Abstract
Expression of late embryogenesis abundant (LEA) proteins is highly correlated with desiccation tolerance in anhydrobiotic animals, selected land plants, and bacteria. Genes encoding two LEA proteins, one localized to the cytoplasm/nucleus (AfrLEA2) and one targeted to mitochondria (AfrLEA3m), were stably transfected into human HepG2 cells. A trehalose transporter was used for intracellular loading of this disaccharide. Cells were rapidly and uniformly desiccated to low water content (<0.12 g H2O/g dry weight) with a recently developed spin-drying technique. Immediately on rehydration, control cells without LEA proteins or trehalose exhibited 0% membrane integrity, compared with 98% in cells loaded with trehalose and expressing AfrLEA2 or AfrLEA3m; surprisingly, AfrLEA3m without trehalose conferred 94% protection. Cell proliferation across 7 d showed an 18-fold increase for cells dried with AfrLEA3m and trehalose, compared with 27-fold for nondried controls. LEA proteins dramatically enhance desiccation tolerance in mammalian cells and offer the opportunity for engineering biostability in the dried state.
Keywords: water stress, biopreservation, intrinsically disordered proteins, osmolyte, Artemia franciscana
It is well established that water is essential for active life (1, 2), yet animals from four different phyla, selected land plants, and certain fungi and bacteria survive severe desiccation for extended periods (3). The mechanisms by which animals protect cellular structure and function in the state of anhydrobiosis, or “life without water” (4–6), are not only of fundamental interest, but also of potential biomedical importance for cell stabilization (7–9). Multiple molecular components may contribute to the intracellular conditions required for successful desiccation in nature, and are often associated with metabolic preconditioning for embryonic stages (10). Many organisms accumulate compatible osmolytes of low molecular weight for osmotic balance during water stress (11, 12), and some have solutes, such as trehalose, that can stabilize biological structures during desiccation (6, 7, 13). More recent investigations underscore the contributions of various protective proteins during drying, including stress proteins (14), late embryogenesis abundant (LEA) proteins, and anhydrin (15, 16), the latter two being intrinsically disordered proteins (17, 18). In the present study, we ectopically expressed two different LEA proteins in human hepatocellular carcinoma (HepG2) cells in the presence and absence of intracellular trehalose. Here we report the profound protection conferred during acute desiccation of these cells using the spin-drying technique.
Late embryogenesis abundant (LEA) proteins were first identified in plant seeds more than 30 y ago (19), and more recently have been reported in several animal groups, including crustaceans, insects, nematodes, tardigrades, and rotifers (15, 16). Multiple LEA proteins are commonly found in a given species, which may be explained in part by targeting to different cellular locations. An organism’s expression levels of LEA protein and mRNA are closely tied to its capacity for desiccation tolerance (20–26). LEA proteins are highly hydrophilic and generally unstructured, with a high percentage of random coils in aqueous solution. Secondary structures, such as α helices, β sheets, and hairpin loops, form as water is removed (16). Multiple functions have been proposed for LEA proteins with varying degrees of experimental support, including action as a “molecular shield” to sterically reduce aggregation of denatured proteins, stabilization of target proteins via chaperone-like activity, protection of cell membranes (particularly for mitochondrial-targeted forms; refs. 27, 28), stabilization of vitrified sugar glasses by increasing the glass-transition temperature (Tg), and sequestration of divalent ions (15, 16). Finally, synergistic interactions have been documented for LEA proteins and sugars such as trehalose, related to their ability to protect target proteins during drying (29).
For the purpose of the present study, we chose to focus on two LEA proteins naturally expressed in embryos of the brine shrimp Artemia franciscana, AfrLEA2 and AfrLEA3m. Several LEA proteins are expressed in these embryos, including group 1 and group 3 LEA proteins (25, 26, 30, 31). Bioinformatic analyses have identified AfrLEA2 in the cytoplasm (25) and AfrLEA3m in mitochondria (26). Expression in HepG2/C3A cells of a chimeric protein composed of the leader sequence from AfrLEA3m and GFP has shown targeting of the construct to mitochondria (26). We developed a HepG2 cell line based on the tetracycline (Tet)-inducible expression system and then stably transfected this line with either Afrlea2 or Afrlea3m under doxycycline (Dox) control. To introduce trehalose into the cytoplasm, we also stably transfected these cells with trehalose transporter 1 (TRET1) from the African chironomid Polypedilum vanderplanki (32) that is expressed constitutively.
To avoid problems commonly encountered with evaporative drying of sessile droplets containing suspended cells, we have developed a spin-drying technique that yields rapid and uniform drying across the sample and low final water contents (33, 34). Droplet drying is inherently slow, and water removal is spatially nonuniform, because a glassy skin forms at the sample interface when solutions of glass-forming solutes such as trehalose are dried (35, 36). This phenomenon slows water removal and prevents desiccation beyond certain limits. The negative biological impact is that cells can be trapped in a partially desiccated and nonvitrified sample and consequently subjected to prolonged hyperionic stress.
In this work, we merged biological and physicochemical strategies in our attempt to enhance the desiccation tolerance of mammalian cells. Specifically, we applied principles gleaned from animals whose evolutionary history has provided the capacity for severe desiccation tolerance (e.g., by means of LEA proteins and protective glass-forming solutes) and combined these with an improved drying method based on thermodynamic and kinetic studies of sugar solutions and relevant thermophysical properties of cells. We report remarkable improvement in the tolerance of mammalian cells to acute desiccation, arguably at the lowest moisture levels yet attained for uniformly dried cells.
Results
HepG2 Tet-On Cell Lines and Inducible Expression of ArfLEA2 and ArfLEA3m.
A Tet-inducible gene expression system in HepG2 cells was developed by stable transfection with a vector encoding the Tet transactivator protein. To verify whether these HepG2 Tet-On cells are competent for efficient expression of the target protein under Dox control, the luciferase gene was transiently transfected into the cells in an appropriate expression vector. Western blot analysis showed strong induction of luciferase protein across the range of 10–1,000 ng/mL Dox (Fig. 1A), with undetectable expression in the uninduced control. A similar pattern was observed when luciferase enzyme activity was plotted as a function of increasing Dox concentrations (Fig. 1B). The induced activities at 10–1,000 ng/mL Dox were significantly higher (P < 0.001) compared with the uninduced control, with the maximum induction at 1,000 ng/mL being at least 500-fold above control. Thus, the Tet-On expression system operates efficiently in HepG2 cells.
Fig. 1.
Construction of HepG2 Tet-On cell lines and the induction of transfected proteins. (A) Representative Western blot of luciferase enzyme expression in the constructed HepG2 Tet-On cell line at 24 h after induction with Dox. α-tubulin served as the loading control. (B) Luciferase catalytic activity measurements (mean ± SEM; n = 8) versus Dox addition. “a” indicates significant differences (P < 0.001) vs. the uninduced control. Both luciferase expression and activity were strongly induced by Dox in a dose-dependent manner. (C) Induction with Dox (1,000 ng/mL for the 120-h period) of AfrLEA2 in HepG2 Tet-On cells as shown by Western blot analysis. (D) AfrLEA2 expression from three individual experiments (mean ± SEM) normalized to α-tubulin. Densitometric quantification was performed using Bio-Rad Quantity One 1-D software. Data are presented as fold increase relative to the uninduced control (hour 0). “a” indicates significant differences (P < 0.005) vs. the uninduced control, and ‘b’ indicates significance (P < 0.005) vs. the 24-h time point. (E) Western blot analysis of AfrLEA3m induction in HepG2 Tet-On cells with 1,000 ng/mL Dox present throughout the 120-h period. (F) AfrLEA3m expression (mean ± SEM; n = 3) normalized to α-tubulin. Quantification and statistical tests are as described above.
The original nucleic acid sequences for Afrlea2 and Afrlea3m cloned from A. franciscana embryos (25, 26) were first optimized for human codon bias, after which each gene was inserted into a Tet-On expression vector (pTRE3G) and stably transfected into HepG2 Tet-On cells. Fig. 1C shows a representative Western blot for AfrLEA2 expression induced with 1,000 ng/mL Dox across a 5-d induction period. Polyclonal antibody raised against recombinant AfrLEA2 exhibited a strong band induced at ∼45 kDa that matched migration of the purified recombinant protein. Quantification of data averaged from three individual experiments (Fig. 1D) indicates that AfrLEA2 induction was significant at day 1 and continued to increase across the 5-d induction period, with maximum induction at ∼11-fold above the uninduced control by 120 h. Western blot analysis for AfrLEA3m expression during a 5-d time course of induction is shown in Fig. 1E. The polyclonal antibody against the recombinant protein recognized a band for AfrLEA3m at ∼34 kDa, consistent with the predicted mass of the mature protein (26) after removal of the mitochondrial targeting sequence (30.9 kDa), allowing for the fact that intrinsically disordered proteins show an increased apparent molecular mass on SDS/PAGE gels owing to reduced binding of SDS (37). Quantification of AfrLEA3m indicates statistically significant induction across the 5-d period (Fig. 1F). The maximum cellular expression (∼20-fold over the uninduced control) occurred at 24 h and declined gradually thereafter. It should be noted that migration and incorporation into the mitochondrial compartment may take longer than 24 h, based on localization of a chimeric protein composed of the AfrLEA3m leader sequence plus GFP (26). Thus, the Tet-On system effectively induces expression of AfrLEA2 and AfrLEA3m in human HepG2 cells. Our ability to document acceptable stability of these LEA proteins in the transfected cells is important, because the uncoiled conformation of intrinsically disordered proteins in aqueous solution can render them susceptible to proteolytic degradation, and, accordingly, they may exhibit reduced half-lives (18).
Subcellular Localization of AfrLEA2 and AfrLEA3m.
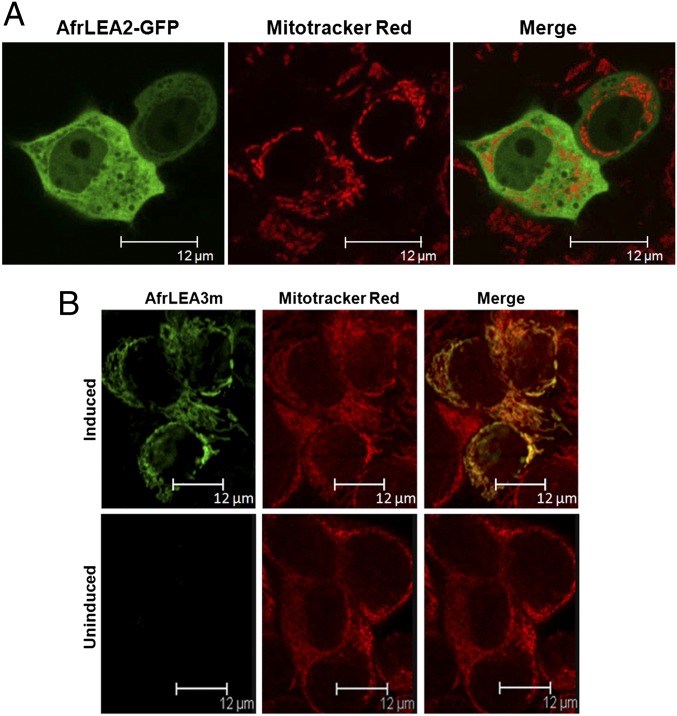
The subcellular localization of both AfrLEA2 and AfrLEA3m proteins in HepG2 Tet-On cells was documented using confocal microscopy. For AfrLEA2, transient transfection was done with a plasmid encoding a chimeric protein composed of AfrLEA2 plus GFP. At 24 h after transfection, AfrLEA2-GFP is localized to the cytoplasm of HepG2 cells (Fig. 2A). Lack of colocalization in the merged image detected by staining with MitoTracker Red (Life Technologies) does not support any mitochondrial targeting for AfrLEA2. This cytoplasmic distribution for AfrLEA2 is consistent with predictions from bioinformatic analysis (25). At 3 d after transfection of HepG2 Tet-On cells, a fraction of the AfrLEA2-GFP was also detected in the nucleus (Fig. S1). Small proteins tagged with GFP are known to readily enter the nucleus without the requirement for a nuclear localization signal (38); apparently this is the case for AfrLEA2-GFP, given that bioinformatic algorithms failed to detect a nuclear localization signal (25).
Fig. 2.
Subcellular localization of AfrLEA2 and AfrLEA3m HepG2 Tet-On cells. (A) Cells were imaged with confocal laser scanning microscopy performed at 24 h after transient transfection with AfrLEA2-GFP. (Left) Distribution of green fluorescence from AfrLEA2-GFP, with nuclei clearly visible as the absence of green fluorescence. (Center) Distribution of mitochondria based on MitoTracker Red staining. (Right) Merged image, confirming the cytoplasmic localization of AfrLEA2-GFP as predicted from bioinformatic data. (B) AfrLEA3m was stably transfected into HepG2 Tet-On cells. The top row presents images obtained from cells induced with Dox for 2 d, and the bottom row shows images for the uninduced control cells. (Left) Immunofluorescent staining with specific AfrLEA3m primary antibody (chicken IgY), followed by secondary antibody (goat anti-chick IgY) tagged with FITC. (Center) Mitochondrial distribution imaged with MitoTracker Red staining. (Right) Merged images clearly showing the yellow color resulting from overlap of the green AfrLEA3m staining with the MitoTracker Red in induced cells, which confirms the mitochondrial location of AfrLEA3m in the HepG2 cell line.
Immunofluorescent staining with primary AfrLEA3m antibody, followed by secondary antibody conjugated to FITC, was used to subcellularly localize AfrLEA3m. Images for both induced and uninduced HepG2 Tet-On-AfrLEA3m cells were captured with confocal microscopy. Fig. 2B definitively shows that AfrLEA3m is targeted to the mitochondrial network of HepG2 cells, based on colocalization with MitoTracker Red These results provide direct evidence of mitochondrial localization of AfrLEA3m using the full-length protein. Thus, we should be able to evaluate the impact of differentially targeted LEA proteins on the protection of HepG2 cells during desiccation.
Cellular Transfection with TRET1 and Kinetics of Sugar Uptake.
In some cases (6, 39), but certainly not all (40–42), animals with natural desiccation tolerance accumulate low molecular weight solutes, such as trehalose, along with protective proteins. Trehalose is a nonreducing sugar with a high Tg value, and consequently it vitrifies readily at biologically relevant temperatures. Vitrification of the intracellular compartment is considered one factor contributing to dehydration tolerance during anhydrobiosis (43). Although trehalose is not permeable to biological membranes, its effectiveness as a stabilizer is greatest when present on both sides of a lipid bilayer (44). Thus, we used a naturally occurring membrane channel TRET1 to load the sugar into HepG2 cells.
A TRET1 construct designed for constitutive expression was stably transfected into HepG2 Tet-On cell lines with and without the capacity for inducible expression of AfrLEA2/AfrLEA3m. Evaluation of TRET1 mRNA expression by RT-PCR revealed expression in TRET1-transfected cells, but not in control cells (Fig. 3A). Next, the functionality of the transporter protein was assessed by measuring trehalose uptake into TRET1-transfected cells and nontransfected control cells that had been preincubated with 50 mM trehalose for up to 24 h (Fig. 3B). HPLC analyses indicated that HepG2-TRET1 cells contained an average of 0.0217 pmol trehalose per cell after 24 h of loading, approximately sevenfold higher than the average level in WT HepG2 cells under the same conditions. The intracellular concentration of trehalose in the HepG2-TRET1 cells was estimated as ∼20 mM. No significant change in cell morphology due to the presence of intracellular trehalose was observed in HepG2 or HepG2-TRET1 cells before spin-drying.
Fig. 3.
TRET1 transporter, trehalose uptake kinetics, and the spin-drying approach. TRET1 is expressed constitutively and is not under Dox control. (A) RT-PCR showing the presence of mRNA for TRET1 in HepG2 Tet-On-TRET1 cells and its absence in HepG2 Tet-On control cells. The TRET1 plasmid was used as template for a positive control (P). A negative control with no added template (N) is shown as well. Actin was used for cell samples as a loading control. (B) Trehalose loading kinetics for HepG2 Tet-On-TRET1 cells and control HepG2 Tet-On cells (without TRET1). Cells were incubated in 50 mM trehalose for the indicated times. Trehalose uptake was more pronounced in the presence of TRET1. Values are mean ± SD (n = 3). When error bars are not visible, they are within the size of the symbol. (C) Schematic of the spin-drying process, in three steps: (1) At the beginning of the spin-drying process, the excess liquid is removed from the sample by radial flow (ν) due to spinning (ω); (2) after the excess liquid is removed by radial flow, the evaporation (E) into an environment continuously purged with dry nitrogen gas causes further drying of the samples; (3) in the final stage of the drying process, a thin film of dried trehalose with very low moisture content is formed.
Biostabilization of HepG2 Cells During Severe Desiccation.
Fluid movements during spin-drying of cells in a glass-forming solution of trehalose are depicted in Fig. 3C. Control HepG2 cells (without LEA protein or intracellular trehalose) exhibited 0% membrane integrity (n = 9) when spin-dried to a final water content of <0.12 g H2O/g dry weight, as confirmed by FTIR microscopy and bulk gravimetric analysis of the water content (Fig. 4A). Membrane integrity after spin-drying and immediate rehydration was based on staining of live (green) and dead (red) cells with Syto-13 and ethidium bromide (Fig. 4B). The membrane integrity of HepG2-TRET1 cells preloaded with trehalose increased to 44.5 ± 22.2% (mean ± SD; n = 3). In contrast, the membrane intergrity of HepG2-AfrLEA2 cells without trehalose was 57.2 ± 13.0% (n = 9), increasing to 98.3 ± 2.2% (n = 9) when these cells were preloaded with trehalose. The most remarkable result is the striking integrity of 93.6 ± 4.6% (n = 9) measured for HepG2-AfrLEA3m cells in the absence of trehalose. Consistent with this finding, evidence indicates that model synthetic peptides composed of several tandem motifs of group 3 LEA proteins (22- to 44-aa residues) vitrify at a high Tg, which suggests that dried LEA proteins themselves may vitrify and act in this way as excellent protectants against desiccation damage (45). It is appropriate to emphasize that although AfrLEA3m is indeed mitochondrial-targeted, synthesis occurs in the cytosol, and importation into mitochondria is time-dependent (26), and thus presumably some fraction of the protein is continuously present in the cytoplasm. In the absence of trehalose, membrane integrity was 0% (n = 3) for both uninduced HepG2-AfrLEA2 and uninduced HepG2-AfrLEA3m cells. The integrity of HepG2-AfrLEA3m cells preloaded with trehalose increased slightly, to 97.7 ± 3.8% (n = 9). Thus, both cellular treatments containing LEA–sugar mixtures display ∼98% membrane integrity after severe desiccation. In vitro studies have shown that mixing LEA-like peptides into trehalose solutions stabilizes the vitrified sugar glasses on drying, as judged by upward shifts in Tg (45).
Fig. 4.
Membrane integrity and long-term growth of HepG2 cells after spin-drying and immediate rehydration. (A) HepG2 cells with TRET1 transporters were incubated in 50 mM trehalose-containing medium for 18 h before spin-drying. Membrane integrity was determined using Syto-13 and ethidium bromide viability dyes. Values are mean ± SD (n = 3–9 independent determinations). “a” indicates statistically significant differences (P < 0.05) vs. control (no trehalose or LEA protein), and “b” indicates statistically significant differences (P < 0.05) vs. cells with intracellular trehalose alone. (B) Fluorescent micrograph of HepG2 cells stained with Syto-13 and ethidium bromide after spin-drying and rehydration (green, live cells; red, dead cells). (Scale bars: 100 μm.) (C) Cells were rehydrated with fully complemented cell culture medium and incubated in a 5% (vol/vol) CO2 environment at 37 °C. Cell counts on days 1, 3, and 7 were obtained with trypan blue exclusion for parallel samples. The error bars indicate ± SEM. By day 7, cell numbers were increased by 18-fold for those with AfrLEA3m plus trehalose, vs. 27-fold for nondried controls during the same growth period (statistically equivalent; P = 0.21). In contrast, the growth kinetics for cells expressing AfrLEA2 (with or without trehalose) and AfrLEA3m (without trehalose) were markedly different compared with the nondried controls (P ≤ 0.05), as indicated by asterisks.
Growth studies conducted across ensuing days after rehydration of dried cells revealed the greatest proliferation for those cells containing AfrLEA3m plus trehalose compared with all other treatment groups (Fig. 4C). By day 7, numbers were increased 18-fold for cells with AfrLEA3m plus trehalose vs. 27-fold for nondried controls during the same growth period (statistically equivalent; P = 0.21). In contrast, the growth kinetics for cells expressing AfrLEA2 (with or without trehalose) and AfrLEA3m (without trehalose) were markedly slower compared with nondried controls (P ≤ 0.05). Plant seeds are known to contain mitochondrial-targeted LEA protein (46). Macherel et al. (47) have argued persuasively that mitochondria play a profound and vital role in desiccation tolerance, and that their protection in the dry state and rapid return to full respiratory activity on rehydration is a matter of life and death in plant cells. The superior protection against desiccation stresses afforded HepG2 cells by AfrLEA3m is consistent with the importance of intact and functional mitochondria during the recovery process.
Discussion
We have successfully applied lessons learned from organisms that are naturally desiccation-tolerant to the protection of a mammalian cell line from very low water activity during acute drying. The significantly improved viability of human HepG2 cells obtained after desiccation below 0.12 g H2O/g dry weight is a very encouraging step toward the ultimate goal of preparing dried cells for storage at ambient temperature. Ectopic expression of LEA proteins cloned from the anhydrobiotic crustacean A. franciscana is a primary contributor to this lyoprotection of human HepG2 cells. LEA proteins are not represented in mammalian genomes, although a short protein motif of 10 amino acids that resembles a LEA protein has been reported in humans (48); however, regions of low sequence complexity characteristic of LEA proteins complicate the assessment of relatedness.
Previous work on air drying of mammalian cells has generally resulted in poor viability at water content below ∼0.35 g H2O/g dry weight obtained by sessile droplet drying (49, 50). A nanoliter droplet approach also yielded low viability (<10%) at a residual water of content of ∼0.1 g H2O/g dry weight (51). Successful air drying to the point of no residual water has been reported for human primary fibroblasts provisioned with intracellular trehalose (52), but a subsequent report from the same group indicated that those water content analyses apparently were inaccurate (53). A group 3 LEA protein from the nematode Aphelenchus avenae transfected into the human cell line T-REx293 (derived from the embryonic kidney cell line HEK293) improved the tolerance to hyperosmotic shock induced by exposure to 100 mM NaCl or 400 mM sugars, but exposure of cells to air drying over periods of hours at different relative humidity levels resulted in mortality (54).
In the present study, the protection of HepG2 cells during drying provided by AfrLEA2 versus AfrLEA3m was statistically identical in the presence of intracellular trehalose, as judged by membrane integrity immediately after rehydration (98.3% vs. 97.7%). Without intracellular trehalose, AfrLEA2 was significantly less effective than AfrLEA3m. This difference was particularly evident in growth studies extended over 7 d, in which proliferation was far lower for cells with AfrLEA2 compared with cells with AfrLEA3m, with or without intracellular trehalose. The reasons for the improved performance of HepG2 cells that expressed AfrLEA3m remain unclear, but this finding points to the critical role in protecting mitochondrial integrity during drying. Insight into this issue has been provided by studies of an LEA protein from pea seed mitochondria (PsLEAm) localized to the matrix (46). Using differential scanning calorimetry and FTIR spectroscopy, Macherel and coworkers (27, 28) found that PsLEAm interacts with liposomes in the dry state. The protein protects liposomes during drying by preventing membrane fusion and lysis. Furthermore, protein modeling studies by that group showed that the amphipathic α helices of PsLEAm formed during drying take on an interesting pattern in which negative amino acid residues form a stripe bordered on either side by positive residue stripes. This pattern may facilitate the protein’s integration with the lipid membrane parallel to its plane (28). Evidence also shows that a LEA protein from a bdelloid rotifer (ArLEA1B) interacts with dried liposomes and decreases the gel-to-liquid crystalline phase-transition temperature (Tm) of dry liposomes (55). Finally, it is noteworthy that mitochondria isolated from embryos of the anhydrobiotic species A. franciscana contain at least one LEA protein (AfrLEA3m), and likely more (16, 30), targeted to the mitochondrion. When these mitochondria are subjected to water stress represented by freezing in a trehalose solution, respiratory control ratios (succinate plus rotenone) for frozen/thawed mitochondria were remarkably well-preserved compared with ratios for control, nonfrozen mitochondria (26). Taken together, these findings add to the mounting evidence that mitochondrial-targeted LEA proteins offer strong protection to organelles against desiccation-induced damage, and this function is important for improving the desiccation tolerance of mammalian cells.
Spin-drying is another key step in the development of an improved dry-processing technique for mammalian cells (34), primarily because of the high degree of spatial uniformity in moisture content obtained in the samples and the rapid drying of cells. To our knowledge, both the short-term membrane integrities and the long-term viabilities obtained after immediate rehydration in this study are the highest yet reported for mammalian cells dried uniformly to a water content below 0.12 g H2O/g dry weight. As we have discussed previously in more detail (34), traditional drying procedures result in substantial spatial nonuniformity, such that regions with higher residual water are prone to higher rates of chemical reactions that can foster unwanted metabolic processes, energy depletion, and cell degradation. Reduced molecular motion, such as that observed in glassy states, is an important requirement for storage of desiccated cells.
A significant challenge that remains is extending the storage time of the spin-dried cells used in this study at ambient temperatures. The HepG2 cells provisioned with intracellular trehalose and LEA proteins are not stable at room temperature in the dried state and begin losing viability if not rehydrated within minutes; the loss of viability can be greatly retarded by immediate storage of the dried cells at liquid nitrogen temperatures (34). Our FTIR measurements for the spin-dried samples indicate a Tg of approximately 4.5 °C, which means that the cells do not exist in a vitrified state at room temperature. Stable, long-term storage of cells requires storage temperatures that are tens of degrees below the Tg. The estimated intracellular concentration of trehalose in these cells is ∼20 mM. Increasing this concentration further would elevate the Tg significantly (56) and predictably improve the storage outcomes at room temperature. The high-capacity trehalose transporter used in our studies promotes facilitated diffusion of sugars (32). Higher levels of TRET1 expression at the plasma membrane would promote increased trehalose loading. We are currently exploring additional approaches for increasing the intracellular content of glass-forming sugars, and mixtures of glass-forming agents, as a means of elevating the Tg for successful storage at room temperature.
In summary, LEA proteins dramatically improve the survivorship of human cells during acute desiccation to low water activity achieved by spin-drying. Spin-drying represents a significant step forward in the processing procedures used for water removal. Adding intracellular trehalose in combination with LEA proteins enhances the long-term growth of cells, as measured across 7 d after rehydration, to proliferation rates approaching those of control cells that were never dried. Successful strategies for long-term storage of dried cells at room temperature may require metabolic preconditioning signals (10), elevated Tg values, and the simultaneous expression of several LEA proteins targeted to various intracellular compartments. In desiccation-tolerant plant tissues, LEA proteins are localized to the nucleus, mitochondrion, chloroplast, endoplasmic reticulum, vacuole, peroxisome, and plasma membrane (15, 16).
Materials and Methods
Detailed information on materials, cell culture, construction of the HepG2 Tet-On cell line, gene transfection, selection of stable cell lines, Western blot analysis, confocal imaging and immunofluorescent staining, trehalose uptake kinetics, spin-drying of cells, and membrane integrity and long-term viability is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Takahiro Kikawada (Japanese National Institute of Agrobiological Sciences) for providing the original vector encoding the trehalose transporter, Daniel Moore (Louisiana State University) for assisting with trehalose measurements, and staff of the Louisiana State University Socolofsky Microscopy Center and Dr. David Burk (Cell Biology and Bioimaging Core, Pennington Biomedical Research Center) for assisting with imaging studies. This work was supported by National Institutes of Health Grant 2R01 DK046270-14A1 and National Science Foundation Grant IOS-0920254.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. EU477187 and FJ592175).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214893109/-/DCSupplemental.
References
- 1.Henderson LJ. The Fitness of the Environment. New York: Macmillan; 1913. [Google Scholar]
- 2.Somero GN, Osmond CB, Bolis CL, editors. Water and Life: Comparative Analysis of Water Relationships at the Organismic, Cellular, and Molecular Levels. Berlin: Springer; 2012. [Google Scholar]
- 3.Crowe JH, Clegg JS. Dry Biological Systems. New York: Academic; 1978. [Google Scholar]
- 4.Keilin D. The problem of anabiosis or latent life: History and current concept. Proc R Soc Lond B Biol Sci. 1959;150(939):149–191. doi: 10.1098/rspb.1959.0013. [DOI] [PubMed] [Google Scholar]
- 5.Crowe JH, Clegg JS. Anhydrobiosis. Stroudsburg, PA: Dowden, Hutchinson & Ross; 1973. [Google Scholar]
- 6.Crowe JH, et al. Anhydrobiosis: Cellular adaptation to extreme dehydration. In: Dantzler WH, editor. Handbook of Physiology. Vol II. Oxford: Oxford Univ Press; 1997. pp. 1445–1477. [Google Scholar]
- 7.Crowe JH, et al. Stabilization of dry mammalian cells: Lessons from nature. Integr Comp Biol. 2005;45(5):810–820. doi: 10.1093/icb/45.5.810. [DOI] [PubMed] [Google Scholar]
- 8.Huang Z, Tunnacliffe A. Desiccation response of mammalian cells: Anhydrosignaling. Methods Enzymol. 2007;428:269–277. doi: 10.1016/S0076-6879(07)28015-2. [DOI] [PubMed] [Google Scholar]
- 9.Hand SC, Hagedorn M. New approaches for cell and animal preservation: Lessons from aquatic organisms. In: Walsh PJ, Smith LE, Fleming LE, Solo-Gabriele H, Gerwick WH, editors. Oceans and Human Health: Risks and Remedies from the Seas. New York: Academic; 2008. pp. 611–629. [Google Scholar]
- 10.Hand SC, et al. Metabolic restructuring during energy-limited states: Insights from Artemia franciscana embryos and other animals. J Insect Physiol. 2011;57(5):584–594. doi: 10.1016/j.jinsphys.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: Evolution of osmolyte systems. Science. 1982;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 12.Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208(Pt 15):2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- 13.Xie G, Timasheff SN. The thermodynamic mechanism of protein stabilization by trehalose. Biophys Chem. 1997;64(1-3):25–43. doi: 10.1016/s0301-4622(96)02222-3. [DOI] [PubMed] [Google Scholar]
- 14.Clegg JS. Stress-related proteins compared in diapause and in activated, anoxic encysted embryos of the animal extremophile, Artemia franciscana. J Insect Physiol. 2011;57(5):660–664. doi: 10.1016/j.jinsphys.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94(10):791–812. doi: 10.1007/s00114-007-0254-y. [DOI] [PubMed] [Google Scholar]
- 16.Hand SC, Menze MA, Toner M, Boswell L, Moore D. LEA proteins during water stress: Not just for plants anymore. Annu Rev Physiol. 2011;73(1):115–134. doi: 10.1146/annurev-physiol-012110-142203. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabortee S, et al. Intrinsically disordered proteins as molecular shields. Mol Biosyst. 2012;8(1):210–219. doi: 10.1039/c1mb05263b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uversky VN, Dunker AK. Understanding protein non-folding. Biochim Biophys Acta. 2010;1804(6):1231–1264. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dure L, 3rd, Greenway SC, Galau GA. Developmental biochemistry of cottonseed embryogenesis and germination: Changing messenger ribonucleic acid populations as shown by in vitro and in vivo protein synthesis. Biochemistry. 1981;20(14):4162–4168. doi: 10.1021/bi00517a033. [DOI] [PubMed] [Google Scholar]
- 20.Blackman SA, Obendorf RL, Leopold AC. Desiccation tolerance in developing soybean seeds: The role of stress proteins. Physiol Plant. 1995;93(4):630–638. doi: 10.1104/pp.100.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battista JR, Park MJ, McLemore AE. Inactivation of two homologues of proteins presumed to be involved in the desiccation tolerance of plants sensitizes Deinococcus radiodurans R1 to desiccation. Cryobiology. 2001;43(2):133–139. doi: 10.1006/cryo.2001.2357. [DOI] [PubMed] [Google Scholar]
- 22.Browne JA, et al. Dehydration-specific induction of hydrophilic protein genes in the anhydrobiotic nematode Aphelenchus avenae. Eukaryot Cell. 2004;3(4):966–975. doi: 10.1128/EC.3.4.966-975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gal TZ, Glazer I, Koltai H. An LEA group 3 family member is involved in survival of C. elegans during exposure to stress. FEBS Lett. 2004;577(1-2):21–26. doi: 10.1016/j.febslet.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 24.Boudet J, et al. Comparative analysis of the heat stable proteome of radicles of Medicago truncatula seeds during germination identifies late embryogenesis abundant proteins associated with desiccation tolerance. Plant Physiol. 2006;140(4):1418–1436. doi: 10.1104/pp.105.074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hand SC, Jones D, Menze MA, Witt TL. Life without water: Expression of plant LEA genes by an anhydrobiotic arthropod. J Exp Zool A Ecol Genet Physiol. 2007;307(1):62–66. doi: 10.1002/jez.a.343. [DOI] [PubMed] [Google Scholar]
- 26.Menze MA, Boswell L, Toner M, Hand SC. Occurrence of mitochondria-targeted late embryogenesis abundant (LEA) gene in animals increases organelle resistance to water stress. J Biol Chem. 2009;284(16):10714–10719. doi: 10.1074/jbc.C900001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolleter D, Hincha DK, Macherel D. A mitochondrial late embryogenesis abundant protein stabilizes model membranes in the dry state. Biochim Biophys Acta. 2010;1798(10):1926–1933. doi: 10.1016/j.bbamem.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Tolleter D, et al. Structure and function of a mitochondrial late embryogenesis abundant protein are revealed by desiccation. Plant Cell. 2007;19(5):1580–1589. doi: 10.1105/tpc.107.050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyal K, Walton LJ, Tunnacliffe A. LEA proteins prevent protein aggregation due to water stress. Biochem J. 2005;388(Pt 1):151–157. doi: 10.1042/BJ20041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner AH, Chakrabortee S, Tunnacliffe A, Clegg JS. Complexity of the heat-soluble LEA proteome in Artemia species. Comp Biochem Physiol Part D Genomics Proteomics. 2012;7(3):260–267. doi: 10.1016/j.cbd.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Warner AH, et al. Evidence for multiple group 1 late embryogenesis abundant proteins in encysted embryos of Artemia and their organelles. J Biochem. 2010;148(5):581–592. doi: 10.1093/jb/mvq091. [DOI] [PubMed] [Google Scholar]
- 32.Kikawada T, et al. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc Natl Acad Sci USA. 2007;104(28):11585–11590. doi: 10.1073/pnas.0702538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakraborty N, et al. A spin-drying technique for lyopreservation of mammalian cells. Ann Biomed Eng. 2011;39(5):1582–1591. doi: 10.1007/s10439-011-0253-1. [DOI] [PubMed] [Google Scholar]
- 34.Chakraborty N, et al. Cryopreservation of spin-dried mammalian cells. PLoS ONE. 2011;6(9):e24916. doi: 10.1371/journal.pone.0024916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams DR, Toner M, Langer R. Microflow and crack formation patterns in drying sessile droplets of liposomes suspended in trehalose solutions. Langmuir. 2008;24(15):7688–7697. doi: 10.1021/la703835w. [DOI] [PubMed] [Google Scholar]
- 36.Aksan A, Irimia D, He X, Toner M. Desiccation kinetics of biopreservation solutions in microchannels. J Appl Phys. 2006;99(6):064703–064709. doi: 10.1063/1.2181280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27(10):527–533. doi: 10.1016/s0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 38.Seibel NM, Eljouni J, Nalaskowski MM, Hampe W. Nuclear localization of enhanced green fluorescent protein homomultimers. Anal Biochem. 2007;368(1):95–99. doi: 10.1016/j.ab.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Madin KAC, Crowe JH. Anhydrobiosis in nematodes: Carbohydrate and lipid-metabolism during dehydration. J Exp Zool. 1975;193(3):335–342. [Google Scholar]
- 40.Tunnacliffe A, Lapinski J. Resurrecting Van Leeuwenhoek’s rotifers: A reappraisal of the role of disaccharides in anhydrobiosis. Philos Trans R Soc Lond B Biol Sci. 2003;358(1438):1755–1771. doi: 10.1098/rstb.2002.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tunnacliffe A, Lapinski J, McGee B. A putative LEA protein, but no trehalose, is present in anhydrobiotic bdelloid rotifers. Hydrobiologia. 2005;546:315–321. [Google Scholar]
- 42.Hengherr S, Heyer AG, Köhler H-R, Schill RO. Trehalose and anhydrobiosis in tardigrades—evidence for divergence in responses to dehydration. FEBS J. 2008;275(2):281–288. doi: 10.1111/j.1742-4658.2007.06198.x. [DOI] [PubMed] [Google Scholar]
- 43.Crowe JH, Carpenter JF, Crowe LM. The role of vitrification in anhydrobiosis. Annu Rev Physiol. 1998;60:73–103. doi: 10.1146/annurev.physiol.60.1.73. [DOI] [PubMed] [Google Scholar]
- 44.Chen T, et al. Beneficial effect of intracellular trehalose on the membrane integrity of dried mammalian cells. Cryobiology. 2001;43(2):168–181. doi: 10.1006/cryo.2001.2360. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu T, et al. Desiccation-induced structuralization and glass formation of group 3 late embryogenesis abundant protein model peptides. Biochemistry. 2010;49(6):1093–1104. doi: 10.1021/bi901745f. [DOI] [PubMed] [Google Scholar]
- 46.Grelet J, et al. Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol. 2005;137(1):157–167. doi: 10.1104/pp.104.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkin OK, Macherel D. The crucial role of plant mitochondria in orchestrating drought tolerance. Ann Bot (Lond) 2009;103(4):581–597. doi: 10.1093/aob/mcn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall BM, Owens KM, Singh KK. Distinct functions of evolutionary conserved MSF1 and late embryogenesis abundant (LEA)-like domains in mitochondria. J Biol Chem. 2011;286(45):39141–39152. doi: 10.1074/jbc.M111.259853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acker JP, Fowler B, Lauman S, Cheley S, Toner M. Survival of desiccated mammalian cells: Beneficial effects of isotonic media. Cell Preserv Technol. 2002;1(2):129–140. [Google Scholar]
- 50.Ma X, et al. A small stress protein acts synergistically with trehalose to confer desiccation tolerance on mammalian cells. Cryobiology. 2005;51(1):15–28. doi: 10.1016/j.cryobiol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Reategui EE, Fowler AJ. Desiccation of nucleated mammalian cells in nanoliter droplets. Chem Eng Res Des. 2008;86(11A):1187–1195. [Google Scholar]
- 52.Guo N, Puhlev I, Brown DR, Mansbridge J, Levine F. Trehalose expression confers desiccation tolerance on human cells. Nat Biotechnol. 2000;18(2):168–171. doi: 10.1038/72616. [DOI] [PubMed] [Google Scholar]
- 53.Gordon SL, et al. Recovery of human mesenchymal stem cells following dehydration and rehydration. Cryobiology. 2001;43(2):182–187. doi: 10.1006/cryo.2001.2361. [DOI] [PubMed] [Google Scholar]
- 54.Chakrabortee S, et al. Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proc Natl Acad Sci USA. 2007;104(46):18073–18078. doi: 10.1073/pnas.0706964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pouchkina-Stantcheva NN, et al. Functional divergence of former alleles in an ancient asexual invertebrate. Science. 2007;318(5848):268–271. doi: 10.1126/science.1144363. [DOI] [PubMed] [Google Scholar]
- 56.Chen T, Fowler A, Toner M. Literature review: Supplemented phase diagram of the trehalose-water binary mixture. Cryobiology. 2000;40(3):277–282. doi: 10.1006/cryo.2000.2244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.