Abstract
It is believed that neurosteroids are produced in the brain and other nervous systems. Here, we show that allopregnanolone (ALLO), a neurosteroid, is exceedingly produced in the pineal gland compared with the brain and that pineal ALLO acts on the Purkinje cell, a principal cerebellar neuron, to prevent apoptosis in the juvenile quail. We first demonstrated that the pineal gland is a major organ of neurosteroidogenesis. A series of experiments using molecular and biochemical techniques has further demonstrated that the pineal gland produces a variety of neurosteroids de novo from cholesterol in the juvenile quail. Importantly, ALLO was far more actively produced in the pineal gland than in the brain. Pinealectomy (Px) decreased ALLO concentration in the cerebellum and induced apoptosis of Purkinje cells, whereas administration of ALLO to Px quail chicks prevented apoptosis of Purkinje cells. We further found that Px significantly increased the number of Purkinje cells that expressed active caspase-3, a key protease in apoptotic pathway, and daily injection of ALLO to Px quail chicks decreased the number of Purkinje cells expressing active caspase-3. These results indicate that the neuroprotective effect of pineal ALLO is associated with the decrease in caspase-3 activity during the early stage of neuronal development. We thus provide evidence that the pineal gland is an important neurosteroidogenic organ and that pineal ALLO may be involved in Purkinje cell survival during development. This is an important function of the pineal gland in the formation of neuronal circuits in the developing cerebellum.
Keywords: steroid, melatonin, HPLC, gas chromatography/mass spectrometry
The cerebellar cortex has been used as an excellent model to study synaptic formation and transmission of neural networks because it forms relatively simple neuronal networks compared with those of other brain regions. The Purkinje cell is a principal cerebellar neuron that integrates the process of memory and learning. It is known that in birds and mammals pinealectomy (Px) induces cell loss in the brain including Purkinje cells during development (1, 2). This observation suggests that a certain component(s) in the pineal gland contributes to Purkinje cell survival during development.
It is now established that steroids can be synthesized de novo in the central and peripheral nervous systems. Such steroids are called “neurosteroids,” and de novo neurosteroidogenesis from cholesterol is a conserved property of the vertebrate brain (for reviews, see refs. 3–7). The Purkinje cell is known as a site of neurosteroidogenesis in the brain (for review, see ref. 8). This cerebellar neuron produces progesterone (PROG) and estradiol-17β (E2) de novo from cholesterol during neonatal life, when cerebellar neuronal circuit formation occurs. Both PROG and E2 promote dendritic growth, spinogenesis, and synaptogenesis via each cognate nuclear receptor in the developing Purkinje cell (9–11). Allopregnanolone (ALLO; 3α,5α-tetrahydroprogesterone), a progesterone metabolite, is also synthesized in the cerebellum and facilitates Purkinje cell survival in the neonate (12).
Until recently, we believed that neurosteroids are produced only in the brain and other nervous systems. On the basis of our previous study of chickens (13), however, the pineal gland may be a major organ in producing neurosteroids de novo from cholesterol. We thus hypothesized that pineal neurosteroid(s) may be involved in Purkinje cell survival during development.
In the present study, we first demonstrated the biosynthetic pathway of neurosteroids in the pineal gland of quail. We found that the pineal gland of quail chicks exceedingly synthesizes ALLO compared with the brain. Px decreased ALLO concentration in the cerebellum and induced apoptosis of Purkinje cells, whereas administration of ALLO to Px quail chicks prevented apoptosis of Purkinje cells. Administration of ALLO to Px quail chicks also decreased the expression of caspase-3, a key protease in the apoptotic pathway, in Purkinje cells. Thus, ALLO rescues developing Purkinje cells in the cerebellum from apoptosis. Our results further point to a role of pineal ALLO in preventing the death of developing Purkinje cells.
Results
De Novo Pregnenolone Formation from Cholesterol in the Quail Chick Pineal Gland.
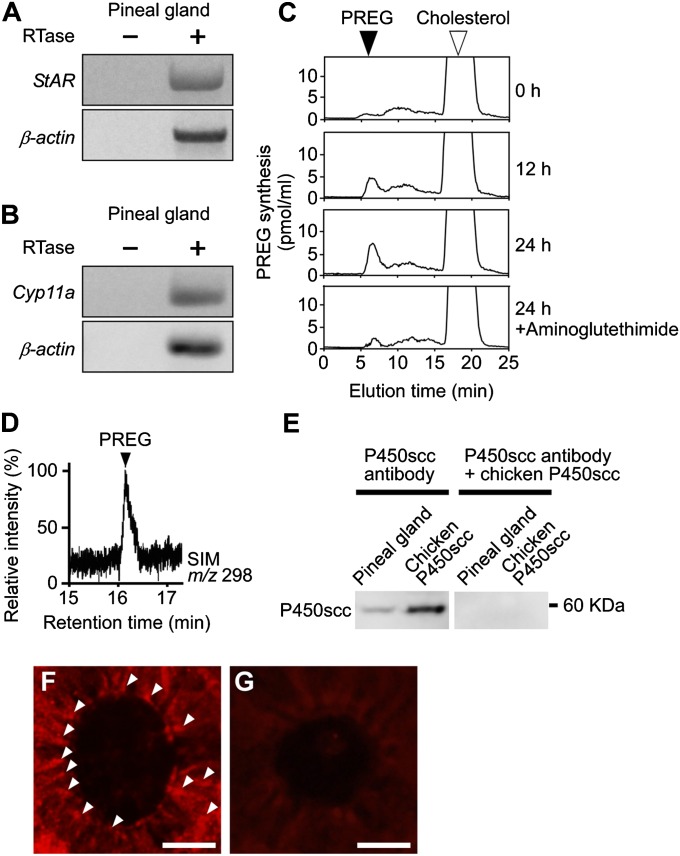
Because pregnenolone (PREG) formation is the first step in steroid synthesis (14, 15), we first investigated whether the pineal gland synthesizes PREG from cholesterol de novo. We used pineal glands of male juvenile quail of posthatch day 7 (P7). Steroidogenic acute regulatory protein (StAR; gene name StAR) delivers cholesterol to the mitochondrial cytochrome P450 side-chain cleavage enzyme (P450scc; gene name Cyp11a) that produces PREG. Reverse transcription PCR (RT-PCR) analyses have demonstrated the expressions of StAR and Cyp11a mRNAs in the pineal gland (Fig. 1 A and B and Table S1). The amplified cDNA bands from the pineal gland were sequenced, and it was verified that they were authentic fragments of StAR (GenBank accession no. NM204686) and Cyp11a (GenBank accession no. NM001001756).
Fig. 1.
De novo PREG formation from cholesterol in the pineal gland. (A and B) RT-PCR analyses of StAR (A) and Cyp11a (B) mRNAs in the pineal gland of male quail chicks. Total RNA extracted from the tissue was reverse-transcribed with (+) or without (−) reverse transcriptase (RTase) followed by PCR amplification. (C) HPLC analysis of PREG formation from cholesterol in the pineal gland of male quail chicks. The pineal gland was incubated with [3H]cholesterol and homogenized after different incubation times, and then each extract was subjected to HPLC. The pineal gland with [3H]cholesterol was also incubated with aminoglutethimide. The arrowheads indicate the elution positions of the substrate cholesterol (open arrowhead) and its metabolite PREG (solid arrowhead). (D) GC-SIM analysis of the metabolite of nonradioactive cholesterol by the pineal gland of male quail chicks. GC-SIM was traced at m/z 298 for PREG as the metabolite of nonradioactive cholesterol. The arrowhead shows the peak corresponding to authentic PREG. (E) Western blot analysis of the pineal gland and extracts of COS-7 cells transfected with chicken Cyp11a cDNA with the anti-human P450scc antibody. Anti-human P450scc antibody was preadsorbed with chicken P450scc protein for control. (F) IHC of P450scc in the pineal gland. Arrowheads indicate immunoreactive cells. (G) IHC using P450scc antibody preadsorbed with a saturating concentration of chicken P450scc protein (10 μg/mL). (Scale bars, 10 μm.) Similar results were obtained in repeated experiments using three different samples.
To investigate PREG formation from cholesterol in the quail pineal gland, pineal glands of male chicks at P7 were incubated with tritiated cholesterol as a precursor, and the radioactive metabolite was analyzed by reversed-phase HPLC. As shown in Fig. 1C, a single radioactive peak was detected, and it exhibited the same retention time as that of tritiated PREG, a reference standard, under the same chromatographic condition. The radioactive peak corresponding to PREG increased in a time-dependent manner from 0 to 24 h of incubation (Fig. 1C). In addition, 50 μM aminoglutethimide, an inhibitor of P450scc, reduced the amplitude of this peak (Fig. 1C).
PREG synthesis in the pineal gland was further demonstrated by gas chromatography/mass spectrometry (GC-MS) as described previously (16–21). Heptafluorobutyrate derivatives of the authentic PREG and the metabolite of nonradioactive cholesterol were prepared and applied to GC-MS analysis. On the basis of GC-MS–selected ion monitoring (SIM) analysis [mass/charge (m/z) 298] (16, 17), it was confirmed that the metabolite had a retention time that was identical to PREG (Fig. 1D).
Immunohistochemical (IHC) analysis using anti-human P450scc antibody was conducted to analyze the cellular localization of P450scc in the pineal gland. To confirm that this antibody recognizes galliformes P450scc protein, we first performed Western blot analysis on the extracts of COS-7 cells transfected with chicken Cyp11a cDNA. A single immunoreactive band (60 kDa) was detected (Fig. 1E). When the pineal gland extracts were analyzed, a single band (60 kDa) was detected at the same position (Fig. 1E). This band disappeared when the antibody was preadsorbed with chicken P450scc protein (Fig. 1E). Clear P450scc immunoreactivity was also observed in the cells forming follicular structures in the quail pineal gland (Fig. 1F). No immunoreactivity was observed when anti-P450scc antibody was preadsorbed with chicken P450scc protein (Fig. 1G).
Neurosteroid Formations from PREG in the Quail Chick Pineal Gland.
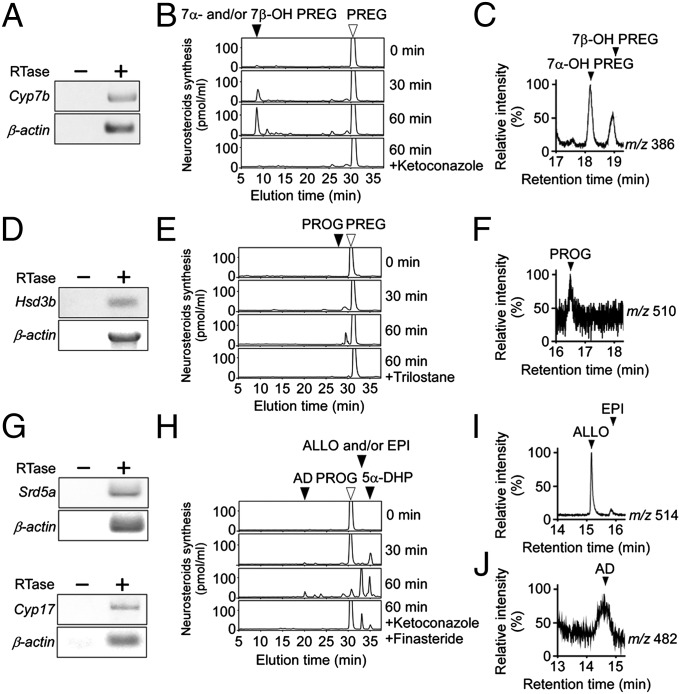
To investigate the biosynthetic pathway of neurosteroids in the pineal gland, pineal glands of male quail chicks at P7 were used for RT-PCR analyses to demonstrate the expressions of steroidogenic enzymes, such as cytochrome P450 7α-hydroxylase (P4507α; gene name Cyp7b), 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase (3β-HSD; gene name Hsd3b), 5α-reductase (gene name Srd5a), cytochrome P450 17α-hydroxylase/c17,20-lyase (P45017α,lyase; gene name Cyp17), 17β-hydroxysteroid dehydrogenase (17β-HSD; gene name Hsd17b), and cytochrome P450 aromatase (P450arom; gene name Cyp19). RT-PCR analyses showed cDNA bands of Cyp7b, Hsd3b, Srd5a, Cyp17, Hsd17b, and Cyp19 (Fig. 2 A, D, and G; Fig. S1 A and D and Table S1). The amplified cDNA bands in the pineal gland were sequenced, and it was verified that they were authentic fragments of Cyp7b (GenBank accession no. AB329632), Hsd3b (GenBank accession no. FJ607242), Srd5a (GenBank accession no. XM001235446), Cyp17 (GenBank accession no. AB281617), Hsd17b (GenBank accession no. NM204943), and Cyp19 (GenBank accession no. AF533667) cDNAs.
Fig. 2.
Neurosteroid formation from PREG in the pineal gland. (A, D, and G) RT-PCR analyses of steroidogenic enzyme Cyp7b (A), Hsd3b (D), Srd5a (G), and Cyp17 (G) mRNAs in the pineal gland of male quail chicks. Total RNA was reverse-transcribed with (+) or without (−) RTase followed by PCR amplification. (B, E, and H) HPLC analyses of neurosteroid formation in the pineal gland of male quail chicks. (B) The pineal gland homogenates were incubated with [3H]PREG + NADPH, and the extracts were subjected to HPLC. The pineal gland homogenates with [3H]PREG were also incubated with ketoconazole. The arrowheads indicate elution positions of the substrate PREG (open arrowhead) and its metabolite 7α- and/or 7β-OH PREG (solid arrowhead). (E) The pineal gland homogenates were incubated with [3H]PREG + NAD+, and the extracts were subjected to HPLC. The pineal gland homogenates with [3H]PREG were also incubated with trilostane. The arrowheads indicate elution positions of the substrate PREG (open arrowhead) and its metabolite PROG (solid arrowhead). (H) The pineal gland homogenates were incubated with [3H]PROG + NADPH, and the extracts were subjected to HPLC. The pineal gland homogenates with [3H]PROG were also incubated with ketoconazole and finasteride. The arrowheads indicate elution positions of the substrate PROG (open arrowhead) and its metabolites 5α-DHP, ALLO and/or EPI and AD (solid arrowheads). (C, F, I, and J) GC-SIM analyses of the metabolites of nonradioactive PREG or PROG by the pineal gland of male quail chicks. GC-SIM was traced at m/z 386 for 7α- and 7β-OH PREG (C) and at m/z 510 for PROG (F), metabolites of nonradioactive PREG, or at m/z 514 for ALLO and EPI (I) and at m/z 482 for AD (J), metabolites of nonradioactive PROG. The arrowheads show the peaks corresponding to authentic 7α- and 7β-OH PREG (C), PROG (F), ALLO and EPI (I), and AD (J). Similar results were obtained in repeated experiments using three different samples.
To demonstrate neurosteroid formation in the quail pineal gland, pineal gland homogenates of male chicks at P7 were incubated with tritiated PREG as a precursor, and radioactive metabolites were analyzed by reversed-phase HPLC as described previously (18–21). The radioactive metabolites corresponding to 7α- and/or 7β-hydroxypregnenolone (7α- and/or 7β-OH PREG; Fig. 2B) and PROG (Fig. 2E) increased in a time-dependent manner. These metabolites were reduced by the treatment of ketoconazole, an inhibitor of cytochrome P450s (Cyps) (Fig. 2B), or by trilostane, an inhibitor of 3β-HSD (Fig. 2E). ALLO and/or epipregnanolone (EPI; 3β,5β-tetrahydroprogesterone), 5α-dihydroprogesterone (5α-DHP), and androstenedione (AD) were produced from the precursor PROG (Fig. 2H). Ketoconazole and finasteride, an inhibitor of 5α-reductase, reduced the productions of these metabolites (Fig. 2H). Testosterone (T) was produced from the precursor AD (Fig. S1B). 5α- and/or 5β-Dihydrotestosterone (5α- and/or 5β-DHT) and E2 were produced from the precursor T (Fig. S1E), and ketoconazole and finasteride reduced these products (Fig. S1E). Isoforms, such as 7α- and 7β-OH PREG; ALLO and EPI; and 5α- and 5β-DHT were not separated by the retention times in HPLC.
Neurosteroid formation in the pineal gland was further confirmed by GC-SIM analysis as described previously (16–21). Derivatives of the authentic 7α- and 7β-OH PREG, PROG, ALLO, EPI, AD, T, 5α- and 5β-DHT, E2, and the metabolites of nonradioactive steroids produced by the pineal gland were applied to GC-SIM analysis (m/z 386 for 7α- and 7β-OH PREG, m/z 510 for PROG, m/z 514 for ALLO and EPI, m/z 482 for AD, m/z 680 for T, m/z 486 for 5α- and 5β-DHT, and m/z 664 for E2). The isoforms 7α- and 7β-OH PREG (Fig. 2C); ALLO and EPI (Fig. 2I); and 5α- and 5β-DHT (Fig. S1F) had different retention times in GC-MS, respectively, unlike HPLC. The neurosteroids produced in the pineal gland were thus identified as 7α- and 7β-OH PREG (Fig. 2C), PROG (Fig. 2F), ALLO and EPI (Fig. 2I), AD (Fig. 2J), T (Fig. S1C), 5α- and 5β-DHT (Fig. S1F), and E2 (Fig. S1G).
ALLO and 7α-OH PREG Are Abundantly Synthesized and Released from the Quail Chick Pineal Gland.
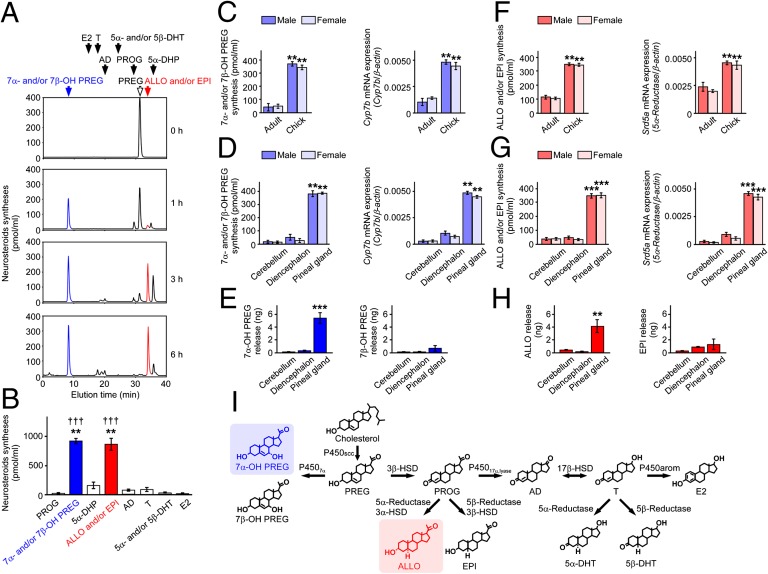
To identify major neurosteroids synthesized in the pineal gland, the pineal glands were cultured in medium 199 with [3H]PREG as a precursor. PREG was converted primarily to 7α- and/or 7β-OH PREG shown in blue and to ALLO and/or EPI shown in red by the pineal gland (Fig. 3 A and B).
Fig. 3.
ALLO and 7α-OH PREG are abundantly synthesized and released from the quail chick pineal gland. (A) Neurosteroids synthesized from PREG in the pineal gland of male quail chicks. The pineal glands were incubated with the substrate [3H]PREG, and the extracts were subjected to HPLC. The arrows indicate elution positions of the substrate PREG (open arrow) and its metabolites (solid arrows). (B) Comparison of the amount of neurosteroids synthesized from PREG in the pineal gland of male quail chicks by HPLC (n = 8). **P < 0.01 vs. 5α-DHP, AD, or T; †††P < 0.001 vs. PROG, 5α- and/or 5β-DHT, or E2. (C) Comparisons of 7α- and/or 7β-OH PREG synthesis and Cyp7b mRNA expression in the pineal gland of adults and chicks of both sexes (n = 8). **P < 0.01 vs. adult. (D) Comparisons of 7α- and/or 7β-OH PREG synthesis and Cyp7b mRNA expression among the pineal gland, cerebellum, and diencephalon of quail chicks of both sexes (n = 8). **P < 0.01 vs. cerebellum or diencephalon. (E) Comparisons of 7α- and 7β-OH PREG releases from the pineal gland, cerebellum, and diencephalon of male quail chicks by GC-MS (n = 6). ***P < 0.001 vs. cerebellum or diencephalon. (F) Comparisons of ALLO and/or EPI synthesis and Srd5a mRNA expression in the pineal gland of adults and chicks of both sexes (n = 8). **P < 0.01 vs. adult. (G) Comparisons of ALLO and/or EPI synthesis and Srd5a mRNA expression among the pineal gland, cerebellum, and diencephalon of quail chicks of both sexes (n = 8). ***P < 0.001 vs. cerebellum or diencephalon. (H) Comparisons of ALLO and EPI releases from the pineal gland, cerebellum, and diencephalon of male quail chicks by GC-MS (n = 6). **P < 0.01 vs. cerebellum or diencephalon. (I) Identified biosynthetic pathways of neurosteroids in the pineal gland. Each column and vertical line in B–H represent the mean ± SEM.
We then compared the syntheses of these major neurosteroids by HPLC and the expressions of their steroidogenic enzyme mRNAs by real-time PCR in the pineal gland among both sexes of adult and juvenile quail. The synthesis of 7α- and/or 7β-OH PREG and the expression of Cyp7b mRNA were detected in both sexes of adults and juvenile quail, but there was a clear age difference in each parameter (Fig. 3C). 7α- and/or 7β-OH PREG synthesis and Cyp7b mRNA expression were greater in juveniles than in adults in both sexes (Fig. 3C). ALLO and/or EPI synthesis and Srd5a mRNA expression were also greater in juveniles than in adults in both sexes (Fig. 3F). The syntheses of these major neurosteroids and the expressions of their steroidogenic enzyme mRNAs in the pineal gland were compared with those of different brain regions. 7α- and/or 7β-OH PREG synthesis and Cyp7b mRNA expression were greater in the pineal gland than in the cerebellum and diencephalon (Fig. 3D). ALLO and/or EPI synthesis and Srd5a mRNA expression were also greater in the pineal gland than in the cerebellum and diencephalon (Fig. 3G).
To investigate neurosteroid release from the pineal gland, the pineal glands were cultured in medium 199, and major neurosteroids were measured by GC-MS. Significant amounts of 7α-OH PREG and ALLO were released from the pineal gland into the culture medium, unlike 7β-OH PREG and EPI (Fig. 3 E and H). In sum, 7α-OH PREG and ALLO were major products secreted by the pineal gland (Fig. 3I).
Pineal ALLO Saves Purkinje Cells from Cell Death in Px Quail Chicks.
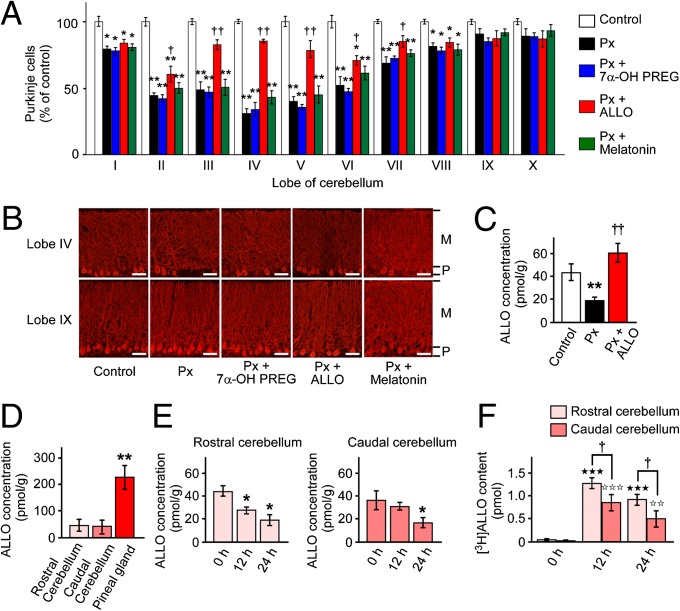
To investigate whether ALLO and 7α-OH PREG, major pineal neurosteroids, or melatonin, a major hormone of the pineal gland, are involved in Purkinje cell survival in the male juvenile quail, Px was performed at P2, and 7α-OH PREG and ALLO were injected daily, or quail chicks were s.c. implanted with a silastic plate containing melatonin (22) from P2 to P7. Px at P2 significantly decreased the number of Purkinje cells in lobes I–VIII at P21 (Fig. 4 A and B and Fig. S2A). Px decreased ALLO concentration in the cerebellum at P7 compared with control (Fig. 4C). Daily injection of ALLO to Px quail chicks from P2 to P7 improved Purkinje cell survival in lobes II–VII at P21 (Fig. 4 A and B and Fig. S2A) and increased ALLO concentration in the cerebellum at P7 compared with Px quail chicks (Fig. 4C). On the contrary, daily injection of 7α-OH PREG or melatonin to Px quail chicks from P2 to P7 did not increase Purkinje cell survival (Fig. 4 A and B and Fig. S2A).
Fig. 4.
Pineal ALLO saves Purkinje cells from cell death in Px quail chicks. Cerebella of control male quail chicks, Px at P2 male quail chicks, and Px at P2 male quail chicks treated with daily injection of 7α-OH PREG (30 ng/5 μL) or ALLO (30 ng/5 μL), or Px at P2 male quail chicks s.c. implanted with a silastic plate containing melatonin (10 mg/plate) from P2 to P7 were analyzed. (A) Number of Purkinje cells in the cerebellar lobes I–X at P21. Purkinje cell number is presented as the percentage of control (n = 12). *P < 0.05 or **P < 0.01 vs. control; †P < 0.05 or ††P < 0.01 Px plus ALLO vs. Px. (B) Morphology of Purkinje cells in the cerebellar lobes IV and IX at P21. M, molecular layer; P, Purkinje cell layer. (Scale bars, 40 μm.) (C) Effects of Px and daily injection of ALLO on ALLO concentration in the cerebellum of male quail chicks at P7 (n = 12). **P < 0.01 vs. control; ††P < 0.01 Px plus ALLO vs. Px. (D) Comparisons of ALLO concentration among the pineal gland, rostral cerebellum, and caudal cerebellum in P2 male quail chicks (n = 7). **P < 0.01 vs. rostral cerebellum or caudal cerebellum. (E) Effects of Px on ALLO concentrations in the rostral and caudal cerebellum of male quail chicks (n = 7). *P < 0.05 vs. 0 h. (F) Contents of [3H]ALLO in the rostral and caudal cerebellum after injection of [3H]ALLO close to the pineal lumen of male quail chicks at P2. [3H]ALLO (20 pmol) was injected close to the pineal lumen, and the content of [3H]ALLO in the rostral and caudal cerebellum was counted in a liquid scintillation counter (n = 7). ★★★P < 0.001 vs. 0 h; ☆☆P < 0.01 or ☆☆☆P < 0.001 vs. 0 h; †P < 0.05 rostral cerebellum vs. caudal cerebellum. Each column and vertical line in A and C–F represent the mean ± SEM.
The effect of ALLO and 7α-OH PREG on Purkinje dendritic length in lobes IV and IX were investigated among the five groups (control, Px, Px + 7α-OH PREG, Px + ALLO, and Px + melatonin) because the number of Purkinje cells in lobe IV was most vulnerable to Px and Px did not affect Purkinje cell numbers in lobe IX (Fig. 4 A and B and Fig. S2A). In contrast to the number of Purkinje cells, there was no significant difference in the maximal dendritic length of Purkinje cells among the five groups in lobes IV and IX (Fig. 4B and Figs. S2A and S3).
To investigate whether melatonin influences the synthesis of ALLO and/or EPI in the cerebellum and circulating ALLO in the male juvenile quail, Px was performed at P2 and the quail chicks were s.c. implanted with a silastic plate containing melatonin or vehicle (22). Px or melatonin administration did not influence the synthesis of ALLO and/or EPI in the cerebellum and circulating ALLO in the male juvenile quail (Fig. S4).
Pineal ALLO Reaches the Adjacent Cerebellar Purkinje Cells by Diffusion.
To better understand the mechanism of how pineal ALLO reaches the adjacent cerebellar Purkinje cells, Px was performed at P2, and ALLO concentration was measured in the rostral (lobes II–V) and caudal (lobes VI–IX) cerebellum of quail chicks. Px decreased ALLO concentration only in the rostral cerebellum and not in the caudal cerebellum 12 h after Px (Fig. 4E). To further investigate whether pineal ALLO reaches the cerebellum by diffusion in the juvenile quail at P2, [3H]ALLO was injected close to the pineal lumen. [3H]ALLO content was significantly higher in the rostral cerebellum than in the caudal cerebellum 12 and 24 h after [3H]ALLO injection (Fig. 4F).
Neuroprotective Effect of Pineal ALLO Is Associated with the Decrease in Caspase-3 Activity in Purkinje Cells.
Finally, we investigated the factor that mediates the neuroprotective effect of pineal ALLO in Purkinje cells. It is well known that caspase-3 plays an important role in Purkinje cell death in vertebrates (23, 24). Caspase-3 is a crucial mediator of apoptosis (23), including in birds (24, 25). Accordingly, the expression of caspase-3 and the fragmentation of nuclear DNA were analyzed in Px quail chicks and Px plus ALLO-administrated quail chicks from P3 to P7.
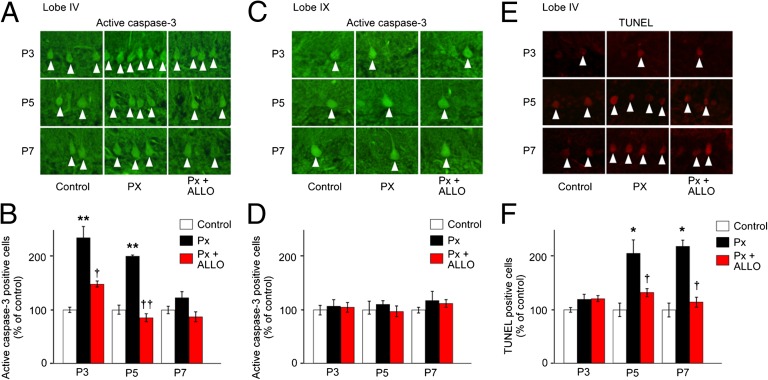
Px significantly increased the number of Purkinje cells that expressed active caspase-3 in lobe IV of P3 and P5 quail chicks compared with control (Fig. 5 A and B). In contrast, daily injection of ALLO to Px chicks decreased the number of Purkinje cells expressing active caspase-3 at P3 and P5 (Fig. 5 A and B). On the other hand, the effect of Px or ALLO administration on active caspase-3 expression was not observed at P7 (Fig. 5 A and B). Px or ALLO administration had no effect on active caspase-3 expression in lobe IX (Fig. 5 C and D).
Fig. 5.
Neuroprotective effect of pineal ALLO is associated with the decrease in caspase-3 activity in Purkinje cells. (A and C) Purkinje cells expressing active caspase-3 in lobes IV (A) and IX (C) of control male quail chicks, Px male quail chicks, and Px plus ALLO male quail chicks at P3, P5, and P7. (B and D) Comparison of the number of Purkinje cells expressing active caspase-3 in lobes IV (B) and IX (D) among control male quail chicks, Px male quail chicks, and Px plus ALLO male quail chicks at P3, P5, and P7 (n = 12). **P < 0.01 vs. control; †P < 0.05 or ††P < 0.01 Px plus ALLO vs. Px. (E) TUNEL-positive Purkinje cells in lobe IV of control male quail chicks, Px male quail chicks, and Px plus ALLO male quail chicks at P3, P5, and P7. (F) Comparison of the number of TUNEL-positive Purkinje cells in lobe IV among control male quail chicks, Px male quail chicks, and Px plus ALLO male quail chicks at P3, P5, and P7 (n = 12). *P < 0.05 vs. control; †P < 0.05 Px plus ALLO vs. Px. Each column and vertical line in B, D, and F represent the mean ± SEM.
DNA fragmentation was further investigated by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) in Purkinje cells in lobe IV. There was no effect of Px or ALLO administration on the number of TUNEL-positive Purkinje cells at P3 (Fig. 5 E and F). However, Px significantly increased the number of TUNEL-positive Purkinje cells compared with control at P5 and P7 (Fig. 5 E and F), and daily injection of ALLO to Px quail chicks decreased the number of TUNEL-positive Purkinje cells compared with Px quail chicks at P5 and P7 (Fig. 5 E and F).
Discussion
A series of experiments using molecular and biochemical techniques has demonstrated that the pineal gland is a major neurosteroidogenic organ that produces a variety of neurosteroids de novo from cholesterol in the juvenile quail, a demonstration of de novo neurosteroidogenesis in the pineal gland in a vertebrate class. Importantly, ALLO and 7α-OH PREG were exceedingly produced in the pineal gland compared with the brain. These major pineal neurosteroids were abundantly released from the pineal gland (Fig. 3 E and H; Fig. S2). These results suggest that pineal ALLO and/or 7α-OH PREG play important roles in the juvenile quail.
It has been reported that Px induces Purkinje cell loss in the developing cerebellum of chicks (2). In this study, we hypothesized that pineal ALLO and/or 7α-OH PREG may facilitate Purkinje cell survival in the juvenile quail. To test this hypothesis, we conducted a series of experiments using Px quail chicks. Px decreased the number of Purkinje cells in the cerebellum of quail chicks at P21, suggesting that pineal ALLO and/or 7α-OH PREG may be involved in the survival of Purkinje cells in the cerebellum during development. Administration of ALLO but not of 7α-OH PREG to Px quail chicks from P2 to P7 facilitated Purkinje cell survival. Accordingly, ALLO secreted by the pineal gland may contribute to Purkinje cell survival during development. Although 7α-OH PREG did not facilitate Purkinje cell survival, this neurosteroid enhances locomotor activities of quail (21) and chickens (13).
Neuronal cell death is an essential feature of developing nervous systems and neurodegenerative diseases (26–28). Most Purkinje cells in cerebellar organotypic culture die when sampled from 1- to 5-d-old mice, whereas they survive when sampled before or after these ages (29, 30). This critical period correlates with a time window when Purkinje cells are engaged in intense synaptogenesis and dendritic remodeling. Our present study suggests that ALLO secreted by the pineal gland is involved in Purkinje cell survival during the critical period of intense synaptogenesis and dendritic remodeling.
Px did not decrease the number of Purkinje cells in the posterior lobes IX and X, unlike the anterior lobes I–VIII. It is well known that cerebellar defects are preferentially localized to the anterior (lobes I–V) and central (lobes VI and VII) lobes (31–33). It was shown in the study of Niemann-Pick type C (NP-C) mice that ALLO is synthesized in the cerebellum and involved in Purkinje and granule cell survival in the developing cerebellum (12). According to Langmade et al. (32), Purkinje cell number was reduced in npc1−/− mice, a model of NP-C disease, compared with WT mice. The greatest loss of Purkinje cells occurred in lobes I–IV, and the loss was not apparent in lobes IX and X (32). Thus, NP-C disease primarily affects the anterior lobes of the cerebellum, which were also affected by Px and ALLO treatment in this study. We found that the pineal gland is an important source of ALLO in the cerebellum (Fig. 4 C–F and Fig. S2). The present and previous studies (12, 32) suggest that pineal ALLO and cerebellar ALLO are involved in Purkinje cell survival during development (Fig. S2).
It is known that in birds and mammals Px induces cell loss in the brain, including loss of Purkinje cells during development (1, 2). Although the neuroprotective action of pineal melatonin is known in birds and mammals (34, 35), it has also been reported that melatonin does not fully ameliorate Purkinje cell loss during development (36). These observations suggest that certain other component(s) in the pineal gland may contribute to Purkinje cell survival during development. In this study, pineal melatonin did not facilitate Purkinje cell survival during development (Fig. 4 A and B) and did not affect cerebellar ALLO and/or EPI synthesis and circulating ALLO level in the male juvenile quail (Fig. S4). These results suggest that pineal ALLO but not melatonin acts as an important component of the pineal gland for Purkinje cell survival during development.
Pineal ALLO concentration (more than 200 pmol/g tissue in the intact pineal gland) was much higher than ALLO concentration in the cerebellum (around 40 pmol/g tissue in the intact cerebellum) (Fig. 4D). These concentrations of ALLO were physiologically relevant compared with ALLO concentration (10–100 pmol/g tissue in the brain) in other vertebrates (37). Px decreased ALLO concentration only in the rostral cerebellum 12 h after Px (Fig. 4E). [3H]ALLO concentration increased in the rostral cerebellum significantly more than in the caudal cerebellum 12 and 24 h after [3H]ALLO injection close to the pineal lumen (Fig. 4F). These results suggest that pineal ALLO reaches the adjacent cerebellar Purkinje cells by diffusion. In addition, the effect of pineal ALLO on the prevention of Purkinje cell death seems to be restricted to the anterior (lobes I–V) and central (lobes VI and VII) lobes of the cerebellum, which might also be taken as evidence that ALLO acts simply by diffusion (Fig. S2). Nevertheless, we cannot exclude the possibility of an anatomical link (e.g., blood vessels) from the pineal gland to the cerebellum.
The intracellular signaling pathway exerting a neuroprotective effect of ALLO in the brain was poorly understood, although the involvement of the GABAA receptor was suggested (12, 38). Our results suggested that pineal ALLO exerts antiapoptotic effects in Purkinje cells by suppressing active caspase-3 expression in the early stage of neuronal development. Px significantly increased the number of Purkinje cells that expressed active caspase-3 in lobe IV of P3 and P5 quail chicks, and daily injection of ALLO to Px quail chicks decreased the number of Purkinje cells expressing active caspase-3 at these ages. The increase in active caspase-3 expression by Px at P3 and P5 may have led to DNA fragmentation at P5 and P7. Apoptosis following DNA fragmentation is one of the mechanisms controlling neuronal cell number in the brain. The massive loss of neurons in many regions of the developing brain, including Purkinje cells, provides quantitative adjustment of populations of interconnecting neurons (39). It is therefore considered that pineal ALLO may play important roles in the prevention of Purkinje cell death and the formation of neuronal circuits in the developing cerebellum.
Materials and Methods
Japanese quail, Coturnix japonica, at various ages were used in this study. Quail were incubated under daily photoperiods of 12-h light/12-h darkness cycles with the light provided by white fluorescent lamps. Pineal glands were isolated from the light-exposed animals at zeitgeber time 6. To analyze the expressions of steroidogenic enzyme mRNAs, RT-PCR analyses were conducted according to our previous methods (10, 11, 13). To assess neurosteroid formation in the quail pineal gland, conversions of substrate steroids were measured biochemically by HPLC and GC-MS according to our previous methods (18–21). To investigate whether pineal neurosteroids or melatonin are involved in Purkinje cell survival during development, Px at P2 and administration of ALLO, 7α-OH PREG, or melatonin from P2 to P7 were conducted using juvenile quail. Px and sham operation on P2 chicks were performed as described previously (22). Quantification of Purkinje cells was performed by counting the number of calbindin-immunoreactive cell bodies in the Purkinje cell layer in each lobe. ALLO concentration after Px was measured by GC-MS. Diffusion of [3H]ALLO administrated close to the pineal lumen was measured by liquid scintillation counter. Furthermore, the factor that mediates the neuroprotective effect of pineal ALLO in Purkinje cells was investigated. Parasagittal cerebellar sections of chicks at P3, P5, and P7 were analyzed by IHC with an antibody against cleaved caspase-3, a key protease in the apoptotic pathway (23–25), and by TUNEL to detect apoptotic cells as described previously (22). Details are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Y. Fukada (University of Tokyo) for his valuable discussion. We also thank S. Ichimaru, M. Kusaka, and H. Takeda (Waseda University) for their technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research 22132004 and 22227002 (to K.T.) and by Grant-in-Aid for Japan Society for the Promotion of Science Fellows 11J10683 (to S. Haraguchi) from the Ministry of Education, Culture, and Science (Tokyo).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210804109/-/DCSupplemental.
References
- 1.Kilic E, Hermann DM, Isenmann S, Bähr M. Effects of pinealectomy and melatonin on the retrograde degeneration of retinal ganglion cells in a novel model of intraorbital optic nerve transection in mice. J Pineal Res. 2002;32(2):106–111. doi: 10.1034/j.1600-079x.2002.1823.x. [DOI] [PubMed] [Google Scholar]
- 2.Tunç AT, et al. Neonatal pinealectomy induces Purkinje cell loss in the cerebellum of the chick: A stereological study. Brain Res. 2006;1067(1):95–102. doi: 10.1016/j.brainres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Baulieu EE. Neurosteroids: Of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- 4.Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 5.Do Rego JL, et al. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30(3):259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. Int Rev Neurobiol. 2001;46:33–78. doi: 10.1016/s0074-7742(01)46058-2. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsui K, Mellon SH. Neurosteroids in the brain neuron: Biosynthesis, action and medicinal impact on neurodegenerative disease. Central Nerv Syst Agents Med Chem. 2006;6(1):73–82. [Google Scholar]
- 8.Tsutsui K. Neurosteroids in the Purkinje cell: Biosynthesis, mode of action and functional significance. Mol Neurobiol. 2008;37(2–3):116–125. doi: 10.1007/s12035-008-8024-1. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto H, Ukena K, Tsutsui K. Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. J Neurosci. 2001;21(16):6221–6232. doi: 10.1523/JNEUROSCI.21-16-06221.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144(10):4466–4477. doi: 10.1210/en.2003-0307. [DOI] [PubMed] [Google Scholar]
- 11.Sasahara K, et al. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci. 2007;27(28):7408–7417. doi: 10.1523/JNEUROSCI.0710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10(7):704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 13.Hatori M, et al. Light-dependent and circadian clock-regulated activation of sterol regulatory element-binding protein, X-box-binding protein 1, and heat shock factor pathways. Proc Natl Acad Sci USA. 2011;108(12):4864–4869. doi: 10.1073/pnas.1015959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsutsui K, Yamazaki T. Avian neurosteroids. I. Pregnenolone biosynthesis in the quail brain. Brain Res. 1995;678(1–2):1–9. doi: 10.1016/0006-8993(95)00116-8. [DOI] [PubMed] [Google Scholar]
- 15.Zuber MX, Mason JI, Simpson ER, Waterman MR. Simultaneous transfection of COS-1 cells with mitochondrial and microsomal steroid hydroxylases: Incorporation of a steroidogenic pathway into nonsteroidogenic cells. Proc Natl Acad Sci USA. 1988;85(3):699–703. doi: 10.1073/pnas.85.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liere P, et al. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;739(2):301–312. doi: 10.1016/s0378-4347(99)00563-0. [DOI] [PubMed] [Google Scholar]
- 17.Meffre D, et al. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: Analysis by gas chromatography/mass spectrometry. Endocrinology. 2007;148(5):2505–2517. doi: 10.1210/en.2006-1678. [DOI] [PubMed] [Google Scholar]
- 18.Haraguchi S, Koyama T, Hasunuma I, Vaudry H, Tsutsui K. Prolactin increases the synthesis of 7α-hydroxypregnenolone, a key factor for induction of locomotor activity, in breeding male newts. Endocrinology. 2010;151(5):2211–2222. doi: 10.1210/en.2009-1229. [DOI] [PubMed] [Google Scholar]
- 19.Haraguchi S, et al. Acute stress increases the synthesis of 7α-hydroxypregnenolone, a new key neurosteroid stimulating locomotor activity, through corticosterone action in newts. Endocrinology. 2012;153(2):794–805. doi: 10.1210/en.2011-1422. [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga M, Ukena K, Baulieu EE, Tsutsui K. 7α-Hydroxypregnenolone acts as a neuronal activator to stimulate locomotor activity of breeding newts by means of the dopaminergic system. Proc Natl Acad Sci USA. 2004;101(49):17282–17287. doi: 10.1073/pnas.0407176101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutsui K, et al. 7α-Hydroxypregnenolone mediates melatonin action underlying diurnal locomotor rhythms. J Neurosci. 2008;28(9):2158–2167. doi: 10.1523/JNEUROSCI.3562-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc Natl Acad Sci USA. 2005;102(8):3052–3057. doi: 10.1073/pnas.0403840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puig B, Ferrer I. Cell death signaling in the cerebellum in Creutzfeldt-Jakob disease. Acta Neuropathol. 2001;102(3):207–215. [PubMed] [Google Scholar]
- 24.Olkowski AA, et al. A study on pathogenesis of sudden death syndrome in broiler chickens. Res Vet Sci. 2008;85(1):131–140. doi: 10.1016/j.rvsc.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Matsunaga E, et al. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004;6(8):749–755. doi: 10.1038/ncb1157. [DOI] [PubMed] [Google Scholar]
- 26.Cheng XS, et al. Neuronal apoptosis in the developing cerebellum. Anat Histol Embryol. 2011;40(1):21–27. doi: 10.1111/j.1439-0264.2010.01033.x. [DOI] [PubMed] [Google Scholar]
- 27.Eccles JC. Neurogenesis and morphogenesis in the cerebellar cortex. Proc Natl Acad Sci USA. 1970;66(2):294–301. doi: 10.1073/pnas.66.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomaidou D, Mione MC, Cavanagh JF, Parnavelas JG. Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J Neurosci. 1997;17(3):1075–1085. doi: 10.1523/JNEUROSCI.17-03-01075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dusart I, Airaksinen MS, Sotelo C. Purkinje cell survival and axonal regeneration are age dependent: An in vitro study. J Neurosci. 1997;17(10):3710–3726. doi: 10.1523/JNEUROSCI.17-10-03710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoumari AM, Wehrlé R, Bernard O, Sotelo C, Dusart I. Implication of Bcl-2 and Caspase-3 in age-related Purkinje cell death in murine organotypic culture: An in vitro model to study apoptosis. Eur J Neurosci. 2000;12(8):2935–2949. doi: 10.1046/j.1460-9568.2000.00186.x. [DOI] [PubMed] [Google Scholar]
- 31.Hess EJ, Wilson MC. Tottering and leaner mutations perturb transient developmental expression of tyrosine hydroxylase in embryologically distinct Purkinje cells. Neuron. 1991;6(1):123–132. doi: 10.1016/0896-6273(91)90127-l. [DOI] [PubMed] [Google Scholar]
- 32.Langmade SJ, et al. Pregnane X receptor (PXR) activation: A mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc Natl Acad Sci USA. 2006;103(37):13807–13812. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levitt P, Noebels JL. Mutant mouse tottering: Selective increase of locus ceruleus axons in a defined single-locus mutation. Proc Natl Acad Sci USA. 1981;78(7):4630–4634. doi: 10.1073/pnas.78.7.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cagnoli CM, Atabay C, Kharlamova E, Manev H. Melatonin protects neurons from singlet oxygen-induced apoptosis. J Pineal Res. 1995;18(4):222–226. doi: 10.1111/j.1600-079x.1995.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 35.Espinar A, et al. Neuroprotection by melatonin from glutamate-induced excitotoxicity during development of the cerebellum in the chick embryo. J Pineal Res. 2000;28(2):81–88. doi: 10.1034/j.1600-079x.2001.280203.x. [DOI] [PubMed] [Google Scholar]
- 36.Edwards RB, Manzana EJ, Chen WJ. Melatonin (an antioxidant) does not ameliorate alcohol-induced Purkinje cell loss in the developing cerebellum. Alcohol Clin Exp Res. 2002;26(7):1003–1009. doi: 10.1097/01.ALC.0000021148.70836.75. [DOI] [PubMed] [Google Scholar]
- 37.Higashi T, Nagura Y, Shimada K, Toyo’oka T. Studies on neurosteroids XXVI. Fluoxetine-evoked changes in rat brain and serum levels of neuroactive androgen, 5α-androstane-3α,17β-diol. Biol Pharm Bull. 2009;32(9):1636–1638. doi: 10.1248/bpb.32.1636. [DOI] [PubMed] [Google Scholar]
- 38.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444(7118):486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 39.Oppenheim RW, Prevette D, Yin QW, Collins F, MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science. 1991;251(5001):1616–1618. doi: 10.1126/science.2011743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.