MICROBIOLOGY Correction for “Carbon and sulfur back flux during anaerobic microbial oxidation of methane and coupled sulfate reduction,” by Thomas Holler, Gunter Wegener, Helge Niemann, Christian Deusner, Timothy G. Ferdelman, Antje Boetius, Benjamin Brunner, and Friedrich Widdel, which appeared in issue 52, December 27, 2011, of Proc Natl Acad Sci USA (108:E1484–E1490; first published December 12, 2011; 10.1073/pnas.1106032108).
The authors note that on page E1484, right column, first full paragraph, line 8, “the anaerobic oxidation of methane with sulfate (AOM) (1)” should instead appear as “the anaerobic oxidation of methane (AOM) with sulfate (1).”
Also on page E1484, right column, Eq. 2 and its explanation appeared incorrectly. The corrected equation and its corrected explanation appear below.
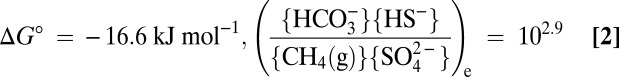 |
(Index e indicates activity ratio applying for equilibrium, viz. ΔG = 0; {H2O} is taken as 1) and thus one of the least exergonic processes sustaining life (ΔG in situ is often between −20 and −40 kJ mol−1) (21–23).
On page E1485, right column, third full paragraph, lines 4–9, “If the subsequent reactions occur with reversibility and are viewed from the molecular perspective (that is, regarding microstates stochastically) at a given moment, the larger fraction of the enzyme molecules of each reaction performs the forward reaction, whereas a smaller fraction simultaneously performs the reverse reaction” should instead appear as “If subsequent reactions occur with reversibility and if the involved population of enzyme molecules is viewed from the molecular perspective (that is, regarding microstates stochastically) within a time interval in the range of an enzymatic turnover, the forward reaction is catalyzed more frequently than the reverse reaction.”
On page E1487, right column, Eq. 11 within the footnote appeared incorrectly. The corrected equation appears below.
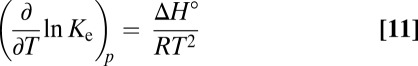 |
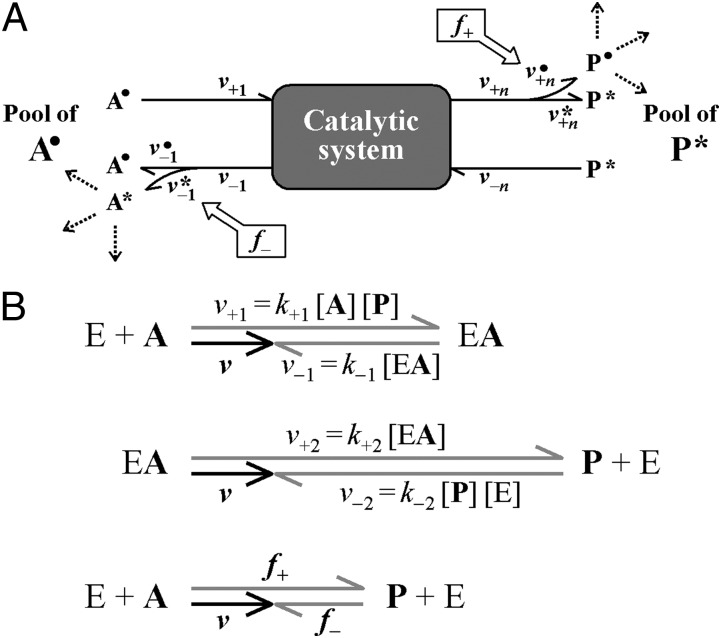
Last, Fig. 3 appeared incorrectly. The corrected figure and its corresponding legend appear below. These errors do not affect the conclusions of the article.
Fig. 3.
Depiction of forward and back flux during the net reaction A → P in a steady state catalytic system with reversible but otherwise unknown internal reactions. Such a system can consist of an entire pathway (A) or a single enzymatic reaction (B; E + A → EA → P + E) (Figs. S4 and S5). All reactions, including uptake or binding and release, are reversible. Arrows indicate rates (velocities). The forward (v+) and back (v−) rates correspond to individual steps in a catalytic system. The index n refers to the number of forward and backward fluxes (B; n = 2 for a single enzymatic reaction) (Fig. S5). A description of abbreviations is given in Table S3. (A) Catalytic system: the fate of substrate and product is followed by the different labels (A•, P*) of the initial pools. Release of A and P includes both the returned fraction that never reached the other side (v•–1, v*+n), and the fraction directly derived from the other side (v•+n, v*–1). Forward (f+) and back (f−) flux are the concentrations (amounts per investigated volume) of P• and A* most recently derived per time from A• and P*, respectively. Hence, return to the side of their origin with progressing reaction is neglected by examining a short time interval (A* in A• and P• in P* remaining very dilute). (B) Vector model for individual rates during net rate (v) of a single enzymatic reversible reaction (E + A ⇄ EA ⇄ P + E) (Fig. S5) in a steady state (pools of A and P are essentially constant). The sizes of the rates are indicated by lengths of arrows. The same net rate (v) is the difference of uptake (binding) and release of substrate (v+1, v−1) and product (v+2, v−2) as well as the difference between forward and back flux (f+, f−). The rate constants for the reaction steps (k+1, k−1, k+2, k−2) and the actual concentrations of E, A, and P determine v+1, v−1, v+2, v−2, respectively, as indicated, and the resulting f+, and f−, which can only be revealed by labeling (Eqs. S35 and S36). The vector model was calculated for an enzymatic reaction with (in rate units) v+1 = 6, v−1 = 4, v+2 = 9, v−2 =7; result: v = 2, f+ = 4.15, f− = 2.15. The associated change in free energy (free energy dissipated) is ΔG = R T ln(f−/f+) = −1.63 kJ (mol A)−1 (formula resulting from Eq. 8; T = 298 K).