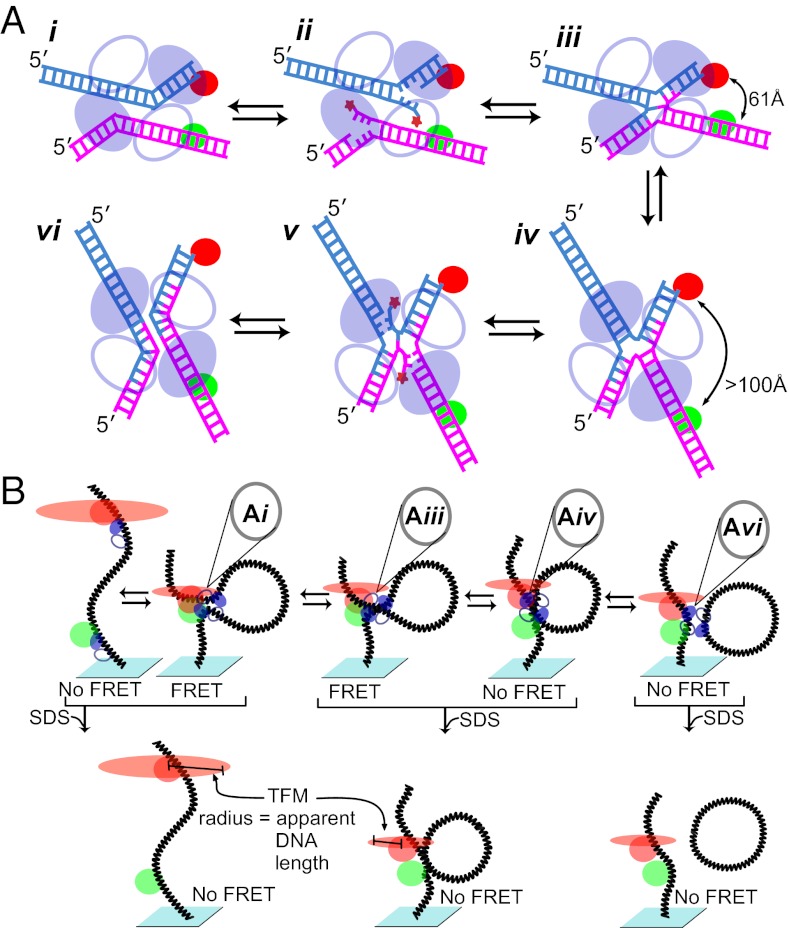
The Cre recombination system, which has been commonly used as a bioengineering tool for altering eukaryotic genomes in a highly efficient and precise manner, is an archetypical member of a large family of tyrosine site-specific DNA recombinases. Different family members are responsible for a wide range of biological pathways such as regulation of gene expression and DNA replication, plasmid copy number maintenance, conjugative transposition, catenated circle resolution, daughter chromosome segregation, and prokaryotic telomere processing. Despite this wide-ranging biology, it is thought that all family members proceed through a common multistep reaction pathway (Fig. 1A) in which a recombinase tetramer executes a sequential pair of strand exchanges that first generate (Fig. 1A, i–iii) and then resolve (Fig. 1A, iv–vi) a four-way DNA Holliday junction (HJ) intermediate. Recombination is accomplished without high-energy cofactors via two pairs of DNA cleavage and ligation reactions staggered by 6 to 8 bp and mediated by covalent 3′-phosphotyrosine linkages (Fig. 1A, ii and iv). By elegantly pairing two single molecule techniques, Pinkney et al. (1) extract several details from this important model system and provide a framework for investigations into other systems.
Fig. 1.
Cre-mediated site-specific recombination and its analysis by dual-aspect single-molecule microscopy. (A) Schematic of the site-specific recombination reaction carried out by the tyrosine family of recombinases. Parental DNAs (loxP in the Cre system; magenta and cyan) are recombined to form two hybrid DNA products. All strand exchanges proceed through two successive transesterification reactions: a strand cleavage reaction (ii and v) in which a tyrosine side chain attacks the DNA backbone and a ligation (iii and vi) in which the resulting phosphotyrosine linkage is attacked by a free DNA 5′ hydroxyl (star). Pairs of DNA-bound recombinase protomers (ovals) synapse to form a tetramer and a pseudo–four-way DNA junction (i) in which only one pair (filled) is activated for DNA cleavage (ii). Following the initial strand exchange, the arms of the resulting HJ (iii) scissor into the alternate bias (iv), and the reactivity of the recombinase pairs is reversed for the second pair of strand exchanges (iv). A pair of fluorescent dyes spectrally suitable for FRET (red and green circles) is placed in strategic positions along the DNA substrate to distinguish the DNA arm position bias of each nucleoprotein complex. The dye configuration depicted yields a FRET signal only when the nucleoprotein complex has a bottom-strand bias, as depicted in i to iii, whereas the dye pair is too far apart in the top-strand bias position. An alternate dye pair placement yields an energy transfer signal only when the DNA structure has a top-strand bias, as shown in iv to vi. (B) Brownian motion of the dye distal to the glass surface signals the apparent length of the tethered DNA, and thus indicates whether the recombination sites remain unpaired (Left) or have synapsed to create a tetramer (Center). Unpaired loxP sites have longer apparent DNA tether lengths with a larger time-averaged fluorescent spot radius (red ovals) than the shorter apparent tether lengths created by synapsis or recombination. An SDS challenge (Lower) is used to determine whether the tetramer has covalently exchanged one or both pairs of DNA strands, creating an HJ intermediate (Lower Center) or recombinant product (Lower Right), respectively.
The study of site-specific recombination has benefited greatly from the use of single-molecule techniques. The reactions are asynchronous and highly reversible, and the nucleoprotein complexes involved are often too massive and hydrophobic to study by using traditional molecular biology techniques such as native gel electrophoresis or surface plasmon resonance. The first single-molecule technique used to study a site-specific recombination reaction used a microscopically visible bead tethered to a slide surface by a DNA containing target sequences necessary for the bacteriophage λ-integrase–mediated excision reaction (2). By tracking the Brownian motion of the tethered particle, the apparent DNA tether length could be determined in real time, revealing various reaction details, including the rate of large nucleoprotein complex assembly, the rate of synapsis or recombinogenic partner joining, the stability of multiple complexes, and the rate of covalent DNA changes when challenged with SDS. Each reaction complex was then validated by its ability to complete the reaction, and off-pathway complexes were discarded.
Pinkney et al. (1) improve on this method by using the Brownian motion of a fluor rather than a microsphere to determine the apparent length of the DNA tether (Fig. 1B), eliminating any forces exerted on the DNA by the tracked particle (3). By using the tethered fluor method (TFM), synapsis of the two Cre-bound loxP recombination sites was found to be rapid and most likely diffusion-limited, as it is in the λ-system. Also in accordance with the previous λ-studies, Pinkney et al. (1) find that the stability of the recombined product complex is very long-lived. Although previous tethered particle motion experiments on the Cre system suggested that the product complex dissociated more rapidly (4), Pinkney et al. (1) use fluorescence correlation spectroscopy to confirm their results; they speculate that the earlier tethered particle motion results were effected by the force exerted on the DNA by the microsphere.
Pinkney et al. (1) further amplify the resolution of their experiments by combining TFM with single-molecule fluorescent resonance energy transfer (smFRET) measurements. As previous work in the field showed (5, 6), inclusion of a pair of dyes (a donor and acceptor) suitable for smFRET allows the authors to detect subtle distance changes between the dye pair within individual recombination intermediates. As an added bonus, the fluorophore used for TFM was also the acceptor for smFRET, and both measurements were made by using only a simple total internal reflection microscope. By marrying the two techniques, the authors are able to determine long-range and subtle DNA structure changes in real time (1).
A central feature of the tyrosine recombinases is a four-way DNA junction whose arms deviate from square planar by ∼11° in two opposite directions (7, 8), and the arm orientation determines which pair of partner recombinases will cleave a DNA strand (Fig. 1A, iii and iv). By using the Cre–HJ cocrystal structures as a guide (7), the authors (1) place the donor and acceptor dyes at strategic positions to distinguish between bottom- and top-strand–biased cruciform nucleoprotein complexes, whether a synapsed pseudo-HJ (Fig. 1A, i) or a covalent HJ (Fig. 1A, iii and iv). With one particular dye configuration, junction structures with a bottom-strand bias yielded FRET whereas complexes with a top-strand bias did not. An alternate configuration of the dye pair confirmed the opposite bias, yielding FRET only when top-strand biased complexes were formed.
The orientation of the pseudojunction arms within the initial synaptic complex (Fig. 1A, i) determines the first pair of DNA strands exchanged (6, 9): “bottom” strands in the Cre reaction. Pinkney et al. (1) determine that this directionality results in the formation of a bottom-strand–biased synaptic structure eight times more frequently than formation of the top-strand–biased structure. Intriguingly, the
The innovative coupling of TFM and smFRET by Pinkney et al. provides a powerful blueprint for investigators of other systems featuring short- and long-range changes in DNA or RNA conformation.
low number of reactions observed to initiate through a top-strand-first mechanism successfully formed recombinant products with nearly the same efficiency as reactions initiated by bottom-strand complexes.
Central to understanding the reaction mechanism of site-specific recombination is isomerization, or the switch between an HJ complex of one bias to a complex of the opposite bias (Fig. 1A, iii and iv). Isomerization is central to the switching of cleavage-active recombinase pairs and precedes rapid resolution of the HJ intermediate (10). It is, or is closely linked to, the rate-limiting step of the overall reaction. When formed with WT Cre, nucleoprotein complexes had a slightly lower mean FRET value than complexes formed with Cre mutants defective in progressing through the isomerization step (11, 12). Given the positions of the dye pair on the HJ arms, such a result suggests that the arms of the WT complex are rapidly interconverting between bottom- and top-strand biases and the FRET signal represents a mean signal between the two HJ biases. Pinkney et al. (1) go further and use a Monte Carlo simulation to determine that the WT complexes oscillate between orientations at rates that favor the bottom-strand bias at a 3:1 ratio. However, this very interesting suggestion is based on the assumption that the complexes assembled with mutant recombinases fail to interconvert between both arm orientations or interconvert with an equilibrium that favors the bottom-strand bias more heavily than a 3:1 ratio.
The rapid interconversion of HJ arm bias before isomerization raises some interesting questions about the nature of isomerization and the rate-limiting step. Does ligation efficiency of the first pair of strand exchanges vary with changes in HJ arm interconversion rates? Is ligation of the first exchanged strand pair the rate-limiting switch, or is a large conformational change in the nucleoprotein complex responsible? Which mechanical or chemical step is rate-limiting? Until now, these question have been almost unthinkably buried within a quagmire of subtle allosteric movement and rapidly oscillating chemical equilibria. We can optimistically anticipate the answers to such questions when these elegant single-molecule techniques (and their descendents) are combined with a host of additional tools, such as DNA-incorporated analogues (13) or peptides (14) that prevent ligation or cleavage.
The innovative coupling of TFM and smFRET by Pinkney et al. (1) provides a powerful blueprint for investigators of other systems featuring short- and long-range changes in DNA or RNA conformation. Researchers in other recombination systems such as homologous recombination and transposition would seem to be the most immediate beneficiaries of the work. However, other potential biological targets, such as transcription initiation complexes, nucleosomes, and spliceosomes, also spring to mind.
Footnotes
The authors declare no conflict of interest.
See companion article on page 20871.
References
- 1.Pinkney JNM, et al. Capturing reaction paths and intermediates in Cre-loxP recombination using single-molecule fluorescence. Proc Natl Acad Sci USA. 2012;109:20871–20876. doi: 10.1073/pnas.1211922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mumm JP, Landy A, Gelles J. Viewing single lambda site-specific recombination events from start to finish. EMBO J. 2006;25(19):4586–4595. doi: 10.1038/sj.emboj.7601325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segall DE, Nelson PC, Phillips R. Volume-exclusion effects in tethered-particle experiments: bead size matters. Phys Rev Lett. 2006;96(8):088306. doi: 10.1103/PhysRevLett.96.088306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan H-F. Real-time single-molecule tethered particle motion experiments reveal the kinetics and mechanisms of Cre-mediated site-specific recombination. Nucleic Acids Res. 2012;40(13):6208–6222. doi: 10.1093/nar/gks274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinney SA, Déclais A-C, Lilley DMJ, Ha T. Structural dynamics of individual Holliday junctions. Nat Struct Biol. 2003;10(2):93–97. doi: 10.1038/nsb883. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh K, Lau C-K, Gupta K, Van Duyne GD. Preferential synapsis of loxP sites drives ordered strand exchange in Cre-loxP site-specific recombination. Nat Chem Biol. 2005;1(5):275–282. doi: 10.1038/nchembio733. [DOI] [PubMed] [Google Scholar]
- 7.Gopaul DN, Guo F, Van Duyne GD. Structure of the Holliday junction intermediate in Cre-loxP site-specific recombination. EMBO J. 1998;17(14):4175–4187. doi: 10.1093/emboj/17.14.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas T, et al. A structural basis for allosteric control of DNA recombination by lambda integrase. Nature. 2005;435(7045):1059–1066. doi: 10.1038/nature03657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee L, Chu LCH, Sadowski PD. Cre induces an asymmetric DNA bend in its target loxP site. J Biol Chem. 2003;278(25):23118–23129. doi: 10.1074/jbc.M302272200. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Voziyanov Y, Pathania S, Jayaram M. Structural alterations and conformational dynamics in Holliday junctions induced by binding of a site-specific recombinase. Mol Cell. 1998;1(4):483–493. doi: 10.1016/s1097-2765(00)80049-0. [DOI] [PubMed] [Google Scholar]
- 11.Hoess R, Wierzbicki A, Abremski K. Isolation and characterization of intermediates in site-specific recombination. Proc Natl Acad Sci USA. 1987;84(19):6840–6844. doi: 10.1073/pnas.84.19.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton DL, Abremski K. Site-specific recombination by the bacteriophage P1 lox-Cre system. Cre-mediated synapsis of two lox sites. J Mol Biol. 1984;178(2):481–486. doi: 10.1016/0022-2836(84)90154-2. [DOI] [PubMed] [Google Scholar]
- 13.Kitts PA, Nash HA. An intermediate in the phage lambda site-specific recombination reaction is revealed by phosphorothioate substitution in DNA. Nucleic Acids Res. 1988;16(14B):6839–6856. doi: 10.1093/nar/16.14.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh K, Lau CK, Guo F, Segall AM, Van Duyne GD. Peptide trapping of the Holliday junction intermediate in Cre-loxP site-specific recombination. J Biol Chem. 2005;280(9):8290–8299. doi: 10.1074/jbc.M411668200. [DOI] [PubMed] [Google Scholar]