Abstract
One of the hurdles for practical application of induced pluripotent stem cells (iPSC) is the low efficiency and slow process of reprogramming. Octamer-binding transcription factor 4 (Oct4) has been shown to be an essential regulator of embryonic stem cell (ESC) pluripotency and key to the reprogramming process. To identify small molecules that enhance reprogramming efficiency, we performed a cell-based high-throughput screening of chemical libraries. One of the compounds, termed Oct4-activating compound 1 (OAC1), was found to activate both Oct4 and Nanog promoter-driven luciferase reporter genes. Furthermore, when added to the reprogramming mixture along with the quartet reprogramming factors (Oct4, Sox2, c-Myc, and Klf4), OAC1 enhanced the iPSC reprogramming efficiency and accelerated the reprogramming process. Two structural analogs of OAC1 also activated Oct4 and Nanog promoters and enhanced iPSC formation. The iPSC colonies derived using the Oct4-activating compounds along with the quartet factors exhibited typical ESC morphology, gene-expression pattern, and developmental potential. OAC1 seems to enhance reprogramming efficiency in a unique manner, independent of either inhibition of the p53-p21 pathway or activation of the Wnt-β-catenin signaling. OAC1 increases transcription of the Oct4-Nanog-Sox2 triad and Tet1, a gene known to be involved in DNA demethylation.
Recent breakthroughs in the development of induced pluripotent stem cells have generated much interest in the therapeutic potential of stem cells in regenerative medicine. Pioneering work by Yamanaka and colleagues identified a transcription factor quartet (4F), octamer-binding transcription factor 4 (Oct4), Sox2, Klf4, and c-Myc, that enables reprogramming of somatic cells to a pluripotent state (1, 2). The induced pluripotent stem cells (iPSCs) closely resemble embryonic stem cells (ESCs) in gene expression, epigenetic signature, and functional pluripotency. The simplicity of this reprogramming approach has opened up tremendous opportunities to generate patient-specific cells for disease modeling and therapeutic applications.
Two issues appear to limit the application of iPSCs, the low efficiency of reprogramming and the integration of transgenes into the somatic genome (3). The low efficiency and slow kinetics of reprogramming methods to generate iPSCs impose major limitations on their biomedical applications and continue to present a problem for ultimate therapeutic applications of iPSCs. There is thus a need for more efficient procedures for iPSC generation, and one approach is the use of small molecule chemicals to reprogram somatic cells with improved efficiency and kinetics.
Substantial effort has been made toward identifying chemical compounds that can enhance the efficiency of reprogramming (4–14). Several small molecules that are known to remodel chromatin and affect epigenetic control are being investigated actively for their effect on reprogramming. It has been shown that DNA methyltransferase inhibitors, histone deacetylase (HDAC) inhibitors, and histone demethylase inhibitors can improve reprogramming efficiency (4, 5, 10, 11, 13). These compounds may act by reducing the epigenetic barriers to reprogramming, and they may potentially improve the efficiency and quality of the derived iPSCs (13). Molecules that act on known signaling pathways involved in ESC self-renewal and pluripotency, including Wnt, TGFβ, and MEK, have also been shown to enhance reprogramming efficiency (8, 9, 11, 15). More recently, retinoic acid receptor (RAR) agonists, vitamin C and lithium have been reported to enhance reprogramming efficiency as well (12, 16, 17).
Oct4 is a key regulator for ESC pluripotency. Reduced expression of Oct4 results in differentiation of ESCs into trophectodermal cells, and ovexpression of Oct4 leads to differentiation of ESCs along the mesodermal and primitive endodermal lineages (18). Because the original report of induced reprogramming using the transcription factor quartet, Oct4, Sox2, Klf4, and c-Myc, the combination of factors used to generate iPSCs have been much studied (4–13, 15, 16, 19–32). However, Oct4 remains as the key, required component of the reprogramming mixture, not replaceable by other factors, except the nuclear receptors NR5a1, NR5a2, and the combination of microRNAs miR-200c, miR-302s, and miR-369s (23, 33, 34). Using neural stem cells that endogenously express Sox2, Klf4, and c-Myc, Oct4 was shown to be sufficient by itself to induce pluripotency (35, 36). The central role of Oct4 in the reprogramming process prompted us to ask whether Oct4-activating compounds may enhance reprogramming efficiency, thus improving iPSC technology.
In this study, we performed high-throughput screening of small-molecule libraries to identify Oct4 promoter-activating compounds using a human Oct4 promoter-driven luciferase reporter. The identified compounds were characterized for their ability to enhance reprogramming efficiency and accelerate the reprogramming process. The derived iPSCs were characterized for their gene expression, epigenetic profile, and pluripotency.
Results
Identification of Oct4-Activating Compounds Using High-Throughput Screening.
We designed a high-throughput screening scheme to identify Oct4 promoter-activating compounds using a luciferase reporter under the control of the human Oct4 promoter (Oct4-luc). The human Oct4 promoter, spanning base pairs −3917 to +55 (relative to transcription start site), contains the Oct4 ESC-specific DNA elements and was able to drive the expression of a GFP reporter gene when transfected into human ESCs (Fig. 1A). A stable cell line (Oct4-luc) that expresses luciferase reporter driven by the exogenous Oct4 promoter was established using hygromycin-resistance as a selection marker. These Oct4-luc cells were used for high-throughput screening of a chemical library of 80,000 compounds. The luciferase reporter activity was measured 24 h after compound treatment. Solvent treatment was included as a negative control. From the primary screening, 812 compounds were identified that activate Oct4-luc activity fivefold or more.
Fig. 1.
Identification of Oct4-activating compounds. (A) Expression of Oct4-GFP reporter in transfected human ESCs. (Scale bar, 30 µm.) (B) Activation of Oct4-luc reporter by OAC1 and nine other compounds. (C) Activation of Nanog-luc reporter. Shown is an example of compound validation using triplicate luciferase reporter assays. DMSO was included as a control in lane 1 for both B and C.
These compounds were confirmed for their ability to activate human Oct4 promoter in triplicate luciferase reporter assays. Oct4, Sox2, and Nanog form a positive regulatory loop to regulate the expression of each other, and Nanog is considered a downstream target gene of Oct4 and Sox2 (37). Therefore, we used activation of Nanog promoter as a validation assay for the identified compounds. Specifically, we tested whether the Oct4 promoter-activating compounds activate a human Nanog promoter-driven luciferase reporter gene in triplicate luciferase reporter assays. Among the identified molecules, compound OAC1 (Oct4-activating compound 1) exhibited considerable activation of both human Oct4 and Nanog reporters (Fig. 1 B and C) and was selected for further characterization.
In addition, 31 structural analogs of OAC1 were characterized for their ability to activate Oct4 and Nanog promoter-driven reporters (Table S1). Fourteen analogs exhibited more than 1.8-fold activation of both Oct4-luc and Nanog-luc and were considered active compounds (Table S1). Structure-activity relationship study revealed that the ring structure at both ends seems important for the Oct4 promoter-activating activity of these compounds, whereas multiple modifications of the ring structure were not favorable for this activity (Table S1). Two of the active compounds that are structurally very close to OAC1 were selected for further analysis and designated as OAC2 and OAC3. These two compounds activated both Oct4 and Nanog reporters to a similar extent as OAC1 (Fig. 2 A and B). Their structure is shown in Fig. 2C.
Fig. 2.
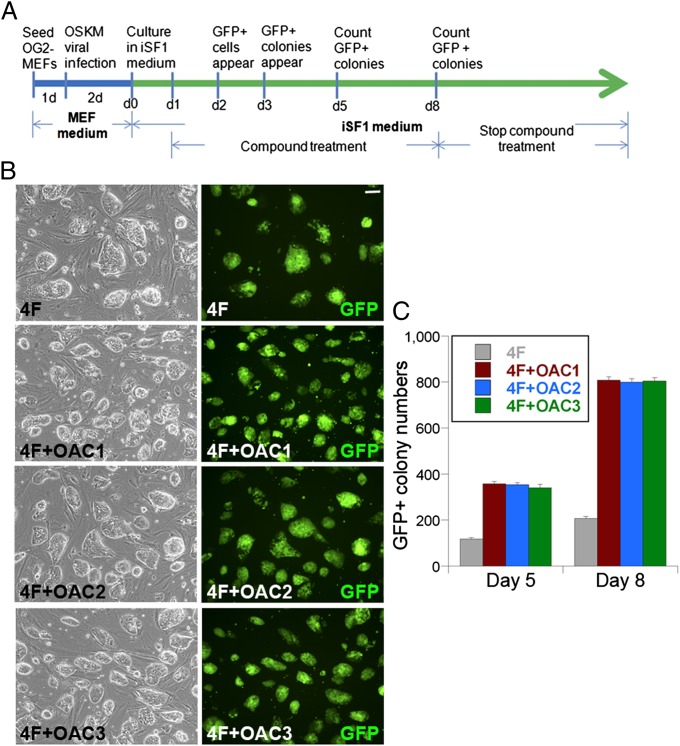
Compound OAC1 and its two structural analogs enhance reprogramming efficiency. (A) Compounds OAC1, OAC2, and OAC3 activated Oct4-luc reporter. (B) OAC1, OAC2, and OAC3 activated Nanog-luc reporter. DMSO treatment was included as a control in lane 1. Eight structural analogs of OAC1 (lanes 2–9) were tested in the reporter assays for both A and B. (C) The structure of OAC1, OAC2, and OAC3. (D) Schematic representation of iPSC generation from MEF in mouse ESC media using 4F (OSKM) + OACs. (E) Images of iPSC clones from reprogramming using 4F, 4F+OAC1, 4F+OAC2, and 4F+OAC3. (Scale bar, 100 µm.) (F) Quantification of iPSC colony numbers in 4F (column 1), 4F+OAC1 (column 2), 4F+OAC2 (column 3), and 4F+OAC3 (column 4) reprogramming. (G) Kinetics of iPSC colony appearance in 4F (column 1), 4F+OAC1 (column 2), 4F+OAC2 (column 3), and 4F+OAC3 (column 4) reprogramming. *P < 0.01 by one-way Anova test for both F and G. Error bars are SD of the mean for all panels.
OAC1 and Analogs Enhance Reprogramming Efficiency.
The generation of iPSCs is a gradual process with relatively low efficiency. Because Oct4 is central to the reprogramming process (35, 36, 38), we hypothesized that compounds that activate Oct4 transcription may facilitate iPSC generation by enhancing reprogramming efficiency. Reprogramming of mouse embryonic fibroblasts (MEFs) was performed with the 4F reprogramming quartet Oct4, Sox2, Klf4, and c-Myc, along with solvent control, OAC1 or its structural analogs OAC2 and OAC3. The same number of starting MEFs and the same viral stocks were used for each treatment. Two days after viral transduction, the 4F-transduced cells were seeded onto feeder cells in mouse ESC culture media, and this day was designated as day 0 (Fig. 2D). Compound treatment started on day 0 and lasted for 7 d. By counting the number of clones with ESC-like morphology at day 18, we found that both OAC1 and its analogs OAC2 and OAC3 enhanced the 4F-induced reprogramming efficiency considerably. The number of colonies with ESC-like morphology (Fig. 2E) increased twofold or more in 4F plus OAC (4F+OAC) treatment, compared with the 4F treatment alone (Fig. 2F and Table S2).
Furthermore, addition of OAC1, OAC2, or OAC3 to the reprogramming mixture considerably accelerated the appearance of iPSC-like colonies. We observed putative iPSC clones derived from the 4F+OAC1 and 4F+OAC2-treated MEFs within 4–6 d after culturing in mouse ESC media. In contrast, the 4F treatment alone did not result in visible clones until 8–10 d after transferring to mouse ESC media. The appearance of putative iPSC colonies was advanced about 3 to 4 d in 4F+OAC-mediated reprogramming, compared with the 4F only reprogramming (Fig. 2G).
Recently, a serum-free media (iSF1) was developed to facilitate the generation of mouse iPSCs (39). We therefore tested the effect of compound OAC1 in the 4F-induced reprogramming in iSF1 media. OG2 MEFs, derived from transgenic mice expressing the Oct4 promoter-driven GFP reporter (Oct4-GFP) (40), were transduced with retroviruses expressing the 4F reprogramming factors, Oct4, Sox2, Klf4, and c-Myc. The 4F-transduced cells were transferred to iSF1 media 2 d after viral transduction and this day was designated as day 0 (Fig. 3A). Treatment of compound OAC1 started on day 1 and lasted for 7 d. Many GFP+ single cells were seen in C1-treated cells at day 2 and the GFP+ colonies started to appear at day 3. At day 5, a more than threefold increase in the number of GFP+ colonies was detected in OAC1-treated, 4F-transduced cells (4F+OAC1), compared with vehicle-treated, 4F-transduced cells (Fig. 3 B and C). A similar increase in the number of GFP+ colonies was observed in OAC2- and OAC3-treated, 4F-transduced cells (4F+OAC2, 4F+OAC3). At day 8, an approximately fourfold increase in the number of GFP+ colonies were observed in 4F+OAC1-treated cells (2.75% reprogramming efficiency), compared with 4F-treated cells (0.68% efficiency) (Table S2). A similar increase was observed in 4F+OAC2 and 4F+OAC3-treated cells (Fig. 3C). The number of GFP+ colonies in 4F-treated cells at day 8 (average of 200) was less than the number of GFP+ colonies in 4F+OAC1-treated cells at day 5 (average of 350) (Fig. 3 B and C). Taken together, these results clearly indicate that compound OAC1 enhances the formation of Oct4-GFP+ colonies and that OAC1 accelerates the dynamics of reprogramming. We next present evidence that these colonies are iPSCs.
Fig. 3.
OAC1 and its structural analogs enhance reprogramming efficiency in iSF1 media. (A) Schematic representation of iPSC generation from MEFs in iSF1 media. (B) 4F (OSKM) infected OG2-MEFs were cultured in iSF1 medium and treated with OAC1, OAC2, or OAC3 (1 µM each) for 7 d. GFP+ colonies are shown in phase contrast and green fluorescence images. (Scale bar, 100 µm.) (C) The number of GFP+ colonies counted at day 5 and day 8. Error bars are SD of the mean.
Colonies Are iPSCs.
ESC-like colonies were picked from either 4F or 4F+OAC treatment and expanded under conventional ESC culture conditions. The 4F-induced clones were labeled as 4F-iPSCs, and the clones generated using the 4F plus the OAC compounds were indicated as 4F+OAC-iPSCs. The stably expanded 4F+OAC1-iPSCs and 4F+OAC2-iPSCs were morphologically indistinguishable from ESCs and 4F-iPSCs (Fig. S1A). These iPSCs expressed the typical mouse ESC cell surface marker SSEA1 and the ESC pluripotency factor Oct4, as revealed by immunostaining analysis (Fig. 4 A and B). The Oct4-GFP reporter was also activated in the 4F+OAC-induced iPSCs (Fig. 4C), consistent with the positive Oct4 immunostaining (Fig. 4 B and C).
Fig. 4.
Characterization of 4F+OAC-iPSCs. (A) SSEA1 immunostaining of 4F, 4F+OAC1, and 4F+OAC2-iPSCs. (Scale bar, 50 µm.) (B) Oct4 immunostaining of 4F, 4F+OAC1, and 4F+OAC2-iPSCs. (Scale bar, 50 µm.) (C) Expression of both the Oct4-GFP reporter (green) and Oct4 (red) in 4F+OAC2-iPSCs. (Scale bar, 25 µm.) (D and E) Bisulfite sequencing analysis of Oct4 (D) and Nanog (E) promoter regions in MEFs, mouse ESCs, 4F+OAC1 and 4F+OAC2-iPSCs. Open and closed circles indicate unmethylated and methylated CpGs, respectively. (F) RT-PCR analysis of endogenous (endo) Oct4, Sox2, and Nanog expression in 4F, 4F+OAC1, and 4F+OAC2-iPSCs. Actin was included as a loading control. MEFs were included as a negative control and mouse ESCs as a positive control. (G) RT-PCR analysis of exogenous (exo) viral genes in 4F, 4F+OAC1 and 4F+OAC2-iPSCs. MEFs were included as a negative control and cells transiently transfected with the viral vector of each gene was included as a positive control (PC).
Bisulfite sequencing analysis revealed that the endogenous Oct4 promoter of both 4F+OAC1-iPSCs and 4F+OAC2-iPSCs was largely demethylated, similar to the hypo-methylated state of the Oct4 promoter in mouse ESCs (Fig. 4D). In contrast, the Oct4 promoter in the parental MEFs was highly methylated (Fig. 4D). Similarly, DNA methylation on the endogenous Nanog promoter was also much lower in the 4F+OAC1 and 4F+OAC2-iPSCs, compared with that in the parental MEFs (Fig. 4E).
Consistent with the hypomethylated state of the Oct4 and Nanog promoters, activation of the endogenous Oct4 and Nanog gene transcription was evident in 4F+OAC1 and 4F+OAC2-iPSCs, similar to that in 4F-iPSCs and mouse ESCs (Fig. 4F). Activation of endogenous Sox2 gene was also detected in 4F+OAC1 and 4F+OAC2-iPSCs, similar to the Sox2 mRNA levels in 4F-iPSCs and mouse ESCs (Fig. 4F). In contrast, the four exogenous reprogramming factors, Oct4, Sox2, Klf4, and c-Myc, were all transcriptionally silenced in 4F+OAC1 and 4F+OAC2-iPSCs (Fig. 4G). The 4F+OAC1 and 4F+OAC2-iPSCs were also positive for alkaline-phosphatase, another marker of pluripotency, similar to mouse ESCs (Fig. S1B).
Differentiation Potential of 4F+OAC-iPSCs.
To examine the developmental potential of 4F+OAC1- and 4F+OAC2-iPSCs, we differentiated these cells in vitro using a standard embryoid body (EB) differentiation approach. Immunostaining revealed that the 4F+OAC1-iPSCs and 4F+OAC2-iPSCs could effectively differentiate into characteristic FoxA2+ endoderm cells, smooth muscle actin (SMA)+ mesodermal cells, and Tuj1+ ectoderm cells (Fig. 5A).
Fig. 5.
Developmental potential of 4F+OAC-induced iPSCs. (A) Differentiation potential of ESCs, 4F+OAC1-iPSCs, and 4F+OAC2-iPSCs in EB formation assays. During EB formation, mouse ESCs, 4F+OAC1-iPSCs and 4F+OAC2-iPSCs were differentiated into FoxA2+ endoderm, SMA+ mesoderm, and Tuj1+ ectoderm cells. (Scale bar, 50 µm.) (B) Teratoma formation of 4F+OAC2-iPSCs. The tissues of all three germ layers, such as epidermis, blood, and intestinal epithelia, were detected in 4F+OAC2-iPSC-derived teratoma sections. (Scale bar, 50 µm.) (C) Chimera mouse production using 4F+OAC2-iPSCs. Adult mice with a high degree of chimerism were developed from 4F+OAC2-iPSCs after blastocyst injection.
To test the in vivo pluripotency of the 4F+compound-induced iPSCs, we transplanted the 4F+OAC2-iPSCs into immunodeficient Nude mice. Four to 6 wk after transplantation, the 4F+OAC2-iPSCs effectively generated typical teratomas containing derivative of all three germ layers, such as intestinal epithelia of endoderm, blood of mesoderm, and epidermis of ectoderm (Fig. 5B).
A more stringent assay for pluripotency is to determine whether iPSCs can generate chimera mice. The developmental potential of the 4F+OAC-induced iPSCs was evaluated by injection of 4F+OAC2-iPSCs into diploid blastocysts. After injection into blastocysts, these iPSCs were able to produce live postnatal animals with high coat-color chimerism (Fig. 5C). The generation of viable chimeras is a further indication of the developmental potential of the 4F+OAC-induced iPSCs.
Collectively, these in vitro and in vivo results demonstrate that a set of structurally related small molecules (OAC1-OAC3) is able to enhance the efficiency of reprogramming somatic cells to iPSCs that are morphologically, molecularly, and developmentally similar to pluripotent ESCs.
Potential Mechanisms.
To uncover the mechanism of OAC1-mediated improvement of reprogramming efficiency, we first tested whether OAC1 functions through regulation of DNA methylation status on the endogenous Oct4 promoter. Human IMR90 fibroblast cells were treated with OAC1 or vehicle control for 2 d. Bisulfite sequencing revealed no significant difference in Oct4 promoter methylation status between OAC1 and DMSO treated cells (Fig. 6A). This result indicates that OAC1 enhanced reprogramming efficiency through a mechanism that is independent of endogenous Oct4 promoter demethylation.
Fig. 6.
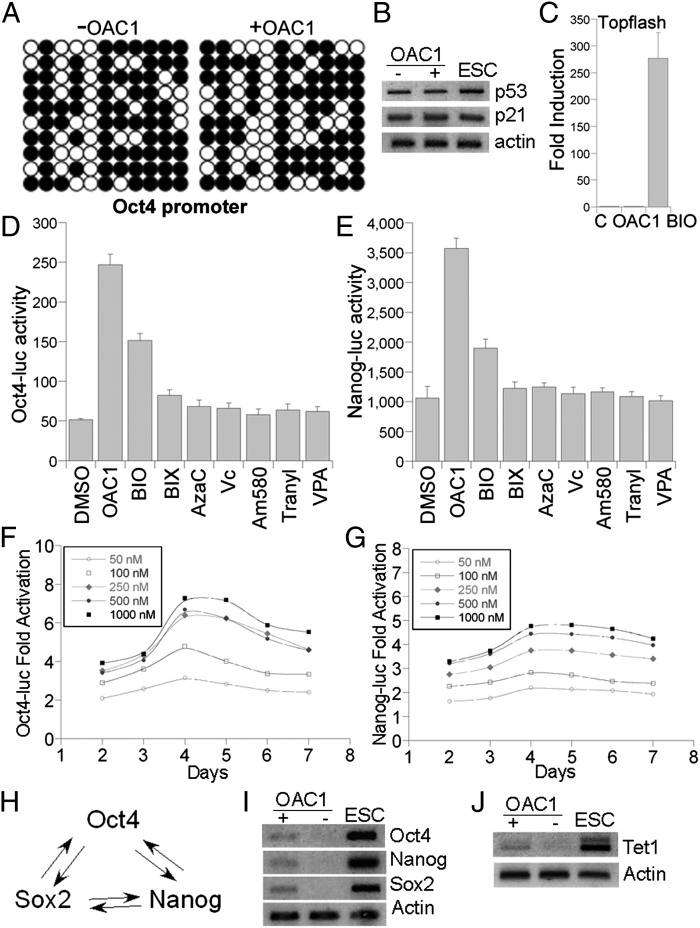
OAC1 activated endogenous Oct4, Nanog, Sox2, and Tet1 expression. (A) Bisulfite genomic sequencing of the promoter regions of OCT4 in human IMR90 fibroblasts treated with or without OAC1 for 2 d. Open and closed circles indicate unmethylated and methylated CpGs, respectively. (B) RT-PCR analyses of p53 and p21 expression in vehicle or OAC1-treated cells. MEFs were treated with OAC1 for 2 d and RNAs were prepared from treated cells for RT-PCR analysis. (C) Topflash luciferase reporter assays in CV1 cells treated with vehicle (C), 1 µM OAC1 or 2 µM BIO. (D and E) OAC1 activated Oct4 and Nanog promoter-driven luciferase reporter genes. Oct4-luc (D) or Nanog-luc (E) stably transfected cells were treated with OAC1 (1 µM), BIO (2 µM), BIX (2 µM), AzaC (2 µM), Vc (25 µg/mL), Am580 (10 nM), Tranylcypromine (Tranyl, 5 µM), and VPA (0.5 mM) for 2 d. (F and G) Dose responsive of OAC1 in Oc4-luc or Nanog-luc stably transfected cells. Oct4-luc (F) or Nanog-luc (G) -transfected cells were treated with OAC1 at concentrations of 50 nM, 100 nM, 250 nM, 500 nM, and 1 μM for 2–7 d. (H) A diagram showing the Oct4, Sox2, and Nanog triad, forming a regulatory feedback circuit. (I) OAC1 activated endogenous Oct4, Sox2, and Nanog genes in MEFs as revealed by RT-PCR analysis. (J) OAC1 activated Tet1 expression in MEFs as revealed by RT-PCR analysis. Actin was included as a loading control.
Recently, the p53-p21 pathway has been shown to serve as a barrier in iPSC generation. Inhibition of the p53-p21 signaling increased reprogramming efficiency (26, 28–31, 41). To investigate whether compound OAC1 enhanced reprogramming efficiency by affecting this pathway, we treated MEFs with vehicle control or OAC1 and determined the expression level of p53 and p21 in the treated cells. RT-PCR analysis revealed no significant difference in the expression levels of both p53 and its downstream target p21 in DMSO and OAC1-treated cells (Fig. 6B). This result suggests that OAC1 enhanced reprogramming efficiency through a mechanism that is distinct from suppressing p53-p21 expression.
We then determined whether OAC1 regulates major signaling pathways that are critical for embryonic stem cell pluripotency. Specifically, we tested whether OAC1 regulates Wnt signaling, a pathway that has been shown to play an important role in ESC maintenance and pluripotency (42). We transfected CV1 cells with the β-catenin–responsive reporter gene Topflash, and the transfected cells were treated with vehicle control or OAC1 (Fig. 6C). The glycogen synthase kinase 3β (GSK3β) inhibitor BIO was included as a positive control (42). Luciferase assay revealed that OAC1 had no effect on Topflash activity, although BIO activated the Topflash reporter potently (Fig. 6C). This result indicates that OAC1 functions through a mechanism that is independent of the Wnt signaling.
To further understand OAC1-induced enhancement of reprogramming efficiency, we compared the effect of OAC1 on the activation of the Oct4 and Nanog promoters to the effect of compounds that were shown to enhance reprogramming efficiency in previous studies, including BIX-01294 (BIX, an inhibitor of the G9a histone methyltransferase) (7), 5′-azacytidine (AzaC, a potent DNA methylation inhibitor) (4), Vitamin C (Vc) (12), Am580 (RAR agonist) (17), tranylcypromine (a lysine-specific histone demethylase inhibitor) (11, 43), and valporic acid (VPA, an HDAC inhibitor) (4, 5). We treated CV1 cells that were stably-transfected with the Oct4-luc or Nanog-luc reporters with OAC1. The GSK-3β inhibitor BIO was shown to maintain Oct4 expression in mouse ESCs (42) and was included as a control. The Oct4-luc and Nanog-luc transfected cells were treated in parallel with BIX, AzaC, Vc, Am580, tranylcypromine, and VPA at the same concentrations that were used to enhance reprogramming efficiency in the previous studies (4, 5, 7, 11, 12, 17, 43). OAC1 exhibited potent induction of both Oct4-luc and Nanog-luc (Fig. 6 D and E). BIO displayed a lower level induction of the two reporters (Fig. 6 D and E). However, no significant effect on either reporter was detected for BIX, AzaC, Vc, Am580, tranylcypromine, and VPA (Fig. 6 D and E). These results revealed that OAC1 is different from other compounds that enhance reprogramming efficiency. OAC1 may directly activate Oct4 and Nanog promoters. Although BIO also activated Oct4 and Nanog promoters, the activation level was much lower (Fig. 6 D and E) and it did not exert any notable effect on reprogramming efficiency (16).
Next, we examined the dose–response and kinetics of OAC1-mediated activation of Oct4 and Nanog promoters. OAC1 activated Oct4-luc considerably at 50 nM concentration, 2 d after compound treatment (Fig. 6F). Activation of Oct4 is dose-dependent, with highest induction at 1 µM of OAC1. The induction is peaked around days 4 and 5 and plateaued at days 6 and 7 (Fig. 6F). A similar dose–response was observed for activation of Nanog-luc by OAC1 (Fig. 6G). A substantial activation of Nanog-luc was seen by OAC1 at 50 nM. The highest induction occurred at 0.5–1 µM of OAC1. The induction is evident by day 2 after compound treatment, peaked around days 4 and 5, and plateaued thereafter (Fig. 6G).
To test whether OAC1 activates endogenous Oct4 and Nanog gene transcription, we treated MEFs with vehicle control or OAC1. Consistent with the results from luciferase reporter assays, RT-PCR analysis revealed that OAC1 activated endogenous Oct4 and Nanog mRNA expression (Fig. 6I). Oct4, Sox2, and Nanog are central to the transcriptional regulatory hierarchy for ESC pluripotency, forming the core transcriptional regulatory circuitry in ESCs (37). Because Oct4, Nanog, and Sox2 have been shown to act in positive regulatory loops to regulate each other expression (37) (Fig. 6H), we also determined Sox2 expression in OAC1-treated cells. OAC1 activated Sox2 mRNA expression, along with Oct4 and Nanog (Fig. 6I).
Tet1 (Ten eleven translocation 1) has been shown to play an important role in mouse ESC maintenance through sustaining the expression of Nanog (44, 45). RT-PCR analysis revealed that Tet1 mRNA expression was also up-regulated in OAC1-treated cells (Fig. 6J). The up-regulation of Tet1 is intriguing, as this is a gene that converts 5-methylcytosine to 5-hydroxymethylcytosine and may be involved in active DNA demethylation.
Discussion
Oct4 and Nanog are two key transcription factors that function as major regulators of pluripotency and self-renewal in ESCs (46–48). Moreover, Oct4 serves as a key pluripotency determinant in reprogramming (38). Induction of endogenous Nanog expression has also been shown to be essential for successful induction of iPSCs (20). In this study, we developed a phenotypic high-throughput screening method and identified OAC1, a small molecule that activates the expression of Oct4 and Nanog promoter-driven luciferase reporters. We then found that transcription of endogenous Oct4 and Nanog are induced by OAC1 treatment. Furthermore, we show that OAC1 and its two structural analogs OAC2 and OAC3 enhanced reprogramming efficiency fourfold, up to as high as 2.75%, and accelerated the appearance of iPSC colonies 3 to 4 d when used in combination with the four reprogramming factors, Oct4, Sox2, Klf4, and c-Myc. These small molecule compounds represent a previously undescribed class of compounds.
The reprogramming efficiency of 4F (OSKM) + OAC1 is more than 20-fold higher than that induced by the 4F alone in the initial Yamanaka study (2). It is also higher than that induced by 4F plus other compounds, such as VPA (4), Aza-C (4), kenpaullone (14), and Repsox2 (9), based on the percentage of colony numbers out of total seeding cell numbers (Table S2). It is worth noting that this is not a direct comparison. The date when the colony numbers were counted and the criteria by which the colonies were scored varied case by case.
The iSF1 medium is proven to facilitate iPSC generation by enhancing the efficiency of reprogramming (39). A screen of 16 compounds, including AzaC, TSA, VPA, and BIX, was performed to evaluate their potential to enhance reprogramming efficiency in iSF1 media (39). Among them, only VPA and TSA led to a twofold increase in reprogramming efficiency at day 8 postinfection of reprogramming factors, no enhancement was detected by other compounds (39). In this study, we showed that OAC1 enhanced reprogramming about fourfold at day 8 postinfection of 4F, suggesting that OAC1 improves reprogramming efficiency more potently than other compounds tested in this reprogramming system.
A number of chemicals have been reported to improve the reprogramming efficiency, including compounds that alter DNA methylation or histone modifications (4, 5, 7, 11–13, 43). When tested in parallel, these compounds, including BIX, AzaC, Vc, Am580, Tranylcypromine, and VPA, did not activate either Oct4 or Nanog promoter-driven luciferase activity 48 h after treatment, in contrast to the potent activation of both Oct4 and Nanog promoters by OAC1. Although BIO also activated Oct4 and Nanog reporter activity in our assays, it activated the reporters at a much lower level and this level of activation seemed not sufficient to enhance reprogramming efficiency (16).
OAC1 and related analogs belong to a structural class of 5-substituted pyrrolo[2,3-b]pyridine- and indole-based benzamides. Although these are known classes of heterocycles, very little is reported about their biological activities. OAC1 was recently identified as a weak luciferase inhibitor with an EC50 of 6.37 µM at 40 °C using loss of enzymatic activity as a measurement (49). However, in a different assay called ATLAS (any target ligand affinity screen), no inhibition was detected (49). This result is consistent with our observation that OAC1 exhibited no inhibition of luciferase activity when tested at 1-µM concentration, although OAC1 induced Oct4 and Nanog reporter activity and mRNA expression at the same concentration.
The Oct4, Sox2, and Nanog triad contributes to ESC pluripotency by forming feedforward and feedback loops to induce their own expression and activate genes encoding components of key signaling pathways governing ESC pluripotency and self-renewal (37). We show here that OAC1 activated the expression of all three factors in the triad. In addition, OAC1 activated the expression of Tet1, a member of the Tet protein family that catalyzes the conversion of 5-methylcytosine (5mC) of DNA to 5-hydroxymethylcytosine (5hmC). Tet1 is expressed at high levels in mouse ESCs and has been shown to play an important role in ESC maintenance and pluripotency (44, 45, 50, 51). Activating the transcription of genes that are key to ESC pluripotency and self-renewal, including Oct4, Nanog, Sox2, and Tet1, provides a mechanism for compound OAC1-induced improvement in reprogramming efficiency and dynamics. The observation of activation of Tet1 is intriguing, but further studies will be necessary to determine the mechanisms by which OAC1 activates the key pluripotency genes.
iPSCs provide great hope not only for basic biology by providing experimental model systems, but also for disease prevention and treatment via stem cell-based cell replacement therapy and stem cell-based drug discovery. However, the low efficiency of iPSC reprogramming is a hurdle to iPSC applications (52). We show here that OAC1 induced iPSC colony formation 3 d after transferring the 4F-transduced cells to the iSF1 reprogramming media and the reprogramming efficiency reached 2.75% by day 8. Our finding that an Oct4-activating small molecule is able to both enhance reprogramming efficiency and accelerate the reprogramming kinetics suggests that this method may be used for large-scale iPSC generation for potential clinical applications. This study also paves the way for detailed mechanistic studies to better understand the reprogramming process.
Materials and Methods
The Oct4-luc cells were seeded at 1.75 × 104 cells per well density into 96-well plates. Compounds were added to cells 1 d after cell seeding at the concentration of 10 µM for 24 h in the High-Throughput Screening core facility at City of Hope. Compounds that induced luciferase activity threefold or more were selected for further validation in both Oct4-luc and Nanog-luc cells.
Additional experimental procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. P. Szabo for providing the OG2 mice. W.L. is a California Institute for Regenerative Medicine Training Grant-supported Postdoctoral Fellow. G.S. is a Herbert Horvitz Postdoctoral Fellow. This work was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke RC1 NS068370 and California Institute for Regenerative Medicine TR2-01832.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219181110/-/DCSupplemental.
References
- 1.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Huangfu D, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26(7):795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huangfu D, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2(6):525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3(5):568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Lin T, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6(11):805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichida JK, et al. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5(5):491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu S, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7(6):651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2011;21(1):196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteban MA, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6(1):71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Mali P, et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010;28(4):713–720. doi: 10.1002/stem.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyssiotis CA, et al. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci USA. 2009;106(22):8912–8917. doi: 10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva J, et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6(10):e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, et al. Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells. Cell Res. 2011;21(10):1424–1435. doi: 10.1038/cr.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc Natl Acad Sci USA. 2011;108(45):18283–18288. doi: 10.1073/pnas.1100893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24(4):372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 19.Marson A, et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3(2):132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva J, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138(4):722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27(5):459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anokye-Danso F, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loewer S, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42(12):1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao B, et al. MicroRNA cluster 302-367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J Biol Chem. 2011;286(19):17359–17364. doi: 10.1074/jbc.C111.235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3(5):475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Banito A, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23(18):2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong H, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamura T, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460(7259):1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460(7259):1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utikal J, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460(7259):1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5(3):237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Heng JC, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6(2):167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Miyoshi N, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8(6):633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Kim JB, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Kim JB, et al. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461(7264):649–3. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 37.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132(4):567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, et al. Towards an optimized culture medium for the generation of mouse induced pluripotent stem cells. J Biol Chem. 2010;285(40):31066–31072. doi: 10.1074/jbc.M110.139436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabó PE, Hübner K, Schöler H, Mann JR. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev. 2002;115(1-2):157–160. doi: 10.1016/s0925-4773(02)00087-4. [DOI] [PubMed] [Google Scholar]
- 41.Marión RM, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460(7259):1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10(1):55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 43.Li W, et al. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27(12):2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito S, et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466(7310):1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freudenberg JM, et al. Acute depletion of Tet1-dependent 5-hydroxymethylcytosine levels impairs LIF/Stat3 signaling and results in loss of embryonic stem cell identity. Nucleic Acids Res. 2012;40(8):3364–3377. doi: 10.1093/nar/gkr1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chambers I, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113(5):643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 47.Mitsui K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113(5):631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 48.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 49.Thompson PA, et al. Identification of ligand binding by protein stabilization: comparison of ATLAS with biophysical and enzymatic methods. Assay Drug Dev Technol. 2008;6(1):69–81. doi: 10.1089/adt.2007.100. [DOI] [PubMed] [Google Scholar]
- 50.Koh KP, et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8(2):200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawlaty MM, et al. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 2011;9(2):166–175. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.González F, Boué S, Izpisúa Belmonte JC. Methods for making induced pluripotent stem cells: Reprogramming à la carte. Nat Rev Genet. 2011;12(4):231–242. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.