Abstract
Ca2+-dependent activator protein for secretion 2 (CAPS2 or CADPS2) potently promotes the release of brain-derived neurotrophic factor (BDNF). A rare splicing form of CAPS2 with deletion of exon3 (dex3) was identified to be overrepresented in some patients with autism. Here, we generated Caps2-dex3 mice and verified a severe impairment in axonal Caps2-dex3 localization, contributing to a reduction in BDNF release from axons. In addition, circuit connectivity, measured by spine and interneuron density, was diminished globally. The collective effect of reduced axonal BDNF release during development was a striking and selective repertoire of deficits in social- and anxiety-related behaviors. Together, these findings represent a unique mouse model of a molecular mechanism linking BDNF-mediated coordination of brain development to autism-related behaviors and patient genotype.
Keywords: psychiatry, mental disorder, dense-core vesicle, exocytosis
Ca2+-dependent activator protein for secretion 2 (CAPS2 or CADPS2) is a member of the CAPS protein family that regulates the trafficking of dense-core vesicles by binding both phosphoinositides and dense-core vesicles (1–5). We initially identified mouse Caps2 as a potent factor promoting the release of brain-derived neurotrophic factor (BDNF) during cerebellar development (6, 7). Our subsequent knockout mouse study showed that Caps2 not only plays a role in neuronal development of the cerebrum and hippocampus as well as the cerebellum, but that it is also associated with social interaction, anxiety, and maternal and circadian behaviors in mice (7, 8). We also showed that the expression of an exon 3-skipped (or -spliced out) form of CAPS2 (designated CAPS2-dex3) (8), which is now known to be a rare alternative splicing variant (9, 10), is increased in a subgroup of patients with autism and is not properly localized in axons (8). Thus, neurons overexpressing dex3 may fail to coordinate local BDNF release from axons properly (8, 9), resulting in improper brain development and function. The human CAPS2 gene locus (7q31.32) is intriguingly located within the autism susceptibility locus 1 (AUTS1) (11) on chromosome 7q31–q33, one of several susceptibility loci for autism (12). Moreover, an association of CAPS2 with autism has been suggested recently, not only by the presence of copy number variations in the CAPS2 gene in autistic patients (13–15), but also by decreased transcription of CAPS2 in the brains of people with autism (16). Thus, clarifying the biological significance of dex3 expression is an important step in elucidating the association of CAPS2 with brain circuit development and behaviors related to autism.
The potential molecular risk factors for autism susceptibility have been increasingly reported (17–26) but are poorly characterized in animal models. In this report, we generated a mouse model expressing dex3 and analyzed the cellular and autistic-like behavioral phenotypes of dex3 mice. Our results support the involvement of the rare dex3 form of Caps2 in defective axonal BDNF secretion, affecting proper brain circuit development and/or function, and thereby contributing to an increased susceptibility to autism. Our results suggest that disturbance of the alternative splicing patterns of Caps2 can directly affect normal brain development and function and could contribute to a genetic background of autism susceptibility.
Results
Generation of a Mouse Line Expressing Exon 3-Skipped Caps2 (dex3).
To clarify the in vivo effect of Caps2 exon 3 skipping, we used a Cre/loxP system to generate a mouse line carrying a deletion of exon 3 (which encodes 111 amino acids) of the Caps2 gene, which expresses the exon 3-skipped Caps2 (Caps2-dex3) protein. A conditional Caps2-dex3 allele was generated by gene targeting in embryonic stem cells (Fig. S1A). The resultant homozygotes, called Caps2Δex3/Δex3 mice, produced a Caps2-immunoreactive protein with a slightly lower molecular weight than that of wild types (Fig. S1B). The Caps2Δex3/Δex3 mice in standard breeding cages exhibited no differences in life expectancy from control mice, and both male and female Caps2Δex3/Δex3 mice had normal reproductive ability.
Dex3 Mice Show a Severe Reduction in Caps2 Immunoreactivity in the Projection Areas of Caps2-Expressing Neurons.
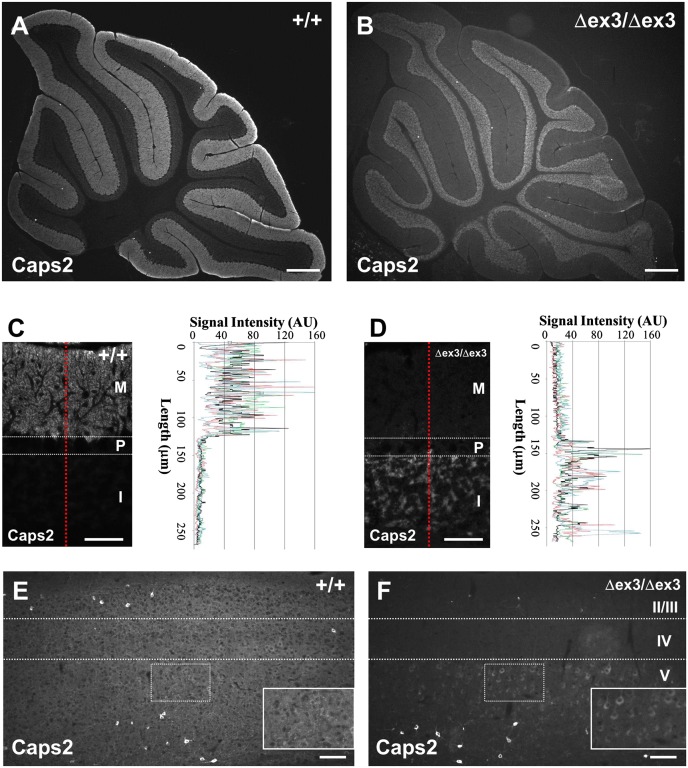
We first examined the intracellular localization of dex3 protein in vivo using an anti-Caps2 antibody. In the cerebellum, wild-type Caps2 protein was mostly localized in the granule cell axons (parallel fibers) extending into the molecular layer (Fig. 1 A and C), whereas dex3 protein was not localized in the axons and instead accumulated in the cell somas in the internal granule cell layer (Fig. 1 B and D). In the cerebrum of wild-type mice, diffuse signal was widely distributed over the cortical layers, and intense signals were observed in the soma of a subset of interneurons scattered throughout layers II/III and V (Fig. 1E). Interestingly, in Caps2Δex3/Δex3 mice, the diffuse signal was almost absent and mostly localized to cell somas in layer V, in addition to some intense signaling seen in interneurons in layers II/III and V (Fig. 1F). This supported our hypothesis that full-length Caps2 protein [exon3-plus; the dominant form in mouse (27) and human (8) brains] can be transported into axons, whereas dex3 protein (exon3-minus) cannot (8).
Fig. 1.
Distribution of Caps2 immunoreactivity in the Caps2Δex3/Δex3 mouse cerebellum and neocortex. (A and B) Sagittal sections of P21 wild-type (A) and Caps2Δex3/Δex3 (B) cerebellum were immunolabeled with an anti-Caps2 antibody. (Scale bars, 300 μm.) (C and D) Sagittal sections of P21 wild-type (C) and Caps2Δex3/Δex3 (D) cerebella were immunolabeled with anti-Caps2 antibody. Immunosignal intensity of red dotted lines is shown by black lines in the graphs. Signal intensity from the other three images is shown in colored lines. M, molecular layer; P, Purkinje cell layer; I, internal granular layer. (Scale bars, 50 μm.) (E and F) Sagittal sections of P21 wild-type (E) and Caps2Δex3/Δex3 (F) neocortex were immunolabeled with an anti-Caps2 antibody. II/III, IV, and V represent cortical layers II/III, IV, and V, respectively. Areas outlined by dotted squares are shown in Insets. (Scale bars, 100 μm.)
In the wild-type mouse hippocampus, Caps2 protein was expressed in dentate gyrus (DG) granule cells and cornu ammonis region 1 (CA1) pyramidal neurons in addition to a subset of interneurons scattered in the DG and CA1 regions (28), as shown in Fig. S2A. The latter immunosignal was strong in the cell soma, whereas Caps2 expressed in DG granule cells was localized in axons of the stratum lucidum. In Caps2Δex3/Δex3 hippocampus, diffuse signal of the stratum lucidum was almost diminished, as shown in Fig. S2 B, D, and E.
Decreased Axonal Localization of BDNF in dex3 Neurons.
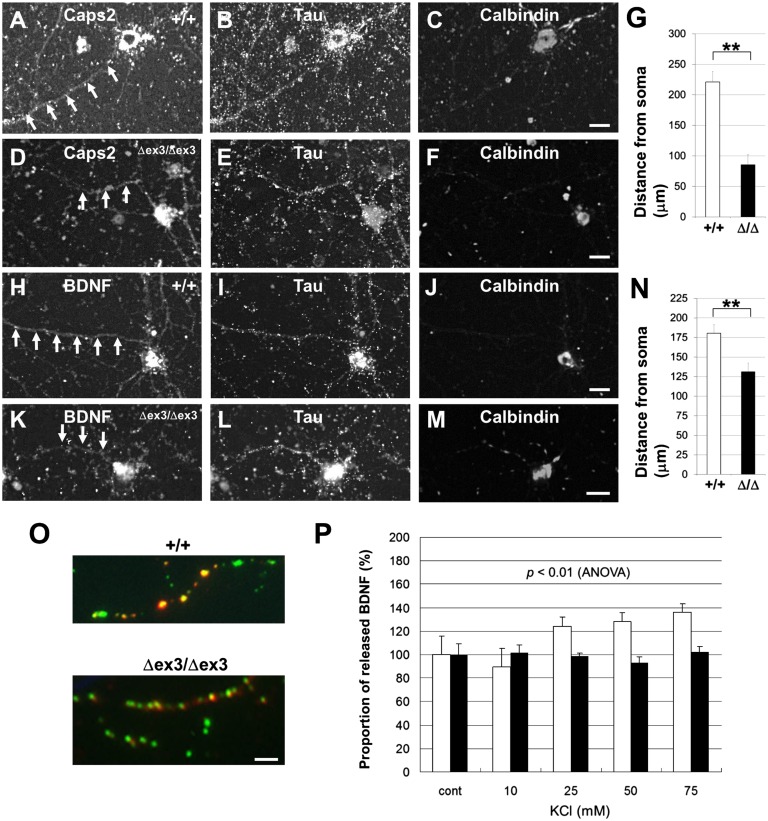
We analyzed the effect of dex3 expression on the axonal localization of BDNF immunoreactivity using primary-cultured hippocampal calbindin-positive granule cells from wild-type and Caps2Δex3/Δex3 mice. In wild-type neurons (Fig. 2 A–C), Caps2-immunopositive puncta were localized in the axon, as shown by colocalization with the axonal marker Tau. However, in Caps2Δex3/Δex3 neurons, Caps2-positive puncta were decreased in the axons (Fig. 2 D–G). Similarly, as shown in Fig. 2 H–J, BDNF-immunopositive puncta were localized in the axon of wild-type neurons, whereas BDNF-positive puncta were decreased in the axon of Caps2Δex3/Δex3 neurons (Fig. 2 K–N). These data suggest that the axonal localization of BDNF is affected in dex3-expressing neurons.
Fig. 2.
Decreased BDNF release from axons in Caps2Δex3/Δex3 hippocampal granule cells. (A–F) Subcellular localization of Caps2, Tau, and calbindin protein in hippocampal primary cultures of wild-type (A–C) and Caps2Δex3/Δex3 (D–F) mice, immunostained for Caps2 (A and D), Tau (B and E), and calbindin (C and F) at 14 d in vitro. Arrows show the position of Caps2 immunoreactivity along the axons. (G) The distance of the Caps2 protein from the soma, indicating axonal transport, is shown for wild-type (white) and Caps2Δex3/Δex3 (black) cells. (H–M) Subcellular localization of BDNF, Tau, and calbindin protein in hippocampal primary cultures from wild-type (H–J) and Caps2Δex3/Δex3 mice (K–M) immunostained for BDNF (H and K), Tau (I and L), and calbindin (J and M) at 14 d in vitro. Arrows show the position of BDNF immunoreactivity along the axons. (Scale bars, 20 μm.) (N) The distance of the BDNF protein from the soma, indicating axonal transport, is shown for wild-type (white) and Caps2Δex3/Δex3 (black) cells. Error bars indicate SEM. **P < 0.01, by Student t test. (O) BDNF–GFP was expressed in hippocampal granule cells and immunostained, without permeabilization, for anti-GFP. GFP fluorescence is shown in green, and GFP immunoreactivity is shown in red. Yellow puncta represent released BDNF. Immunostaining for MAP2(a+b) was performed to identify axons and dendrites. (Scale bars, 5 μm.) (P) The proportion of released BDNF puncta per total BDNF puncta in unstimulated neurons (cont) was normalized to 100%. Proportions of released BDNF puncta per total BDNF puncta upon KCl stimulation of axons are shown for wild-type (white) and Caps2Δex3/Δex3 (black) cells. There was a significant difference in the proportion of released BDNF between wild-type and Caps2Δex3/Δex3 cells (P < 0.01, repeated measures ANOVA). Error bars indicate SEM.
To exclude the possibility of a defect in cell polarization, we examined the immunoreactivity of MAP2 (a dendritic marker) and Tau (an axonal marker). Both were localized normally in Caps2Δex3/Δex3 hippocampal cells (Fig. S3).
Decreased Axonal Secretion of BDNF in dex3 Neurons.
To analyze the axonal release of BDNF in Caps2Δex3/Δex3 neurons, a BDNF–green fluorescent protein (GFP) fusion construct was transfected into hippocampal dentate granule cell cultures prepared from either wild-type or Caps2Δex3/Δex3 mice. KCl-induced (i.e., depolarization-induced) BDNF–GFP release from transfected neurons was evaluated by immunostaining with an anti-GFP antibody before cell permeabilization and by counting immunopositive puncta on axons (Fig. 2O), as previously reported (29–31). The number of cell-surface (i.e., released) BDNF–GFP puncta in KCl-stimulated cells was normalized to that in unstimulated control cells. A graded increase in KCl concentration revealed an activity (depolarization)-dependent release of BDNF–GFP from the axons of wild-type but not Caps2Δex3/Δex3 cells (Fig. 2P). These results showed that regulated release of BDNF was impaired in the axons of Caps2Δex3/Δex3 neurons.
Deficits in Hippocampal and Cortical Interneurons and Dendritic Spines in dex3 Mice.
There is a report showing disrupted architecture in the gamma-aminobutyric acid (GABA)ergic interneuron circuit of the neocortex in autism (32). It was also shown that differentiation of a subset of neocortical parvalbumin-positive GABAergic neurons is regulated by BDNF (33). Thus, we analyzed parvalbumin- and calbindin-positive interneurons in Caps2Δex3/Δex3 mice. In the hippocampus at postnatal days 17 (P17) and P21, there was no significant difference in the number of parvalbumin-positive interneurons among wild-type, Caps2+/Δex3, and Caps2Δex3/Δex3 mice (Figs. S4 A and B and S5 A–D). On the other hand, in the neocortex, there were significantly fewer parvalbumin-positive interneurons in Caps2Δex3/Δex3 mice than in their wild-type littermates (Figs. S4 C and D and E–H), which showed a phenotype similar to that of Bdnf−/− mice (33) and Caps2-null mutants (8).
Fewer calbindin-positive interneurons were observed at P7 in the hippocampus of both Caps2+/Δex3 and Caps2Δex3/Δex3 mice compared with wild types (Figs. S4E and S5 I and J). In contrast, there was no significant difference in the P21 hippocampus among wild-type, Caps2+/Δex3, and Caps2Δex3/Δex3 mice (Figs. S4F and S5 K and L). In the neocortex at either P7 or P21, no difference was observed in the number of calbindin-positive neurons between wild-type and Caps2Δex3/Δex3 mice (Figs. S4 G and H and S5 M–P). Moreover, there was no significant difference among wild-type (14.02 ± 2.17 cells/mm2, mean ± SEM, n = 12), Caps2+/Δex3 (10.83 ± 2.98 cells/mm2, mean ± SEM, n = 10), and Caps2Δex3/Δex3 (13.45 ± 2.25 cells/mm2, mean ± SEM, n = 10) mice in calretinin(+) interneurons of the P21 neocortex. In addition, there was no significant difference in neuronal-specific nuclear protein (NeuN)-positive neurons of the neocortex among the wild-type, Caps2+/Δex3, and Caps2Δex3/Δex3 genotypes at P21 (Fig. S6).
Spine distributions are altered in the developing brain of a large number of genetic conditions that result in mental disorders (34). Because BDNF affects dendritic protrusion (35), we next analyzed the formation of dendritic protrusions in hippocampal dentate granule cells using Golgi staining (Fig. S7 A and B). Dentate granule cells are one of the major neuronal types that secrete BDNF. The total number of dendritic protrusions was decreased in Caps2Δex3/Δex3 mice compared with wild types (Fig. S7C). Furthermore, Caps2Δex3/Δex3 mice displayed a decreased number of spines on the dendrites of dentate granule cells (Fig. S7C), suggesting that excitatory neurons and synapses are also affected in Caps2Δex3/Δex3 mice.
Impairments in Behavioral Phenotypes in dex3 Mice.
We asked the question whether these cytological and physiological changes in Caps2Δex3/Δex3 mice affected autism-related behaviors. For the following behavioral studies, we used male mice except for the analysis of maternal behavior.
Motor coordination.
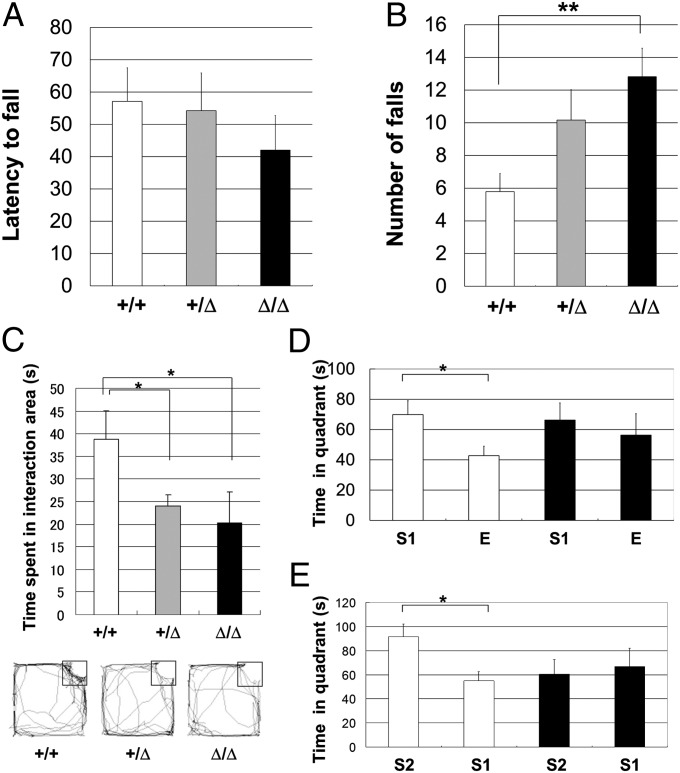
First, to assess the cerebellar functions of Caps2Δex3/Δex3 mice, we tested their coordinated motor performance on a rotarod treadmill. There was no difference in the latency to first fall among wild-type, Caps2+/Δex3, and Caps2Δex3/Δex3 mice (Fig. 3A). However, for the average number of falls within a 3-min period at 24 rpm, Caps2Δex3/Δex3 mice showed low-to-moderate rotarod performance compared with that of their wild-type littermates (Fig. 3B). In addition, there was no significant difference in performance in a grip traction test among the genotypes (Fig. S8). These findings suggest that the drastically altered axodendritic polarity of Caps2 localization in the cerebellum of Caps2Δex3/Δex3 mice (Fig. 1) impairs their motor coordination ability.
Fig. 3.
Impaired motor coordination and social behavior in dex3 mutant mice. (A) Mice were placed on a rotarod at 24 rpm. The time (s) taken for wild-type (white, n = 21), Caps2+/Δex3 (gray, n = 25), and Caps2Δex3/Δex3 (black, n = 17) littermates to fall off was measured. (B) Average number of falls within 3 min at 24 rpm. P < 0.05, one-factor ANOVA. (C) Each mouse was placed into an open-field apparatus for 15 min. A small cage containing a stranger C57BL/6J mouse was placed in one corner. The time spent in the interaction area (black square) in 15 min is shown for wild-type (white; n = 10), Caps2+/Δex3 (gray; n = 13), and Caps2Δex3/Δex3 (black; n = 10) mice. Representative movement traces are shown below. P < 0.05, one-factor ANOVA. (D) Three-chamber test. A C57BL/6J stranger mouse was placed in a small cage in one of the side chambers and a small empty cage was placed in the opposite chamber. Comparison of the time spent in the quadrants around the stranger mouse (S1) and the empty cage (E) is shown for wild-type (open bars; n = 12) and Caps2Δex3/Δex3 (closed bars; n = 10) mice. (E) Novel stranger mouse (S2) was placed in a cage in one side chamber, and a familiar mouse (S1), which was used in the test shown in D, was placed in the other side chamber. Error bars indicate SEM. *P < 0.05; **P < 0.01, by post hoc t test.
Impaired social behavior.
An impairment in social interaction is one of the characteristic features of individuals with autism (36, 37). To investigate the in vivo effect of the Caps2-dex3 variant on social behavior, we tracked the movements of wild-type, Caps2+/Δex3, and Caps2Δex3/Δex3 mice placed in the center of an open field followed by placing a stranger mouse (C57BL/6J) in a small cage in one corner. When the stranger mouse was introduced, both Caps2+/Δex3 and Caps2Δex3/Δex3 mice showed a significant reduction, compared with wild-type mice, in the time spent approaching or interacting with the stranger mouse cage (Fig. 3C).
We also performed a three-chamber social interaction test (Fig. 3 D and E). The mouse being tested was placed in the central chamber and could move freely among the three chambers. A stranger mouse was placed in one of the side chambers in a small cage, and an empty cage was placed in the opposite chamber. Wild-type mice tended to contact the stranger mouse, and the time spent in the quadrant containing the stranger mouse was significantly higher than the time spent in the corresponding quadrant in the opposite chamber containing the empty cage. Caps2Δex3/Δex3 mice also spent a little more time with the stranger mouse than with the empty cage, but the difference was not significant (Fig. 3D). It is possible that this is because of an impairment in spatial recognition memory. Therefore, we tested Caps2Δex3/Δex3 mice in a Y-maze test. This indicated no impairment in spatial recognition memory in Caps2Δex3/Δex3 mice (Fig. S9A).
Next, we compared social interactions with either novel (unfamiliar) or familiar mice. Wild-type mice tended to contact a stranger mouse rather than a familiar mouse, whereas there was no significant difference for Caps2Δex3/Δex3 mice (Fig. 3E). This result might be because of an impairment in olfaction. Therefore, we measured the olfactory ability of Caps2Δex3/Δex3 mice by measuring the time to find hidden cookies (Materials and Methods). This indicated no impairment in basic olfactory function in Caps2Δex3/Δex3 mice (Fig. S9B).
Our data suggest that wild-type mice are more interested in a novel mouse than a familiar mouse, and that Caps2Δex3/Δex3 mice have decreased sociability compared with the wild types; this may be analogous to the impairment in appropriate social interactions often seen in autistic patients (38).
Exploratory and anxiety-related behavior.
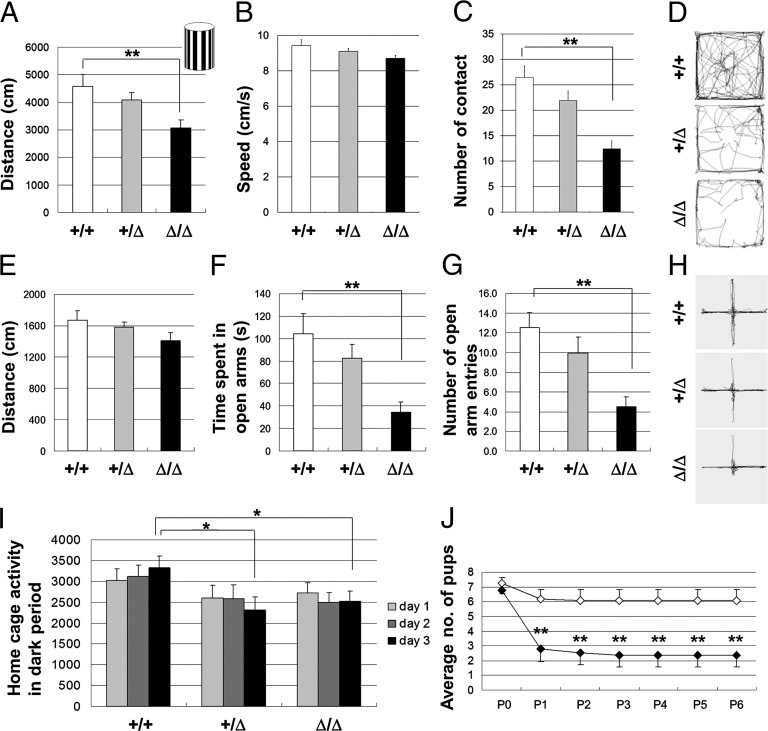
The Caps2Δex3/Δex3 mice showed decreased exploratory behavior and/or increased anxiety in a novel environment. Caps2Δex3/Δex3 mice placed in an open field showed normal locomotor activity (Fig. S10A) and speed (Fig. S10B) compared with their wild-type littermates. However, when placed in an open field containing a novel object in the central area (the black-and-white vertical object shown in the Inset of Fig. 4A), Caps2Δex3/Δex3 mice became less active (Fig. 4A) without changing their speed of movement (Fig. 4B) and tended to contact the novel object less frequently than wild-type mice did (Fig. 4C). Representative traces from wild-type, Caps2+/Δex3, and Caps2Δex3/Δex3 mice are shown in Fig. 4D.
Fig. 4.
Caps2Δex3/Δex3 mice showed increased anxiety-like behavior in an unfamiliar environment, and Caps2Δex3/Δex3 mothers displayed impaired maternal behavior in nurturing their newborns. (A–D) The horizontal movement distance (A), speed (B), and the number of contacts with a novel object (C) in an open field (15 min) are shown for wild-type (white; n = 11), Caps2+/Δex3 (gray; n = 13), and Caps2Δex3/Δex3 (black; n = 12) mice in the presence of the novel object shown in the Inset of A, placed in the center. Representative movement traces are shown in D. (E–H) Elevated plus maze test. The horizontal movement distance (E), time spent in the open arms (F), and number of open-arm entries (G) in an elevated plus maze (10 min) is shown for wild-type (white; n = 11), Caps2+/Δex3 (gray; n = 12), and Caps2Δex3/Δex3 (black; n = 12) mice. Representative movement traces are shown in H. (A, F, and G) P < 0.01, one-factor ANOVA. **P < 0.01, by post hoc t test. (I) Caps2Δex3/Δex3 mice displayed impaired habituation to a fresh cage. After habituation to a fresh cage for 24 h, the locomotor activity of wild-type (n = 11), Caps2+/Δex3 (n = 13), and Caps2Δex3/Δex3 (n = 12) mice was measured for 3 d (12-h light/dark period). Bars show the mean number of photobeam interruptions in the dark period of the 24-h cycle. P < 0.05, one-factor ANOVA. *P < 0.05, by post hoc t test. (J) Maternal neglect of newborns by Caps2Δex3/Δex3 mothers. Wild-type females were mated with Caps2Δex3/Δex3 males, and vice versa. Graph shows the survival of pups born to primiparous wild-type (open diamonds; n = 12) and Caps2Δex3/Δex3 (black diamonds; n = 13) females. Error bars indicate SEM. P < 0.01, repeated measures ANOVA. **P < 0.01, by post hoc u test.
In the elevated plus maze test, there was no difference in horizontal distance moved, suggesting that locomotor activity in this test was not affected by exon 3 skipping (Fig. 4E). However, the Caps2Δex3/Δex3 mice spent less time in the open arms than did the wild types (Fig. 4F). In addition, the number of open-arm entries was decreased (Fig. 4G). Representative traces from wild-type, Caps2+/Δex3, and Caps2Δex3/Δex3 mice are shown in Fig. 4H. However, Caps2Δex3/Δex3 mice showed no significant anxiety-like behavior in the light/dark transition test (Fig. S11 A–C), indicating that their tendency to display increased anxiety-like behavior might depend on the particular behavioral test used; for example, they may be more sensitive to novelty or unfamiliarity than to a light/dark preference.
In the home cage, locomotor activity was similar between Caps2Δex3/Δex3 mice and their wild-type littermates when measured during a 3-d period (12-h light/dark cycle) after habituation to a fresh home cage for 24 h (Fig. S12). However, in the dark period of the 24-h cycle, even on the third day, both Caps2Δex3/Δex3 and Caps2+/Δex3 mice exhibited reduced locomotion activity compared with wild-type mice (Fig. 4I), suggesting that Caps2Δex3/Δex3 and Caps2+/Δex3 mice do not adapt well to a new environment.
Decreased dam–pup interaction and communication.
To evaluate whether the maternal behavior of Caps2Δex3/Δex3 mice was affected, we monitored the nurturing of newborns by their mothers, to assess maternal care as an important social interaction between dams and pups. To exclude the effect of pup genotype, Caps2+/Δex3 pups were used for this experiment. In many cages, the newborns of a Caps2Δex3/Δex3 dam and a wild-type sire rarely survived beyond P1 (Fig. 4J). In contrast, the newborns of a Caps2Δex3/Δex3 sire and a wild-type dam normally survived beyond P1 (Fig. 4J). These results indicated that Caps2Δex3/Δex3 mothers display defective maternal behavior.
To investigate communication ability from pups to their dam, we studied the ultrasound vocalization (USV) of neonates using Caps2+/Δex3 mice. To exclude the effect of sire genotype, wild-type or Caps2Δex3/Δex3 males were mated with wild-type females. The resultant wild-type or Caps2+/Δex3 pups were used to monitor USV. We found that the duration of USVs decreased from P5 to P10 in wild-type pups, but that Caps2+/Δex3 pups emitted very few USVs throughout this period (Fig. S13). This suggests that the skipping of exon 3 affects the dam–pup interaction by USV calls.
Impaired circadian rhythm.
Autism is frequently accompanied by an abnormal sleep–wake rhythm (39, 40). Regarding the circadian rhythm of locomotor activity under a 12-h light/dark cycle, we detected no differences in the sleep–wake rhythm among wild-type, Caps2+/Δex3, and Caps2Δex3/Δex3 mice. Under constant dark conditions, the sleep–wake rhythm of the Caps2Δex3/Δex3 mice showed a shorter period than that of wild-type mice. However, there was no statistically significant difference among the three genotypes (Fig. S14A), because some Caps2Δex3/Δex3 mice (4/14) showed impaired circadian rhythmicity (Fig. S14C) compared with wild types and this resulted in a large variance (Fig. S14B).
Discussion
In this report, we developed a mouse line expressing exon 3-deleted Caps2 (dex3), the same as a rare alternatively spliced variant of human CAPS2 that was identified in some individuals with autism (8). Caps2-dex3 mice displayed deficits in axonal localization in cerebral, hippocampal, and cerebellar neurons, resulting in decreased local secretion of BDNF from axons. This local loss of BDNF was associated with a development-dependent decrease in the number of hippocampal and cortical parvalbumin- and calbindin-positive interneurons, as well as a decreased number of dendritic spines in the dentate granule cells. Moreover, we showed that Caps2-dex3 mice displayed increased anxiety in an unfamiliar environment and impaired social behavior and circadian rhythmicity. These lines of in vivo evidence support the idea that disturbance in the proper levels of local BDNF release, which we assume to be caused here by aberrant subcellular targeting of Caps2, contributes to the abnormal circuit connectivity observed in autism (41).
Previous studies reported that decrease of the BDNF-tyrosine kinase B (TrkB) signal cascade caused reduction of parvalbumin(+) neurons (33, 42). Our results showed a decreased number of parvalbumin(+) neurons in P21 homozygote neocortex, although the number of other interneurons and NeuN(+) neurons was unchanged, suggesting that parvalbumin(+) interneurons, a minor cell type, were specifically affected in the neocortex of homozygotes. However, it cannot be ruled out that the apparent reduction in the number of parvalbumin(+) neurons was due to a decrease in the expression level of parvalbumin, without loss of neurons.
One of the symptoms of autism is augmented anxiety and/or reduced environmental exploration in a novel environment. Novel stimuli, such as unfamiliar environments or objects, are theorized to create conflict in rodents by concurrently evoking both approach and avoidance behaviors (43). Approach behavior or “exploration” reflects an animal’s tendency to explore novel stimuli or environments, whereas avoidance behavior or “anxiety-related behavior” is thought to reflect an animal’s fear of novelty. In this report, Caps2Δex3/Δex3 mice showed decreased locomotor activity compared with wild-type mice when placed in an open field containing a novel object. Moreover, Caps2Δex3/Δex3 mice tended not to contact a novel object. Overall, Caps2Δex3/Δex3 mice tended to show augmented anxiety or reduced environmental exploration in a novel environment.
Caps2Δex3/Δex3 mice showed impairments in both social interaction and social novelty preference. Caps2+/Δex3 mice also exhibited a significant reduction in interaction activity, suggesting that a social behavioral deficit might be attributable to the approximately equal balance of dex3-minus vs. dex3-plus isoform expression (Fig. S1B) that was found in some autistic patients (8). The link between social behavior in rodents and humans is difficult to establish. Our model may provide a powerful tool to explore its mechanisms.
Recently, it was suggested that splicing dysregulation is an underlying mechanism of neuronal dysfunction in autism (16). However, it remains unclear how the increased removal of exon 3 of CAPS2 is specifically caused in certain autistic patients. We did not find any nucleotide sequence differences in the three common splicing cis-elements (the 5′ donor and 3′ acceptor sites and the branch point) of the CAPS2 gene in autistic patients displaying exon 3 skipping (8). We also examined the sequence of introns 2 and 3 in the autistic subjects, and we found some variants specifically identified in patients displaying increased exon 3 skipping. We are currently investigating the association of these variants with exon 3 skipping. We must also consider the possibility that there is a polymorphism(s) in an unknown gene(s), which alters the splicing of CAPS2 mRNA (10). This scenario would predict the abnormal splicing of multiple genes besides CAPS2. However, the Caps2Δex3/Δex3 mice, in which only Caps2 was mutated, showed autistic-like phenotypes, arguing against the involvement of other abnormally spliced genes. This causality is reminiscent of that between mutation of Mecp2 (the cause) and altered expression of BDNF (the effect) in Rett syndrome. Bdnf is one of the affected genes in Mecp2-knockout mice; however, BDNF is strongly associated with the symptoms of Rett syndrome (44, 45). Clearly, the genetic involvement for autism is complex, but our approach of model validity—reverse engineering a mouse model for autistic patients with overexpression of a dex3 isoform of CAPS2, which shows relevant phenotypes—is certainly promising.
Materials and Methods
Animals.
All experimental protocols were approved by the RIKEN Institutional Animal Care and Use Committee. Mice were housed on a 12-h light/dark cycle, with the dark cycle from 20:00–08:00.
Generation of Caps2 Exon 3-Skipped Targeting Vector.
An 11-kb genomic fragment containing exon 3 of the mouse Caps2 gene was obtained from the genomic DNA of C57BL/6 mice and was used to construct the targeting vectors (Fig. S1A). One loxP sequence was inserted at the BtrI site, and the phosphoglycerate kinase–neomycin resistance (neo) cassette, flanked by another loxP sequence and a pair of frt sequences, was inserted into the AatII site upstream of exon 3, to generate a loxP–frt–neo–frt–exon3–loxP cassette. For negative selection, the diphtheria toxin A-fragment gene cassette was added to the 3′ end of the targeting vector.
Generation of Caps2 exon 3-skipped mice, primaryculture, immunohistochemistry, immunocytochemistry, and behavioral tests are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Charles Yokoyama (RIKEN Brain Science Institute) for help in improving our manuscript. This study was supported by Grants-in-Aid for Scientific Research from Japan Foundation for Neuroscience and Mental Health; the Naito Foundation; the Narishige Neuroscience Research Foundation; the Uehara Memorial Foundation, the Nakajima Foundation; the Yamada Science Foundation; the Mother and Child Health Foundation, the NOVARTIS Foundation for the Promotion of Science; the Hamaguchi Foundation for the Advancement of Biochemistry; Scientific Research on Innovative Areas “Foundation of Synapse and Neurocircuit Pathology;” the Japan Science and Technology Agency (JST)/Core Research for Evolutional Science and Technology (CREST); Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grants 23110524 and 23700454; Japan Society for the Promotion of Science Grants 23300137 and 23240062; the Institute of Physical and Chemical Research (RIKEN); and the Program to Disseminate Tenure Tracking System of MEXT granted to Gunma University.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210055109/-/DCSupplemental.
References
- 1.Binda AV, Kabbani N, Levenson R. Regulation of dense core vesicle release from PC12 cells by interaction between the D2 dopamine receptor and calcium-dependent activator protein for secretion (CAPS) Biochem Pharmacol. 2005;69(10):1451–1461. doi: 10.1016/j.bcp.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Speidel D, et al. CAPS1 regulates catecholamine loading of large dense-core vesicles. Neuron. 2005;46(1):75–88. doi: 10.1016/j.neuron.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Tandon A, et al. Differential regulation of exocytosis by calcium and CAPS in semi-intact synaptosomes. Neuron. 1998;21(1):147–154. doi: 10.1016/s0896-6273(00)80522-x. [DOI] [PubMed] [Google Scholar]
- 4.Grishanin RN, et al. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43(4):551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Sadakata T, et al. Interaction of calcium-dependent activator protein for secretion 1 (CAPS1) with the class II ADP-ribosylation factor small GTPases is required for dense-core vesicle trafficking in the trans-Golgi network. J Biol Chem. 2010;285(49):38710–38719. doi: 10.1074/jbc.M110.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadakata T, et al. The secretory granule-associated protein CAPS2 regulates neurotrophin release and cell survival. J Neurosci. 2004;24(1):43–52. doi: 10.1523/JNEUROSCI.2528-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadakata T, et al. Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci. 2007;27(10):2472–2482. doi: 10.1523/JNEUROSCI.2279-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadakata T, et al. Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J Clin Invest. 2007;117(4):931–943. doi: 10.1172/JCI29031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadakata T, Furuichi T. Developmentally regulated Ca2+-dependent activator protein for secretion 2 (CAPS2) is involved in BDNF secretion and is associated with autism susceptibility. Cerebellum. 2009;8(3):312–322. doi: 10.1007/s12311-009-0097-5. [DOI] [PubMed] [Google Scholar]
- 10.Eran A, et al. Comment on “Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients.”. J Clin Invest. 2009;119(4):679–680. doi: 10.1172/JCI38620. author reply 680–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Molecular Genetic Study of Autism Consortium (IMGSAC) Further characterization of the autism susceptibility locus AUTS1 on chromosome 7q. Hum Mol Genet. 2001;10(9):973–982. doi: 10.1093/hmg/10.9.973. [DOI] [PubMed] [Google Scholar]
- 12.Cisternas FA, Vincent JB, Scherer SW, Ray PN. Cloning and characterization of human CADPS and CADPS2, new members of the Ca2+-dependent activator for secretion protein family. Genomics. 2003;81(3):279–291. doi: 10.1016/s0888-7543(02)00040-x. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto N, Hatsukawa Y, Shimojima K, Yamamoto T. Submicroscopic deletion in 7q31 encompassing CADPS2 and TSPAN12 in a child with autism spectrum disorder and PHPV. Am J Med Genet A. 2011;155A(7):1568–1573. doi: 10.1002/ajmg.a.34028. [DOI] [PubMed] [Google Scholar]
- 14.Christian SL, et al. Novel submicroscopic chromosomal abnormalities detected in autism spectrum disorder. Biol Psychiatry. 2008;63(12):1111–1117. doi: 10.1016/j.biopsych.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szatmari P, et al. Autism Genome Project Consortium Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voineagu I, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474(7351):380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nat Rev Genet. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bangash MA, et al. Enhanced polyubiquitination of Shank3 and NMDA receptor in a mouse model of autism. Cell. 2011;145(5):758–772. doi: 10.1016/j.cell.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Durand CM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39(1):25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19(2):231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Peça J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318(5847):71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkel S, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42(6):489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 24.Jamain S, et al. Paris Autism Research International Sibpair Study Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34(1):27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoghbi HY. Postnatal neurodevelopmental disorders: Meeting at the synapse? Science. 2003;302(5646):826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- 26.Pinto D, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466(7304):368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadakata T, Washida M, Furuichi T. Alternative splicing variations in mouse CAPS2: Differential expression and functional properties of splicing variants. BMC Neurosci. 2007;8:25. doi: 10.1186/1471-2202-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadakata T, et al. Differential distributions of the Ca2+ -dependent activator protein for secretion family proteins (CAPS2 and CAPS1) in the mouse brain. J Comp Neurol. 2006;495(6):735–753. doi: 10.1002/cne.20947. [DOI] [PubMed] [Google Scholar]
- 29.Kuczewski N, et al. Backpropagating action potentials trigger dendritic release of BDNF during spontaneous network activity. J Neurosci. 2008;28(27):7013–7023. doi: 10.1523/JNEUROSCI.1673-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiorentino H, et al. GABA(B) receptor activation triggers BDNF release and promotes the maturation of GABAergic synapses. J Neurosci. 2009;29(37):11650–11661. doi: 10.1523/JNEUROSCI.3587-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinoda Y, et al. Calcium-dependent activator protein for secretion 2 (CAPS2) promotes BDNF secretion and is critical for the development of GABAergic interneuron network. Proc Natl Acad Sci USA. 2011;108(1):373–378. doi: 10.1073/pnas.1012220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: Implications for autisim. Neuroscientist. 2003;9(6):496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- 33.Jones KR, Fariñas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76(6):989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: Cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39(1):29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- 35.Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11(2):172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization 1992. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines (WHO, Geneva) pp XII, 362.
- 37.American Psychiatric Association Task Force on DSM-IV 1994. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV (American Psychiatric Association, Washington, DC) 4th Ed pp XXVII, 886.
- 38.Nakatani J, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137(7):1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filipek PA, et al. Practice parameter: Screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55(4):468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- 40.Richdale AL, Prior MR. The sleep/wake rhythm in children with autism. Eur Child Adolesc Psychiatry. 1995;4(3):175–186. doi: 10.1007/BF01980456. [DOI] [PubMed] [Google Scholar]
- 41.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Zheng K, et al. TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc Natl Acad Sci USA. 2011;108(41):17201–17206. doi: 10.1073/pnas.1114241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48(4):254–260. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- 44.Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49(3):341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 45.Chen WG, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302(5646):885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.