Abstract
Paclitaxel (PTX) is one of the most effective chemotherapeutic agents for a wide spectrum of cancers, but its therapeutic benefit is often limited by severe side effects. We have developed a micelle-based PTX formulation based on a simple conjugate derived from polyethylene glycol 5000 (PEG5K) and embelin (EB). Embelin is a natural product and exhibits antitumor activity through blocking the activity of X-linked inhibitor of apoptosis protein (XIAP). PEG5K-EB2 conjugate self-assembles to form stable micelles in aqueous solution and efficiently encapsulates hydrophobic drugs such as PTX. PEG5K-EB2 micelles have a relatively low CMC of 0.002mg/mL (0.35μM) with sizes in the range of 20 ~ 30 nm with or without loaded PTX. In vitro cell uptake study showed that the PEG5K-EB2 micelles were efficiently taken up by tumor cells. In vitro release study showed that PTX formulated in PEG5K-EB2 micelles was slowly released over 5 days with much slower release kinetics than that of Taxol formulation. PTX formulated in PEG5K-EB2 micelles exhibited more potent cytotoxicity than Taxol in several cultured tumor cell lines. Total body near infrared fluorescence (NIRF) imaging showed that PEG5K-EB2 micelles were selectively accumulated at tumor site with minimal uptake in major organs including liver and spleen. PTX-loaded PEG5K-EB2 micelles demonstrated an excellent safety profile with a maximum tolerated dose (MTD) of 100–120 mg PTX/kg in mice, which was significantly higher than that for Taxol (15–20 mg PTX/kg). Finally, PTX formulated in PEG5K-EB2 micelles showed superior anti-tumor activity compared to Taxol in murine models of breast and prostate cancers.
Keywords: Nanomicelles, Embelin, Controlled drug release, Drug delivery, Paclitaxel, Cancer therapy
1. Introduction
Paclitaxel (PTX) is one of the first-line chemotherapeutic agents that are effective for the treatment of a wide range of cancers, including lung, ovarian, breast, prostate, head and neck cancer, and advanced forms of Kaposi’s sarcoma. It works through interfering with normal breakdown of microtubules during cell division. The main challenge with PTX therapy is its poor solubility in aqueous solutions. Therefore, it is of tremendous incentive to develop effective delivery systems for PTX to enhance its accumulation at tumor site to maximize its therapeutic efficacy while minimizing the side effects. Taxol® and Abraxane® are two FDA approved PTX formulations. Taxol® is an alcohol/Cremophor formulation of PTX, which is irritating and can cause hyperactivity reactions. Abraxane® is PTX-loaded human albumin nanoparticles that have a size around 130 nm, which is within the range that can penetrate well-vascularized solid tumors via an enhanced permeability and retention (EPR) effect [1,2]. It is now known that for less vascularized tumors, particles with smaller size (≤ 64 nm) were needed for effective penetration through neovasculatures to reach tumor cells [3]. There have been continuous efforts to develop various types of new formulations to improve targeted delivery of PTX to different types of tumors. Among all drug delivery systems being investigated, polymeric micelles have gained considerable attention and are rapidly becoming a powerful nanomedicine platform for cancer therapeutics applications due to their simplicity, small sizes (10–100nm), ability to solubilize water insoluble anticancer drugs, and prolonged drug retention time [4–6]. However, most of the carrier materials in lipidic or polymeric drug delivery systems utilize “inert” excipients that lack therapeutic effect. The presence of large amount of carrier materials not only adds to the cost, but also imposes additional safety concerns [7]. One interesting strategy in formulation design is that components of carriers have therapeutic effects and can be freed from the delivery systems following intracellular delivery to achieve synergistic or additive effect with co-delivered drugs. One example is pegylated vitamin E, D-α-tocopheryl polyethylene glycol succinate (Vitamin E TPGS or TPGS) [8–10]. Vitamin E is linked to PEG via a biodegradable ester linkage and forms a hydrophobic core in this micellar system to solubilize other water-insoluble drugs. Vitamin E itself shows antitumor effect against different types of cancers through a variety of mechanisms [11,12]. Synergistic antitumor activity has been demonstrated in a number of in vitro and in vivo studies for TPGS-based formulations of PTX and other anticancer agents [6,13].
Our group has previously developed PEG-derivatized embelin as another dual-functional carrier for the delivery of poorly water-soluble anti-cancer drugs [14]. This system was constructed by coupling two embelin molecules to polyethylene glycol PEG 3500 (PEG3.5K) through an ester linkage (PEG3.5K-EB2). Embelin is a naturally occurring alkyl substituted hydroxyl benzoquinone compound and a major constituent of Embelia ribes BURM. It exhibits various biological effects including antidiabetic, anti-inflammatory, and hepatoprotective activities [15–17]. Embelin also shows antitumor activity in various types of cancers via inhibiting the activity of X-linked inhibitor of apoptosis protein (XIAP) [15,18–22]. XIAP is overexpressed in various types of cancers cells, particularly drug-resistant cancer cells and inhibition of XIAP has been employed as a new strategy for the treatment of cancers [23,24]. We demonstrated that PEG3.5K-EB2 formed small-sized micelles (20–30 nm) and solubilized various hydrophobic agents including PTX [14]. Preliminary study showed that the antitumor activity of embelin was well retained following coupling to PEG3.5K. More importantly, PEG3.5K-EB2 synergized with PTX in antitumor activity in several cancer cell lines in vitro. In this study, we showed that a similar PEG derivative of embelin with a longer PEG, PEG5K-embelin2 formed stable micelles with PTX at lower carrier/PTX molar ratios. We further characterized the biophysical properties of the improved micellar system including size, loading capacity, and drug release kinetics. The in vitro cytotoxicity of PTX-loaded PEG5K-embelin2 was also studied in several cancer cell lines. Finally, the in vivo antitumor activity of PTX-loaded PEG5K-embelin2 was investigated in both breast cancer and prostate cancer models.
2. Experimental section
2.1. Materials
Paclitaxel (98%) was purchased from AK Scientific Inc. (CA, USA). 2,5-dihydroxy-3-undecyl-1,4-benzoquinone (embelin 98%) was purchased from 3B Scientific Corporation (IL, USA). Dulbecco’s phosphate buffered saline (DPBS) was purchased from Lonza (MD, USA). Methoxy-PEG5,000-OH, dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), trypsin-EDTA solution, Triton X-100, and Dulbecco’s Modified Eagle’s Medium (DMEM) were all purchased from Sigma-Aldrich (MO, USA). Fetal bovine serum (FBS), penicillin-streptomycin solution, and DiD (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate, D-307) were from Invitrogen (NY, USA). All solvents used in this study were HPLC grade.
2.2. Synthesis of PEG5K-EB2
PEG5k-EB2 was similarly synthesized according to our reported method for PEG3.5k-EB2 [14]. This involves the synthesis of benzoquinone followed by coupling to Boc-aspartic acid. Undecyl side chains were then installed onto each of the two benzoquinone rings. Finally, PEG was coupled to aspartic acid-EB2 through the deprotected amino group. The final product was analyzed by 1NMR and MALDI-TOF.
2.3. Preparation and characterization of PTX- and DiD-loaded PEG5K-EB2 micelles
PTX-solubilized micelles were prepared by the following method. PTX (10 mM in chloroform) was added to PEG5K-EB2 (10 mM in chloroform) with various carrier/drug ratios. The organic solvent was first removed by nitrogen flow to form a thin dry film of drug/carrier mixture. The film was further dried under high vacuum for 2 h to remove any traces of remaining solvent. Drug-loaded micelles were formed by suspending the film in DPBS. The drug-free micelles and DiD-loaded micelles were similarly prepared as described above. The mean diameter of PEG5K-EB2 micelles with or without loaded drug was assessed by dynamic light scattering (DLS). The morphology and size distribution of drug-free or PTX-loaded PEG5K-EB2 micelles were observed using transmission electron microscopy (TEM) after negative staining. The CMC of PEG5K-EB2 was determined by employing pyrene as a fluorescence probe as described before [14]. The concentration of PTX loaded in PEG5K-EB2 micelles was evaluated by HPLC as described previously [14]. The drug loading capacity (DLC) and drug loading efficiency (DLE) were calculated according to the following formula:
2.4. In vitro drug release study
An in vitro drug release study was carried out by dialysis using DPBS (PH = 7.4) containing 0.5% (w/v) Tween 80 as the release medium. Taxol formulation was employed as a control. Two mL of PTX-loaded PEG5K-EB2 micelles or Taxol (1 mg PTX/mL) were sealed in dialysis tubes (MWCO = 12 KDa, Spectrum Laboratories) which were then immersed in 200 mL release medium in a beaker covered with parafilm. The beakers were placed in an incubator shaker at 100 rpm and 37°C. The concentration of PTX remaining in the dialysis tubes at various time points was measured by HPLC with the detector set at 227 nm. Values were reported as the means from triplicate samples.
2.5. Cell culture
DU145 and PC-3 are two androgen-independent human prostate cancer cell lines. 4T1.2 is a mouse metastatic breast cancer cell line. All cell lines were cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin in a humidified environment at 37 °C with 5% CO2.
2.6. Cellular uptake of Nile red-loaded PEG5K-EB2 micelles
The cellular uptake study was conducted with Nile red as a hydrophobic fluorescence probe [25]. Nile red-loaded PEG5K-EB2 micelles (7.5:1, m/m, PEG5K-EB2: Nile red) were prepared via a solvent evaporation method as described above. PC-3 cells were seeded in 24-well plates at 2×104 cells per well in 1 mL complete DMEM and cultured for 24 h, followed by removal of culture medium and addition of Nile red-loaded PEG5K-EB2 micelles at the Nile red concentration of 1μg/mL. The cells were incubated at 37°C with 5% CO2 for 2 h. Subsequently, the nuclei of cells were stained with Hoechst33342 for 5 min. Cells were then washed with DPBS three times and fixed with 4% paraformaldehyde for 30 min at room temperature. Finally, the slides were rinsed with DPBS three times and mounted with cover slips and observed under a fluorescence microscope (Eclipse TE300 Microscope).
2.7. In vitro cytotoxicity study
The cytotoxicity of PTX formulated in PEG5K-EB2 micelles was assessed with three cancer cell lines (DU145, PC-3, and 4T1.2) and compared to Taxol formulation. Briefly, DU145, PC-3 or 4T1.2 cells were seeded in 96-well plates followed by 24 h of incubation in DMEM with 10% FBS and 1% streptomycin-penicillin. Various dilutions of PTX-loaded PEG5K-EB2 and Taxol (at the equivalent concentrations of PTX) were added to cells. Controls include PEG5K-EB2 and Cremophor/ethanol and they were added to cells at concentrations equivalent to those of carriers in the corresponding PTX formulation groups. Cells were incubated for 72 h and cell viability was assessed by MTT assay as described previously [14].
2.8. Hemolytic effect of PEG5K-EB2 micelles
Fresh blood samples were collected through cardiac puncture from rats. EDTA-Na2 was immediately added into 10 mL of blood to prevent coagulation. Red blood cells (RBCs) were separated from plasma by centrifugation at 1500 rpm for 10 min at 4°C. The RBCs were washed three times with 30 mL ice-cold DPBS. RBCs were then diluted to 2% w/v with ice-cold DPBS and utilized immediately for the hemolysis assay. One mL of diluted RBC suspension was treated with various concentrations (0.2 and 1.0 mg/mL) of PEG5k-EB2 and PEI, respectively, and then incubated at 37 °C in an incubator shaker for 4 h. The samples were centrifuged at 1500 rpm for 10 min at 4 °C, and 100 μL of supernatant from each sample was transferred into a 96-well plate. The release of hemoglobin was determined by the absorbance at 540 nm using a microplate reader. RBCs treated with Triton X-100 (2%) and DPBS were considered as the positive and negative controls, respectively. Hemoglobin release was calculated as (ODsample-ODnegative control)/(ODpositive control-ODnegative control) × 100%
2.9. Animals
Female BALB/c mice, 10–12 weeks were purchased from Charles River (Davis, CA). Male nude mice, 6–8 weeks ages, were purchased from Harlan (Livermore, CA). All animals were housed under pathogen-free conditions according to AAALAC guidelines. All animal-related experiments were performed in full compliance with institutional guidelines and approved by the Animal Use and Care Administrative Advisory Committee at the University of Pittsburgh.
2.10. Maximum tolerated dose (MTD)
Groups of 4 BALB/c mice were administered intravenously with Taxol (15, 20, 25 mg PTX/kg body weight), or PTX-loaded PEG5K-EB2 micelles (30, 50, 75, 100, 120 mg PTX/kg body weight), respectively. Changes in body weight and survival of mice were followed daily for two weeks. The MTD was defined as the dose that causes neither mouse death due to the toxicity nor greater than 15% of body weight loss or other remarkable changes in the general appearance within the entire period of the experiments.
2.11. Biodistribution of PEG5K-EB2 micelles via NIRF optical imaging
The in vivo biodistribution and tumor targeting efficiency of PEG5K-EB2 micelles were investigated by using a near infrared fluorescence dye, DiD. Two nude mice bearing bilateral s.c. PC-3 xenografts were used in this study. Two-hundred μL of DiD-loaded PEG5K-EB2 micelles were i.v. injected into each mouse and the concentration of DiD in the formulation was 0.4mg/mL. At indicated times, the two mice were scanned using a Carestream Molecular Imaging System, In-Vivo Multispectral FX PRO, with the excitation at 630 nm and the emission at 700 nm using a 30 second exposure time. Prior to and during each imaging, the mice were anesthetized by isoflurane inhalation. X-ray images were also taken for tumor location and overlaid with corresponding NIR images. After imaging, the mice were euthanized by CO2 overdose.
2.12. In vivo therapeutic study
Two mouse tumor models were used to examine the therapeutic effect of PTX formulated in PEG5K-EB2 micelles: a syngeneic murine breast cancer model (4T1.2) and a human prostate cancer (PC-3) xenograft model.
For the breast cancer model, 2 x 105 4T1.2 cells in 200 μL PBS were inoculated s.c. at the right flank of female BALB/c mice. Treatments were initiated when tumors in the mice reached a tumor volume around 50 mm3 and this day was designated as day 1. On day 1, mice were randomly divided into six groups (n=5) and received i.v. administration of free PEG5K-EB2 micelles, Taxol (10 mg PTX/kg), PTX-loaded PEG5K-EB2, and saline, respectively on days 1, 4, 7, 10, and 13. PTX-loaded PEG5K-EB2 micelles were given at two different dosages, 10 mg/kg and 20 mg PTX/kg, respectively. Free PEG5K-EB2 micelles were given at the equivalent dosage of the carrier in the group of PTX-loaded PEG5K-EB2 micelles (20 mg PTX/kg). Tumor sizes were measured with digital caliper twice a week and calculated according to the following formula: (L×W2)/2, where L is the longest and W is the shortest in tumor diameters (mm). To compare between groups, relative tumor volume (RTV) was calculated at each measurement time point (where RTV equals the tumor volume at a given time point divided by the tumor volume prior to first treatment). Mice were sacrificed when tumor reached 2000 mm3 or developed ulceration.
To monitor the potential toxicity, the body weights of all mice from different groups were measured every three days. In addition, serum level of transaminases (AST, ALT) in the mice treated with PTX/PEG5K-EB2 (20 mg PTX/kg) and PBS groups was investigated at the completion of the study.
For establishment of PC-3 xenograft tumor model, 2×106 PC-3 cells in 200 μL PBS were inoculated s.c. at the right flank in male nude mice. Treatments were started when tumors in the mice reached a volume around 50 mm3 and different groups (n = 6) were similarly treated as described above on days 1, 3, 7, 10, 13, 24, and 28. Tumor size and body weight were monitored as described above.
2.13. Statistical analysis
In all statistical analysis, the significance level was set at a probability of P < 0.05. All results were reported as the mean ± standard error (SEM) unless otherwise indicated. Statistical analysis was performed by Student’s t-test for two groups, and one-way ANOVA for multiple groups, followed by Newman-Keuls test if P < 0.05.
3. Results and discussion
3.1. Preparation and characterization of PTX-loaded PEG5K-EB2 micelles
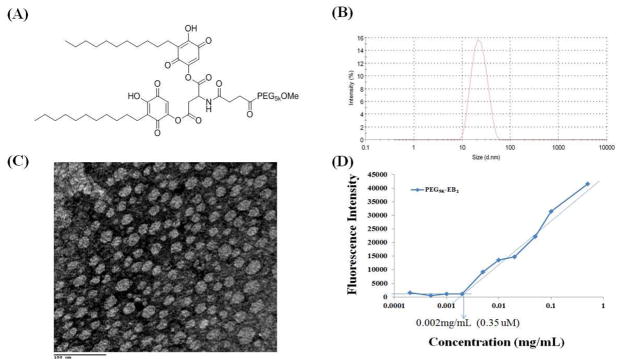
We previously developed a PEG3.5K-EB2 conjugate and preliminary in vitro study suggested that it functioned as a dual delivery system and showed synergistic activity with co-delivered PTX against several cancer cell lines [14]. In this report we developed a similar PEG-derivatized embelin conjugate with a PEG of higher MW (PEG5K) as parts of our efforts to improve the stability and loading capacity of this micellar system. The chemical structure of PEG5K-EB2 conjugate, in which two embelin molecules were linked to one molecule of PEG5K through a bridge of aspartic acid, is shown in Fig. 1A. The PEG5K-EB2 conjugate was synthesized via stepwise solution-phase condensation reactions using MeO-PEG-OH, succinic anhydride, Boc-aspartic acid and embelin as building blocks. HPLC shows that the final product (PEG5K-EB2) is at least 95.57% pure (Fig. S1). 1H NMR spectrum of PEG5K-EB2 shows signals at 3.63 ppm attributed to the methylene protons of PEG, the embelin proton signals at 8.14 and 6.72 ppm and the carbon chain signals at 1.05—1.25 ppm. The aspartate signals were identified at 5.57, 4.98 and 2.60 ppm (Fig. S2). The molecular weight of the PEG5K-EB2 conjugate from MALDI-TOF MS (5701) is similar to the theoretical value (5703) (Fig. S3), indicating the successful synthesis of PEG5K-EB2 conjugate.
Fig. 1.
(A) The chemical structure of PEG5K-EB2, (B) The size distribution of free PEG5K-EB2 nanoparticles in PBS measured by dynamic light scanning (DLS), (C) Transmission electron microscopy of PEG5K-EB2 micelles, and (D) critical micelle concentration (CMC) using pyrene as a fluorescence probe.
In aqueous solution, PEG5K-EB2 readily self-assembles to form micellar nanoparticles with the particle size of around 20 nm as determined by DLS analysis (Fig. 1B). Fig. 1C shows the TEM images of PEG5K-EB2 micelles after staining with 1% uranyl acetate. Spherical particles of uniform size were observed and the sizes of the micelles observed under TEM were consistent with those measured by DLS.
Fig. 1D shows the CMC of PEG5K-EB2 micelles using pyrene as a fluorescence probe. Upon incorporation into the micelles, the fluorescence intensity of pyrene increases substantially at the concentration of micelles above the CMC [26]. Based on the partition of the pyrene, the CMC of PEG5K-EB2 was obtained by plotting the fluorescence intensity versus logarithm concentration of the polymer. The CMC of PEG5K-EB2 was determined from the crossover point at the low concentration range. The CMC of the PEG5K-EB2 conjugate is 0.35 μM, which is much lower than most single chain micelle surfactants used in drug delivery systems (mM). The relatively low CMC may render the micelles stable upon dilution in vivo, which is important for effective delivery to tumors.
PEG5K-EB2 effectively solubilized PTX in aqueous solution. Tab. 1 compares PEG5K-EB2 with PEG3.5K-EB2 with respect to the sizes of PTX-loaded micelles, the drug loading capacity (DLC), and the drug loading efficiency (DLE) under various drug/carrier molar ratios. For PEG3.5K-EB2 micelles, a minimal 2.5/1 of carrier/PTX molar ratio was required to form stable PTX-loaded micelles. Under this ratio, the size of the drug-loaded micelles was around 143 nm, which was significantly larger than the size of drug-free micelles. Increasing the carrier/PTX ratios resulted in a decrease in the sizes of PTX-formulated micelles. At a carrier/PTX ratio of 7.5/1, the size of PTX-loaded PEG3.5K-EB2 micelles was similar to that of drug-free micelles.
Compared to PEG3.5K-EB2, PEG5K-EB2 conjugate requires much lower carrier/PTX ratios to form stable and small-sized PTX-loaded micelles. PTX-loaded PEG5K-EB2 micelles still maintained the small size (25 nm) even at the carrier/PTX ratio of 0.75:1 and PTX concentration of 1 mg/mL. Further increase in carrier/drug ratios was associated with an increase in the drug loading efficiency and the PTX concentrations at which PTX-loaded PEG5K-EB2 micelles remained stable. The improved stability and loading capacity for PEG5K-EB2 micelles compared to PEG3.5K-EB2 micelles is likely due to longer PEG brushes capable of providing better steric hindrance and stabilizing effect for micelle nanoparticles.
The size of drug carriers plays a key role in effective targeted delivery to tumors. It has been long known that particles in the size range of 100–200 nm can effectively penetrate solid tumors via an EPR effect [1,2]. However, a recent study reported that particles with a size of 154 nm were significantly taken up by liver and lungs with limited accumulation at tumor sites [3]. In contrast, particles with respective size of 17 and 64 nm were much more effective in passive targeting to the solid tumor in a subcutaneous model of human ovarian cancer xenograft [3]. The small size of PEG5K-EB2 micelles (20 ~ 30 nm) may explain their effective in vivo targeting as discussed later.
3.2. Release kinetics of PTX-loaded micelles
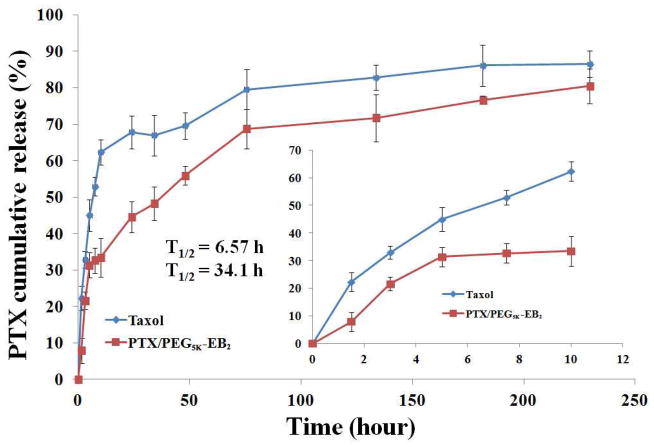
A dialysis method was used to assess the kinetics of release of PTX from PEG5K-EB2 micelles with DPBS (pH = 7.4) containing 0.5% Tween 80 (w/v) as the release medium. Taxol, a clinically used PTX formulation was included as a control. As shown in Fig. 2, PTX formulated PEG5K-EB2 exhibited significantly better stability than Taxol formulation. For the first 10 h, there was only 33.42% of PTX released from the PEG5K-EB2 micellar formulation in comparison to the 62.32% release in Taxol formulation. PTX-loaded PEG5K-EB2 micellar formulation displayed a much slower PTX release compared to Taxol formulation during the entire experimental period. The T1/2 of PTX release is 34.1 h for PEG5K-EB2 micelles, which is significantly longer than that for Taxol formulation (6.57 h). The relatively slower and sustained release in PTX-loaded PEG5K-EB2 micelle formulation may be ascribed to the strong interaction between the carriers and PTX. Embelin has a benzoquinone ring and a long alkyl chain. In addition to hydrophobic interaction with PTX, the π-π stacking and the hydrogen bonding also contribute to the overall carrier/PTX interaction. The close proximity of two embelins in PEG5K-EB2 conjugate is likely to facilitate the interaction of the carrier with PTX. Indeed, PEG-embelin conjugates of 1:1 molar ratio were much weaker solubilizer for hydrophobic drugs including PTX (data not shown). More studies on the structure-activity relationship (SAR) may lead to the development of an improved carrier for in vivo applications.
Fig. 2.
Cumulative PTX release profile from PTX-loaded PEG5K-EB2 micelles and Taxol. DPBS (PH = 7.4) containing 0.5% (w/v) Tween 80 was used as the release medium. T1/2 means the time needed to release half of the PTX from the formulations. Values reported are the means ± SD for triplicate samples.
3.3. Hemolysis assay
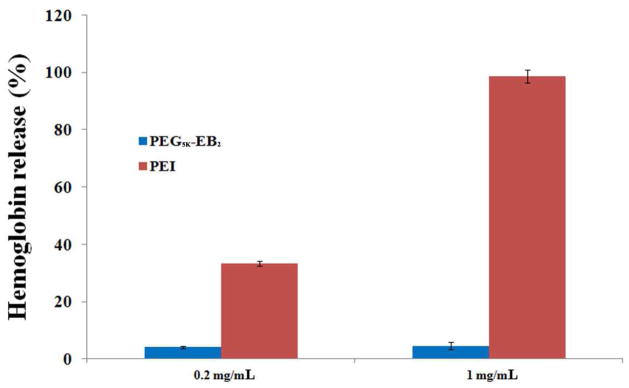
A major concern for micelle systems is whether or not the surface activity of the surfactant molecules affects cell membrane integrity. Therefore, we examined the hemolytic activity of drug-free PEG5K-EB2 micelles and compared to polyethylenimine (PEI), a cationic polymer with potent cell surface activity [27]. As shown in Fig. 3, treatment of RBCs with PEI resulted in significant hemolysis in a dose-dependent manner. In contrast, no significant hemolysis was observed for blank PEG5K-EB2 micelles. The negligible hemolytic activity suggests that PEG5K-EB2 conjugate is a mild surfactant that is suitable for in vivo drug delivery.
Fig. 3.
In vitrohemolysis assay of PEG5K-EB2 compared with PEI. Both PEG5K-EB2 and PEI with two different concentrations (0.2, 1 mg/mL) were incubated with rat red blood cells (RBCs) for 4 h at 37 °C in an incubator shaker. The degree of RBCs lysis was measured spectrophotometrically (λ=540 nm) according to the release of hemoglobin. (2%Triton X-100 and DPBS were used as a positive and negative control, respectively). Values reported are the means ± SD for triplicate samples
3.4. Cellular uptake study
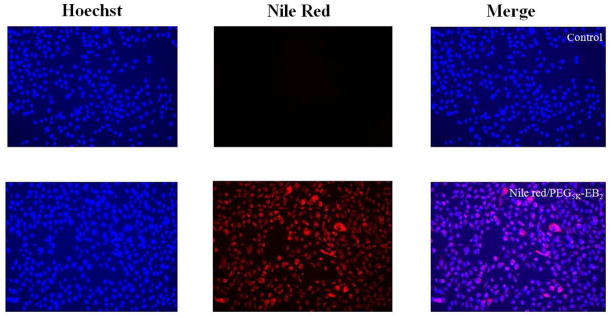
The cellular uptake of Nile red-loaded PEG5K-EB2 micelles in prostate cancer cell line PC-3 was investigated by fluorescence microscopy. PC-3 cells were cultured with Nile red-loaded PEG5K-EB2 micelles (equivalent concentration of Nile red at 1 μg/mL) at 37°C for 2 h. The nucleus was then stained with Hochest 33342 for 5 mins prior to observation under a fluorescence microscope. As shown in Fig 4, fluorescence was observed both on the cell membrane and inside the cells with most of the signals located intracellularly. Both perinuclear punctuate and diffuse distribution was observed, suggesting that Nile red-loaded PEG5K-EB2 was largely taken up by cells via endocytosis and partially released into cytoplasm. Escape of the delivered cargos from endosome into cytoplasm is important as this is where the drug target(s) is located. Although more studies are needed to understand the intracellular trafficking and the underlying mechanism, our data did suggest that PEG5K-EB2 micelles were capable of effectively mediating intracellular delivery of formulated drugs.
Fig. 4.
Fluorescence microscope images of PC-3 cells that incubated with Nile red-loaded PEG5K-EB2 for 2 h. Cell nuclei were stained with Hoechest 33342 prior to observation.
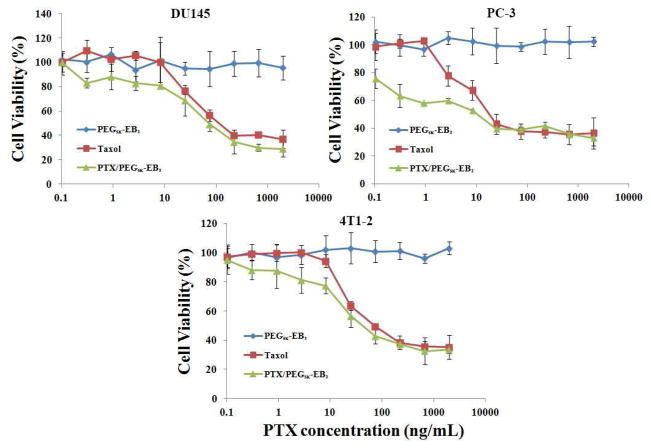
3.5. In vitro cytotoxicity of PTX-loaded PEG5K-EB2 micelles
In vitro cytotoxicity of PTX formulated in PEG5K-EB2 micelles was examined with three cancer cell lines (DU145, PC-3, and 4T1.2) and compared to Taxol formulation. PEG5K-EB2 alone showed minimal cytotoxic effect to human prostate cancer cells DU145 at the concentrations used to deliver PTX (Fig. 5A). It is also apparent from Fig. 5A that PTX formulated in PEG5K-EB2 micelles showed higher levels of cytotoxicity to DU145 cells compared to Taxol formulation, particularly at low PTX concentrations. Similar results were obtained in PC-3 (Fig. 5B) and 4T1.2 (Fig. 5C) tumor cells. Most of the reported PTX micellar formulations showed lower or similar levels of cytotoxicity compared to Taxol [3,28–30]. The improved in vitro cytotoxicity of PTX formulated in PEG5K-EB2 micelles may be due to the improved bioavailability of PTX inside the tumor cells. It remains to be tested whether there is also a synergistic effect between PEG5K-EB2 micelles and the co-delivered PTX. It has been reported that under the subeffective doses, embelin sensitized tumor cells to various types of therapies including chemotherapy and radiotherapy [14,31,32]. Embelin is coupled to PEG via a cleavable ester linkage, embelin may be freed from the conjugate following intracellular delivery and synergizes with co-delivered PTX in antitumor activity. It should be noted that PEG5K-EB2 itself is less active in antitumor activity than PEG3.5K-EB2 [14]. This might be due to less effective release of embelin from PEG5K-EB2 due to a more pronounced steric hindrance imposed by PEG5K. More studies are needed to better understand the mechanism involved in the antitumor effect of PTX-loaded PEG5K-EB2 micelles.
Fig. 5.
Cytotoxicity of Taxol, free PEG5K-EB2, and PTX-loaded PEG5K-EB2 nanoparticles against two androgen-independent human prostate cancer cell lines DU145 and PC-3, the 4T1-2 mouse breast cancer cell line. Cells were treated for 72 h and cytotoxicity was determined by MTT assay. Values reported are the means ± SD for triplicate samples
3.6. Maximum tolerated dose study
The maximum tolerated dose for a single i.v. administration of PTX-loaded PEG5K-EB2 micelles was assessed in tumor-free mice and compared to Taxol. The mice were injected i.v. with different doses of PTX-loaded PEG5K-EB2 or Taxol followed by daily body weight measurement and observation of general signs of toxicity. As shown in Tab. 2, Taxol was well tolerated at the dose of 15 mg PTX/kg. However, increasing the PTX dosage to 20 mg/kg resulted in the death of 2 mice among the 4 treated mice. For the mice treated with PTX-loaded PEG5K-EB2 micelles, there were only 8.7% weight loss and no noticeable changes in normal activity at a PTX dosage as high as 100 mg/kg. At the dosage of 120 mg PTX/kg, two out of 4 treated mice died of toxicity. Based on these data it was estimated that the single i.v. MTD for Taxol was 15~20 mg PTX/kg while that for PTX-loaded PEG5K-EB2 micelles was 100~120 mg PTX/kg. The MTD for PTX-loaded PEG5K-EB2 micelles is higher than most of the reported PTX formulations [33–35]. The high MTD for PTX/PEG5K-EB2 is likely due to the slow release kinetics for PTX (Fig. 2), low levels of nonselective uptake by major organs (see later), and the excellent safety profile of embelin. Embelin has antiinflammatory and hepatoprotective activity [15,17]. In addition, normal tissues are less sensitive to embelin compared to tumor cells due to the significantly lower levels of XIAP expression in normal tissues. The significantly improved safety of our delivery system over Taxol formulation will allow high dosage of PTX to be given to achieve maximal therapeutic effect.
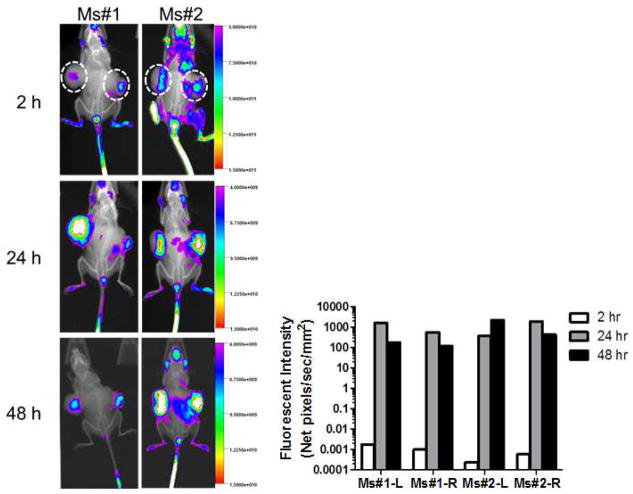
3.7. Biodistribution of PEG5K-EB2 micelles via NIRF optical imaging
Biodistribution and tumor targeting efficiency of PEG5K-EB2 micelles were evaluated in a mouse xenograft model of human prostate cancer (PC-3), using a hydrophobic near infrared fluorescence (NIRF) dye, DiD. Two hundred μL of micelles co-loaded with PTX and DiD was intravenously injected into two mice bearing bilateral PC-3 tumors, respectively. The two mice were then followed over time by the scanning with Carestream Molecular Imaging System. Fig. 6 shows the imaging of the tumor-bearing mice at 2, 24, 48 h following i.v. injection of PTX/PEG5K-EB2 mixed micelles carrying DiD. A noticeable signal in tumor was observed as early as 2 h post injection; the signal peaked around 24 h and remained clearly visible 48 h after injection. Interestingly, little fluorescence signal was observed in liver and spleen, the two major internal organs that are involved in the nonspecific clearance of nanoparticles by the reticuloendothelial system (RES). The effective targeting of PEG5K-EB2 micelles to the tumors and the minimal uptake by RES system are largely due to the very small-sized particles, excellent PEG shielding effect, and a likely excellent stability in the blood circulation. Our results were consistent with the studies with other micellar systems of similar particle sizes [3,35,36].
Fig. 6.
In vivo NIRF imaging over time as indicated in prostate cancer PC-3-xenograft-bearing mice at 2, 24, 48 h following i.v. injection of PEG5K-EB2 micelles co-loaded with PTX and DiD.
3.8 In vivo therapeutic study
The in vivo therapeutic activity of PTX formulated in PEG5K-EB2 micelles was investigated in two mouse tumor models: a syngeneic murine breast cancer model (4T1.2) and a human prostate cancer xenograft model (PC-3).
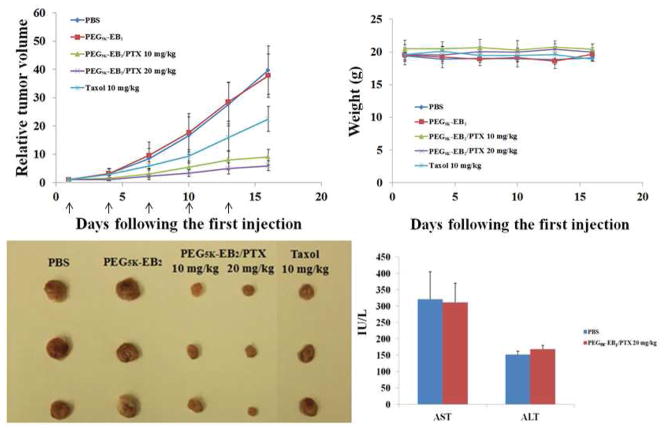
4T1.2 is a highly metastatic breast cancer cell line and was chosen in this study to stringently assess the therapeutic efficacy of our new delivery system. As shown in Fig. 7A, PEG5K-EB2 alone showed no effect in inhibiting the tumor growth. This is likely due to a low concentration of embelin in this group. Taxol formulation showed a modest effect in inhibiting the tumor growth at a dose of 10 mg PTX/kg. In contrast, PTX formulated in PEG5K-EB2 micelles showed a much more pronounced antitumor activity at the same dosage. Increasing the PTX dosage to 20 mg/kg resulted in a further improvement in the therapeutic effect. No significant changes in body weight were noticed in all treatment groups compared to PBS control group (Fig. 7B). In addition, serum levels of transaminases in the mice treated with the high dose of PTX-loaded PEG5K-EB2 micelles were comparable to those in PBS control group (Fig. 7C), suggesting that significant therapeutic effect can be achieved with minimal toxicity using our new delivery system.
Fig. 7.
(A) Enhanced antitumor activity of PTX formulated in PEG5K-EB2 micelles. BABL/c mice were inoculated s.c. with 4T1-2 cells (2 x 105 cells/mouse). Five days later, mice received various treatments on days 1, 4, 7, 10, and 13, and tumor growth was monitored and plotted as relative tumor volume. P < 0.01 (20 mg/kg PTX/PEG5K-EB2 vs. Taxol), P < 0.02 (10 mg/kg PTX/PEG5K-EB2 vs. Taxol), P < 0.05 (20 mg/kg PTX/PEG5K-EB2 VS 10 mg/kg PTX/PEG5K-EB2 ). N = 5. (B) Changes of body weight in mice receiving different treatments (C): Serum level of transaminase in the mice treated with PTX/PEG5K-EB2 (20 mg PTX/kg) at the end of the study.
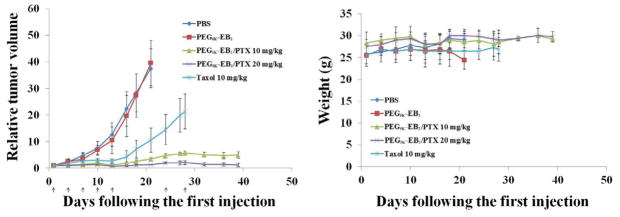
Following the demonstration of effective antitumor activity in the syngeneic murine breast cancer model, the in vivo therapeutic effect of PTX-loaded PEG5K-EB2 micelles was further investigated in a human prostate cancer xenograft model (PC-3). PC-3 tumor-bearing mice were similarly treated as described in the study with the 4T1.2 tumor model and the data are shown in Fig. 8A. It is apparent that tumor growth was more effectively controlled by PTX/PEG5K-EB2 micelles in PC-3 model compared to 4T1.2 tumor model. By day 16 after the first treatment, the tumor growth was completely suppressed with a RTV of 0.84 in the group treated with a high dose (20 mg PTX/kg) of PTX-loaded PEG5K-EB2 micelles. Tumor growth was also significantly slowed in the group treated with a low dose (10 mg PTX/kg), in which the tumors only reached a RTV of 1.75. This compared very favorably to Taxol group, in which RTV reached 4.24. Although the tumors started to recover slightly after day 24 in the two groups treated with PTX/PEG5K-EB2 mixed micelles, RTV was reduced back to 1.15 at day 39 in the high dose group following two additional treatments at days 24 and 28. In fact, two out of 6 mice in this group became tumor-free after day 32 without further treatment. The growth of tumor in the low dose group also became static after two additional treatments. In contrast, tumors in Taxol group continued to grow at a steady and fast rate. No noticeable changes in weight were shown from direct measurement of tumor-bearing mice in all groups (Fig. 8B). The superior anti-tumor efficacy along with the minimal toxicity of PTX/PEG5K-EB2 micelles could be ascribed to their high efficiency in tumor-targeting and minimal nonspecific uptake by RES (Fig. 6). The slow release kinetics of PTX/PEG5K-EB2 micelles may also contribute to the enhanced antitumor activity. More studies are needed to better understand the mechanism for the antitumor activity of PTX-loaded PEG5K-EB2 micelles.
Fig. 8.
(A) Enhanced antitumor activity of PTX formulated in PEG5K-EB2 micelles. Nude mice were inoculated s.c. with PC-3 cells (2 x 106 cells/mouse). A week later, mice received various treatments on days 1, 3, 7, 10, 13, 24, and 28, and tumor growth was monitored and plotted as relative tumor volume. P < 0.005 (20 mg/kg PTX/PEG5K-EB2 vs. Taxol), P < 0.01(10 mg/kg PTX/PEG5K-EB2 vs. Taxol), P < 0.05 (20 mg/kg PTX/PEG5K-EB2 VS 10 mg/kg PTX/PEG5K-EB2 ), N = 6. (B) Changes of body weight in mice receiving different treatments
Conclusion
A conjugate of PEG5K with two embelin molecules (PEG5K-EB2) forms small sized micelles (20 ~ 30 nm) that effectively solubilize hydrophobic drugs such as PTX. Compared to a similar conjugate with a lower MW PEG (PEG3.5K-EB2), PEG5K-EB2 gives increased drug loading capacity and forms stable drug-loaded micelles at lower carrier/drug ratios. PEG5K-EB2 micelles have a low CMC and are effective in mediating intracellular delivery of loaded agents. PTX-loaded PEG5K-EB2 micelles show a kinetics of sustained release and are effectively targeted to tumors in vivo with minimal nonspecific uptake by RES. PTX formulated in PEG5K-EB2 micelles exhibited potent cytotoxicity to several cultured cancer cell lines. In vivo, PTX-loaded PEG5K-EB2 micelles demonstrated an excellent safety profile with a MTD of 100 ~ 120 mg PTX/kg, which was significantly higher than that (15 ~ 20 mg PTX/kg) for Taxol. Furthermore, superior antitumor activity over Taxol formulation was demonstrated in both breast cancer and prostate cancer models. In a human prostate cancer xenograft model (PC-3), complete inhibition of tumor growth was achieved with minimal toxicity to the animals. Our results suggest that PEG5K-EB2 is a safe and effective drug delivery system that warrants more studies in the future.
Supplementary Material
Table 1.
Biophysical characterizations of free and drag-loaded PEG-Embelin micelles
| Micelles | Molar ratio | Size(nm) | PDI | Conc. of PTX in micelles (mg/ml) | DLC (%) | DLE (%) |
|---|---|---|---|---|---|---|
| PEG3.5k-EB2 | — | 22.8±0.3 | 0.09 | — | — | — |
| PEG3.5K-EB2:PTX | 2.5:1 | 143±17 | 0.23 | 1 | 7.5 | 79.9 |
| 5:1 | 58.7±0.5 | 0.32 | 1 | 3.9 | 96.7 | |
| 7.5:1 | 27.5±0.2 | 0.23 | 1 | 2.6 | 98.6 | |
|
| ||||||
| PEG5K-EB2 | — | 20.6±0.1 | 0.05 | — | — | — |
| PEG5K-EB2:PTX | 0.75:1 | 25.5±1.0 | 0.06 | 1 | 16.6 | 63.7 |
| 1:1 | 21.7±0.4 | 0.25 | 1 | 13.0 | 70.8 | |
| 2 | 13.0 | 62.4 | ||||
| 2.5:1 | 22.0±0.28 | 0.04 | 1 | 5.6 | 93.1 | |
| 2 | 5.6 | 90.8 | ||||
| 5:1 | 21.9±0.32 | 0.01 | 1 | 2.9 | 98.6 | |
| 2 | 2.9 | 94.9 | ||||
| 7.5:1 | 22.2±0.14 | 0.11 | 1 | 2.0 | 96.6 | |
| 2 | 2.0 | 94.9 | ||||
| 3 | 2.0 | 84.4 | ||||
PDI = polydispersity index. DLC = drug loading capacity. DLE = drug loading efficiency. PEG3.5k-EB2 = PEG3.5K-Embelin2. PEG5K-Eb2 = PEG5K-Embelin2. PTX = paclitaxel.
PTX concentrations in micelles were kept at 1 mg/mL.
Blank micelle concentration was 20 mg/mL.
Values reported are the means ± SD for triplicate samples.
Table 2.
Animal deaths and weight loss in the MTD study
| Formulations | Does (mg/kg) | Animal death | Weight loss (%) |
|---|---|---|---|
| Taxol | 15 | 0/4 | 5.4 |
| 20 | 2/4 | N/A | |
| 25 | 4/4 | N/A | |
|
| |||
| PTX-loaded PEG5K-EB2 micelles | 30 | 0/4 | −1.2 |
| 50 | 0/4 | 1.6 | |
| 75 | 0/4 | 6.5 | |
| 100 | 0/4 | 8.7 | |
| 120 | 2/4 | N/A | |
Acknowledgments
This work was supported in part by NIH grants (R21CA128415 and R21CA155983) and a DOD grant (BC09603). We would like to thank Drs. Donna Stolz and Ming Sun for their help with negative EM study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumouritropic accumulation of proteins and the antitumour agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 2.Gao Z, Lukyanov AN, Singhal A, Torchilin VP. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2:979–82. [Google Scholar]
- 3.Luo J, Xiao K, Li Y, Lee JS, Shi L, Tan YH, et al. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjugate Chem. 2010;21:1216–24. doi: 10.1021/bc1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 5.Sutton D, Nasongkla N, Blanco E, Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res. 2007;24:1029–46. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 6.Mi Y, Liu Y, Feng SS. Formulation of docetaxel by folic acid-conjugated D-α-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS2k) micelles for targeted and synergistic chemotherapy. Biomaterials. 2011;32:4058–66. doi: 10.1016/j.biomaterials.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Croy SR, Kwon GS. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12:4669–84. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Feng SS. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials. 2006;27:262–70. doi: 10.1016/j.biomaterials.2005.05.104. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Lee SH, Gan CW, Feng SS. In vitro and in vivo investigation on PLA-TPGS nanoparticles for controlled and sustained small molecule chemotherapy. Pharm Res. 2008;8:1925–35. doi: 10.1007/s11095-008-9611-6. [DOI] [PubMed] [Google Scholar]
- 10.Prashant C, Dipak M, Yang CT, Chuang KH, Jun D, Feng SS. Superparamagnetic iron oxide--loaded poly(lactic acid)-D-alpha-tocopherol polyethylene glycol 1000 succinate copolymer nanoparticles as MRI contrast agent. Biomaterials. 2010;31:5588–97. doi: 10.1016/j.biomaterials.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 11.Ji X, Wang Z, Geamanu A, Sarkar FH, Gupta SV. Inhibition of cell growth and induction of apoptosis in non-small cell lung cancer cells by delta-tocotrienol is associated with notch-1 down-regulation. J Cell Biochem. 2011;112:2773–83. doi: 10.1002/jcb.23184. [DOI] [PubMed] [Google Scholar]
- 12.Husain K, Francois RA, Yamauchi T, Perez M, Sebti SM, Malafa MP. Vitamin E δ-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-κB activation in pancreatic cancer. Mol Cancer Ther. 2011;10:2363–72. doi: 10.1158/1535-7163.MCT-11-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Huang L, Liu F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol Pharm. 2010;7:863–9. doi: 10.1021/mp100012s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Lu J, Gao X, Li J, Zhao W, Sun M, et al. PEG-derivatized embelin as a dual functional carrier for the delivery of paclitaxel. Bioconjug Chem. 2012;23:1443–51. doi: 10.1021/bc3000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chitra M, Sukumar E, Suja V, Devi CS. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. 1994;40:109–13. doi: 10.1159/000239181. [DOI] [PubMed] [Google Scholar]
- 16.Bhandari U, Jain N, Pillai KK. Further studies on antioxidant potential and protection of pancreatic beta-cells by embelia ribes in experimental diabetes. Exp Diabetes Res. 2007;2007:15803–08. doi: 10.1155/2007/15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh D, Singh R, Singh P, Gupta RS. Effect of embelin on lipid peroxidation and free radical scavenging activity against liver damage in rats. Basic Clin Pharmacol Toxicol. 2009;105:243–8. doi: 10.1111/j.1742-7843.2009.00429.x. [DOI] [PubMed] [Google Scholar]
- 18.Danquah M, Li F, Duke CB, Miller DD, Mahato RI. Micellar delivery of bicalutamide and embelin for treating prostate cancer. Pharm Res. 2009;26:2081–92. doi: 10.1007/s11095-009-9903-5. [DOI] [PubMed] [Google Scholar]
- 19.Sreepriya M, Bali G. Chemopreventive effects of embelin and curcumin against N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in wistar rats. Fitoterapia. 2005;76:549–55. doi: 10.1016/j.fitote.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Dai Y, Qiao L, Chan KW, Yang M, Ye J, Ma J, et al. Peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of embelin on colon carcinogenesis. Cancer Res. 2009;69:4776–83. doi: 10.1158/0008-5472.CAN-08-4754. [DOI] [PubMed] [Google Scholar]
- 21.Heo JY, Kim HJ, Kim SM, Park KR, Park SY, Kim SW, et al. Embelin suppresses STAT3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase PTEN. Cancer Lett. 2011;308:71–80. doi: 10.1016/j.canlet.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Nikolovska-Coleska Z, Xu L, Hu Z, Tomita Y, Li P, Roller PP, et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three dimensional structure database. J Med Chem. 2004;47:2430–40. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 23.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–803. [PubMed] [Google Scholar]
- 24.Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–61. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 25.Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescence stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–73. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabanov AV, Nazarova IR, Astafieva IV, Batrakova EV, Alakhov VY, Yaroslavov AA, et al. Micelle formation and solubilization of fluorescent probes in poly-(oxyethylene-b-oxypropylene-b-oxyethylene) solutions. Macromolecules. 1995;28:2303–14. [Google Scholar]
- 27.Reul R, Nguyen J, Kissel T. Amine-modified hyperbranched polyesters as non-toxic, biodegradable gene delivery systems. Biomaterials. 2009;30:5815–24. doi: 10.1016/j.biomaterials.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, He Y, Ma G, Song C, Sun H. Paclitaxel-loaded polymeric micelles based on poly( ε-caprolactone)-poly(ethyleneglycol)-poly( ε-caprolactone) triblock copolymers: in vitro and in vivo evaluation. Nanomedicine. 2012;8:925–34. doi: 10.1016/j.nano.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Xiao K, Luo J, Lee JS, Pan S, Lam KS. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. J Control Release. 2010;144:314–23. doi: 10.1016/j.jconrel.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Xiao K, Luo J, Xiao W, Lee JS, Gonik AM, et al. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 2011;32:6633– 45. doi: 10.1016/j.biomaterials.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai Y, Desano J, Qu Y, Tang W, Meng Y, Lawrence TS, et al. Natural IAP inhibitor embelin enhances therapeutic efficacy of ionizing radiation in prostate cancer. Am J Cancer Res. 2011;1:128–43. [PMC free article] [PubMed] [Google Scholar]
- 32.Danquah M, Duke CB, 3rd, Patil R, Miller DD, Mahato RI. Combination therapy of antiandrogen and XIAP inhibitor for treating advanced prostate cancer. Pharm Res. 2012;29:2079–91. doi: 10.1007/s11095-012-0737-1. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Wang Y, Wang Y, Fan M, Luo F, Qian Z. Characterization, pharmacokinetics and disposition of novel nanoscale preparations of paclitaxel. Int J Pharm. 2011;414:251–9. doi: 10.1016/j.ijpharm.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Danhier F, Magotteaux N, Ucakar B, Lecouturier N, Brewster M, Préat V. Novel self assembling PEG-p-(CL-co-TMC) polymeric micelles as safe and effective delivery system for paclitaxel. Eur J Pharm Biopharm. 2009;73:230–8. doi: 10.1016/j.ejpb.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Xiao K, Luo J, Fowler WL, Li Y, Lee JS, Xing L, et al. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30:6006–16. doi: 10.1016/j.biomaterials.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng N, Dai W, Du W, Zhang H, Lei L, Zhang H, et al. A novel lanreotide-encoded micelle system targets paclitaxel to the tumors with overexpression of somatostatin receptors. Mol Pharm. 2012;9:1175–88. doi: 10.1021/mp200464x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.