Abstract
Glycine receptors (GlyRs) are found in most areas of the brain, and their dysfunction can cause severe neurological disorders. While traditionally thought of as inhibitory receptors, presynaptic-acting GlyRs (preGlyRs) can also facilitate glutamate release under certain circumstances, although the underlying molecular mechanisms are unknown. In the current study, we sought to better understand the role of GlyRs in the facilitation of excitatory neurotransmitter release in mouse visual cortex. Using whole-cell recordings, we found that preGlyRs facilitate glutamate release in developing, but not adult, visual cortex. The glycinergic enhancement of neurotransmitter release in early development depends on the high intracellular to extracellular Cl− gradient maintained by the Na+–K+–2Cl− cotransporter and requires Ca2+ entry through voltage-gated Ca2+ channels. The glycine transporter 1, localized to glial cells, regulates extracellular glycine concentration and the activation of these preGlyRs. Our findings demonstrate a developmentally regulated mechanism for controlling excitatory neurotransmitter release in the neocortex.
Key points
Cortical glycine receptors (GlyRs) flux chloride based on a local chloride gradient set by chloride transporters; this gradient changes with age.
In acute slices from the developing mouse visual cortex, tonic GlyR activity increases spontaneous excitatory neurotransmitter release in a calcium-dependent manner.
Glycine transporters, localized mainly to astrocytes, regulate this tonic activity.
After a critical period of early development, GlyRs are no longer tonically active and become hyperpolarizing, inhibiting spontaneous neurotransmitter release.
These results define mechanisms that contribute to baseline neurotransmission during critical periods of neuronal development, and help identify synaptic functions that could be impacted by GlyR dysfunction.
Introduction
Glycine receptors (GlyRs) are found throughout the CNS. They are localized to pre- and postsynaptic membranes (Dahan et al. 2003; Danglot et al. 2004), where they play a critical role in both inhibitory and excitatory neurotransmission. GlyR function is controlled by both subunit composition and local glycine concentrations (Schmieden et al. 1992; Yoon et al. 1998). The importance of glycinergic transmission is underscored by findings that disruptions in glycine homeostasis, such as hyperglycinemia, contribute to severe neurological disorders that manifest during early development (Singer et al. 1989; Steiner et al. 1996; Hoover-Fong et al. 2004). Despite this critical role, the developmental mechanisms that regulate glycinergic functions are poorly understood.
GlyRs primarily flux Cl−; thus, whether these receptors are depolarizing or hyperpolarizing depends on the local Cl− gradient (Wang & Xu, 2006). Two major Cl− transporters help to establish this gradient; the Na+–K+–Cl− cotransporter 1 (NKCC1) transports Cl− into the cell, whereas the K+–Cl− cotransporter 2 (KCC2) pumps Cl− out of the cell (Payne et al. 2003). During early development, NKCC1 expression is high and KCC2 expression is low, creating a high intracellular ([Cl−]i) to extracellular ([Cl−]o) Cl− concentration gradient. A developmental increase in the ratio of KCC2 to NKCC1 causes an increase in the relative [Cl−]o (Payne et al. 2003). Consequently, GlyRs are generally depolarizing during early development and become progressively more hyperpolarizing with maturation, as dictated by the Cl− gradient (Turecek & Trussell, 2001; Ye et al. 2004; Song et al. 2006; Lee et al. 2009).
GlyRs can act presynaptically to modulate release of neurotransmitters in several systems (Turecek & Trussell, 2001; Jeong et al. 2003; Kawa, 2003; Kubota et al. 2010; Waseem & Fedorovich, 2010). The developmental regulation of these presynaptic-acting GlyRs (preGlyRs) suggests that by modulating neurotransmitter release they might play an important role in establishing synaptic function during formative periods of cortical development. The primary visual cortex provides an attractive model to study postnatal development, but the contribution of preGlyRs to the maintenance of basal neurotransmitter release has not been addressed.
In the present study we took advantage of the ability of GlyRs to regulate spontaneous, action potential-independent neurotransmitter release (Turecek & Trussell, 2001; Jeong et al. 2003; Kawa, 2003; Lee et al. 2009; Kubota et al. 2010; Waseem & Fedorovich, 2010) to investigate mechanisms underlying preGlyR function. Our data indicate that preGlyRs are tonically activated by glycine, most likely from astrocytes, and that these preGlyRs activate voltage-gated calcium channels (VGCCs) to enhance neurotransmitter release during visual cortex development.
Methods
Ethical approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Subjects
C57BLJ/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and bred in-house. Mice (n= 163) were examined between postnatal day (P)13 and P40. Mice were maintained on a 12:12 h light:dark cycle and fed ad libitum.
Brain slice preparation
Slices of visual cortex were prepared from mice using standard methods (Corlew et al. 2007; Yashiro et al. 2009). Briefly, mice were anaesthetized with pentobarbital sodium (40 mg kg−1, i.p.) and decapitated upon disappearance of the corneal reflex. Brains were rapidly removed and immersed in oxygenated ice-cold dissection buffer (in mm: NaCl, 87; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; sucrose, 75; dextrose, 10; ascorbic acid, 1.3; MgCl2, 7; CaCl2, 0.5). The visual cortex was cut in 300 μm coronal slices using a vibrating microtome (Leica VT1200S, Buffalo Grove, IL, USA). Slices were allowed to recover for 30 min in a submersion chamber at 35°C filled with standard artificial cerebrospinal fluid (ACSF; in mm: NaCl, 124; KCl, 3; Na2PO4, 1.25; NaHCO3, 26; MgCl2, 1; CaCl2, 2; dextrose, 20; saturated with 95% O2, 5% CO2; ∼315 mosmol l−1 and pH ∼7.25), and were then kept at room temperature for a minimum of 30 min. Visual cortex slices were transferred to a submersion-recording chamber and continuously perfused at 2 ml min−1 with standard ACSF warmed to 30°C.
Voltage-clamp recordings
Patch pipettes (3–5 MΩ tip resistance) were filled with an internal solution containing (in mm): KCl, 20; potassium gluconate, 100; Hepes, 10; Mg-ATP, 4; Na-GTP, 0.3; sodium phosphocreatine, 10; 0.01% Alexa 488 (wt/vol), with the pH adjusted to 7.25 and osmolarity adjusted to ∼295 mosmol l−1. 4,4′-Diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS; 1 mm) was included in the internal solution, to block postsynaptic Cl− channels (Albertson et al. 1996; Hollrigel et al. 1998; Leewanich et al. 1998). Cells were voltage-clamped in the whole-cell configuration using a Multiclamp 700B amplifier. Data were digitized using a Digidata 1440A (Molecular Devices, Sunnyvale, CA, USA), and acquired and analysed using pCLAMP 10.2 software (Molecular Devices). Only cells that had confirmed pyramidal morphology with Rseries < 30 MΩ and exhibiting <20% change in Rinput, Rseries and Iholding for the duration of the experiment were included in the analysis.
AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) were recorded at −70 mV in the presence of tetrodotoxin (TTX; 200 nm) and bicuculline methiodide (BMI; 30 μm) to block voltage-gated sodium channels and GABAA receptors, respectively. mEPSC amplitude and frequency were measured before, during and after drug bath application. Events were identified and detected by their rapid rise time (<3 ms) and characteristic shape using an automatic template detection program (pCLAMP; Molecular Devices; Clements & Bekkers, 1997). All events were manually verified; only events with a monotonic rise time and exponential decay were included in the analysis. Normalized values for frequency and amplitudes were used for mEPSC data analysis. An average of ∼1300 events was analysed for each neuron.
Data collection and statistics
Experiments were conducted in a manner that roughly interleaved experimental and control conditions, and were analysed blinded to experimental condition. Two-tailed paired or unpaired Student's t tests were employed where appropriate; for comparison of multiple groups, we used a one-way ANOVA. For all tests, the level of significance was P < 0.05.
Electron microscopy
Material was from three P17 mice, perfusion-fixed with 4% depolymerized paraformaldehyde; in one mouse 0.1% glutaraldehyde was added to the fixative. Following perfusion, brains were removed and stored in phosphate-buffered saline (pH 7.4); in two mice, the brain was first postfixed in 4% depolymerized paraformaldehyde overnight. Fifty micrometer coronal sections through the visual cortex were cut with a Vibratome and stored in phosphate buffer at 4°C. After pretreatment for 10 min in 3% hydrogen peroxide (to inactivate endogenous peroxidase activity) and then 10% normal donkey serum (to block non-specific binding), sections were incubated on a shaker overnight in primary polyclonal anti-glycine transporter 1 (GlyT1) antiserum, raised in goat (1:2000; Millipore, AB1770; Billerica, MA, USA). After rinsing and additional blocking with 2% normal donkey serum, sections were incubated for 1 h in biotinylated anti-goat IgG secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA), then for 1 h in ExtraAvidin (Sigma, St Louis, MO, USA), 1:5000 dilution. After visualization of antibody binding sites with a nickel-enhanced diaminobenzidine (DAB) reaction, sections in phosphate buffer were treated for 40 min in 0.5% OsO4, rinsed in 0.1 m maleate buffer, pH 6.0, stained en bloc with 1% uranyl acetate for 45 min, then dehydrated in graded ethanols and infiltrated in plastic (Spurr epoxy resin, Electron Microscopy Sciences, Hatfield, PA, USA). Sections were flat-embedded between two sheets of ACLAR plastic sandwiched by glass slides and heat-polymerized for 48 h at 60°C. Chips of embedded tissue were cut out from the cured wafer and glued to epoxy blanks. Thin sections (∼60 nm) were cut on an ultramicrotome, collected on copper mesh grids, post-stained with uranyl acetate and Sato's lead, and examined on a Tecnai 12 transmission electron microscope. Images were collected with a 12 bit Gatan 1024 × 1024 cooled CCD camera. Brightness and contrast of final images were adjusted using Adobe Photoshop.
Chemicals
Drugs were made as 100×–1000× stock solutions in water or DMSO, and diluted immediately before use in ACSF. All drugs were obtained from Sigma, except: TTX, BMI and d,l-2-amino-5-phosphonopentanoic acid (d,l-APV) were obtained from Ascent Scientific (Princeton, NJ, USA); N-[3-(4′-fluorophenyl)-3-(4′phenylphenoxy)propyl] sarcosine (NFPS) and sarcosine were obtained from Tocris (Ellisville, MO, USA); and cyclopiazonic acid (CPA) was obtained from Calbiochem (La Jolla, CA, USA).
Results
GlyR activity enhances excitatory neurotransmitter release in developing visual cortex
Changes in mEPSC frequency, but not amplitude, by GlyR agonists and antagonists have been used as a readout of preGlyR function (Jeong et al. 2003; Kawa, 2003; Lee et al. 2009). We therefore prepared visual cortex slices from P13–18 mice, and examined the effects of GlyR agonists and antagonists on mEPSC frequency and amplitude in layer (L)2/3 pyramidal neurons. We included 1 mm DIDS in the internal recording solution to block postsynaptic GlyR function (30 ± 8% of baseline-evoked glycine current, n= 13, P < 0.001; paired Student's t test; Inoue, 1985; Parker et al. 1988; Song et al. 2006).
Glycine transiently increased the frequency of mEPSCs in neurons in a dose-dependent manner (Fig. 1A and B; 100 nm: 98 ± 6% of baseline, P > 0.05; 1 μm: 121 ± 5% of baseline, P < 0.05; 1 mm: 142 ± 7% of baseline, P < 0.001; ANOVA). We wanted to use the lowest concentration that reliably gave us an increase in mEPSC frequency; therefore, we used 1 μm glycine for further experiments. mEPSC amplitude was unchanged with glycine application (Fig. 1C), further supporting that these effects are not postsynaptic in origin. These data demonstrate that glycine can promote action potential-independent neurotransmitter release. We next wanted to determine if glycine could enhance action potential-driven neurotransmitter release. We thus tested the effect of glycine (1 μm) on synaptic responses recorded in L2/3 and evoked by L4 stimulation. Under these conditions, we failed to see an effect of glycine on evoked EPSC amplitudes (Fig. 2A and B). We also found that glycine failed to alter the paired-pulse ratio (PPR), a gauge of neurotransmitter release (Dobrunz & Stevens, 1997), at any stimulus interval tested (Fig. 2C). Together, these findings indicate that glycine is depolarizing at P13–18 and can cause a large increase in spontaneous, action potential-independent transmitter release, but under our recording conditions we were not able to detect a role for GlyRs in action potential-driven neurotransmitter release.
Figure 1. Glycine promotes neurotransmitter release in developing V1.
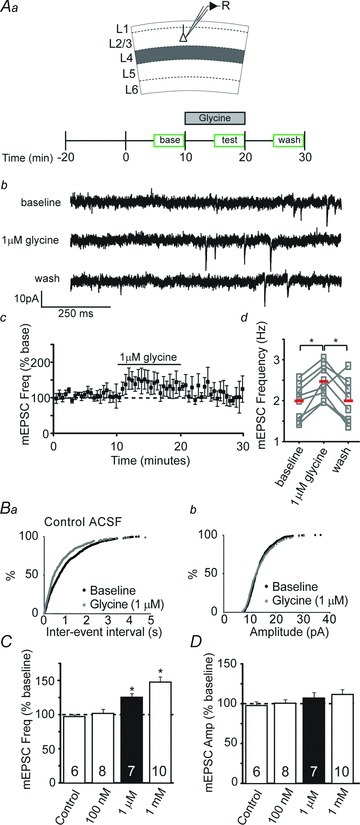
Aa, schematic of recording and experimental design. Glycine was acutely applied after a 10 min baseline period and then washed out after an additional 10 min. Ab, example whole-cell recording from a L2/3 pyramidal cell depicting miniature excitatory postsynaptic currents (mEPSCs) recorded at baseline, during bath application of 1 μm glycine, and after washout of glycine in a visual cortex slice from a P15 mouse. Ac, group data show the time course over which glycine reversibly enhanced mEPSC frequency. A4, scatterplot demonstrating that glycine reversibly enhances mEPSC frequency in 7 individual L2/3 pyramidal neurons (boxes). Red lines represent averages. B, cumulative probability distributions of mEPSC frequency (Ba) and amplitude (Bb) following glycine application (1 μm). Glycine shortened inter-event interval, with no obvious effects on amplitude. C, averaged normalized mEPSC frequency to illustrate acute effects of various glycine concentrations (100 nm–1 mm), expressed as a percentage of baseline. Asterisks denote bars significantly different from control (P < 0.05). D, averaged normalized data illustrate that glycine did not significantly alter mEPSC amplitude. Sample sizes in C and D are given within the bars. The mEPSC frequencies and amplitudes were normalized to the averaged baseline levels before acute drug application. ACSF, artificial cerebrospinal fluid.
Figure 2. Exogenous glycine does not detectably alter evoked excitatory transmission in the developing visual cortex.
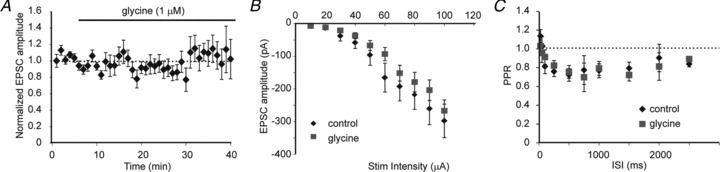
A, averaged data showing the effects of glycine (1 μm) on the amplitude of excitatory postsynaptic currents (EPSCs) evoked by L4 stimulation and recorded in L2/3 pyramidal neurons in the presence of BMI (30 μm) and d,l-APV (200 nm; n= 10, P > 0.05; paired t test). B, averaged data showing the input–output relationship of evoked L4 to L2/3 synaptic currents recorded in the presence or absence of glycine (1 μm; n= 9 for each group, P > 0.05; RMANOVA). C, averaged paired-pulse ratio (PPR) of evoked L2/3 EPSCs recorded in the presence or absence of glycine (1 μm; n= 11 for each group, P > 0.05; RMANOVA). ISI, inter-stimulus interval.
To test if GlyRs tonically regulate spontaneous, action potential-independent neurotransmitter release onto L2/3 pyramidal neurons, we recorded the effects of bath application of the GlyR antagonist strychnine on mEPSC frequency and amplitude. Strychnine (3 μm) significantly reduced mEPSC frequency (Fig. 3B; 81 ± 4% of baseline, P < 0.01; t test) without changing mEPSC amplitude (Fig. 3C; 99 ± 3% of baseline, P > 0.6). This suggests that glycine at ambient levels promotes GlyR-mediated spontaneous neurotransmitter release.
Figure 3. Strychnine-sensitive GlyRs promote neurotransmitter release.
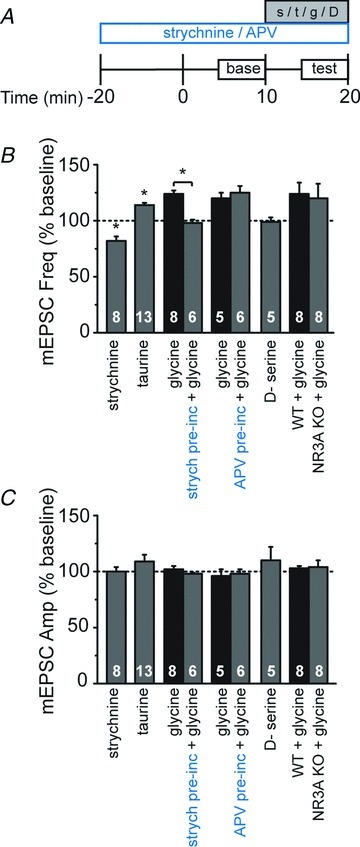
A, schematic of experimental design. Pre-incubation of strychnine and d,l-2-amino-5-phosphonopentanoic acid (d,l-APV; indicated in blue) was initiated 20 min prior to the start of whole-cell recordings and maintained for the duration of the recording, while acute drugs (strychnine, s; taurine, t; glycine, g; or d-serine, D) were applied after a 10 min baseline period and then washed out after an additional 10 min. B, averaged data of normalized miniature excitatory postsynaptic current (mEPSC) frequency demonstrates acute effects of indicated drugs as a percentage of baseline. Asterisks denote significance (P < 0.05). C, averaged normalized data demonstrate that the pharmacological manipulations did not significantly alter mEPSC amplitude. Sample sizes in B and C are given within the bars. The mEPSC frequencies and amplitudes were normalized to the averaged baseline levels before acute drug application.
At the concentration used here, strychnine has a high specificity for blocking GlyRs (Okabe et al. 2004). However, glycine can also influence neurotransmitter release indirectly, by binding to presynaptic NMDA receptors (NMDARs; Johnson & Ascher, 1987), which can promote neurotransmitter release at these young ages (Woodhall et al. 2001; Corlew et al. 2007; Brasier & Feldman, 2008). We therefore tested the effects of 100 μm taurine, which has been reported to exhibit high specificity for GlyRs when used at this concentration (Yu et al. 2008). Taurine significantly increased mEPSC frequency (Fig. 3B; 115 ± 3% of baseline, P < 0.01; t test) with only modest and non-significant effects on amplitude (Fig. 3C; 107 ± 5% of baseline, P > 0.1). In a subset of experiments, we included d,l-APV (100 μm), an NMDAR-selective antagonist, in the extracellular solution to prevent any possible taurine activation of NMDARs, and saw similar results (frequency: 114 ± 2% of baseline; amplitude: 109 ± 6% of baseline).
Strychnine, but not d,l-APV, pre-incubation occluded the glycine-mediated increase in mEPSC frequency (Fig. 3B; strychnine; 98 ± 3% of baseline vs. control 124 ± 3% of baseline, P < 0.02: d,l-APV; 125 ± 6% of baseline vs. control 120 ± 5% of baseline, P > 0.05; unpaired t tests), further supporting that strychnine-sensitive GlyR activation, not NMDAR activation, underlies glycine-induced increases in neurotransmitter release. As additional evidence, we bath-applied the agonist d-serine (50 μm), which binds with high affinity to glycine sites on NMDARs, but not GlyRs. d-Serine had no effect on mEPSC frequency (Fig. 3B; 99 ± 4% of baseline, P > 0.5). Thus, multiple pharmacological manipulations indicated that glycine promotes spontaneous neurotransmitter release in developing visual cortex via GlyRs, and not via NMDARs.
It remains possible that the glycine-mediated effects on neurotransmitter release occur through a novel class of excitatory GlyRs composed of the NMDAR subunits NR1 and NR3A, as these diheteromers act as GlyRs and show little block by classic NMDAR antagonists, including d,l-APV (Das et al. 1998; Chatterton et al. 2002). To test whether NR1/NR3A diheteromers might underlie the glycine-mediated effects on neurotransmitter release, we applied glycine to visual cortical slices from NR3A knock-out mice (Das et al. 1998) bathed in d,l-APV. Similar to recordings in wild-type mice, L2/3 neurons in NR3A knock-out mice exhibited a glycine-induced enhancement of mEPSC frequency (Fig. 3B; 120 ± 13% of baseline, compared with 124 ± 9% of baseline in wild-type mice, P > 0.9), without affecting mEPSC amplitude (Fig. 3C; 104 ± 6% of baseline, compared with 102 ± 2% of baseline in wild-type mice, P > 0.2). We conclude that glycine promotes neurotransmitter release in the developing cortex by activating strychnine-sensitive GlyRs, rather than NMDARs or NR1/NR3A-containing excitatory GlyRs.
These experiments, which show that multiple glycine agonists/antagonists alter mEPSC frequency in the presence of postsynaptic GlyR blockade but do not alter mEPSC amplitude, strongly suggest that the effects are presynaptic. Taken together, our data indicate that preGlyRs tonically facilitate excitatory, spontaneous, action potential-independent transmission in developing mouse visual cortex.
PreGlyRs enhance neurotransmitter release via heteromeric GlyRs and a depolarizing Cl− gradient
The gradient of [Cl−]i relative to [Cl−]o determines whether GlyRs are depolarizing or hyperpolarizing (Rivera et al. 1999; Stein & Nicoll, 2003). In early development, high expression of NKCC1 maintains a high [Cl−]i, which favours depolarizing GlyR currents. NKCC1 expression decreases and KCC2 expression increases during development, reducing the [Cl−]i/[Cl−]o ratio, thus favouring hyperpolarizing currents (Wang et al. 2002). To establish the role of Cl− transport in glycine modulation of neurotransmitter release during the visual cortex critical period, we tested the effects of the selective NKCC1 inhibitor bumetanide (10 μm; Haas, 1989) on spontaneous neurotransmission. Bumetanide pre-incubation occluded the increase in neurotransmitter release caused by glycine application (Fig. 4C; 102 ± 6% of baseline compared with control 128 ± 8% of baseline, P < 0.01; t test) without affecting mEPSC amplitude (Fig. 4D; bumetanide + glycine, 103 ± 2% of baseline compared with control 105 ± 6% of baseline, P > 0.6). Together these results suggest that preGlyRs are depolarizing in early development, at least in part due to the high Cl− gradient established by NKCC1.
Figure 4. Heteromeric GlyRs require a favourable Cl− gradient to promote neurotransmitter release.
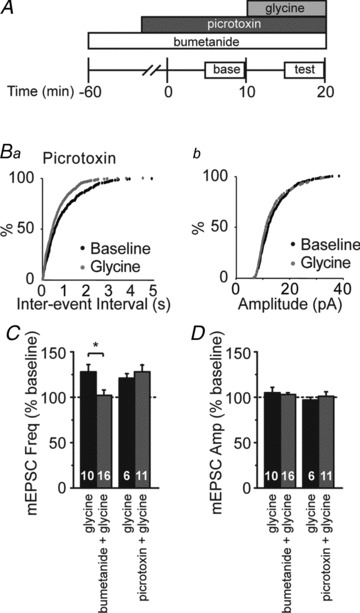
A, schematic of experimental design. Antagonists were applied 20 min (picrotoxin) or 60 min (bumetanide) prior to beginning whole-cell recordings. After a 10 min baseline period, glycine was applied for 10 min. B, cumulative probability distributions of miniature excitatory postsynaptic current (mEPSC) frequency (Ba) and amplitude (Bb) following glycine application (1 μm). Glycine shortened inter-event interval, but not amplitude, in the presence of picrotoxin. C, averaged normalized mEPSC frequency demonstrating that pre-incubation with bumetanide (10 μm) blocked facilitation by glycine (1 μm), whereas pre-incubation with picrotoxin (50 μm) had little effect on glycine-induced increase in mEPSC frequency. Asterisks denote significance (P < 0.05). D, averaged normalized data demonstrate that no significant changes in mEPSC amplitude were observed with any of the pharmacological manipulations. Sample sizes in C and D are given within the bars. The mEPSC frequencies and amplitudes were normalized to the averaged baseline levels before acute drug application.
A developmental switch has been reported in the subunit composition of GlyRs, from homomeric receptors containing only α subunits, to heteromeric receptors containing both α and β subunits (Aguayo et al. 2004). This subunit switch might alter the role of GlyRs in regulating spontaneous neurotransmitter release. To examine this possibility, we pre-incubated slices with picrotoxin (50 μm), which at this dosage blocks homomeric but not heteromeric GlyRs (Yoon et al. 1998), finding that picrotoxin failed to prevent the glycine-mediated increase in mEPSC frequency (Fig. 4C; 126 ± 8% of baseline compared with control 121 ± 5% of baseline, P > 0.3; t test). We saw similar results when we added d,l-APV to the picrotoxin in a subset of interleaved experiments (131 ± 8% of baseline, P > 0.1). These data suggest that the glycine-responsive receptors are heteromeric GlyRs containing both α and β subunits.
PreGlyR-mediated enhancement of neurotransmitter release requires extracellular Ca2+
Excitatory neurotransmitter release generally relies on increases in presynaptic intracellular Ca2+ levels (Adam-Vizi, 1992; Sudhof, 2008). GlyR activity has been linked to Ca2+ signaling in immature neurons of the spinal cord and neocortex (Flint et al. 1998; Kirsch & Betz, 1998). To establish a role for Ca2+ in preGlyR facilitation, we examined the effects of glycine in nominally ‘Ca2+-free’ ACSF with the addition of the calcium chelator EGTA (3 mm;‘zero Ca2+’). The loss of external Ca2+ greatly reduced mEPSC frequency prior to application of glycine (33 ± 10% of baseline, P < 0.004; paired t test). Under this zero Ca2+ condition, subsequent application of glycine had no effect on neurotransmitter release, as measured by mEPSC frequency (Fig. 5C; 101 ± 7% of baseline, P > 0.5). This suggests that preGlyRs facilitate glutamate release at these ages via a calcium-dependent increase in neurotransmitter release.
Figure 5. Glycine-dependent neurotransmitter release requires Ca2+ entry through VGCCs.
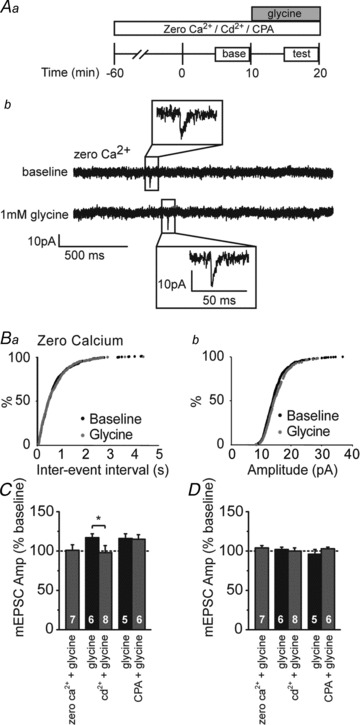
Aa, schematic of experimental design. Antagonists (Cd2+ or cyclopiazonic acid (CPA)) or a nominally Ca2+-free bath solution (zero Ca2+) were applied 60 min prior to the start of whole-cell recordings. After a 10 min baseline period, glycine was applied for 10 min. Ab, example whole-cell recording from a L2/3 pyramidal cell bathed in zero Ca2+ ACSF depicting miniature excitatory postsynaptic currents (mEPSCs) recorded at baseline and during bath application of 1 μm glycine. B, cumulative probability distributions of mEPSC frequency (Ba) and amplitude (Bb) following glycine application (1 μm); glycine had no effect in zero Ca2+ ACSF. C, averaged normalized mEPSC frequency demonstrating pre-incubation in zero Ca2+ ACSF containing the calcium chelator EGTA (3 mm) prevents glycine facilitation. Inclusion of Cd2+ (200 μm) in the ACSF also prevents glycine facilitation of mEPSC frequency. CPA (30 μm) incubation has no effect on glycine enhancement of mEPSC frequency. Asterisk denotes significance (P < 0.05). D, averaged normalized mEPSC amplitude; no significant changes were observed with any of the pharmacological manipulations. Sample sizes in C and D are given within the bars. The mEPSC frequencies and amplitudes were normalized to the averaged baseline levels before glycine application.
To test whether preGlyR activation enhances neurotransmitter release by activating VGCCs, we examined the effects of the non-selective VGCC inhibitor Cd2+ (200 μm) on glycine-induced changes in neurotransmitter release. Pre-incubation with Cd2+ prevented glycine facilitation of transmitter release (Fig. 5C; 98 ± 9% of baseline compared with control 117 ± 5% of baseline, P < 0.01; unpaired t test), suggesting that GlyR-mediated depolarization enhances neurotransmitter release by activating VGCCs. Calcium influx can trigger neurotransmitter release either by directly activating vesicular release machinery or by triggering the release of Ca2+ from intracellular stores (calcium-induced calcium release; Galante & Marty, 2003; Neher & Sakaba, 2008). To examine the latter possibility, we tested the effects of glycine on neurotransmitter release after depleting intracellular Ca2+ stores by bath-applying CPA (30 μm), a Ca2+ store ATPase inhibitor (Seidler et al. 1989; Emptage et al. 2001). Pre-incubation with CPA did not prevent glycine-mediated increases in mEPSC frequency (Fig. 5C; 115 ± 6% of baseline compared with control 116 ± 6% of baseline, P > 0.2). These data suggest that, by depolarizing presynaptic terminals, GlyRs activate VGCCs, and the ensuing Ca2+ entry directly enhances spontaneous glutamatergic release.
GlyT1 activity supports tonic preGlyR activity
Extracellular glycine levels are tightly regulated by the glycine transporters GlyT1 and GlyT2, which are predominantly expressed in astrocytes and neurons, respectively (Zafra et al. 1995a,b; Aragon & Lopez-Corcuera, 2003; Eulenburg et al. 2005; although see Raiteri & Raiteri, 2010). To determine whether glycine transporters maintain extracellular glycine levels sufficient for tonic enhancement of neurotransmitter release, we used transporter-selective inhibitors. GlyT1 is blocked by sarcosine (500 μm) or NFPS (100 nm), while GlyT2 can be blocked by Alx 1393 (200 nm; Xu et al. 2005; Harsing et al. 2006). We pre-incubated slices in GlyT inhibitors for a minimum of 1 h, and tested whether these manipulations impaired tonic preGlyR activity by measuring the effects of the GlyR antagonist strychnine on mEPSC frequency and amplitude during continuous application of sarcosine, NFPS or Alx 1393. Blockade of GlyT1, but not GlyT2, prevented reductions in mEPSC frequency by strychnine (Fig. 6C; sarcosine, 103 ± 7% of baseline; NFPS, 97 ± 3% of baseline; Alx 1393, 82 ± 7% of baseline, P < 0.02; ANOVA), suggesting that GlyT1 activity is necessary to support tonic preGlyR-mediated neurotransmitter release. None of these manipulations altered mEPSC amplitude (Fig. 6D; P > 0.05; ANOVA).
Figure 6. The glial GlyT1 regulates extracellular glycine levels and glycine-dependent neurotransmitter release.
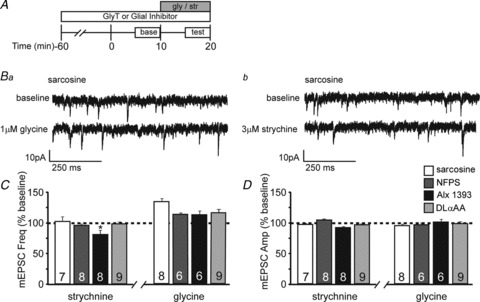
A, schematic of experimental design to test whether pre-incubation with GlyT antagonists or a gliotoxin blocks effects of glycine and strychnine. Antagonists/toxin were applied 60 min prior to the start of recordings. After a 10 min baseline period, glycine (gly) or strychnine (str) was applied for 10 min. B, example whole-cell recordings from L2/3 pyramidal cell bathed in sarcosine depicting miniature excitatory postsynaptic currents (mEPSCs) recorded at baseline and during bath application of 1 μm glycine (Ba) or 3 μm strychnine (Bb). C, averaged normalized mEPSC frequency demonstrates that pre-incubation with sarcosine, N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl] sarcosine (NFPS) or d,l-α-aminoadipic acid (d,l-αAA), but not the GlyT2-specific antagonist Alx 1393, prevents the strychnine-induced reduction in mEPSC frequency. Pre-incubation with sarcosine, NFPS, Alx 1393 or d,l-αAA had no effect on glycine-dependent facilitation. D, averaged normalized mEPSC amplitude; no significant changes were observed with any of the pharmacological manipulations. The mEPSC frequencies and amplitudes in C and D were normalized to the averaged baseline levels before glycine or strychnine application.
Because most GlyT1s are found on glia (Zafra et al. 1995a,b), we wanted to explore the possible involvement of glia in supporting glycine-mediated neurotransmitter release. To do this, we tested the effects of the astrocytic toxin d,l-α-aminoadipic acid (d,l-αAA; 200 μm; Casper & Reif-Lehrer, 1983; Huck et al. 1984; Khurgel et al. 1996) on strychnine-sensitive spontaneous activity. d,l-αAA pre-incubation (>1 h) prevented strychnine-induced changes in spontaneous activity (Fig. 6C; 99 ± 3% of baseline, P > 0.05; ANOVA). Taken together, these data suggest that GlyT1, apparently associated with glial cells, regulates extracellular glycine levels and thereby facilitates excitatory spontaneous, action potential-independent neurotransmitter release in the developing cortex.
To confirm that these manipulations did not affect strychnine-sensitive neurotransmitter release by directly altering GlyRs, we tested the effects of glycine following pre-incubation with sarcosine, NFPS, Alx 1393 or d,l-αAA. Glycine increased mEPSC frequency to a similar degree even in the presence of GlyT1 and GlyT2 inhibitors (Fig. 6C; sarcosine, 132 ± 5% of baseline; NFPS, 114 ± 3% of baseline; Alx 1393, 117 ± 6% of baseline, P > 0.05; ANOVA), without affecting amplitude (Fig. 6D; P > 0.05; ANOVA). Glycine also enhanced mEPSC frequency to a similar degree in the presence of d,l-αAA (Fig. 6C; d,l-αAA, 119 ± 5% of baseline, P > 0.05; ANOVA), suggesting that preGlyRs can function despite disruption of GlyT or astrocytic function. Thus, multiple pharmacological manipulations support a role for GlyT1, likely on astrocytes, for maintaining tonic glycine-mediated spontaneous neurotransmitter release.
To provide direct evidence regarding the distribution of GlyT1 in the visual cortex, we performed electron microscopy with specific antibodies to determine its subcellular localization. Because GlyT1 staining was incompatible with classical glutaraldehyde fixation, the ultrastructure was unavoidably compromised, but remained interpretable. To minimize difficulties associated with antibody penetration, attention was focused on a zone ∼1–2 μm beneath the section surface that displayed highly selective immunostaining. DAB reaction product was predominantly in fine processes within the neuropil. GlyT1 staining was predominantly astrocytic, though occasional thin dendritic processes were also stained. These immunopositive astrocytic profiles were commonly situated in close association with axospinous synapses (Fig. 7A–C).
Figure 7. GlyT1 are localized primarily on astrocytic processes.
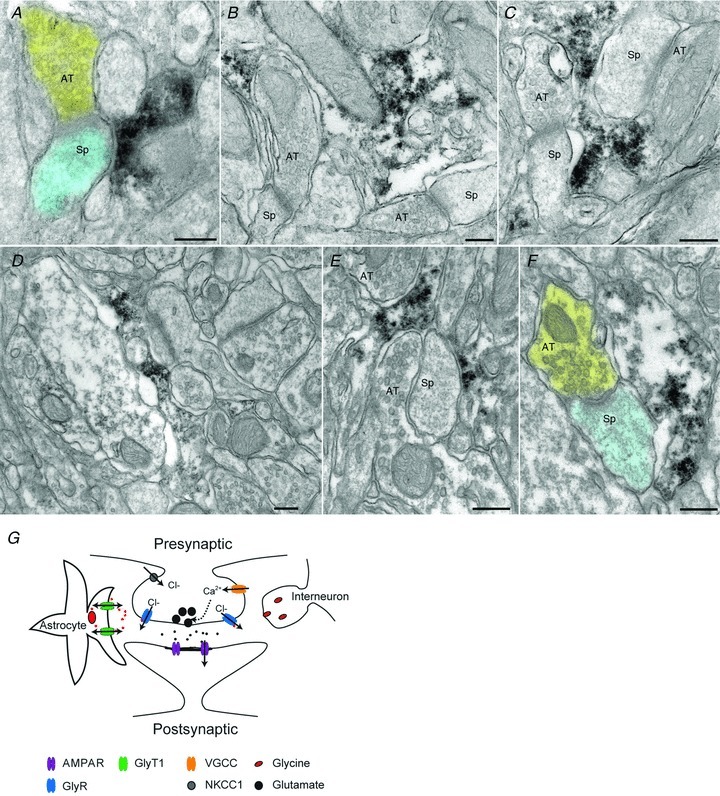
A, axon terminal (shaded yellow) makes synaptic contact with a dendritic spine (shaded blue). Electron-dense DAB reaction product coding for GlyT1 is visible within an astrocytic profile lying adjacent to the spine. B, an irregular astrocytic profile immunopositive for GlyT1 lies adjacent to two asymmetric synapses in the same electron microscopic field. The astrocytic cytoplasm has been disrupted by the immune processing and the relatively weak fixation necessary to preserve antigenicity. C, electron-dense reaction product in astrocytic profile lies between two axodendritic synapses. The small lightly-immunostained profile on the bottom left is likely also to be astrocytic. D, low-magnification montage shows immunostained astrocytic profile in a field of neuropil. E, axon terminal and spine are enveloped by immunostained astrocytic processes. F, axospinous synapse lies adjacent to immunopositive astrocytic process; false-colour as in A. G, illustration depicting the mechanism of preGlyR facilitation of neurotransmitter release. Abbreviations: AMPAR, AMPA receptor; AT, axon terminal; GlyR, glycine receptor; GlyT, glycine transporter; NKCC1, Na+–K+–2Cl− cotransporter; Sp, spine; VGCC, voltage-gated calcium channel. Scale bars: 200 nm.
PreGlyRs fail to enhance excitatory neurotransmitter release after the critical period
Previous studies have found that chloride-fluxing channels can shift from depolarizing to hyperpolarizing, although there are regional differences in the timing of this shift (Ben-Ari et al. 1994; Chen et al. 1996; Backus et al. 1998; Tapia & Aguayo, 1998; Wang et al. 2005). Therefore, we tested whether preGlyRs remain depolarizing and tonically active in the visual cortex after the end of the critical period. The data presented above were from P13–18 mice. In contrast, glycine in more mature mice (P30–40) no longer increased mEPSC frequency, but instead significantly reduced it (Fig. 8D; 79 ± 6% of baseline, P < 0.003; paired t test). We conclude that the effect of glycine on neurotransmitter release switches during development, sometime between P18 and P30 under our recording conditions. We also asked whether preGlyRs were tonically active at P30–40, as was observed in younger mice. Strychnine application had no effect on neurotransmitter release (Fig. 8D; 99 ± 2% of baseline, P > 0.7), indicating that the tonic activation of GlyRs was also lost with age.
Figure 8. Strychnine-sensitive GlyRs decrease neurotransmitter release but lack tonic effects in older mice.
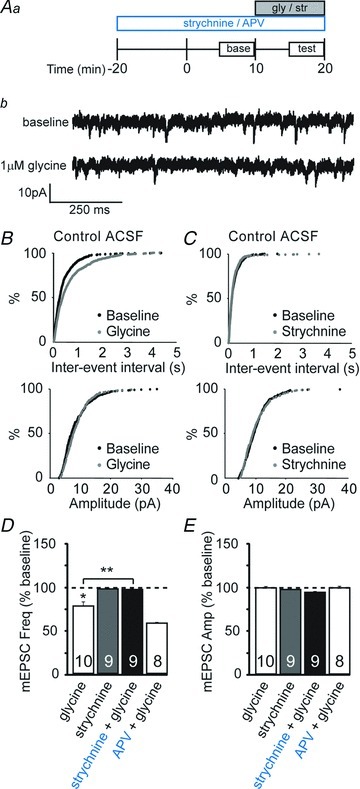
Aa, schematic diagram of experimental design. Antagonists (strychnine or d,l-2-amino-5-phosphonopentanoic acid (d,l-APV)) were applied 20 min prior to the start of recordings. After a 10 min baseline period, glycine (gly) or strychnine (str) was applied for 10 min. Ab, example whole-cell recording from a L2/3 pyramidal cell depicting miniature excitatory postsynaptic currents (mEPSCs) recorded at baseline and during bath application of 1 μm glycine in a visual cortex slice from a P32 mouse. B, cumulative probability distributions show effect of glycine (1 μm) on mEPSC frequency, but not amplitude. C, cumulative probability distributions show no effect of strychnine (3 μm) on mEPSC frequency or amplitude. D, averaged normalized data from P28–35 mice demonstrating that glycine (1 μm) reduced mEPSC frequency; application of strychnine (3 μm) had no effect on mEPSC frequency at this age. Strychnine (3 μm) but not d,l-APV (100 μm) prevented the glycine-dependent reduction in mEPSC frequency. Asterisks denote significance (P < 0.05). E, averaged normalized mEPSC amplitude; no significant changes were observed with any of the pharmacological manipulations. Sample sizes in D and E are given within the bars. The mEPSC frequencies and amplitudes were normalized to the averaged baseline levels before glycine or strychnine application.
To confirm that the hyperpolarizing effect of glycine in the adult was due to GlyR and not NMDAR activation, we measured the effects of glycine after pre-incubating slices in the GlyR antagonist strychnine or the NMDAR antagonist d,l-APV. Strychnine, but not d,l-APV, abolished the effect of glycine application on mEPSC frequency (Fig. 8D; strychnine + glycine, 99 ± 2% of baseline, P < 0.006 compared with glycine alone; d,l-APV + glycine, 60 ± 2% of baseline, P > 0.05 compared with glycine alone; ANOVA). There was no effect of these manipulations on mEPSC amplitude (Fig. 8E; P > 0.05), suggesting that GlyRs exert an inhibitory presynaptic influence on neurotransmitter release in the adult. Thus, while preGlyRs tonically facilitate glutamate release in younger animals, they are hyperpolarizing in older animals and no longer contribute to spontaneous, action potential-independent neurotransmission.
Discussion
The present study demonstrates that preGlyRs tonically enhance glutamate release in the visual cortex of young mice (P13–18). This GlyR-mediated enhancement of neurotransmitter release requires a depolarizing Cl− gradient established by NKCC1 activity, and subsequent calcium entry through VGCCs. These preGlyRs are tonically activated by ambient glycine, which is regulated through GlyT1 activity. In contrast, preGlyRs in adult visual cortex lack tonic activity and instead exhibit a hyperpolarizing effect. These data demonstrate an age-dependent role for GlyRs in regulating glutamate release in the neocortex.
Glycine-mediated effects on glutamate release
Glycine could influence neurotransmitter release in a number of ways, but the two most parsimonious explanations are through activation of NMDARs, where glycine acts as an important co-agonist (Ahmadi et al. 2003), or through GlyRs located on the presynaptic bouton (Dahan et al. 2003; Danglot et al. 2004). Indeed, NMDAR activity can tonically facilitate neurotransmitter release in the developing cortex (Woodhall et al. 2001; Corlew et al. 2007; Brasier & Feldman, 2008; Larsen et al. 2011). Our results, together with previous literature showing that presynaptic NMDARs are saturated at their glycine binding sites (Li & Han, 2007), suggest that glycine enhances glutamate release by activating GlyRs, not NMDARs.
How do GlyRs trigger neurotransmitter release? Neurotransmitter release is typically triggered by calcium entry into the presynaptic terminal through VGCCs (Augustine et al. 1987; Turner et al. 1992); thus we were not surprised to find that glycine promotes neurotransmitter release in the developing visual cortex by activating VGCCs. Whether preGlyRs depolarize presynaptic membranes sufficiently to activate VGCCs has been debated (Takahashi & Momiyama, 1993; Wu et al. 1999; Ishikawa et al. 2005). However, work addressing the influence of GABA on neurotransmission has long supported a role for presynaptic chloride currents in bouton depolarization in the cerebral cortex (Trigo et al. 2008; Ruiz et al. 2010), as well as in the spinal cord (for review, see Hochman et al. 2010). Our conclusion that preGlyRs activate VGCCs in the developing visual cortex is consistent with previous observations in other brain regions (Turecek & Trussell, 2001), and raises the possibility that these receptors are localized to the presynaptic bouton, near VGCCs. However, we cannot rule out other possible locations for GlyRs. For example, it is conceivable that somatic GlyRs can trigger subthreshold depolarizations sufficient to induce VGCC activity and presynaptic transmitter release, as has been shown with direct somatic depolarizations in the cerebellum (Christie et al. 2011). Future studies are needed to identify both the cell types that have preGlyRs and the precise subcellular localization of these receptors. To date, GlyRs have been localized to L3 and L5 pyramidal cells, cortical subplate neurons, and Cajal-Retzius cells (Naas et al. 1991; Okabe et al. 2004; and current study); we speculate that the ability of preGlyR-mediated currents to activate VGCCs may be common throughout the developing CNS.
GlyT1 contribution to preGlyR function
Ambient and released glycine must be tightly regulated for normal neural function, and a number of disease states are caused by dysregulation of glycine levels (discussed below). Glycine is predominantly biosynthesized in the mammalian liver (Sato et al. 1969); its regulation in the brain is largely dictated by the glycine transporters GlyT1 and GlyT2. These transporters have distinct mechanisms of function and cellular localization; GlyT1 transports glycine bidirectionally based on its concentration gradient, whereas GlyT2 packages glycine by the vesicular inhibitory amino acid transporter for later release (Eulenburg et al. 2005). We took advantage of the distinct pharmacology of glycine transporters to show that GlyT1 is likely the critical transporter in setting the ambient extracellular glycine levels that promote preGlyR-mediated glutamate release. Physiologically, circulating glycine has been measured to be in the low micromolar range (Westergren et al. 1994), but GlyT1 activity is capable of reducing cleft glycine concentrations to ∼150 nm (Attwell et al. 1993; Roux & Supplisson, 2000). During periods of glial depolarization and in some pathophysiological conditions (e.g. ischemia or hypoxia), glycine can reach concentrations of 20–100 μm or more (Baker et al. 1991; Phillis et al. 1994; Roux & Supplisson, 2000); in these circumstances preGlyR activity could increase spontaneous neurotransmitter release by up to 42% (current study), potentially synchronizing neurotransmitter release across active zones to generate postsynaptic action potentials (Sharma & Vijayaraghavan, 2003).
In the brain, GlyT1 is found mainly on glia, whereas GlyT2 is restricted to neurons (Zafra et al. 1995a,b; Aragon & Lopez-Corcuera, 2003; Eulenburg et al. 2005; Raiteri & Raiteri, 2010). Both neurons and astrocytes are capable of glycine release (Jonas et al. 1998; Huang et al. 2004), but they must first take up glycine from extracellular spaces via transporters. We found that both GlyT1 antagonists and a glial toxin prevent tonic preGlyR-mediated enhancement of neurotransmitter release in the developing visual cortex. These data collectively suggest that GlyT1 on astrocytes controls ambient glycine levels and tonic preGlyR activity. In light of our findings, glycine may need to be added to the list of gliotransmitters, which now include glutamate, ATP and d-serine (Perea & Araque, 2009).
PreGlyR activity in the adult
Similar to the depolarizing role of GABA receptors during early neuronal development (Ben-Ari, 2002), preGlyRs tonically facilitate neurotransmitter release in the developing visual cortex. In contrast, preGlyRs are not tonically active in more mature cortex and, when acutely activated, they have an inhibitory effect on glutamate release. The significance of this developmental shift is not yet known, although it may reflect the need for increased inhibitory drive to terminate the critical period of cortical plasticity (Fagiolini & Hensch, 2000; Feldman, 2000; Hensch, 2004; Harauzov et al. 2010). While it is likely that all GlyRs undergo a developmental shift in their function, we cannot rule out the possibility that there are also distinct microdomains where GlyRs have differential hyperpolarizing versus depolarizing effects at any given age.
Importance of GlyRs
Glycine is a primary inhibitory neurotransmitter in the spinal cord and brainstem (Rajendra et al. 1997), and contributes to inhibition in the hippocampus (Chattipakorn & McMahon, 2003; Song et al. 2006). Postsynaptic GlyRs are also important for some forms of short-term plasticity (Zhang et al. 2006), the induction of long-term depression (Chen et al. 2011), shunting inhibition (Keck et al. 2008) and the osmoregulation of neuronal activity (Deleuze et al. 2005). However, the contribution of depolarizing preGlyRs to shaping neurotransmitter release has only recently been appreciated. Spontaneous activity-independent neurotransmitter release has recently been shown to stabilize dendritic spines (McKinney et al. 1999), regulate dendritic protein synthesis (Sutton et al. 2004) and shape the chemistry (locally and globally) of postsynaptic cells (Otsu & Murphy, 2003; Sharma & Vijayaraghavan, 2003), suggesting that the maintenance of spontaneous activity by preGlyRs in the developing visual cortex likely contributes to proper circuit formation. While we were unable to identify a role for preGlyRs in adjusting action potential-driven transmitter release under our recording conditions, such a role might be identified with other parameters or conditions. In support of this idea, previous studies have shown that presynaptic NMDARs can enhance spontaneous neurotransmitter release in the presence of brain-derived neurotrophic factor, but these receptors failed to enhance evoked transmitter release unless VGCC activity was reduced (Madara & Levine, 2008). Future experiments are needed to determine whether there are conditions that unmask a role for preGlyRs in evoked transmitter release.
The importance of glycinergic transmission is underscored by the severe disease states and behavioral deficits caused by mutations that alter GlyR subunits or glycine homeostasis. Many of these clinical signs likely reflect glycine's key role as inhibitory transmitter in the brain stem and spinal cord; for example, improper GlyR clustering and synapse formation results in deficits in touch-evoked responses and escape behaviors in zebrafish (Ogino et al. 2011). Likewise, GlyR mutations in mice reduce the ethanol sensitivity to motor incoordination and righting reflexes (Findlay et al. 2002). However, other effects target higher levels of the neuraxis. For example, GlyR mutations not only increase startle responses in mice (Harvey et al. 2008), but also disrupt amygdala-associated anxiety-like behaviors (McCool & Chappell, 2007).
Evidence from clinical practice strongly implicates glycine in neocortical development and function. Infantile and late-onset hyperglycinemia present with mild to moderate mental retardation, agitated delirium, vertical gaze palsy, behavioral hyperirritability, aggressive outbursts and seizures (Singer et al. 1989; Steiner et al. 1996; Dinopoulos et al. 2005). In addition to these chronic changes in glycine homeostasis, acute manipulations that can affect glycinergic signaling also have profound neurological consequences. For example, elevations in circulating glycine concentration (which can occur with surgical complications) have been correlated with a decrease in cognition and spatial acuity, as well as visual disturbances that reverse once glycine returns to baseline levels (Mizutani et al. 1990; Nilsson & Hahn, 1994; Radziwill et al. 1997). Glycine disturbances have also been suggested in other diseases, including schizophrenia and epilepsy, where some common treatments targeted at the glycine-binding site of NMDARs affect glycine homeostasis (Waziri, 1988; Birdsall, 1998; de Koning et al. 1998; Farber et al. 1999; Javitt, 2009), or have been shown to alter GlyR functions directly (Rigo et al. 2002).
Despite the fact that GlyRs are important targets for many therapeutic drugs (Deutsch et al. 1983; Jaeken, 2002), surprisingly few studies have considered the possibility that preGlyRs can influence neurotransmitter release (Chepkova et al. 2002; Zhang et al. 2006; Lee et al. 2009). Our results provide mechanistic insight into the role of preGlyRs in facilitating excitatory neurotransmitter release, and the switch of preGlyRs to an inhibitory role with maturation. Besides providing new insights into development of cortical circuits, our findings identify synaptic functions likely to be impacted by GlyR dysfunction.
Acknowledgments
We thank T. Kash, R. Larsen, K. McCarthy, E. McCoy, B. Walters and members of the Philpot laboratory for critically reading the manuscript and for helpful discussions. We thank K. Phend and S. Burette for histological assistance. This work was supported by NSF (Award # 0822969, B.D.P.) and NIH (T32-HD40127, P.A.K.; R01NS039444, R.J.W.; and R01EY018323, B.D.P.).
Glossary
- d,l-αAA
d,l-α-aminoadipic acid
- ACSF
artificial cerebrospinal fluid
- d,l-APV
d,l-2-amino-5phosphonopentanoic acid
- BMI
bicuculline methiodide
- CPA
cyclopiazonic acid
- DAB
diaminobenzidene
- DIDS
4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid
- GlyR
glycine receptor
- GlyT1
glycine transporter 1
- GlyT2
glycine transporter 2
- KCC2
K+-Cl− cotransporter 2
- L
layer
- mEPSC
miniature excitatory postsynaptic currents
- NFPS
N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl] sarcosine
- NKCC1
Na+–K+–2Cl− cotransporter
- NMDAR
NMDA receptor
- P
postnatal day
- PPR
paired-pulse ratio
- preGlyR
presynaptic-acting glycine receptor
- TTX
tetrodotoxin
- VGCC
voltage-gated Ca2+ channels
Author contributions
P.A.K. conceptualized and designed the experiments, collected, analysed, and interpreted electrophysiological data, and drafted/revised the manuscript. B.D.P. assisted with experimental design, data interpretation and manuscript revision. A.C.B. and R.J.W. collected, analysed and interpreted immunocytochemical data to localize GlyT1, and assisted with manuscript revision. All experiments were performed at the University of North Carolina in Chapel Hill, USA, and all authors approved the final version of the manuscript. None of the authors has any conflict of interest to declare.
References
- Adam-Vizi V. External Ca(2+)-independent release of neurotransmitters. J Neurochem. 1992;58:395–405. doi: 10.1111/j.1471-4159.1992.tb09736.x. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, van Zundert B, Tapia JC, Carrasco MA, Alvarez FJ. Changes on the properties of glycine receptors during neuronal development. Brain Res Brain Res Rev. 2004;47:33–45. doi: 10.1016/j.brainresrev.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Ahmadi S, Muth-Selbach U, Lauterbach A, Lipfert P, Neuhuber WL, Zeilhofer HU. Facilitation of spinal NMDA receptor currents by spillover of synaptically released glycine. Science. 2003;300:2094–2097. doi: 10.1126/science.1083970. [DOI] [PubMed] [Google Scholar]
- Albertson TE, Walby WF, Stark LG, Joy RM. The effect of propofol on CA1 pyramidal cell excitability and GABAA-mediated inhibition in the rat hippocampal slice. Life Sci. 1996;58:2397–2407. doi: 10.1016/0024-3205(96)00243-3. [DOI] [PubMed] [Google Scholar]
- Aragon C, Lopez-Corcuera B. Structure, function and regulation of glycine neurotransporters. Eur J Pharmacol. 2003;479:249–262. doi: 10.1016/j.ejphar.2003.08.074. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP, Smith SJ. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Backus KH, Deitmer JW, Friauf E. Glycine-activated currents are changed by coincident membrane depolarization in developing rat auditory brainstem neurones. J Physiol. 1998;507:783–794. doi: 10.1111/j.1469-7793.1998.783bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AJ, Zornow MH, Grafe MR, Scheller MS, Skilling SR, Smullin DH, Larson AA. Hypothermia prevents ischemia-induced increases in hippocampal glycine concentrations in rabbits. Stroke. 1991;22:666–673. doi: 10.1161/01.str.22.5.666. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tseeb V, Raggozzino D, Khazipov R, Gaiarsa JL. gamma-Aminobutyric acid (GABA): a fast excitatory transmitter which may regulate the development of hippocampal neurones in early postnatal life. Prog Brain Res. 1994;102:261–273. doi: 10.1016/S0079-6123(08)60545-2. [DOI] [PubMed] [Google Scholar]
- Birdsall TC. Therapeutic applications of taurine. Altern Med Rev. 1998;3:128–136. [PubMed] [Google Scholar]
- Brasier DJ, Feldman DE. Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. J Neurosci. 2008;28:2199–2211. doi: 10.1523/JNEUROSCI.3915-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper DS, Reif-Lehrer L. Effects of alphaaminoadipate isomers on the morphology of the isolated chick embryo retina. Invest Ophthalmol Vis Sci. 1983;24:1480–1488. [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chattipakorn SC, McMahon LL. Strychnine-sensitive glycine receptors depress hyperexcitability in rat dentate gyrus. J Neurophysiol. 2003;89:1339–1342. doi: 10.1152/jn.00908.2002. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol. 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RQ, Wang SH, Yao W, Wang JJ, Ji F, Yan JZ, Ren SQ, Chen Z, Liu SY, Lu W. Role of glycine receptors in glycine-induced LTD in hippocampal CA1 pyramidal neurons. Neuropsychopharmacology. 2011;36:1948–1958. doi: 10.1038/npp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepkova AN, Doreulee N, Yanovsky Y, Mukhopadhyay D, Haas HL, Sergeeva OA. Long-lasting enhancement of corticostriatal neurotransmission by taurine. Eur J Neurosci. 2002;16:1523–1530. doi: 10.1046/j.1460-9568.2002.02223.x. [DOI] [PubMed] [Google Scholar]
- Christie JM, Chiu DN, Jahr CE. Ca(2+)-dependent enhancement of release by subthreshold somatic depolarization. Nat Neurosci. 2011;14:62–68. doi: 10.1038/nn.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys J. 1997;73:220–229. doi: 10.1016/S0006-3495(97)78062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- Danglot L, Rostaing P, Triller A, Bessis A. Morphologically identified glycinergic synapses in the hippocampus. Mol Cell Neurosci. 2004;27:394–403. doi: 10.1016/j.mcn.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- de Koning TJ, Duran M, Dorland L, Gooskens R, Van Schaftingen E, Jaeken J, Blau N, Berger R, Poll-The BT. Beneficial effects of L-serine and glycine in the management of seizures in 3-phosphoglycerate dehydrogenase deficiency. Ann Neurol. 1998;44:261–265. doi: 10.1002/ana.410440219. [DOI] [PubMed] [Google Scholar]
- Deleuze C, Alonso G, Lefevre IA, Duvoid-Guillou A, Hussy N. Extrasynaptic localization of glycine receptors in the rat supraoptic nucleus: further evidence for their involvement in glia-to-neuron communication. Neuroscience. 2005;133:175–183. doi: 10.1016/j.neuroscience.2005.01.060. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Stanley M, Peselow ED, Banay-Schwartz M. Glycine: a possible role in lithium's action and affective illness. Neuropsychobiology. 1983;9:215–218. doi: 10.1159/000117967. [DOI] [PubMed] [Google Scholar]
- Dinopoulos A, Kure S, Chuck G, Sato K, Gilbert DL, Matsubara Y, Degrauw T. Glycine decarboxylase mutations: a distinctive phenotype of nonketotic hyperglycinemia in adults. Neurology. 2005;64:1255–1257. doi: 10.1212/01.WNL.0000156800.23776.40. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Farber NB, Newcomer JW, Olney JW. Glycine agonists: what can they teach us about schizophrenia. Arch Gen Psychiatry. 1999;56:13–17. doi: 10.1001/archpsyc.56.1.13. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Inhibition and plasticity. Nat Neurosci. 2000;3:303–304. doi: 10.1038/73849. [DOI] [PubMed] [Google Scholar]
- Findlay GS, Wick MJ, Mascia MP, Wallace D, Miller GW, Harris RA, Blednov YA. Transgenic expression of a mutant glycine receptor decreases alcohol sensitivity of mice. J Pharmacol Exp Ther. 2002;300:526–534. doi: 10.1124/jpet.300.2.526. [DOI] [PubMed] [Google Scholar]
- Flint AC, Liu X, Kriegstein AR. Nonsynaptic glycine receptor activation during early neocortical development. Neuron. 1998;20:43–53. doi: 10.1016/s0896-6273(00)80433-x. [DOI] [PubMed] [Google Scholar]
- Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J Neurosci. 2003;23:11229–11234. doi: 10.1523/JNEUROSCI.23-35-11229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. Properties and diversity of (Na-K-Cl) cotransporters. Annu Rev Physiol. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, Viegi A, Berardi N, Maffei L. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci. 2010;30:361–371. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsing LG, Jr, Juranyi Z, Gacsalyi I, Tapolcsanyi P, Czompa A, Matyus P. Glycine transporter type-1 and its inhibitors. Curr Med Chem. 2006;13:1017–1044. doi: 10.2174/092986706776360932. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Topf M, Harvey K, Rees MI. The genetics of hyperekplexia: more than startle! Trends Genet. 2008;24:439–447. doi: 10.1016/j.tig.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hochman S, Shreckengost J, Kimura H, Quevedo J. Presynaptic inhibition of primary afferents by depolarization: observations supporting nontraditional mechanisms. Ann N Y Acad Sci. 2010;1198:140–152. doi: 10.1111/j.1749-6632.2010.05436.x. [DOI] [PubMed] [Google Scholar]
- Hollrigel GS, Ross ST, Soltesz I. Temporal patterns and depolarizing actions of spontaneous GABAA receptor activation in granule cells of the early postnatal dentate gyrus. J Neurophysiol. 1998;80:2340–2351. doi: 10.1152/jn.1998.80.5.2340. [DOI] [PubMed] [Google Scholar]
- Hoover-Fong JE, Shah S, Van Hove JL, Applegarth D, Toone J, Hamosh A. Natural history of nonketotic hyperglycinemia in 65 patients. Neurology. 2004;63:1847–1853. doi: 10.1212/01.wnl.0000144270.83080.29. [DOI] [PubMed] [Google Scholar]
- Huang H, Barakat L, Wang D, Bordey A. Bergmann glial GlyT1 mediates glycine uptake and release in mouse cerebellar slices. J Physiol. 2004;560:721–736. doi: 10.1113/jphysiol.2004.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck S, Grass F, Hortnagl H. The glutamate analogue alpha-aminoadipic acid is taken up by astrocytes before exerting its gliotoxic effect in vitro. J Neurosci. 1984;4:2650–2657. doi: 10.1523/JNEUROSCI.04-10-02650.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I. Voltage-dependent chloride conductance of the squid axon membrane and its blockade by some disulfonic stilbene derivatives. J Gen Physiol. 1985;85:519–537. doi: 10.1085/jgp.85.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Kaneko M, Shin HS, Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J Physiol. 2005;568:199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken J. Genetic disorders of gamma-aminobutyric acid, glycine, and serine as causes of epilepsy. J Child Neurol. 2002;17(Suppl 3):3S84–3S87. discussion 83S88. [PubMed] [Google Scholar]
- Javitt DC. Glycine transport inhibitors for the treatment of schizophrenia: symptom and disease modification. Curr Opin Drug Discov Devel. 2009;12:468–478. [PubMed] [Google Scholar]
- Jeong HJ, Jang IS, Moorhouse AJ, Akaike N. Activation of presynaptic glycine receptors facilitates glycine release from presynaptic terminals synapsing onto rat spinal sacral dorsal commissural nucleus neurons. J Physiol. 2003;550:373–383. doi: 10.1113/jphysiol.2003.041053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kawa K. Glycine facilitates transmitter release at developing synapses: a patch clamp study from Purkinje neurons of the newborn rat. Brain Res Dev Brain Res. 2003;144:57–71. doi: 10.1016/s0165-3806(03)00159-7. [DOI] [PubMed] [Google Scholar]
- Keck T, Lillis KP, Zhou YD, White JA. Frequency-dependent glycinergic inhibition modulates plasticity in hippocampus. J Neurosci. 2008;28:7359–7369. doi: 10.1523/JNEUROSCI.5618-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurgel M, Koo AC, Ivy GO. Selective ablation of astrocytes by intracerebral injections of alpha-aminoadipate. Glia. 1996;16:351–358. doi: 10.1002/(SICI)1098-1136(199604)16:4<351::AID-GLIA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- Kubota H, Alle H, Betz H, Geiger JR. Presynaptic glycine receptors on hippocampal mossy fibers. Biochem Biophys Res Commun. 2010;393:587–591. doi: 10.1016/j.bbrc.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Larsen RS, Corlew RJ, Henson MA, Roberts AC, Mishina M, Watanabe M, Lipton SA, Nakanishi N, Perez-Otano I, Weinberg RJ, Philpot BD. NR3A-containing NMDARs promote neurotransmitter release and spike timing-dependent plasticity. Nat Neurosci. 2011;14:338–344. doi: 10.1038/nn.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EA, Cho JH, Choi IS, Nakamura M, Park HM, Lee JJ, Lee MG, Choi BJ, Jang IS. Presynaptic glycine receptors facilitate spontaneous glutamate release onto hilar neurons in the rat hippocampus. J Neurochem. 2009;109:275–286. doi: 10.1111/j.1471-4159.2009.05960.x. [DOI] [PubMed] [Google Scholar]
- Leewanich P, Tohda M, Matsumoto K, Subhadhirasakul S, Takayama H, Aimi N, Watanabe H. A possible mechanism underlying corymine inhibition of glycine-induced Cl− current in Xenopus oocytes. Eur J Pharmacol. 1998;348:271–277. doi: 10.1016/s0014-2999(98)00147-2. [DOI] [PubMed] [Google Scholar]
- Li YH, Han TZ. Glycine binding sites of presynaptic NMDA receptors may tonically regulate glutamate release in the rat visual cortex. J Neurophysiol. 2007;97:817–823. doi: 10.1152/jn.00980.2006. [DOI] [PubMed] [Google Scholar]
- Madara JC, Levine ES. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J Neurophysiol. 2008;100:3175–3184. doi: 10.1152/jn.90880.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell A. Strychnine and taurine modulation of amygdala-associated anxiety-like behaviour is ‘state’ dependent. Behav Brain Res. 2007;178:70–81. doi: 10.1016/j.bbr.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- Mizutani AR, Parker J, Katz J, Schmidt J. Visual disturbances, serum glycine levels and transurethral resection of the prostate. J Urol. 1990;144:697–699. doi: 10.1016/s0022-5347(17)39558-7. [DOI] [PubMed] [Google Scholar]
- Naas E, Zilles K, Gnahn H, Betz H, Becker CM, Schroder H. Glycine receptor immunoreactivity in rat and human cerebral cortex. Brain Res. 1991;561:139–146. doi: 10.1016/0006-8993(91)90758-n. [DOI] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Hahn RG. Mental status after transurethral resection of the prostate. Eur Urol. 1994;26:1–5. doi: 10.1159/000475333. [DOI] [PubMed] [Google Scholar]
- Ogino K, Ramsden SL, Keib N, Schwarz G, Harvey RJ, Hirata H. Duplicated gephyrin genes showing distinct tissue distribution and alternative splicing patterns mediate molybdenum cofactor biosynthesis, glycine receptor clustering, and escape behaviour in zebrafish. J Biol Chem. 2011;286:806–817. doi: 10.1074/jbc.M110.125500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe A, Kilb W, Shimizu-Okabe C, Hanganu IL, Fukuda A, Luhmann HJ. Homogenous glycine receptor expression in cortical plate neurons and Cajal-Retzius cells of neonatal rat cerebral cortex. Neuroscience. 2004;123:715–724. doi: 10.1016/j.neuroscience.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Otsu Y, Murphy TH. Miniature transmitter release: accident of nature or careful design. Sci STKE. 2003;2003:pe54. doi: 10.1126/stke.2112003pe54. [DOI] [PubMed] [Google Scholar]
- Parker I, Sumikawa K, Miledi R. Responses to GABA, glycine and beta-alanine induced in Xenopus oocytes by messenger RNA from chick and rat brain. Proc R Soc Lond B Biol Sci. 1988;233:201–216. doi: 10.1098/rspb.1988.0019. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. GLIA modulates synaptic transmission. Brain Res Rev. 2009;63:93–102. doi: 10.1016/j.brainresrev.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Smith-Barbour M, O’Regan MH, Perkins LM. Amino acid and purine release in rat brain following temporary middle cerebral artery occlusion. Neurochem Res. 1994;19:1125–1130. doi: 10.1007/BF00965145. [DOI] [PubMed] [Google Scholar]
- Radziwill AJ, Vuadens P, Borruat FX, Bogousslavsky J. Visual disturbances and transurethral resection of the prostate: the TURP syndrome. Eur Neurol. 1997;38:7–9. doi: 10.1159/000112895. [DOI] [PubMed] [Google Scholar]
- Raiteri L, Raiteri M. Functional ‘glial’ GLYT1 glycine transporters expressed in neurons. J Neurochem. 2010;114:647–653. doi: 10.1111/j.1471-4159.2010.06802.x. [DOI] [PubMed] [Google Scholar]
- Rajendra S, Lynch JW, Schofield PR. The glycine receptor. Pharmacol Ther. 1997;73:121–146. doi: 10.1016/s0163-7258(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Rigo JM, Hans G, Nguyen L, Rocher V, Belachew S, Malgrange B, Leprince P, Moonen G, Selak I, Matagne A, Klitgaard H. The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br J Pharmacol. 2002;136:659–672. doi: 10.1038/sj.bjp.0704766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Campanac E, Scott RS, Rusakov DA, Kullmann DM. Presynaptic GABAA receptors enhance transmission and LTP induction at hippocampal mossy fibre synapses. Nat Neurosci. 2010;13:431–438. doi: 10.1038/nn.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Kochi H, Motokawa Y, Kawasaki H, Kikuchi G. Glycine metabolism by rat liver mitochondria. I. Synthesis of two molecules of glycine from one molecule each of serine, bicarbonate and ammonia. J Biochem. 1969;65:63–70. [PubMed] [Google Scholar]
- Schmieden V, Kuhse J, Betz H. Agonist pharmacology of neonatal and adult glycine receptor alpha subunits: identification of amino acid residues involved in taurine activation. EMBO J. 1992;11:2025–2032. doi: 10.1002/j.1460-2075.1992.tb05259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- Singer HS, Valle D, Hayasaka K, Tada K. Nonketotic hyperglycinemia: studies in an atypical variant. Neurology. 1989;39:286–288. doi: 10.1212/wnl.39.2.286. [DOI] [PubMed] [Google Scholar]
- Song W, Chattipakorn SC, McMahon LL. Glycine-gated chloride channels depress synaptic transmission in rat hippocampus. J Neurophysiol. 2006;95:2366–2379. doi: 10.1152/jn.00386.2005. [DOI] [PubMed] [Google Scholar]
- Stein V, Nicoll RA. GABA generates excitement. Neuron. 2003;37:375–378. doi: 10.1016/s0896-6273(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Steiner RD, Sweetser DA, Rohrbaugh JR, Dowton SB, Toone JR, Applegarth DA. Nonketotic hyperglycinemia: atypical clinical and biochemical manifestations. J Pediatr. 1996;128:243–246. doi: 10.1016/s0022-3476(96)70399-2. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neurotransmitter release. Handb Exp Pharmacol. 2008:1–21. doi: 10.1007/978-3-540-74805-2_1. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Tapia JC, Aguayo LG. Changes in the properties of developing glycine receptors in cultured mouse spinal neurons. Synapse. 1998;28:185–194. doi: 10.1002/(SICI)1098-2396(199803)28:3<185::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Trigo FF, Marty A, Stell BM. Axonal GABAA receptors. Eur J Neurosci. 2008;28:841–848. doi: 10.1111/j.1460-9568.2008.06404.x. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Turner TJ, Adams ME, Dunlap K. Calcium channels coupled to glutamate release identified by omega-Aga-IVA. Science. 1992;258:310–313. doi: 10.1126/science.1357749. [DOI] [PubMed] [Google Scholar]
- Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res Dev Brain Res. 2002;139:59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- Wang F, Xiao C, Ye JH. Taurine activates excitatory non-synaptic glycine receptors on dopamine neurones in ventral tegmental area of young rats. J Physiol. 2005;565:503–516. doi: 10.1113/jphysiol.2005.085423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xu TL. Chloride homeostasis differentially affects GABA(A) receptor- and glycine receptor-mediated effects on spontaneous circuit activity in hippocampal cell culture. Neurosci Lett. 2006;406:11–16. doi: 10.1016/j.neulet.2006.06.064. [DOI] [PubMed] [Google Scholar]
- Waseem TV, Fedorovich SV. Presynaptic glycine receptors influence plasma membrane potential and glutamate release. Neurochem Res. 2010;35:1188–1195. doi: 10.1007/s11064-010-0174-7. [DOI] [PubMed] [Google Scholar]
- Waziri R. Glycine therapy of schizophrenia. Biol Psychiatry. 1988;23:210–211. doi: 10.1016/0006-3223(88)90093-5. [DOI] [PubMed] [Google Scholar]
- Westergren I, Nystrom B, Hamberger A, Nordborg C, Johansson BB. Concentrations of amino acids in extracellular fluid after opening of the blood-brain barrier by intracarotid infusion of protamine sulfate. J Neurochem. 1994;62:159–165. doi: 10.1046/j.1471-4159.1994.62010159.x. [DOI] [PubMed] [Google Scholar]
- Woodhall G, Evans DI, Cunningham MO, Jones RS. NR2B-containing NMDA autoreceptors at synapses on entorhinal cortical neurons. J Neurophysiol. 2001;86:1644–1651. doi: 10.1152/jn.2001.86.4.1644. [DOI] [PubMed] [Google Scholar]
- Wu LG, Westenbroek RE, Borst JG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu TX, Gong N, Xu TL. Inhibitors of GlyT1 and GlyT2 differentially modulate inhibitory transmission. Neuroreport. 2005;16:1227–1231. doi: 10.1097/00001756-200508010-00019. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, Weinberg RJ, Ehlers MD, Philpot BD. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci. 2009;12:777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Wang F, Krnjevic K, Wang W, Xiong ZG, Zhang J. Presynaptic glycine receptors on GABAergic terminals facilitate discharge of dopaminergic neurons in ventral tegmental area. J Neurosci. 2004;24:8961–8974. doi: 10.1523/JNEUROSCI.2016-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KW, Wotring VE, Fuse T. Multiple picrotoxinin effect on glycine channels in rat hippocampal neurons. Neuroscience. 1998;87:807–815. doi: 10.1016/s0306-4522(98)00158-4. [DOI] [PubMed] [Google Scholar]
- Yu X, Xu Z, Mi M, Xu H, Zhu J, Wei N, Chen K, Zhang Q, Zeng K, Wang J, Chen F, Tang Y. Dietary taurine supplementation ameliorates diabetic retinopathy via anti-excitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem Res. 2008;33:500–507. doi: 10.1007/s11064-007-9465-z. [DOI] [PubMed] [Google Scholar]
- Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995a;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur J Neurosci. 1995b;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Xu L, Xu TL. Glycine receptor activation regulates short-term plasticity in CA1 area of hippocampal slices of rats. Biochem Biophys Res Commun. 2006;344:721–726. doi: 10.1016/j.bbrc.2006.03.198. [DOI] [PubMed] [Google Scholar]


