A major advance in the field of cannabinoid research was the discovery of the endocannabinoid system, which is currently thought to consist of two G protein-coupled receptors (cannabinoid CB1 and CB2 receptors) and endogenous compounds such as arachidonoylethanolamide (i.e., anandamide; AEA; Fig. 1) and 2-arachidonoyl glycerol (2-AG) that can activate these receptors and are known as endocannabinoids (1). This system of receptors and endogenous agonists, which is also made up of enzymes that catalyze endocannabinoid biosynthesis or metabolic degradation, and of processes responsible for the cellular uptake of endocannabinoids, is thought to have numerous roles in both health and disease (2, 3). Some of these are “autoprotective” in nature and hence beneficial, with examples including the amelioration of inflammatory pain, multiple sclerosis, and Parkinson disease; whereas a few of its other roles, for example, in obesity, are “autoimpairing,” and therefore unwanted. AEA, 2-AG, and other “direct” cannabinoid receptor agonists are thought to trigger G protein-mediated signaling of CB1 and CB2 receptors by targeting orthosteric sites on these receptors (1). There is evidence, however, that the CB1 receptor also contains one or more “allosteric” sites that can be targeted by allosteric modulators in a manner that can enhance or reduce the efficacy with which direct agonists activate this receptor orthosterically (4–7). Just as the discovery of the CB1 receptor prompted a search for endogenous ligands for this receptor (8), so too the discovery that CB1 receptors contain allosteric sites has prompted a need to look for an endogenous CB1 allosteric modulator. This need has now been met by Pamplona et al. (9), who, in PNAS, present evidence that the endogenous anti-inflammatory ligand lipoxin A4 (LXA4; Fig. 1) can allosterically enhance AEA-induced activation of CB1 receptors within the brain when it is administered exogenously and when it is produced endogenously. This is a ligand that is already known to target the FPR2/ALX receptor as an agonist, mainly outside the brain, and, like AEA and 2-AG, to be an eicosanoid that is formed from arachidonic acid (10–12).
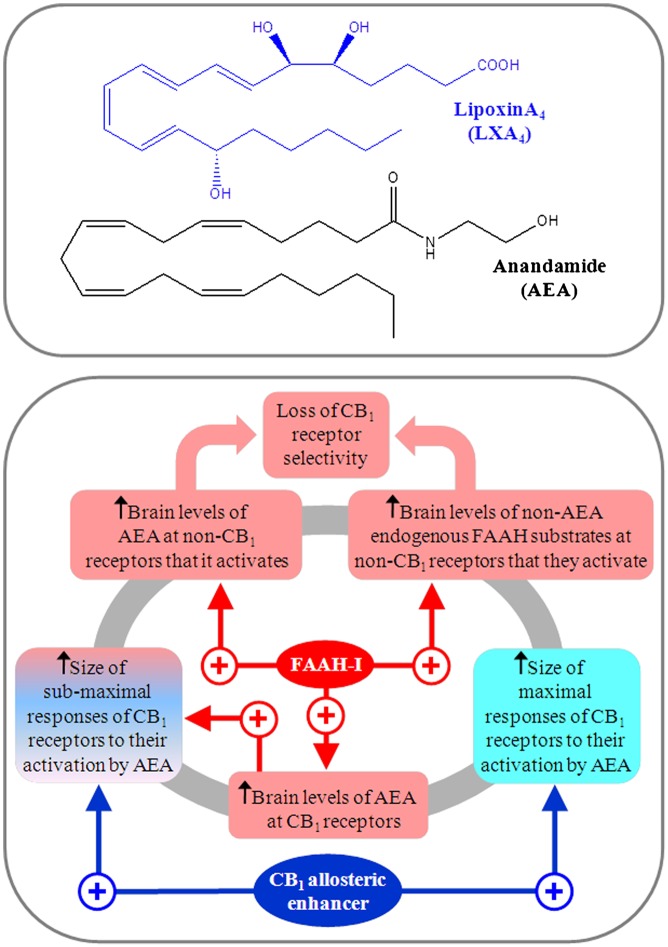
Fig. 1.
Upper: Structures of LXA4 and AEA. Lower: Comparison of how an FAAH inhibitor (FAAH-I) and a CB1 receptor allosteric enhancer could affect the potency, efficacy, and selectivity with which endogenously released AEA targets cannabinoid CB1 receptors in the brain. Further details are provided in the text.
In their PNAS paper, Pamplona et al. (9) present data showing that, when administered to mice intracerebroventricularly at doses of 0.1 and 1 pmol, LXA4 can act in an FPR2/ALX receptor-independent manner to produce a set of four effects: hypolocomotion, catalepsy, hypothermia, and antinociception in a hotplate test. These effects of LXA4 all appeared to be CB1 receptor-mediated because they (i) could be prevented by the CB1-selective antagonist/inverse agonist SR141716A, (ii) were not detectable in mice from which the cannabinoid CB1 receptor had been genetically deleted, and (iii) are known to be induced by established CB1 receptor agonists (1). Importantly, the results obtained in this investigation (9) also suggest that LXA4 did not induce this “tetrad” of effects through direct activation of the CB1 receptor, as it did not share the well known ability of established CB1 receptor agonists to produce a complete displacement of [3H]SR141716A from specific binding sites in mouse brain membranes, or to inhibit forskolin-induced stimulation of cAMP production by mouse CB1-transfected HEK cells. Instead, it most likely acted by potentiating the activation of CB1 receptors by AEA, as (i) intracerebroventricular injections of doses of AEA and LXA4 that were subeffective by themselves produced catalepsy in mice when they were coadministered, (ii) LXA4 produced a marked leftward shift in the log concentration–response curve of AEA for its inhibition of forskolin-induced stimulation of cAMP production by mouse CB1-HEK cells, and (iii) LXA4 also augmented AEA-induced increases in inward K+ currents in CB1 receptor-containing Xenopus laevis oocytes. Pamplona et al. (9) also find that, at a concentration at which LXA4 potentiated AEA in vitro (100 nM), it also slows the dissociation of [3H]CP55940 from specific binding sites in mouse brain membranes, which is widely accepted to be a strong indication of ligand-induced allosteric modulation (13). Other experiments that Pamplona et al. (9) perform show that LXA4 is present in significant amounts in mouse hippocampus, cortex, and cerebellum. Consequently, they postulate that AEA is potentiated by LXA4 not only when this lipoxin is administered exogenously but also when it has been produced endogenously. This hypothesis is supported by their findings, first, that intracerebroventricularly injected AEA produces much less catalepsy in mice from which the LXA4-synthesizing enzyme 5-lipoxygenase has been genetically deleted than in WT mice, and, second, that this effect of AEA can also be attenuated by the 5-lipoxygenase inhibitor MK-886. Because, when administered alone, LXA4 produces behavioral effects in mice that appear to be mediated by CB1 receptors, it is also likely that it can increase the ability of endogenously released AEA to activate these receptors.
It has long been known that AEA-induced activation of CB1 receptors can also be enhanced by drugs that inhibit its metabolism by fatty acid amide hydrolase (FAAH) (3). However, there are three important differences between the ways in which an allosteric enhancer and an FAAH inhibitor increase the activation of the CB1 receptor by endogenously released AEA (Fig. 1). First, an FAAH inhibitor will produce such an increase by elevating the concentration of AEA at the CB1 receptor, whereas an allosteric enhancer will produce it by increasing the potency and/or efficacy with which AEA activates this receptor. Second, by raising the levels of endogenously released AEA, an FAAH inhibitor is expected to increase AEA-induced activation not only of CB1 receptors but also of other receptors and ion channels that this endocannabinoid targets (14), whereas a highly specific CB1 allosteric enhancer would be expected only to increase AEA-induced activation of CB1 receptors. Third, FAAH catalyzes the metabolism not only of AEA but also of 2-AG, and of a number of nonendocannabinoid endogenous fatty acid ethanolamides, the levels and lifespans of which can therefore also be increased by inhibitors of this enzyme (2, 3). It could well be, therefore, that LXA4 is more selective than an FAAH inhibitor as a potentiator of AEA, a possibility that merits further investigation. In the meantime, what has already been found by Pamplona et al. (9) is that LXA4 is not an inhibitor of FAAH or the 2-AG–metabolizing enzyme monoacylglycerol lipase, and also that, when administered exogenously, LXA4 does not alter mouse brain levels of AEA. They also find that, although LXA4 enhances the ability of AEA to induce catalepsy, there is no such detectable potentiation of 2-AG (9). This may be because 2-AG activates the CB1 receptor with much higher efficacy than AEA in the absence of LXA4, and so is much less susceptible than AEA to any efficacy-enhancing effect of LXA4. It should be borne in mind, however, that, although Pamplona et al. (9) show that LXA4 can potentiate AEA, it is currently unclear whether it can also increase the maximum size of any AEA-induced effect. It is noteworthy as well that, whereas LXA4 increases the potency with which AEA produces signs of CB1 receptor agonism in the cAMP assay performed with mouse CB1-HEK cells, it decreases the stimulatory effect of AEA on [35S]GTPγS binding to mouse brain membranes. Clearly, further research is required to investigate this apparent discrepancy, and indeed to explore more fully the effect of LXA4 on agonist-induced CB1 receptor signaling.
The discovery that the ability of AEA to activate CB1 receptors appears to be allosterically enhanced by endogenously produced LXA4, in at least some brain
Pamplona et al. present convincing evidence that LXA4 is an endogenous CB1 receptor allosteric enhancer.
areas, has therapeutic implications that merit further investigation. More specifically, it will be important to establish whether endogenous LXA4 enhances any of the proposed autoprotective effects of endogenously released AEA (2, 3), and, if it does, whether it would be therapeutically beneficial to boost this effect with an inhibitor of LXA4 metabolism. Importantly, such enhancement might well be very selective, as it is likely to be restricted to effects of AEA that (i) are CB1 receptor-mediated rather than, for example, CB2 receptor- or TRP-mediated (14), and (ii) occur in brain areas in which both it and LXA4 are present at appropriate concentrations. The possibility that this kind of enhancement of one or more of the autoprotective effects of AEA could be further enhanced by simultaneously boosting (i) the potency/efficacy of AEA with an inhibitor of LXA4 metabolism and (ii) the brain levels of AEA with an FAAH inhibitor also warrants further investigation. It will be important as well to establish whether any known autoimpairing effects, such as obesity, that are thought to be induced or exacerbated by the endogenous release of AEA (2, 3), could be ameliorated by administering a selective inhibitor of LXA4 biosynthesis to oppose any ongoing endogenous potentiation of AEA by LXA4 at CB1 receptors that mediate one or more of these unwanted effects. In contrast to a selective CB1 receptor antagonist, such an inhibitor would, according to the findings of Pamplona et al. (9), be expected to lessen CB1-mediated effects of AEA but not of 2-AG, and to produce a reduction of this kind only at CB1 receptors that are being simultaneously exposed to AEA and LXA4. In view of these possibilities, it is noteworthy that Pamplona et al. (9) have already obtained evidence that, when it is administered exogenously, LXA4 can enhance the ability of endogenously released AEA to oppose β-amyloid–induced memory impairments in mice, raising the possibility that an inhibitor of LXA4 metabolism might ameliorate at least some unwanted symptoms of Alzheimer’s disease. It will also be important, of course, to seek out any toxicological consequences of suppressing or augmenting any of the actions of LXA4 through inhibition of its biosynthesis or metabolic degradation, to obtain some indication of the benefit-to-risk ratios of these potential therapeutic strategies.
In conclusion, Pamplona et al. (9) present convincing evidence that LXA4 is an endogenous CB1 receptor allosteric enhancer, and hence a member of the allosteric branch of the ever-expanding endocannabinoid family. Further research is now required to explore more fully the impact of endogenous LXA4 on the endocannabinoid system in different parts of the brain in both health and disease. As well as advancing our knowledge of this system, such research would hopefully also reveal much-needed new therapeutic strategies for ameliorating one or more serious brain disorders.
Footnotes
The author declares no conflict of interest.
See companion article on page 21134.
References
- 1.Howlett AC, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 2.Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005;7(3):E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Marzo V. Targeting the endocannabinoid system: To enhance or reduce? Nat Rev Drug Discov. 2008;7(5):438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 4.Adam L, et al. 17th Annual Symposium on the Cannabinoids. Burlington, VT: International Cannabinoid Research Society; 2007. Positive allosteric modulators of CB1 receptors; p. 86. [Google Scholar]
- 5.Horswill JG, et al. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br J Pharmacol. 2007;152(5):805–814. doi: 10.1038/sj.bjp.0707347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price MR, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68(5):1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- 7.Ross RA. Allosterism and cannabinoid CB1 receptors: The shape of things to come. Trends Pharmacol Sci. 2007;28(11):567–572. doi: 10.1016/j.tips.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 9.Pamplona FA, et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci USA. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(suppl 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell R, et al. Lipoxin A4 is a novel estrogen receptor modulator. FASEB J. 2011;25(12):4326–4337. doi: 10.1096/fj.11-187658. [DOI] [PubMed] [Google Scholar]
- 12.Ye RD, et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61(2):119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54(2):323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 14.Pertwee RG, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]