Abstract
Endogenous neurosteroids are among the most potent and efficacious potentiators of activation of GABAA receptors. It has been proposed that a conserved glutamine residue in the first membrane-spanning region (TM1 region) of the α subunits is required for binding of potentiating neurosteroids. Mutations of this residue can reduce or remove the ability of steroids to potentiate function. However, it is not known whether potentiation requires that a steroid interact with the α subunit, or not. To examine this question we mutated the homologous residue in the β2 and γ2L subunits to glutamine, and found that these mutations could not confer potentiation by allopregnanolone (3α5αP) when expressed in receptors containing ineffective α1 subunits. However, potentiation is restored when the entire TM1 region from the α1 subunit is transferred to the β2 or γ2L subunit. Mutations in the TM1 region that affect potentiation when made in the α1 subunit have similar effects when made in transferred TM1 region. Further, the effects of 3α5αP on single-channel kinetics are similar for wild-type receptors and receptors with moved TM1 regions. These results support the idea that steroids bind in the transmembrane regions of the receptor. The observations are consistent with previous work indicating that neurosteroid potentiation is mediated by an action that affects the receptor as a whole, rather than an individual subunit or pair of subunits, and in addition demonstrate that the mechanism is independent of the nature of the subunit that interacts with steroid.
Key points
Neuroactive steroids are endogenous compounds that are potent and efficacious potentiators of GABAA receptors.
Neuroactive steroids have been shown to act after interacting with amino acids in the first transmembrane-spanning region (TM1 region) of the α subunit.
We show that the TM1 region can be moved from the α1 subunit to other subunits of the GABAA receptor (the β2 or γ2 subunits) and confer potentiation.
Our data show that neurosteroid potentiation does not require that the steroid interacts with a particular subunit of the GABAA receptor, nor that it interacts with a subunit that also binds GABA.
Our data also indicate that a steroid binding site is located in the TM1 region.
Introduction
Endogenous neurosteroids are among the most potent and efficacious potentiators of activation of GABAA receptors. Steroids act on these receptors in regions exposed to the cell membrane (Akk et al. 2005), and a particular residue in the first membrane-spanning region (TM1 region) of the α1 subunit (α1Gln241) has been shown to be required for potentiation (Hosie et al. 2006). Examination of a series of mutations and of steroids of different structures resulted in the proposal that this residue is directly involved in steroid binding (Hosie et al. 2006), although further studies have demonstrated that the interactions between a potentiating steroid and the receptor are likely to be complex (Akk et al. 2008; Li et al. 2009). All GABAA receptor α subunits have a glutamine residue at this position, and mutation of this glutamine to leucine in other α subunits also removes neurosteroid potentiation from receptors containing the mutated subunit (Hosie et al. 2009). These observations suggest that steroid potentiation requires the presence of this residue in the α subunit.
To test this idea, and to explore the role of homologous regions in other subunits, we mutated the corresponding residue in the β2 and γ2L subunits (tryptophan in both cases) to glutamine, and found that potentiation was not restored for receptors containing a mutated (steroid-insensitive) α1 subunit. However, a study of potentiation of the nicotinic α4β2 receptor by estradiol (Jin & Steinbach, 2011) raised the possibility that a larger region might be required. Indeed, we find that when the entire TM1 region is transplanted from the α1 subunit to the β2 or γ2L subunit, steroid potentiation is restored in receptors with steroid-insensitive α1 subunits.
Methods
Receptors were expressed using GABAA receptor subunits from rat: α1 (NCBI Reference Sequence: NP_899155.1); β2 (NP_037089.1); and γ2L (NP_899156.1). Subunits were inserted in the expression vector pcDNA3 (Invitrogen, San Diego, CA, USA). HEK cells were plated at a density of 200,000 cells dish−1 1 day before transfection. A total of 0.4 μg of plasmid cDNAs at 1:1:1 ratio was mixed with 10 μl of Effectene reagent and 3.2 μl of Enhancer of Effectene (QIAGEN, Valencia, CA, USA) per dish. Fifteen–twenty hours later, cells were washed with culture medium. Recordings were made 1–3 days after transfection.
The amino acid residues in the first transmembrane regions for the three subunits are shown in Fig. 1. The polynucleotide sequence coding for the entire region shown was transferred; that is, the residues shown for the α1 sequence were substituted for the residues shown for the β2 or γ2L sequence. Point mutations were made using QuikChange site directed mutagenesis (Stratagene, San Diego, CA, USA). The entire coding sequence for all constructs was sequenced to ascertain that only the desired mutations were made.
Figure 1. The regions of subunits studied.
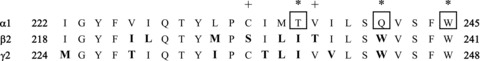
The figure shows the aligned TM1 regions, which were the regions swapped from the α1 subunit to the other subunits. All residues shown for the α1 sequence were directly substituted for the residues shown for the β2 or γ2L sequence. The critical residues in the α1 subunit are boxed and marked with asterisks (α1(T236), α1(Q241) and α1(W245)). Aligned residues that differ from the sequence in the α1 subunit are shown in bold type. The sequences shown begin at α1(I222), β2(I218) and γ2L(M224) (numbering for the mature subunit).
3α5αP ((3α, 5α)-3-hydroxypregnan-20-one, allopregnanolone) was purchased from Sigma-Aldrich (St Louis, MO, USA) and prepared as a 10 mm stock solution in DMSO and diluted on the day of the experiment. Concentrations of steroid above 10 μm were not used, as the steroid solubility is not known precisely but is likely to be less than 30 μm.
The α1 subunit is epitope (FLAG)-tagged (Ueno et al. 1996) in the amino-terminal end of the subunit. Cells expressing high levels of surface receptors were selected for physiological recordings using a bead-binding technique in which the presence of the FLAG peptide was detected with a mouse monoclonal antibody to the FLAG epitope (M2; Sigma-Aldrich), which had been adsorbed to beads with a covalently attached goat anti-mouse IgG antibody (Invitrogen).
Whole-cell patch-clamp records were obtained and analysed as described (Li et al. 2006). To characterize activation by GABA, six–eight concentrations of GABA were applied and the peak response measured. Normalized concentration–response curves for each cell were fit with the Hill equation to estimate the [GABA] producing a half-maximal response (EC50). Potentiation was assessed by the ratio of the response of a cell to a low concentration of GABA + steroid to the response of that cell to the same concentration of GABA in the absence of steroid (the ‘potentiation ratio’). Concentrations of GABA were selected to produce ∼15% of the maximal response, from the concentration–response relationship for that particular combination of subunits. Direct activation by steroid was assessed by determining the response of a cell to the application of 10 μm steroid in the absence of GABA, and normalized to the response of the same cell to 1 mm GABA. This ratio is essentially the fraction of maximal activation that 10 μm steroid can elicit. Two statistical tests were made of the potentiation ratio. In the first, the ratios for a given construct and condition (e.g. wild-type receptors using 1 μm 3α5αP) were compared with 1 (no effect) using a paired t test. This test indicated whether a significant effect was seen. In the second, potentiation ratios were compared across sets of constructs using one-way ANOVA with a Dunnett post hoc correction. This test indicated whether responses of a given construct differed from the base construct in the set.
Single-channel recordings were made in the cellattached mode, and analysed as described previously (Akk & Steinbach, 2000; Akk et al. 2008). In the single-channel recordings of cells expressing α1(I&L) +β2(TM1) +γ2L subunits we observed some clusters of activity in the presence of 3α5αP that appeared to be unaffected by 3α5αP. These clusters were assumed to result from incorporation of wild-type β subunits that are endogenously expressed in HEK cells (Ueno et al. 1996), and were not analysed.
Results
Potentiation can be transferred by moving the entire TM1 region
The first transmembrane regions of the rat GABAAα1, β2 and γ2L subunits are shown in Fig. 1. Previous work (Hosie et al. 2006; Akk et al. 2008) has demonstrated that mutations of α1Gln241 can reduce or remove the ability of neurosteroids to potentiate responses of the α1β2γ2L receptor to low concentrations of GABA. To produce a receptor that is neither potentiated nor activated by neurosteroids, we introduced two mutated residues in the α1 TM1: α1Q241L to remove potentiation; and α1T236I to remove direct activation (Hosie et al. 2006). This construct will be abbreviated as α1(I&L). We used the potentiating neurosteroid 3α5αP to test for neurosteroid modulation. Receptors expressed after transfection of α1(I&L) +β2 +γ2L subunits were not potentiated by 1 μm 3α5αP and weakly potentiated by 10 μm (Fig. 2; Table 1).
Figure 2. Potentiation of responses to GABA.
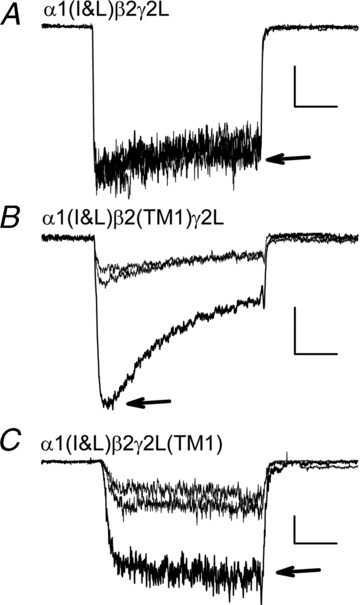
Each panel presents three traces showing responses to 4 s applications of low [GABA] immediately before and after a response to the same [GABA]+ 1 μm 3α5αP (indicated by arrow). A, responses from a cell transfected with α1(I&L) +β2 +γ2L to 50 μm GABA (scale bars: 1 s, 40 pA). Note the absence of potentiation. B, responses from a cell transfected with α1(I&L) +β2(TM1) +γ2L to 10 μm GABA (scale bars: 1 s, 100 pA). C, responses from a cell transfected with α1(I&L) +β2 +γ2(TM1) to 10 μm GABA (scale bars: 1 s, 20 pA). Note the presence of potentiation in B and C. There was some variability in the decline of current (‘apparent desensitization’) between cells expressing a given set of subunits, and this has not been studied quantitatively as of yet. The concentrations of GABA were chosen to elicit ∼15% of maximal current: 19 ± 3% (7 cells) for α1(I&L) +β2 +γ2L; 15 ± 2% (14) for α1(I&L) +β2(TM1) +γ2L; and 9 ± 2% (7) for α1(I&L) +β2 +γ2(TM1) (mean ± SEM (number of cells)).
Table 1.
Effects of mutations on potentiation and gating by 3α5αP
| Potentiation by 0.1 μm 3α5αP | Potentiation by 1 μm 3α5αP | Potentiation by 10 μm 3α5αP | Gating by 10 μm 3α5αP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subunits | mean ± SEM (N) | P to 1 | P in set | mean ± SEM (N) | P to 1 | P in set | mean ± SEM (N) | P to 1 | P in set | mean ± SEM (N) | P to 0 | P to wt |
| α1 β2 γ2L | 2.67 ± 0.26 (5) | ** | 4.98 ± 0.61 (10) | *** | 4.81 ± 0.42 (5) | *** | 0.034 ± 0.009 (10) | ** | — | |||
| α1(I&L) β2 γ2L | ND | 1.19 ± 0.15 (7) | NS | 1.25 ± 0.06 (6) | ** | — | 0.001 ± 0.001 (13) | NS | *** | |||
| α1(I&L) β2(TM1) γ2L | 2.18 ± 0.25 (5) | ** | 3.39 ± 0.30 (11) | *** | *** | 2.53 ± 0.23 (11) | *** | *** | 0.003 ± 0.002 (7) | NS | *** | |
| α1(I&L) β2 γ2L(TM1) | 1.57 ± 0.18 (6) | * | 2.08 ± 0.16 (13) | *** | * | 2.91 ± 0.26 (6) | *** | *** | 0.007 ± 0.004 (7) | NS | ** | |
| α1(I&L) β2(W237Q) γ2L | ND | 1.01 ± 0.03 (5) | NS | NS | ND | 0.003 ± 0.002 (5) | NS | ** | ||||
| α1(I&L) β2(I232T&W237Q) γ2L | ND | 1.03 ± 0.09 (6) | NS | NS | ND | 0.003 ± 0.002 (6) | NS | *** | ||||
| α1(I&L) β2 γ2L(W252Q) | ND | 0.92 ± 0.05 (5) | NS | NS | ND | 0.008 ± 0.004 (5) | NS | ** | ||||
| α1(I&L) β2(TM1 Q237L) γ2L | ND | ND | 1.06 ± 0.04 (15) | NS | NS | 0.001 ± 0.001 (4) | NS | ** | ||||
| α1(I&L) β2(CTVQ) γ2L | ND | 2.37 ± 0.13 (7) | *** | ** | ND | 0.001 ± 0.000 (5) | NS | *** | ||||
| α1(I&L) β2(TM1 W241L) γ2L | ND | ND | 1.12 ± 0.14 (11) | NS | NS | 0.004 ± 0.004 (5) | NS | ** | ||||
| α1(W245L) β2 γ2L | ND | 1.04 ± 0.10 (5) | NS | — | ND | 0.001 ± 0.001 (5) | NS | *** | ||||
| α1(W245L) β2(TM1) γ2L | ND | 2.18 ± 0.15 (5) | ** | *** | ND | 0.004 ± 0.001 (5) | * | ** | ||||
| α1(W245L) β2 γ2(TM1) | ND | 1.14 ± 0.16 (8) | NS | NS | ND | 0.001 ± 0.000 (8) | ** | *** | ||||
The first column presents the subunits transfected. The first group of columns first shows the potentiation ratio for data from cells tested with a GABA concentration producing 15—20% of the maximal response ± 0.1 μm 3α5αP. The column headed ‘P to 1’ summarizes the probability that the ratio is the same as a value of 1 (no potentiation; paired two-tailed t test). ‘P in set’ summarizes the probability that the ratio is the same as that for receptors in the first row of the set (e.g. α1(I&L) β2 γ2L; one-way ANOVA with Dunnett correction). The next two groups of columns show similar data for potentiation by 1 and 10 μm 3α5αP. The final group of columns shows data for direct gating by 10 μm 3α5αP in the absence of GABA, normalized to the response to 1 mm GABA in the same cell (i.e. fraction of maximal GABA-elicited response). ‘P to 0’ summarizes the probability that the ratio is equal to 0 (no gating; two-tailed paired t test), and ‘P to wt’ the probability that the value is the same as that for receptors containing wild-type subunits (first row; one-way ANOVA with Dunnett correction). Probabilities summarized as: NS: P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. ND: experiment not done. All data are presented as mean ± SEM (N cells).
We first sought to determine whether it was possible to restore potentiation by mutating the homologous residue in the β2 or γ2L subunit from tryptophan to glutamine. When the mutated β2 (β2(W237Q)) or γ2L (γ2L(W252Q)) subunits were expressed with the α1(I&L) subunit, potentiation was still absent (Fig. 3; Table 1).
Figure 3. Summary of potentiation of GABA responses.
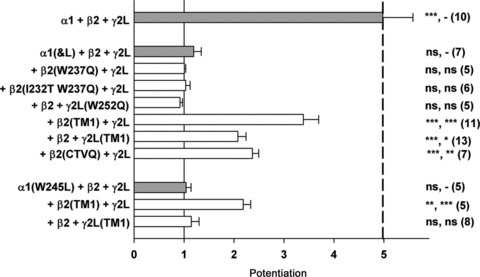
The mean (± SEM) potentiation by 1 μm 3α5αP is shown. The combinations of subunits are grouped as follows: top combination is all wild-type subunits; the next group all contain α1(I&L) with various constructs; and the bottom group all contain α1(W245L). The thin vertical continuous line shows a value of 1 (no potentiation), and the thick dashed line shows the mean potentiation for wild-type receptors. The symbols to the right of the bars indicate probability that potentiation differs significantly from 1 (that is no effect; two-tailed paired t test) and from the potentiation from the initial combination in the group (e.g. α1(I&L) +β2 +γ2L; one-way ANOVA with Dunnett's post hoc correction) (initial combination, NS P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001). Data values are presented in Table 1.
A recent study examined the ability of 17β-estradiol to potentiate nicotinic α4β2 receptors (Jin & Steinbach, 2011). In this case, potentiation had been mapped to the final six residues of the nicotinic α4 receptor. It was found that potentiation could be restored in receptors containing mutated nicotinic α4 subunits by transferring these six residues to the carboxy terminus of the nicotinic β2 subunit. By analogy to this result, we reasoned that perhaps it was necessary to transfer a larger region of the α1 TM1 region to restore neurosteroid potentiation to the GABAA receptor.
Accordingly, we transferred the entire TM1 region from the GABAAα1 subunit. In this case, 3α5αP potentiation was restored to GABAA receptors containing α1(I&L) subunits (Figs 2 and 3; Table 1). Potentiation was restored when the transferred TM1 region was present in the β2 subunit (β2(TM1)) or the γ2L subunit (γ2(TM1)).
We routinely used 1 μm 3α5αP to potentiate responses, as this is a saturating concentration for wild-type receptors (Akk et al. 2008). However, we tested 10 μm 3α5αP on some constructs to determine whether major changes had occurred in apparent potency (Table 1). We did not use higher concentrations of steroid due to low aqueous solubility. As can be seen, there were only small increases in potentiation between 1 and 10 μm steroid, which is consistent with previous studies that indicate 1 μm is a saturating concentration. For α1 +β2 +γ2L and α1(I&L) +β2(TM1) +γ2L receptors, the potentiation ratio did not differ at 10 μm from 1 μm; while for α1(I&L) +β2 +γ2L(TM1) receptors the increase was significant (P < 0.01, two-tailed t test).
Inspection of the region transferred (see Fig. 1) shows a total of eight differences between β2 and α1 in this region. We mutated both β2(I231T) and β2(W237Q) as these residues were implicated in steroid actions (Hosie et al. 2006). However, this did not confer potentiation when expressed with α1(I&L) and γ2L (Table 1). Among the eight differing residues, four are differences that occur relatively rarely in related proteins (as assessed from the BLOSUM62 matrix; Henikoff & Henikoff, 1992). These are the pairs β2(S229)/α1(C233), β2(I232)/α1(T236), β2(T233)/α1(V237) and β2(W237)/α1(Q241). Accordingly, we tested the consequences of mutating all four of these residues in the β2 TM1 region (β2(CTVQ)), and found that this reduced number of mutations was capable of conferring potentiation (Table 1; Fig. 3). This observation indicates that the entire TM1 region is not required, but further work is necessary to define the roles of amino acids in underlying steroid potentiation.
Consequences of mutations
We then examined whether the transferred TM1 regions showed some properties for potentiation that are similar to those when the region is located in the α1 subunit. In the first case, we mutated the critical glutamine in the transferred TM1 region to leucine. This removed potentiation: β2(TM1 Q237L) had a potentiation ratio of 1.06 ± 0.04 (15) when GABA was applied with 10 μm 3α5αP, which did not differ from no effect (Table 1).
We also mutated a tryptophan residue in the α1 TM1 region (α1(W245L)), that we have previously shown to remove potentiation by neurosteroids (Akk et al. 2008; see Fig. 1 for position). We confirmed that expression of α1(W245L) with wild-type β2 and γ2L subunits resulted in receptors that were not potentiated by 3α5αP (Fig. 3; Table 1). However, expression of α1(W245L) with β2(TM1) and wild-type γ2L did result in significant potentiation of the receptors (Fig. 3; Table 1), although expression with β2 +γ2L(TM1) did not. The α1(W245L) mutation reduced the amount of potentiation compared with the α1(I&L) mutation (P < 0.01 for either the β2(TM1) +γ2L or β2 +γ2L(TM1) constructs; two-tailed t test). We do not have an explanation for this difference between the effects of the two α1 mutations. The inverse experiment, mutating the transferred tryptophan in β2 (β2(TM1 W241L)) abolished potentiation when expressed with α1(I&L) (Table 1). These results demonstrate that critical residues in the transferred TM1 regions have roles similar to those in the α1 TM1 region.
Single-channel kinetics
One possibility is that steroid potentiation of receptors with transferred TM1 regions occurs by a different kinetic mechanism than for wild-type receptors. To examine this, we performed cell-attached single-channel recordings of activity elicited by 100 μm GABA in the absence and presence of 10 μm 3α5αP, for receptors comprising α1(I&L) +β2(TM1) +γ2L subunits. In the absence of 3α5αP the open time distributions contained only two components (Fig. 4; Table 2), rather than the three components seen in wild-type receptors activated by GABA (Akk et al. 2008). We have already reported that receptors composed of α1(Q241L) with wild-type β2 and γ2L subunits also show only two open time components (Akk et al. 2008), so it is likely that this observation results from a dominant kinetic effect of the α1Q241L mutation. In the presence of 3α5αP, three components were seen in the open time distributions. The duration of the second component was prolonged, and a new longer duration component appeared (Fig. 3; Table 2). Similarly, the longest intracluster closed time was greatly reduced in prevalence in the presence of 3α5αP (Fig. 4; Table 2). These changes are similar to wild-type receptors potentiated by 3α5αP, for which there is an increase in both the prevalence and duration of the longest duration open time component, and a reduction in the prevalence of the longest intracluster closed time (Akk et al. 2004). It is interesting to note that wild-type receptors activated by piperidine-4-sulfonic acid (P4S), a low efficacy agonist, show only two components in the open time distributions in the absence of potentiating neurosteroid, similar to α1(Q241L)-containing receptors activated by GABA (Akk et al. 2008). When wild-type receptors activated by P4S are exposed to 3α5αP, a third open time component appears and the prevalence of the long duration closed component is reduced (Akk et al. 2008), as we see with GABA activation of α1(Q241L)-containing receptors expressed with potentiation-conferring subunits. In sum, the kinetic mechanisms by which neurosteroids potentiate receptors containing transferred TM1 regions appear indistinguishable from those for wild-type receptors.
Figure 4. Potentiation of single-channel currents.
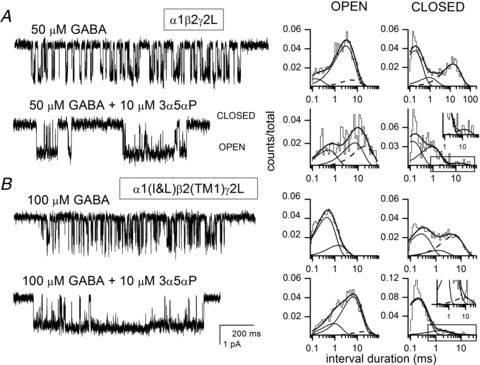
Sample single-channel clusters from cell-attached patches from HEK cells expressing rat α1 +β2 +γ2L (A) or α1(I&L) +β2(TM1) +γ2L receptors (B). The receptors were activated by 50 μm or 100 μm GABA, in the absence and presence of 10 μm 3α5αP. Openings are shown as downward deflections. The scale bars in A (1 pA, 200 ms) apply to A and B. The open and closed time histograms from the respective patches are shown next to the data traces. The lines show the fits to the data: the thick lines show the sum of all components fit; individual components are shown with thin continuous lines; while the dashed lines show the components discussed in the Results (OT3 and CT3). A, for GABA alone, the mean open times are 0.1 ms (15%), 2.8 ms (73%) and 5.2 ms (12%), and the mean closed times are 0.2 ms (55%), 0.8 ms (13%) and 12.7 ms (32%). For GABA + 3α5αP, the mean open times are 0.5 ms (29%), 6.6 ms (40%) and 17.9 ms (31%), and the mean closed times are 0.1 ms (53%), 0.8 ms (41%) and 12.2 ms (6%). B, for GABA alone, the open times were fit with two components, with mean durations of 0.4 ms (79%) and 1.3 ms, and the closed times with three components of 0.2 ms (49%), 1.2 ms (13%) and 4.6 ms. For GABA + 3α5αP, the open times required three components of 0.8 ms (26%), 5.3 ms (68%) and 17.2 ms (6%), and the closed times are 0.2 ms (88%), 1.4 ms (11%) and 7.9 ms (1%). 3α5αP enhances the mean open duration of the mutant receptor by introducing a new, long-lived open state (dashed line) and prolonging the mean duration of the second open time component. 3α5αP also acts on the closed time distribution by decreasing the prevalence of the longest-lived closed time component (dashed line). The inset in the closed time distribution in the presence of 3α5αP shows a magnified view of the longest duration closed time component (boxed in main histogram), to demonstrate the very small component. The mean values for kinetic parameters are shown in Table 2.
Table 2.
Properties of single-channel activity from cells transfected with α1 +β2 +γ2L (WT) or α1(I&L) +β2(TM1) +γ2L subunits (mutant)
| Receptor | GABA | 3α5αP | OT1 | Fr OT1 | OT2 | Fr OT2 | OT3 | Fr OT3 | N |
|---|---|---|---|---|---|---|---|---|---|
| WT | 50 | 0.31 ± 0.14 | 0.20 ± 0.05 | 3.0 ± 0.5 | 0.67 ± 0.06 | 6.3 ± 2.6 | 0.13 ± 0.05 | 7 | |
| WT | 50 | 10 | 0.29 ± 0.13 NS | 0.29 ± 0.05* | 3.0 ± 2.6 NS | 0.31 ± 0.11*** | 16.5 ± 6.2** | 0.40 ± 0.09*** | 4 |
| mutant | 100 | 0.39 ± 0.03 | 0.80 ± 0.07 | 1.2 ± 0.1 | 0.20 ± 0.07 | — | 0 | 4 | |
| mutant | 100 | 10 | 0.53 ± 0.32 NS | 0.24 ± 0.07*** | 5.5 ± 0.5** | 0.61 ± 0.10** | 27.8 ± 10.7 | 0.15 ± 0.08** | 3 |
| Receptor | GABA | AP | CT1 | Fr CT1 | CT2 | Fr CT2 | CT3 | Fr CT3 | N |
|---|---|---|---|---|---|---|---|---|---|
| WT | 50 | 0.19 ± 0.10 | 0.56 ± 0.09 | 1.5 ± 0.6 | 0.15 ± 0.07 | 13.0 ± 3.5 | 0.29 ± 0.06 | 7 | |
| WT | 50 | 10 | 0.12 ± 0.01 NS | 0.55 ± 0.07 NS | 1.0 ± 0.3 NS | 0.35 ± 0.07** | 10.7 ± 3.5 NS | 0.10 ± 0.04*** | 4 |
| mutant | 100 | 0.24 ± 0.05 | 0.50 ± 0.06 | 1.3 ± 0.5 | 0.14 ± 0.02 | 6.2 ± 2.3 | 0.37 ± 0.04 | 4 | |
| mutant | 100 | 10 | 0.18 ± 0.04 NS | 0.81 ± 0.09* | 1.2 ± 0.4 NS | 0.16 ± 0.07 NS | 11.2 ± 6.1 NS | 0.04 ± 0.03*** | 3 |
Single-channel activity in clusters of openings was analysed to determine the numbers of components present, and their mean durations and overall fraction of the total events. Mean values are presented for the mean open time durations (OT1, OT2 and OT3) and the fractions of the total number of openings in each component. The longest duration component (OT3) was not present for activity from the mutant receptor in the absence of 3α5αP, as is also true for receptors including the α1(Q241L) subunit (Akk et al. 2008), but appeared in the presence of 3α5αP. The longest duration closed time component (CT3) was significantly less common in the presence of 3α5αP. (Significance of the difference between control and +3α5αP by two-tailed t test for each receptor type; NS P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001.) We compared the ability of kinetic models to describe the data by comparing the log-likelihoods for the respective fits (Horn, 1987). We fit open time distributions with either 2 or 3 open states, for both control (no 3α5αP) and plus steroid for responses from α1(I&L) +β2(TM1) +γ2L receptors. The differences in log-likelihood values for control recordings were 0, 4, 2 and 1 unit, while for recordings plus steroid they were 10, 11 and 16. For an assumed probability of 0.05 that the increased log-likelihood would arise by chance, the difference in log-likelihood for each added state should be greater than 3. We conclude that a 3 open state model does not provide a significantly better description for control records, but does for recordings in the presence of steroid.
Direct activation by 3α5αP
Although potentiation by 3α5αP was restored, direct activation was not restored by any of the constructs when expressed with the α1(I&L) subunit (Table 1). Direct activation was tested with 10 μm 3α5αP, to use a high concentration but within the aqueous solubility of the steroid. We note that direct gating is quite low in our hands, even for the wild-type receptor, so it is possible that potency, rather than efficacy, is affected. In any case, the absence of direct gating might result from the fact that activation has been proposed to involve bridging of a steroid between two adjacent subunits (Hosie et al. 2006), which might be abrogated by the altered neighbour relationships.
Effects of mutations on other receptor properties
We note that the TM1 region also appears to be important for activation of receptors by GABA. As found earlier (Hosie et al. 2006; Akk et al. 2008), the concentration of GABA required for half-maximal activation (EC50) was significantly increased for receptors comprising α1(I&L) +β2 +γ2L subunits (Table 3). Similarly, transferring the TM1 region to other subunits altered the EC50 (Table 3), as did mutations in the transferred domains. The mean response elicited by 1 mm GABA was reduced for many of the subunit combinations, compared with wild-type subunits (Table 3). This likely reflects a reduction in receptor assembly and transport, although some reduction in maximal probability of being open may occur (Akk et al. 2008).
Table 3.
Effects of mutations on receptor properties
| EC50 for GABA activation (μm) | Response to 1 mm GABA (−60 mV, pA) | |||||
|---|---|---|---|---|---|---|
| Subunits | mean ± SEM (N) | P to wt | P in set | mean ± SEM (N) | P to wt | P in set |
| α1 β2 γ2L | 8 ± 2 (11) | — | — | −1908 ± 205 (22) | — | — |
| α1(I&L) β2 γ2L | 270 ± 47 (6) | *** | — | −976 ± 202 (18) | * | — |
| α1(I&L) β2(TM1) γ2L | 34 ± 8 (4) | NS | * | −2259 ± 395 (21) | NS | ** |
| α1(I&L) β2 γ2L(TM1) | 356 ± 108 (6) | *** | NS | −1371 ± 322 (13) | NS | NS |
| α1(I&L) β2(W237Q) γ2L | 102 ± 20 (6) | NS | NS | −187 ± 40 (7) | ** | NS |
| α1(I&L) β2(I232T&W237Q) γ2L | 140 ± 50 (5) | NS | NS | −252 ± 52 (6) | ** | NS |
| α1(I&L) β2 γ2L(W252Q) | 145 ± 21 (5) | NS | NS | −469 ± 123 (6) | * | NS |
| α1(I&L) β2(TM1 Q237L) γ2L | 116 ± 30 (3) | NS | NS | −396 ± 114 (15) | *** | NS |
| α1(I&L) β2(CTVQ) γ2L | 170 ± 30 (8) | ** | NS | −1607 ± 314 (13) | NS | NS |
| α1(I&L) β2(TM1 W241L) γ2L | 98 ± 21 (5) | NS | NS | −248 ± 72 (11) | *** | NS |
| α1(W245L) β2 γ2L | 63 ± 5 (5) | NS | — | −2019 ± 492 (7) | NS | — |
| α1(W245L) β2(TM1) γ2L | 4 ± 1 (5) | NS | ** | −2235 ± 342 (5) | NS | NS |
| α1(W245L) β2 γ2(TM1) | 46 ± 10 (7) | NS | NS | −2426 ± 197 (7) | NS | NS |
The first column presents the subunits transfected. The subsequent columns show data for activation by GABA: the mean EC50 for fits of data with the Hill equation and the mean response to 1 mm GABA (at or close to the maximal response for all combinations). The results of statistical tests are given as in Table 1.
We examined the correlations between pairs of values, to determine whether there were possible relationships (e.g., a low maximal response might reflect a low maximal probability of being open, which might result in enhanced apparent potentiation). However, there was little correlation between pairs of values. The rank correlation coefficients were not significantly different from 0 for potentiation and EC50, gating and EC50, gating and potentiation, or gating and maximal response (P > 0.12 for each). The lack of correlation of potentiation with EC50 is consistent with the idea that the effects on activation by GABA and on potentiation by 3α5αP are independent of each other.
The regression of rank potentiation on rank maximal response was weakly significant (slope = 0.6, P= 0.03), and of rank EC50 on rank maximal response (slope =−0.6, P= 0.04). That is, constructs that gave small maximal responses tended to have less potentiation and larger EC50 values. The correlation of potentiation with maximal response is of unknown significance, but the nature of the correlation is the opposite of that expected if, for example, small maximal responses resulted from low maximal probability of being open. In addition, we note that the data in Tables 1 and 3 show a group of constructs with both low maximal response and low potentiation. For constructs with maximal currents of 1.5 nA or more, potentiation ranged from none (potentiation ratio = 1.04, maximal response 1.8 nA) to maximal (4.98, 1.8 nA). The correlation of EC50 with maximal response is perhaps more robust, as there is a group of constructs with maximal response <1 nA with EC50 values greater than 97 μm, and a second group with responses >1 nA and EC50 values less than 64 μm. Some of the correlation might be explained by the possibility that our estimate of maximal response (response to 1 mm GABA) was actually submaximal for constructs with the largest EC50 values, but this seems unlikely to account for all of it. However, the significance of the correlation is unclear at present.
Discussion
These findings support the idea that the primary structure of the TM1 region is critical for steroid interaction with the GABAA receptor. It was proposed that the steroid molecule also interacts with two residues in the TM4 region of the α1 subunit (Hosie et al. 2006). Both the β2 and γ2L subunits have identical residues to α1 at the homologous positions, so this transfer does not address the question of additional binding regions.
It can be difficult to disentangle binding from transduction, particularly when the region of interest is close to the gating region of the receptor and the drug (steroid) is likely to affect interactions of the transmembrane helices. However, the observation that potentiation can be transferred among subunits of the GABAA receptor provides circumstantial support to the idea that this domain of the receptor participates in steroid recognition (binding), rather than being solely involved in transduction. If steroids bound elsewhere on the receptor, it seems less likely that the mechanism coupling the binding site to the transduction region would be normally operative when the transduction region was moved to a different subunit. Still, the fact that mutations in the TM1 region affect gating by GABA suggests that this region is involved not just in binding steroids but also in transduction of binding to potentiation. We also note that evolutionarily related subunits might diverge from a common ancestor in such a way that both α and non-α subunits contain binding sites for steroids, but the non-α subunits have lost the transduction mechanism. Distinguishing binding from transduction will require further work. It is clear that the interactions between steroid and receptor are relatively complicated and may involve an extended interface rather than a point-to-point, specific interaction (Akk et al. 2008; Li et al. 2009).
Previous work has indicated that steroid potentiation is mediated by an overall effect on receptor function, rather than a direct effect delimited by an action on a single subunit (Akk et al. 2009; Bracamontes & Steinbach, 2009; Bracamontes et al. 2011). That is, we have been able to produce receptors with wild-type or mutated TM1 regions, and wild-type or mutated GABA-binding sites in all combinations, and have found that the intact sites do not have to occur on the same α subunit to subserve potentiation. The present results extend this observation, by demonstrating that the TM1 region can be moved to subunits other than the α subunit and result in a receptor that shows steroid potentiation.
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number GM47969. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.H.S. is the Russell and Mary Shelden Professor of Anesthesiology.
Glossary
- 3α5αP
(3α, 5α)-3-hydroxypregnan-20-one, allopregnanolone
- P4S
piperidine-4-sulfonic acid
- TM1 region
first membrane-spanning region
Author contributions
Conception and design of the experiments: J.R.B., G.A. and J.H.S.; collection, analysis and interpretation of data: J.R.B., P.L., G.A. and J.H.S.; drafting the article or revising it critically for important intellectual content: J.R.B., P.L., G.A. and J.H.S. All authors approved the final version for publication.
References
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol. 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the GABA-A receptor alpha1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol. 2008;74:614–627. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Steinbach JH. Activation and modulation of concatemeric GABA-A receptors expressed in human embryonic kidney cells. Mol Pharmacol. 2009;75:1400–1411. doi: 10.1124/mol.108.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Activation and block of recombinant GABA(A) receptors by pentobarbitone: a single-channel study. Br J Pharmacol. 2000;130:249–258. doi: 10.1038/sj.bjp.0703335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracamontes J, McCollum M, Esch C, Li P, Ann J, Steinbach JH, Akk G. Occupation of either site for the neurosteroid allopregnanolone potentiates the opening of the GABAA receptor induced from either transmitter binding site. Mol Pharmacol. 2011;80:79–86. doi: 10.1124/mol.111.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracamontes JR, Steinbach JH. Steroid interaction with a single potentiating site is sufficient to modulate GABA-A receptor function. Mol Pharmacol. 2009;75:973–981. doi: 10.1124/mol.108.053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys J. 1987;51:255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABA A receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Jin X, Steinbach JH. A portable site: a binding element for 17{beta}-estradiol can be placed on any subunit of a nicotinic {alpha}4{beta}2 receptor. J Neurosci. 2011;31:5045–5054. doi: 10.1523/JNEUROSCI.4802-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bandyopadhyaya AK, Covey DF, Steinbach JH, Akk G. Hydrogen bonding between the 17beta-substituent of a neurosteroid and the GABA(A) receptor is not obligatory for channel potentiation. Br J Pharmacol. 2009;158:1322–1329. doi: 10.1111/j.1476-5381.2009.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Covey DF, Steinbach JH, Akk G. Dual potentiating and inhibitory actions of a benz[e]indene neurosteroid analog on recombinant alpha1beta2gamma2 GABAA receptors. Mol Pharmacol. 2006;69:2015–2026. doi: 10.1124/mol.106.022590. [DOI] [PubMed] [Google Scholar]
- Ueno S, Zorumski C, Bracamontes J, Steinbach JH. Endogenous subunits can cause ambiguities in the pharmacology of exogenous gamma-aminobutyric acidA receptors expressed in human embryonic kidney 293 cells. Mol Pharmacol. 1996;50:931–938. [PubMed] [Google Scholar]


