, Soltesz I. Slow kinetics of miniature IPSCs during early postnatal development in granule cells of the dentate gyrus. J Neurosci. 1997;17:5119–5128. doi: 10.1523/JNEUROSCI.17-13-05119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschneider K, Mulert C. Neuroimaging in anxiety disorders. Dialogues Clin Neurosci. 2011;13:453–461. doi: 10.31887/DCNS.2011.13.4/kholzschneider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T. The development of the human hippocampal fissure. J Anat. 1967;101:655–676. [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Tamamaki N, Owada H, Kakizaki T, Kume N, Totsuka M, Yamamoto T, Yawo H, Yagi T, Obata K, Yanagawa Y. Noradrenergic excitation of a subpopulation of GABAergic cells in the basolateral amygdala via both activation of nonselective cationic conductance and suppression of resting K+ conductance: a study using glutamate decarboxylase 67-green fluorescent protein knock-in mice. Neuroscience. 2008;157:781–797. doi: 10.1016/j.neuroscience.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Esclapez M, Caillard O, Bernard C, Khalilov I, Tyzio R, Hirsch J, Dzhala V, Berger B, Ben-Ari Y. Early development of neuronal activity in the primate hippocampus in utero. J Neurosci. 2001;21:9770–9781. doi: 10.1523/JNEUROSCI.21-24-09770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Seress L, Mrzljak L, Judas M. Early onset of synapse formation in the human hippocampus: a correlation with Nissl-Golgi architectonics in 15- and 16.5-week-old fetuses. Nueroscience. 1989;30:105–116. doi: 10.1016/0306-4522(89)90357-6. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurons. J Physiol. 1987;393:743–762. doi: 10.1113/jphysiol.1987.sp016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manko M, Geracitano R, Capogna M. Functional connectivity of the main intercalated nucleus of the mouse amygdala. J Physiol. 2011;589.8:1911–1925. doi: 10.1113/jphysiol.2010.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Ozaki M, Wada K, Sekiguchi M. Noradrenaline-induced spontaneous inhibitory postsynaptic currents in mouse basolateral nucleus of amygdala pyramidal neurons: comparison with dopamine-induced currents. Neurosci Lett. 2010;480:167–172. doi: 10.1016/j.neulet.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Nowakowski RG, Rakic P. The mode of migration of neurons to the hippocampus: a Golgi and electron microscopic analysis in foetal rhesus monkey. J Neurocytol. 1979;8:697–718. doi: 10.1007/BF01206671. [DOI] [PubMed] [Google Scholar]
- Okada M, Onodera K, Van Renterghem C, Sieghart W, Takahashi T. Functional correlation of GABAA receptor subunits expression with the properties of IPSCs in the developing thalamus. J Neurosci. 2000;20:2202–2208. doi: 10.1523/JNEUROSCI.20-06-02202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paldino AM, Purpura DO. Branching pattern of hippocampal neurons of human fetus during dendritic differentiation. Exp Neurol. 1979;64:620–631. doi: 10.1016/0014-4886(79)90236-x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala, Second Edition: a Functional Analysis. Oxford: Oxford University Press; 2000. pp. 31–115. [Google Scholar]
- Pouzat C, Hestrin S. Developmental regulation of basket/stellate cell → Purkinje cell synapses in the cerebellum. J Neurosci. 1997;17:9104–9112. doi: 10.1523/JNEUROSCI.17-23-09104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology. 2011;214:33–53. doi: 10.1007/s00213-010-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C, Spengler M, Weber E, Weston A, Jongen-Relo A. Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur J Neurosci. 2005;21:1521–1535. doi: 10.1111/j.1460-9568.2005.04003.x. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez de Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Seidel K, Helmeke C, Poeggel G, Braun K. Repeated neonatal separation stress alters the composition of neurochemically characterized interneuron subpopulations in the rodent dentate gyrus and basolateral amygdala. Dev Neurobiol. 2008;68:1137–1152. doi: 10.1002/dneu.20651. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Zushida K, Yoshida M, Maekawa M, Kamichi S, Yoshida M, Sahara Y, Yuasa S, Takeda S, Wada K. A deficit of brain dystrophin impairs specific amygdala GABAergic transmission and enhances defensive behavior in mice. Brain. 2009;132:124–135. doi: 10.1093/brain/awn253. [DOI] [PubMed] [Google Scholar]
- Seress L, Ribak CE. Postnatal development of CA3 pyramidal neurons and their afferents in the Ammon's horn of rhesus monkeys. Hippocampus. 1995;5:217–231. doi: 10.1002/hipo.450050308. [DOI] [PubMed] [Google Scholar]
- Soma M, Aizawa H, Ito Y, Maekawa M, Osumi N, Nakahira E, Okamoto H, Tanaka K, Yuasa S. Development of the mouse amygdala as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J Comp Neurol. 2009;513:113–128. doi: 10.1002/cne.21945. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Zhang L, Carlen PL. Patch-clamp study of postnatal development of CA1 neurons in rat hippocampal slices: membrane excitability and K+ currents. J Neurophysiol. 1992;68:55–69. doi: 10.1152/jn.1992.68.1.55. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kohno Y, Nakagawa R, Ide Y, Takeda S, Nagasaki N, Noda Y. Regional characteristics of stress-induced increase in brain noradrenaline release in rats. Pharmacol Biochem Behav. 1983;19:543–547. doi: 10.1016/0091-3057(83)90132-6. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor a6 subunit. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Holmes GL, Ben-Ari Y, Khazipov R. Timing of the developmental switch in GABA(A) mediated signaling from excitation to inhibition in CA3 rat hippocampus using gramicidin perforated patch and extracellular recordings. Epilepsia. 2007;48:96–105. doi: 10.1111/j.1528-1167.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Ivanov A, Bernard C, Holmes GL, Ben-Ari Y, Khazipov R. Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. J Neurophysiol. 2003;90:2964–2972. doi: 10.1152/jn.00172.2003. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Minlebaev M, Rheims S, Ivanov A, Jorquera I, Holmes GL, Zilberter Y, Ben-Ari Y, Khazipov R. Postnatal changes in somatic g-aminobutyric acid signalling in the rat hippocampus. Eur J Neurosci. 2008;27:2515–2528. doi: 10.1111/j.1460-9568.2008.06234.x. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn J. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABAA receptor a1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Age-specific threats induce CRF expression in the paraventricular nucleus of the hypothalamus and hippocampus of young rats. Horm Behav. 2005;47:139–150. doi: 10.1016/j.yhbeh.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Nakamura K, Kohsaka S. Stereotaxic Atlas of the Marmoset Brain. Japan: National Institute of Neuroscience, National Center of Neurology and Psychiatry; 2010. [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


