Abstract
Germinal centers (GCs) are specialized microenvironments in secondary lymphoid organs where high-affinity antibody-producing B cells are selected based on B-cell antigen receptor (BCR) signal strength. BCR signaling required for normal GC selection is uncertain. We have found that protein kinase N1 (PKN1, also known as PRK1) negatively regulates Akt kinase downstream of the BCR and that this regulation is necessary for normal GC development. PKN1 interacted with and inhibited Akt1 kinase and transforming activities. Pkn1−/− B cells were hyperresponsive and had increased phosphorylated Akt1 levels upon BCR stimulation. In the absence of immunization or infection, Pkn1−/− mice spontaneously formed GCs and developed an autoimmune-like disease with age, which was characterized by autoantibody production and glomerulonephritis. More B cells, with fewer somatic BCR gene V region hypermutations were selected in Pkn1−/− GCs. These results indicate that PKN1 down-regulation of BCR-activated Akt activity is critical for normal GC B-cell survival and selection.
Keywords: humoral immunity, immunoglobulin, affinity maturation
Class IA phosphotydilinositl-3-OH kinases (PI3Ks) are heterodimeric enzymes composed of a p85α, p85β, or p55γ regulatory subunit and a p110α, p110β, or p110δ catalytic subunit. PI3Ks generate PtdIns(3,4,5)P3 lipid messengers by phosphorylating PtdIns(4,5)P2 at the plasma membrane inner leaflet (1–3). PtdIns(3,4,5)P3 interacts with intracellular enzymes that have a pleckstrin-homology (PH) domain (2). Among PtdIns(3,4,5)P3-interacting molecules, Akt, the cell homolog of v-akt, a murine thymoma virus oncogene, is a major class I PI3K downstream effector (4). The PI3K–Akt pathway has a pivotal role in normal and cancer cell proliferation and survival, by regulating cell cycle and antiapoptotic versus proapoptotic protein expression or activity (5).
The PI3K–Akt pathway is also important for immune responses (6, 7). In B cells, PI3K can be activated by the BCR, the CD19–CD21 coreceptor complex, the B-cell PI3K adaptor protein (BCAP), or by cytokine growth factor receptors (8). Mice deficient in PI3K p85α or p110δ have defects in marginal zone B and B-1 cell development (9–13). In contrast, mice with B-cell–specific Akt Phosphatase and tensin homolog deleted from Chromosome 10 (PTEN) phosphatase deficiency have increased PtdIns(3,4,5)P3, expanded B cells, and antibody-mediated autoimmune disease (14). Surprisingly, mature B cells were rescued in B-cell antigen receptor (BCR)-negative mice by ectopically expressing a constitutively active PI3K or specifically ablating B-cell PTEN expression (15). Moreover, ectopic Akt1 expression in mouse lymphocytes results in peripheral B and T cells (16), whereas bone marrow chimera mice lacking Akt1 and Akt2 in hematopoietic cells have substantially reduced marginal zone B and B1 cells, similar to PI3K-deficient mice (17). A human lacking the PI3K p85α subunit had a much earlier block in B-cell development than was evident in p85α-deficient mice (18). These studies have demonstrated the central importance of the PI3K–Akt pathway in B-cell development and maintenance. After recruitment to the plasma membrane by PtdIns(3,4,5)P3, Akt is activated by phosphoinositide-dependent kinase I (PDK1) phosphorylation of Akt serine 473 and by a kinase that phosphorylates Akt threonine 308. PKN 1, 2, and 3, also known as protein kinase C-related kinases (PRKs), may negatively regulate Akt by inhibiting PDK1 activation of Akt (19–24). Apoptotic stimulation can also induce PKN1 and PKN2 proteolytic processing and the cleaved C termini may bind and inhibit Akt (19, 25), whereas PKN3 is required for malignant PI3K effects in prostate cell growth (26). The studies reported here were undertaken to investigate the role of PKN1 in the PI3K–Akt pathway mediated germinal center B-cell antigen-induced activation, hypermutation, and selection for high avidity antibody production.
To determine whether PKN1 is involved in the PI3K–Akt signaling, we determined the effects of PKN1 in B cells before and after BCR stimulation on Akt activity and generated PKN1-deficient mice to elucidate the physiological roles of PKN1, in vivo. These experiments revealed PKN1 to be indispensable for physiologically appropriate GC B-cell selection through PKN1 affects on BCR-induced Akt activation.
Results
Akt1 and PKN1 Interact in B Cells.
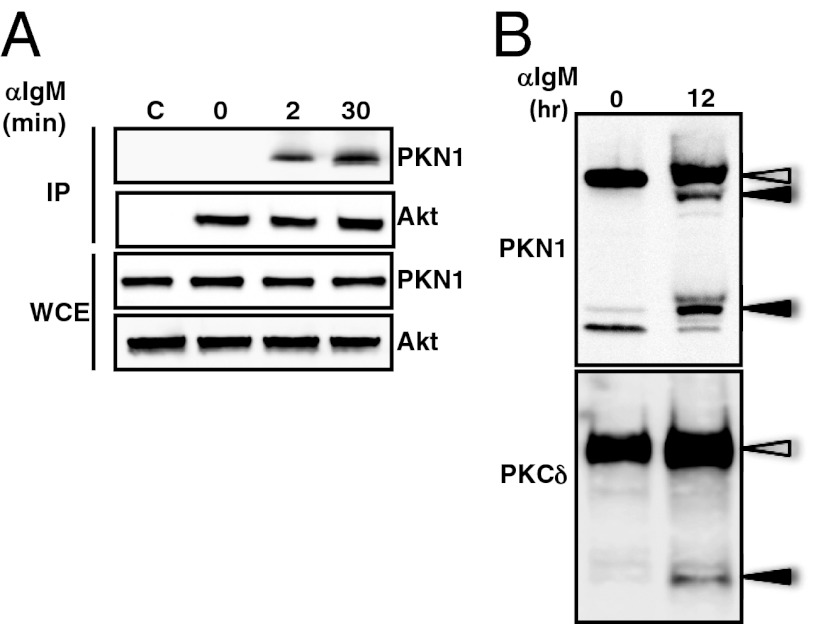
Consistent with a role for PKN1 in immune cell survival, growth, or activation, PKN1 mRNA was abundantly expressed in C57BL/6 mouse splenic lymphocytes (SPL), bone marrow (BM), CD4 T cells (CD4), mesenteric lymph node (LYM), peritoneal cavity (PEC), purified spleen B cells (B), and to a lesser extent in thymus (THY) from C57BL/6 mice. Murine embryonic fibroblasts were a nonimmune cell control (Fig. S1). To determine if PKN1 associated with Akt following B-cell receptor activation, Akt1 in Ramos Burkitt lymphoma cells was immune precipitated before and after anti–Ig-mediated (anti-IgM) BCR cross-linking. PKN1 coprecipitated with Akt1 from cell lysates stimulated for at least 2 min but not from unstimulated cell lysates (Fig. 1A). PKN1 and PKN2 C-terminally truncated proteins also precipitated with Akt from cells receiving apoptotic stimuli (19, 25). Some members of the protein kinase C (PKC) family are also activated by caspase-dependent cleavage after apoptotic stimulation (27). When B cells were stimulated with anti-IgM antibody for >12 h, PKN1 and PKCδ proteolytically processed forms were detected (Fig. 1B). Full-length PKN1 coprecipitated with Akt1 (Fig. 1A). No interaction was detected between processed PKN1 and Akt1, even after longer stimulation.
Fig. 1.
BCR-dependent association of PKN1 with Akt and cleavage. (A) Ramos cells, a human Burkitt’s lymphoma cell line, were stimulated with an antihuman IgM F(ab)2 for the indicated time periods. Cell lysates were immunoprecipitated (IP) with an anti-Akt or control antibody (shown as C) and immunoblotted as indicated. Whole cell extracts (WCE) correspond to 10% of the total cell lysates. (B) Ramos cells were stimulated with immobilized antihuman IgM F(ab)2 for 12 h. The cell lysates were immunoblotted with either an anti-PKN1 or anti-PKCδ antibody. Open arrowheads indicate full-length proteins. Closed arrowheads show the processed form of the proteins.
PKN1 Inhibits Akt1 Kinase and Transforming Activities.
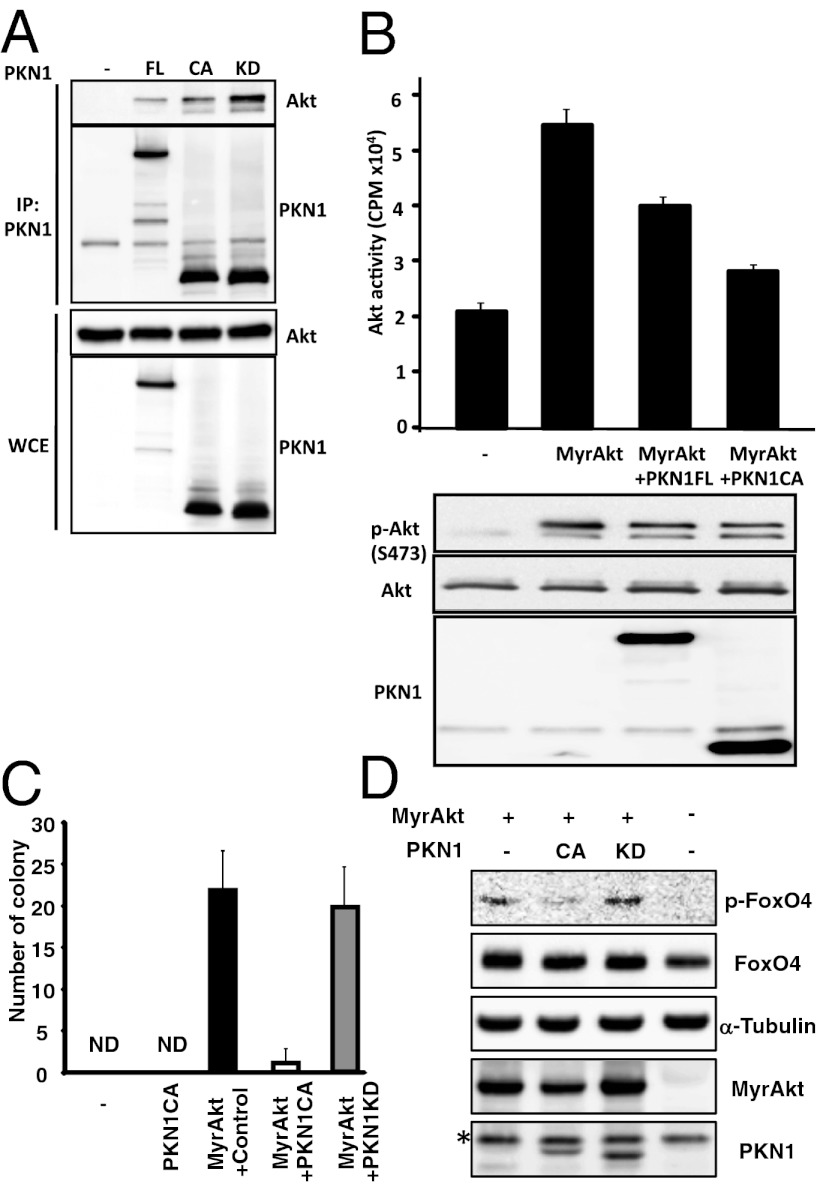
When full-length PKN1 (PKN1FL), kinase dead (PKN1KD), or constitutively active PKN1 (PKN1CA) lacking the N-terminal regulatory region were coexpressed with Akt1 in 293T cells, PKN1FL, PKN1CA, and PKN1KD coprecipitated Akt1 with efficiencies that correlated with PKN1FL, -CA, or -KD expression (Fig. 2A). Furthermore, PKN1FL expression inhibited myristoylated, highly plasma membrane associated, and constitutively activated Akt1 (MyrAkt), and PKN1CA, which was slightly more efficiently expressed, further inhibited MyrAkt activity (Fig. 2B). HA-MyrAkt expressed in 293T cells, HA-immune precipitated and assayed for Akt/Serum and glucocorticoid-inducible kinase (SGK) substrate peptide phosphorylation, in vitro, resulted in vector control transfected cells with 20,000 cpm SGK signal (Fig. 2B). HA-MyrAkt increased SGK to ∼55,000 cpm, 35,000 cpm above vector control, and wtPKN1FL reduced cotransfected HA-MyrAkt activity to ∼40,000 cpm (15,000 cpm less than HA-MyrAkt), without substantially decreasing HA-MyrAkt by Western blot (Fig. 2B). Moreover, similarly expressed, catalytically active, PKN1CA, reduced HA-MyrAkt activity to 28,000 cpm, only 8,000 cpm above vector control. These experiments done in triplicate and repeated three times, with narrow error bars and Student’s t test, P < 0.01, indicate that PKN1 significantly negatively regulates Akt.
Fig. 2.
PKN1 interacts with and inhibits Akt. (A) 293T cells were transfected with expression vectors for HA-tagged Akt1, FLAG-tagged full-length (FL), and a constitutively activate (CA) or kinase-dead (KD) PKN1. Cell lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose and then immunoblotted with an anti-HA antibody or anti-FLAG antibody. (B) Akt activity was assessed using an in vitro Akt kinase assay that measured the phosphorylation levels of the Akt SGK substrate peptide. Data are representative of several different experiments. (C) Immortalized rat fibroblast 3Y1 cells were retrovirally transduced with myristoylated Akt1 (MyrAkt) together with GFP. Then, the MyrAkt (GFP)-positive cells were superinfected with retrovirus carrying PKN1CA or PKN1KD and plated in soft agar. Three weeks after plating, the number of colonies was determined. ND, not detected. (D) FoxO4 phosphorylation by MyrAkt in 3Y1 cells from C. *, nonspecific bands.
To assess the impact of PKN1 on Akt-mediated cell effects, 3Y1 rat fibroblast cells were retrovirally cotransduced with MyrAkt and PKN1CA and cultured in soft agar. Colonies appeared 21 d after MyrAkt retroviral transduction, but not after control vector (Fig. 2C). Coexpressing PKN1CA with MyrAkt significantly reduced colony number and size, whereas kinase-inactive PKN1, (PKN1KD), failed to inhibit MyrAkt-induced transformation, even though PKN1KD associated with Akt1 (Fig. 2C). Moreover, FoxO4a phosphorylation, a downstream target of Akt, was suppressed in cells cotransfected with PKN1CA, but not PKN1KD (Fig. 2D) (28). These data indicate that PKN1 is an Akt inhibitor.
PKN1 Deficiency Results in Spontaneous GC Formation and Autoantibody Production.
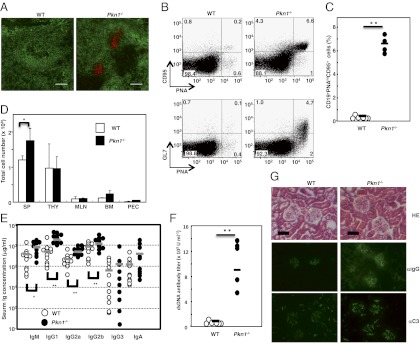
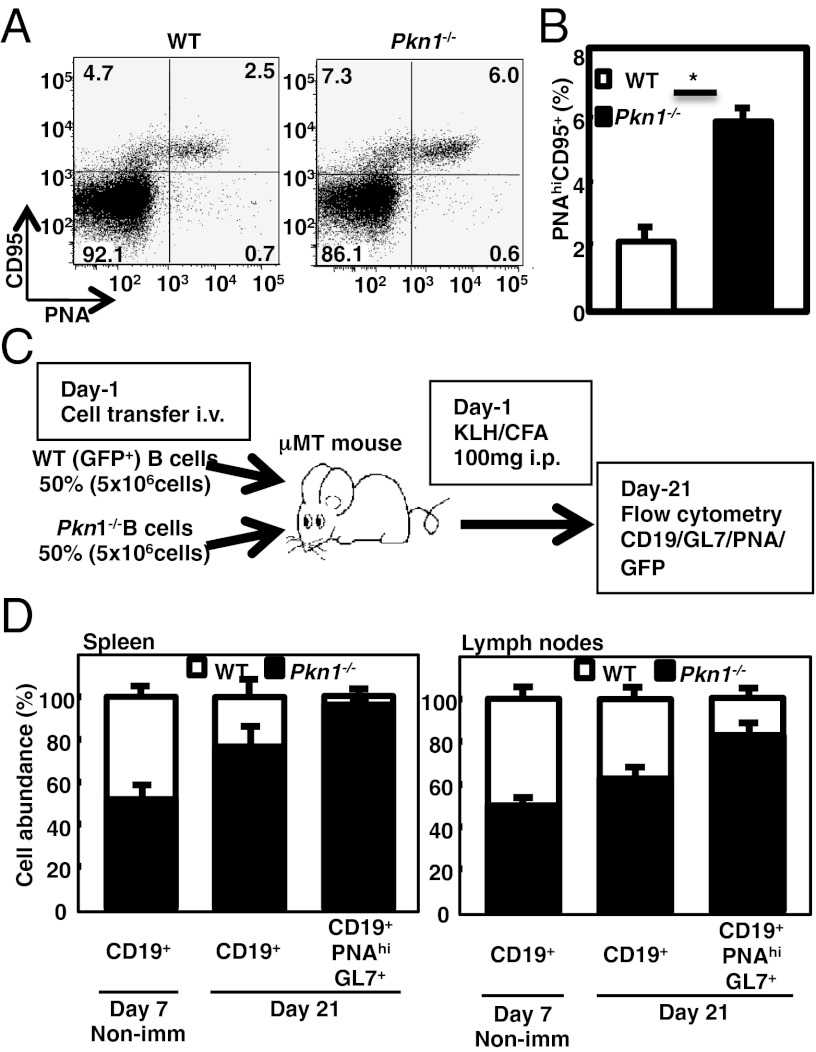
To clarify the physiologic PKN1 role, in vivo, PKN1-deficient (Pkn1−/−) mice were made (Fig. S2). Pkn1−/− mice were born in a Mendelian ratio and appeared normal. Defects in lymphocyte development, as assessed by differentiation antigen expression were not detected in Pkn1−/− mice <12 wk of age. However, spontaneous splenic GC formation was evident in comparative studies of Pkn1−/− and normal mouse spleens more than 30 wk of age, even in the absence of immunization or infection (Fig. 3A). CD95+ peanut agglutinin (PNA)+ and GL7+PNA+ GC B cells were also increased in Pkn1−/− mice by flow cytometric analyses (Fig. 3 B and C). Furthermore, Pkn1−/− mice also had significantly increased spleen cell numbers (Fig. 3D) and substantially elevated serum Ig levels (Fig. 3E). Moreover, aged Pkn1−/− mice had elevated antidouble-stranded (ds) DNA antibody serum levels (Fig. 3F). Glomeruli from some Pkn1−/− kidneys also had increased mesangial cell numbers and thickened basement membranes, characteristic of proliferative glomerulonephritis (Fig. 3G, Top). In addition, IgG and complement component C3 deposition was evident in mesangial regions of Pkn1−/− mice and not in control mice of the same age (Fig. 3G, Middle and Bottom).
Fig. 3.
Immunological abnormalities in Pkn1−/− mice. Spontaneous germinal center (GC) formation in Pkn1−/− mice. (A) Spleen sections from WT and Pkn1−/− mice over 30 wk of age were stained with either an anti-IgM antibody (green) or peanut agglutinin (red). Magnification 100×. (B) Flow cytometric analysis of GC B cells from splenocytes that were stained with CD19, PNA, GL7, and CD95. (C) Statistical analysis of PNAhiCD95+ B cells in splenic CD19+ B cells. **P < 0.01, based on Student’s t test. (D) Total cell numbers in the spleen (SP), thymus (THY), mesenteric lymph node (MLN), bone marrow (BM), and peritoneal cavity (PEC) from either WT or Pkn1−/− mice. Data are from five mice per group. *P < 0.05 based on Student’s t test. (E) The Ig levels in sera from either WT (open circle) or Pkn1−/− (filled circle) mice were examined by ELISA. The bar in the figure shows the mean of 10–11 mice per group. *P < 0.05 and **P < 0.01, based on Student’s t test. (F) Anti-dsDNA antibody production in 1-y-old WT (n = 6) and Pkn1−/− mice (n = 5). (G) Development of glomerulonephritis in Pkn1−/− mice. Haematoxylin−eosin staining (HE, Top) and immunofluorescence staining with anti-IgG antibodies (αIgG, Middle) and anti-C3 antibodies (αC3, Bottom) of kidney sections from 6-mo-old WT and Pkn1−/− mice.
Pkn1−/− B Cells Are Hyperactive After BCR Stimulation.
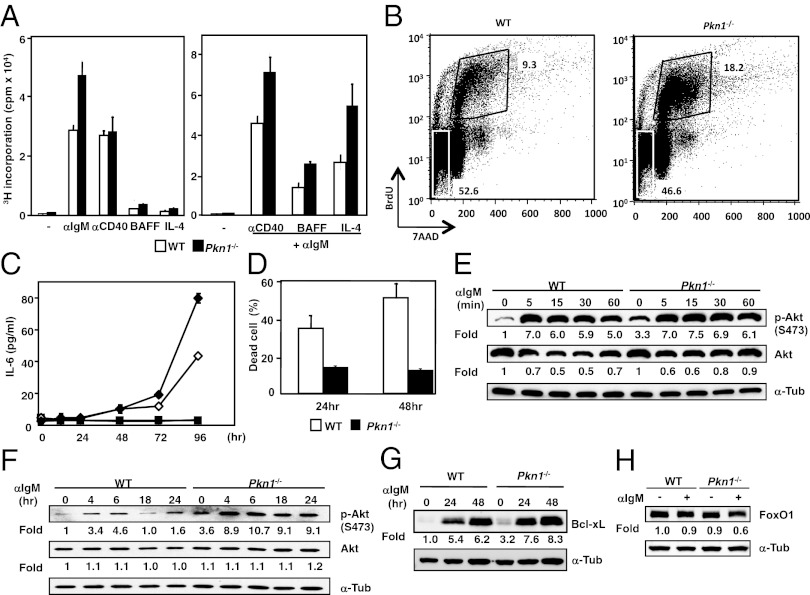
We considered whether spontaneous GC formation in Pkn1−/− mice was due to intrinsic hyperactivation or impaired immune cell apoptosis. To examine these possibilities, B cells and T cells from Pkn1−/− and wild-type (WT) littermates were activated by various stimuli. When anti-IgM antibody F(ab′)2 fragment stimulated B cells alone or in combination with an anti-CD40 antibody were treated with B-cell–activating factor belonging to the tumor necrosis factor family (BAFF), or interleukin 4 (IL-4), Pkn1−/− B cells had higher proliferative [3H]thymidine incorporation responses than WT B cells. However, PKN1 deficiency did not affect B-cell–proliferative responses stimulated by anti-CD40 antibody, BAFF, or IL-4 alone (Fig. 4A). Furthermore, PKN1-deficient B-cell hyperresponsiveness to anti-IgM antibody only or anti-IgM plus anti-CD40 antibody was confirmed using an in vitro 5-bromo-2′-deoxy-uridine (BrdU) incorporation assay and IL-6 production ELISAs (Fig. 4 B and C). Whereas B lymphocytes normally undergo activation-induced cell death (AICD) after BCR cross-linking with immobilized anti-IgM antibodies, PKN1-deficient B cells were resistant to AICD when stimulated by anti-IgM antibody coated plates (Fig. 4D). These findings indicate that PKN1 deficiency affects BCR signaling required for cell survival, but not the threshold required for BCR activation. When purified splenic CD4+ T cells were stimulated with anti-CD3 and anti-CD28 antibody or by phorbol ester and a calcium ionophore, WT and Pkn1−/− cell proliferative responses were not different, indicating that PKN1 is dispensable for T-cell activation induced by T-cell–antigen receptor ligation (Fig. S3).
Fig. 4.
PKN1 regulates the proliferation and survival of B cells by inhibiting Akt. (A) Purified B cells were cultured for 72 h with the indicated stimuli. Cultures were pulsed with 3[H]thymidine for 12 h. Error bars show the mean ± SEM. Data are representative of three independent experiments. (B) B cells purified from splenocytes were stimulated with an anti-IgM F(ab)2 for 24 h and pulsed with 10 nM BrdU for 12 h. The cells were harvested and stained with an anti-BrdU antibody and 7-Aminoactinomycin D (7-AAD) for FACS analysis. The numbers in the figure show the percentage of gated cells relative to the total cell number. Data are representative of three independent experiments. (C) Splenic B cells from either WT (open) or Pkn1−/− mice (closed) were stimulated with (diamonds) or without (squares) an anti-IgM F(ab)2 and anti-CD40 antibody for the indicated time periods. IL-6 production was examined by ELISA. Representative data from three independent experiments are shown. (D) BCR-mediated AICD. B cells purified from WT and Pkn1−/− spleens were stimulated with an immobilized anti-IgM F(ab)2 for the indicated time periods. (E and F) Akt phosphorylation in B cells is mediated by BCR signaling. Numbers represent the fold induction normalized to the protein levels of nonstimulated WT. (G) Bcl-xL protein expression induced by BCR ligation. Numbers represent the fold change normalized to the WT protein level without BCR stimulation. (H) B cells were stimulated with an anti-IgM F(ab)2 for 8 h. FoxO1 protein level was determined by Western blotting. Fold change denotes the values that were normalized to the protein level from WT lysates that were stimulated in the absence of an anti-IgM antibody.
We next investigated the basis for PKN1-deficiency effects on BCR signaling. As expected, Akt1 phosphorylation patterns differed between Pkn1−/− B cells and WT B cells. Pkn1−/− B cells consistently had enhanced “basal” Akt1 S473 phosphorylation before BCR engagement, although Akt1 phosphorylation was similarly robustly induced within 5 min and maintained for 60 min after BCR stimulation in both WT and Pkn1−/− B cells (Fig. 4E). However, Pkn1−/− B cells consistently had higher late phase Akt S473 phosphorylation from 4 to 24 h after BCR stimulation (Fig. 4F). Despite dramatic effects on Akt1 regulation, WT and Pkn1−/− B cells were not significantly different in phosphorylation of other signaling molecules downstream of BCR, including IKKα/β, Erk, JNK, and p38 (Fig. S4 A and B), indicating that PKN1 deficiency specifically affects Akt activation, among various downstream BCR signaling molecules. Indeed, Bcl-xL induction, a GC B-cell–specific antiapoptotic effect of PI3K–Akt signaling (12, 29), was enhanced in PKN1-deficient B cells stimulated by anti-IgM antibody (Fig. 4G). Moreover, FoxO1, which is directly degraded after Akt-mediated phosphorylation (28, 30, 31), was down-regulated more robustly in PKN1-deficient B cells than WT B cells (Fig. 4H).
Humoral Immune Responses in Pkn1−/− Mice.
To evaluate the impact of the PKN1 deficiency on humoral immune responses, young Pkn1−/− mice that had not formed GCs were immunized with 4-hydroxyl-3-nitrophenyl-acetyl (NP)-conjugated chicken γ-globulin (CGG). Pkn1−/− mice developed much larger GCs than WT mice after immunization. GC B-cell numbers were several-fold higher in Pkn1−/− mice than WT mice (Fig. 5 A and B). Most GC B cells in Pkn1−/− mice weakly expressed surface IgG, whereas surface Ig expression was down-regulated in most GC B cells in WT mice (Fig. S5). However, serum levels of NP-specific antibodies were not significantly different between Pkn1−/− and WT mice (Fig. S6), indicating that antigen-specific serum antibody titers do not reflect GC B-cell numbers in PKN1-deficient animals.
Fig. 5.
Accelerated GC reaction of Pkn1−/− mice is B-cell intrinsic. Eleven-week-old WT and Pkn1−/− mice were immunized with NP-CGG/CFA. (A) Flow cytometric analysis of GC B cells (B220+PNAhiCD95+) was performed 7 d after immunization. (B) Statistical analyses were performed using four to five mice. **P < 0.01, based on Student’s t test. (C and D) Naïve Pkn1+/+ B cells expressing GFP along with equal numbers of Pkn1−/− B cells were transferred into B-cell–deficient (μMT) mice, after which the mice were immunized with KLH/CFA. Splenocytes and mesenteric lymph node cells were prepared from recipient mice on either day 7 or day 21, and the number of B cells was determined. (C) Schematic representation of the transfer experiment. (D) Fraction of total (CD19+) and GC (CD19+PNAhiGL7+) B cells from transferred WT (open columns) and Pkn1−/− (closed columns) cells in the spleen and mesenteric lymph nodes. Day 7 mice were unimmunized control mice that were examined 7 d after the cell transfer (day 7 nonimmunized). Representative data are shown for at least four mice per group.
Abnormal GC Formation Is Intrinsic to Pkn1−/− B Cells.
To determine whether aberrant GC reactions observed in Pkn1−/− mice were intrinsic to B cells, equal numbers of naïve Pkn1−/− B cells and GFP-expressing Pkn1+/+ B cells were transferred into B cell-deficient (μMT) mice that selectively lack mature B cells. Both groups of mice were immunized with keyhole limpet hemocyanin (KLH) (32). Three weeks after immunization, Pkn1−/− B cells significantly outcompeted Pkn1+/+ B cells in the spleen and mesenteric lymph nodes, although Pkn1−/− and Pkn1+/+ B cells had comparable ability to home to lymphoid tissues in μMT mice 7 d after transfer (Fig. 5C). Specifically, most GL-7+ GC B cells after transfer were Pkn1−/− in origin (96.3% in the spleen and 82.7% in the mesenteric lymph nodes), strong evidence that accelerated GC reactions in Pkn1−/− mice were due to intrinsic B-cell hyperactivation (Fig. 5D).
Ig Locus of Pkn1−/− GC B Cells Undergoes Reduced Somatic Hypermutation.
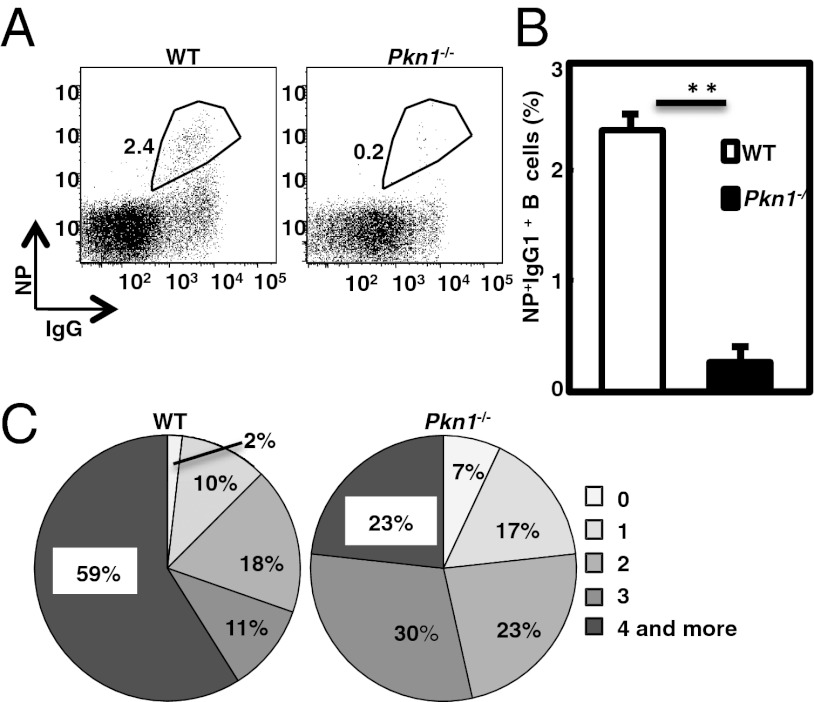
The proceeding observations indicate that PKN1 deficiency results in B-cell–antigen hyperresponsiveness and aberrant GC formation. To evaluate GC quality, antigen-specific GC B cells from Pkn1−/− mice immunized with NP-CGG/alum were compared with the frequency of NP-binding+ GC B cells was significantly lower in Pkn1−/− mice than WT mice after immunization (Fig. 6 A and B), suggesting that antigen-specific B-cell selection is impaired in the GCs of Pkn1−/− mice, even though GC formation is significantly enhanced. Sorting of single NP-binding+ B cells from immunized mice and sequencing the Ig heavy chain VH186.2-DFL16.1 gene, which is preferentially used in NP-specific B cells in C57BL/6 mice revealed no differences in the frequencies of NP-specific GC B cells with VH186.2 between Pkn1−/− and WT mice (Table S1). However, the frequency of B cells expressing the VH186.2 gene with more than four mutations was significantly lower in Pkn1−/− mice than in control mice (59% vs. 23%) (Fig. 6C), although there were no differences in VH186.2-DFL16.1 use between the two groups (93% vs. 80%) (Table S1). Pkn1−/− mice also had a lower frequency of B cells carrying the tryptophan (W) to leucine (L) mutation at position 33 of the VH186.2 gene, a signature of a high affinity for NP hapten. In addition, the ratio of replacement to silent mutations (R/S) in the entire complementarity-determining region (CDR) of the Ig heavy chain gene was also decreased in Pkn1−/− mice (Table S1). However, Pkn1−/− mice did not have decreased serum titers for high-affinity antibodies, which were detected using a low-valency antigen (Fig. S6). This result is likely due to the serological method and may not have sufficient sensitivity to detect a 50% reduction in antibodies with W to L mutation at position 33, consistent with the hypothesis that PKN1 is critical for negative selection of B cells that produce less mutated, low-affinity antibodies.
Fig. 6.
Pkn1−/− mice have impaired antigen-specific B-cell generation and somatic hypermutation of IgH genes. Young (within 12 wk of age) WT and Pkn1−/− mice were immunized with NP-CGG/alum. (A) Flow cytometric analysis of NP-specific B cells (CD3−CD90.2−CD4−CD8−CD11b−Gr-1−IgM−IgD−NP+IgG+) was performed 14 d after immunization. Numbers in the gates indicate the frequency of NP-binding+IgG1+ B cells. (B) Statistical analysis of the frequencies of NP-specific B cells from WT and Pkn1−/− mice. Means are shown from five mice per group. **P < 0.01, based on Student’s t test. (C) Number of amino acid mutations in the CDR1 and CDR2 regions of the VH186.2 genes from NP-specific B cells 14 d after NP-CGG immunization. Numbers indicate the frequency of the mutation.
Discussion
PKN1 Negatively Regulates Signals Downstream of the BCR.
PKN1 and PKN2 have been previously suggested to indirectly or directly negatively regulate Akt kinase activity (19, 20). The present study has confirmed these previous findings and found that PKN1 down-regulates Akt transforming activity, which is a major Akt biological activity. Moreover, analysis of Pkn1−/− mice also revealed that PKN1 inhibition of Akt is critical for regulated B-cell activation and survival, particularly in GCs. The inhibitory pathway appears to be regulated by BCR signals, such as PI3K-mediated activation, because PKN1 physically interacted with Akt in B cells within a few minutes after BCR stimulation.
Apoptotic stimuli have been shown to induce PKN2 proteolytic processing and the processed C-terminal region but not full-length PKN2 directly binds to and inhibits Akt (19). Unlike apoptotic stimuli, BCR stimulation induced an interaction between Akt1 and full-length PKN1, but not the processed form, although PKN1 processing was observed 12 h after stimulation. PKN1 and PKN2 can inhibit PDK1, an important Akt activator (20, 22–24). However, we failed to detect interaction between PDK1 and Akt1 or PKN1 before or after BCR stimulation. Instead, our findings are more compatible with PKN1-mediated inhibition of Akt1 being regulated by BCR signals, although the detailed mechanism remains to be determined.
PKN1 Deficiency Results in B-Cell Hyperreactivity.
PKN1 deficiency selectively enhanced Akt activity in B cells stimulated with an anti-IgM antibody. Aside from Akt activation, there were no detectable differences in the activation of other signaling molecules downstream of the BCR in Pkn1−/− versus WT B cells. Selective Akt hyperactivation may explain the resistance of Pkn1−/− B cells to AICD. Indeed, antiapoptotic Bcl-xL, which is an Akt downstream target, was up-regulated in Pkn1−/− B cells. Also noteworthy is that Pkn1−/− B cells show no hyperreactivity when stimulated with anti-CD40 antibody or BAFF, which also activate Akt. These findings indicate that PKN1-mediated down-regulation of Akt occurs downstream of the BCR but not downstream of CD40 or BAFF receptor.
Like Pkn1−/− B cells, PTEN-deficient B cells have enhanced Akt activity that results in resistance to apoptotic stimuli (14). However, B-cell–specific PTEN-deficient mice have expanded marginal zone B-cell and B-1 cell numbers, reduced follicular B-cell numbers, and decreased GC formation, substantially different from the Pkn1−/− mouse phenotype. Possible explanations for this discrepancy include: (i) Accumulated PtdIns(3,4,5)P3 may recruit and activate not only Akt but also many other PH domain-containing downstream molecules in PTEN-deficient B cells, which may contribute to a more complex phenotype. (ii) PTEN is more abundantly expressed in immature cells, particularly bone marrow cells than in mature lymphocytes, whereas PKN1 mRNA is abundantly detected in splenic B and T cells, but not in primary lymphoid organs, such as bone marrow and thymus. B-cell differentiation stages affected by each mutation may differ between PKN1-deficient and PTEN-deficient mice. (iii) Enhanced Akt phosphorylation is detectable a few minutes after BCR stimulation in Pten−/− B cells. On the other hand, enhanced Akt activation is more evident at later phases of B-cell activation. Therefore, differences in the kinetics of Akt activation may explain different phenotypes between Pten−/− and Pkn1−/− two mutant mice.
PKN1 Regulates the Survival of GC B Cells.
One of the most striking phenotypes of Pkn1−/− mice is spontaneous GC formation. The proliferation and survival of GC B cells are strictly dependent not only on antigens presented by follicular dendritic cells, but also on signals from follicular helper T (Tfh) cells (33–35). Although PKN1 mRNA expression is almost comparable between T cells and B cells, the responsiveness of Pkn1−/− and WT T cells to various stimuli are highly similar. We did not detect any differences in the numbers of Tfh cells between Pkn1−/− and WT mice (Fig. S7). Moreover, when Pkn1−/− and WT B cells were cotransferred into μMT mice, Pkn1−/− B cells preferentially expanded and dominated GCs after immunization, indicating that Pkn1−/− B cells have a survival advantage over WT B cells in GCs. These observations indicate that spontaneous and accelerated GC formation can be attributed to enhanced Akt activity in B cells that is caused by the PKN1 deficiency.
PKN1-Mediated Regulation of Akt Activity Is Necessary for B-Cell Maintenance of GC Quality.
Although the proportion of NP-binding+ GC B cells was lower in Pkn1−/− mice than in WT mice, the total number of antigen-specific GC B cells may be comparable between the two groups when the larger sizes of GCs in Pkn1−/− mice are taken into consideration. Consistent with this, there were no detectable differences in the serum titers of antigen-specific antibodies. Thus, it appears that the enlarged GCs of Pkn1−/− mice are due to abnormally selected B cells that express low-affinity antibodies. Indeed, the proportion of B cells carrying fewer mutations in the VH186.2 gene was higher in NP-binding+ GC B cells from Pkn1−/− mice than those of WT controls. These abnormally selected B cells probably contain self-reactive clones because aged Pkn1−/− mice had high serum levels of autoantibodies.
BCR signals are transduced through multiple pathways, including PLCγ and PI3K–Akt pathways (36). However, it was unclear which BCR signaling pathway is critical for affinity maturation and GC B-cell selection. Our findings substantiate the importance of the PI3K–Akt pathway in GC B-cell survival and selection. Furthermore, we clearly demonstrate that PKN1-mediated Akt control has a critical role in quality control of GC B cells by establishing a selection threshold based on BCR signal strength. The present study therefore provides unique insight into control mechanisms of GC B-cell selection and the escape mechanisms of self-reactive B cells.
Materials and Methods
Pkn1−/− mice were generated by disrupting the gene within E14 (129) embryonic stem cells using standard procedures and then backcrossing these mice to C57BL/6 mice. Mice were maintained in pathogen-free animal facilities at the Research Institute for Microbial Diseases, Osaka University and immunized intraperitoneally with 100 μg NP-CGG (Biosearch Technologies) in alum under the guidelines of Osaka University for animal studies; this study was approved by the institutional animal care and use committee (permission number: H21-28-0). Western blotting, immunoprecipitation, ELISA, and the in vitro kinase assay were performed following standard or published protocols. For colony transforming assay, Rat Akt1 and PKN1 cDNA were cloned into murine stem cell virus-based retroviral vectors and retrovirally transduced into 3Y1 rat fibroblast cells. Cell growth in soft agar was examined as previously described. For DNA sequence analysis of Ig genes, total RNA was isolated from single NP-binding+ B cells that were sorted using a flow cytometer and then cDNA was synthesized. Nested PCR for the VH186.2-DFL16.1 gene was performed, followed by DNA sequencing. For more details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Okabe for providing GFP transgenic mice, W. Pear and Y. Kawaguchi for the retroviral vectors, T. Kato for the PKN1 and Akt1 cDNA, K. Nakamura and Y. Kabumoto for help with the flow cytometric analysis, A. Muraguchi for technical advice of B-cell sorting, and M. Luftig for critically reading the manuscript. We also thank members of the H.K. laboratory, M. Mizui, and O. Simma for critical discussions; J. Yoshida, C. Hasui, M. Ishiguro, K. Shiozaki, and T. Sugimoto for technical assistance; and K. Kubota, K. Inada, and F. Hotta for secretarial support. The work was supported in part by Grants-in-Aids for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (to T.Y. and H.K.); a Grant-in-Aid for the Global Center of Excellence program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a Grant-in-Aid for JSPS fellow (to K.S.-Y.); and National Institutes of Health Grant R01 CA0085180 (to E.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218925110/-/DCSupplemental.
References
- 1.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 2.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 4.Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three Akts. Genes Dev. 1999;13(22):2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 5.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 6.Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4(4):313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- 7.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 8.Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111(3):1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- 9.Clayton E, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196(6):753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fruman DA, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283(5400):393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 11.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297(5583):1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, et al. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat Immunol. 2003;4(3):280–286. doi: 10.1038/ni890. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, et al. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 1999;283(5400):390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197(5):657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsons MJ, et al. Expression of active protein kinase B in T cells perturbs both T and B cell homeostasis and promotes inflammation. J Immunol. 2001;167(1):42–48. doi: 10.4049/jimmunol.167.1.42. [DOI] [PubMed] [Google Scholar]
- 17.Calamito M, et al. Akt1 and Akt2 promote peripheral B-cell maturation and survival. Blood. 2010;115(20):4043–4050. doi: 10.1182/blood-2009-09-241638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conley ME, et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85α subunit of PI3K. J Exp Med. 2012;209(3):463–470. doi: 10.1084/jem.20112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh H, et al. Inhibition of Akt and its anti-apoptotic activities by tumor necrosis factor-induced protein kinase C-related kinase 2 (PRK2) cleavage. J Biol Chem. 2000;275(44):34451–34458. doi: 10.1074/jbc.M001753200. [DOI] [PubMed] [Google Scholar]
- 20.Wick MJ, Dong LQ, Riojas RA, Ramos FJ, Liu F. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 2000;275(51):40400–40406. doi: 10.1074/jbc.M003937200. [DOI] [PubMed] [Google Scholar]
- 21.Mukai H. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J Biochem. 2003;133(1):17–27. doi: 10.1093/jb/mvg019. [DOI] [PubMed] [Google Scholar]
- 22.Biondi RM, et al. Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA. EMBO J. 2000;19(5):979–988. doi: 10.1093/emboj/19.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dettori R, et al. Regulation of the interaction between protein kinase C-related protein kinase 2 (PRK2) and its upstream kinase, 3-phosphoinositide-dependent protein kinase 1 (PDK1) J Biol Chem. 2009;284(44):30318–30327. doi: 10.1074/jbc.M109.051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balendran A, et al. A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Czeta (PKCzeta) and PKC-related kinase 2 by PDK1. J Biol Chem. 2000;275(27):20806–20813. doi: 10.1074/jbc.M000421200. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi M, Mukai H, Toshimori M, Miyamoto M, Ono Y. Proteolytic activation of PKN by caspase-3 or related protease during apoptosis. Proc Natl Acad Sci USA. 1998;95(20):11566–11571. doi: 10.1073/pnas.95.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leenders F, et al. PKN3 is required for malignant prostate cell growth downstream of activated PI 3-kinase. EMBO J. 2004;23(16):3303–3313. doi: 10.1038/sj.emboj.7600345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emoto Y, et al. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 1995;14(24):6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 29.Vikstrom I, et al. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330(6007):1095–1099. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinman RM, Bushanam JN, Nichols WA, Satterthwaite AB. B cell receptor signaling down-regulates forkhead box transcription factor class O 1 mRNA expression via phosphatidylinositol 3-kinase and Bruton’s tyrosine kinase. J Immunol. 2007;178(2):740–747. doi: 10.4049/jimmunol.178.2.740. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf I, Zhu X, Kharas MG, Chen J, Fruman DA. Optimal B-cell proliferation requires phosphoinositide 3-kinase-dependent inactivation of FOXO transcription factors. Blood. 2004;104(3):784–787. doi: 10.1182/blood-2003-09-3071. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura D, Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356(6365):154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 33.Allen CDC, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27(2):190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein U, Dalla-Favera R. Germinal centres: Role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8(1):22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 35.MacLennan IC, et al. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 36.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.