Abstract
Hypersecretion of cytokines by innate immune cells is thought to initiate multiple organ failure in murine models of sepsis. Whether human cytokine storm also plays a similar role is not clear. Here, we show that human hematopoietic cells are required to induce sepsis-induced mortality following cecal ligation and puncture (CLP) in the severely immunodeficient nonobese diabetic (NOD)/SCID/IL2Rγ−/− mice, and siRNA treatment to inhibit HMGB1 release by human macrophages and dendritic cells dramatically reduces sepsis-induced mortality. Following CLP, compared with immunocompetent WT mice, NOD/SCID/IL2Rγ−/− mice did not show high levels of serum HMGB1 or murine proinflammatory cytokines and were relatively resistant to sepsis-induced mortality. In contrast, NOD/SCID/IL2Rγ−/− mice transplanted with human hematopoietic stem cells [humanized bone marrow liver thymic mice (BLT) mice] showed high serum levels of HMGB1, as well as multiple human but not murine proinflammatory cytokines, and died uniformly, suggesting human cytokines are sufficient to induce organ failure in this model. Moreover, targeted delivery of HMGB1 siRNA to human macrophages and dendritic cells using a short acetylcholine receptor (AchR)-binding peptide [rabies virus glycoprotein (RVG)-9R] effectively suppressed secretion of HMGB1, reduced the human cytokine storm, human lymphocyte apoptosis, and rescued humanized mice from CLP-induced mortality. siRNA treatment was also effective when started after the appearance of sepsis symptoms. These results show that CLP in humanized mice provides a model to study human sepsis, HMGB1 siRNA might provide a treatment strategy for human sepsis, and RVG-9R provides a tool to deliver siRNA to human macrophages and dendritic cells that could potentially be used to suppress a variety of human inflammatory diseases.
Sepsis is an important cause of mortality in intensive-care units, with more than 750,000 individuals developing severe sepsis in North America annually and a mortality rate varying between 35 and 50% (1, 2). The pathogenesis of sepsis includes countless disturbances of the host immune system starting with a harmful, infection-triggered exaggerated inflammatory cascade that results in tissue injury and rapidly leads to massive apoptosis of immune cells (2, 3). This is followed by a secondary immune paralysis phase accompanied by uncontrolled growth of bacteria and tissue damage. Although therapy to suppress the immediate cytokine response, such as treatment with TNF and IL-1β antibodies, have failed in clinical trials (4–6), it has now come to be recognized that, at least in animal models, high-mobility group protein 1 (HMGB1), which is secreted from macrophages and dendritic cells (DCs) but not lymphocytes late in the disease, acts as a master regulator of late and sustained cytokine storm, up-regulating many cytokines including TNF-α, IL-6, IL-1β, and IL-8 (reviewed in refs. 7–11). In fact, injection of mice with HMGB1 is enough to induce the lethal organ damage seen in sepsis (12), whereas treatment with neutralizing HMGB1 antibody can rescue mice and rats from experimental sepsis (13, 14). However, although HMGB1 is also secreted in human sepsis (12), its role in sepsis pathogenesis or the impact of its neutralization on human cells remain unclear.
RNA interference can be used to silence virtually any gene, including multiple genes, as long as a way can be found to introduce small interfering (si)RNAs into relevant cell types in vivo without toxicity. Several advances have been made in developing methods to deliver siRNA in vivo to different cell types, most successfully to the liver cells (reviewed in refs. 15–17). A lipid-like nanoparticle called C12-200, which had been developed for liver-specific delivery of siRNA, was recently also shown to deliver siRNA to murine monocytes, and silencing C-C chemokine receptor type 2 (CCR2) in monocytes using this reagent was effective in reducing atherosclerosis, islet transplantation and tumors (18). Whether this reagent also targets human DCs and monocytes/macrophages is unclear. We have reported previously that a short 29-aa peptide derived from the rabies virus glycoprotein (RVG), fused to 9R residues (RVG-9R), can deliver siRNA to murine macrophages and brain cells by specific binding to its ligand acetylcholine receptor (AchR) (19, 20). Because AchR is also expressed on human macrophages and DCs (21) and also because the acetylcholine-binding site on the α7 subunit is highly conserved, we reasoned that RVG-9R might also be used to target human macrophages and DCs. In this study, we validate this hypothesis in vitro, as well as in vivo, using human hematopoietic stem cell–engrafted nonobese diabetic NOD/SCID/IL2Rγ−/− mice that lack mouse innate and adaptive immune systems (22). More importantly, we also show that silencing human HMGB1 using this delivery reagent in this mouse model substantially reduces human lymphocyte apoptosis and cytokine storm and protects mice from sepsis-induced mortality.
Results
Human Cytokines Are Necessary to Induce Sepsis Following CLP in Humanized Mice.
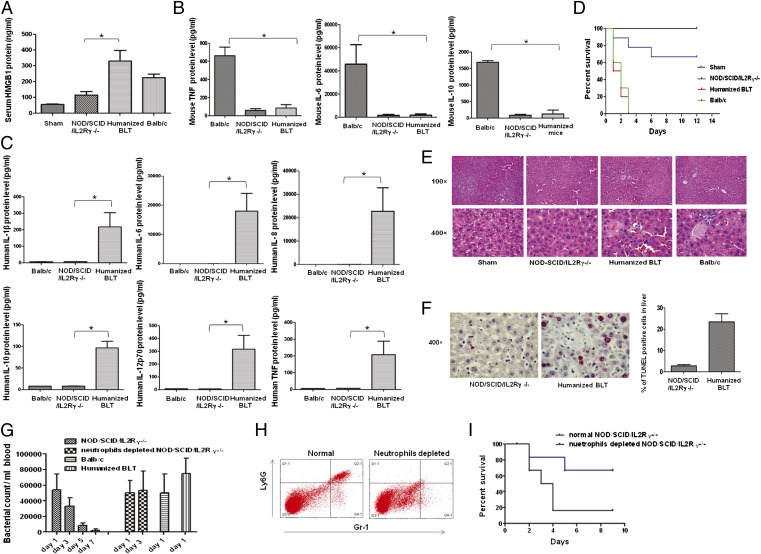
NOD/SCID/IL2Rγ−/− mice lack a functional immune system and are also defective in multiple cytokine signaling (22). However, whether the residual defective monocytes/macrophages secrete murine cytokines that could cause tissue injury during sepsis is not known. If they are incapable of secreting murine cytokines, then the only source of cytokines in NOD/SCID/IL2Rγ−/− mice transplanted with human hematopoietic stem cells (HSCs) would be human cells and this would allow us to test the impact of human cytokines in sepsis. Therefore, we compared immunocompetent WT BALB/c mice, immunodeficient NOD/SCID/IL2Rγ−/− mice, and NOD/SCID/IL2Rγ−/− mice transplanted with human HSCs [humanized bone marrow liver thymic mice (BLT) mice] for sepsis induction following CLP. Twenty hours after CLP, blood was tested for the key late inflammatory mediator HMGB1, as well as other downstream mouse and human proinflammatory cytokines, and the mice were followed for survival. Whereas high levels of HMGB1 (Fig. 1A) and murine cytokines (Fig. 1B) could be detected in the sera of WT mice, NOD/SCID/IL2Rγ−/− mice showed minimal levels of these cytokines. In contrast, humanized BLT mice showed elevated levels of human HMGB1 and multiple human, but not murine cytokines (Fig. 1 A–C). Correspondingly, both WT and humanized mice died quickly, with all mice dying within 3 d after CLP, whereas ∼70% of NOD/SCID/IL2Rγ−/− mice survived symptom-free for up to a 12-d period of observation, and in the few mice that died, the death was delayed by 2–4 d (Fig. 1D). Histological examination of liver and lungs showed evidence of disseminated intravascular coagulation with marked congestion and fibrin thrombi in the blood vessels, focal vacuolar changes, chromatin disruption, and spotty apoptosis following CLP in BALB/c and humanized BLT mice but not in NOD/SCID/IL2Rγ−/− mice (Fig. 1E). We also performed TUNEL staining of liver sections to detect apoptotic cells. Whereas high levels of TUNEL+ cells were detected in humanized mice, TUNEL+ cells were substantially reduced in NOD/SCID/IL2Rγ−/− mice (Fig. 1F). Taken together, these results suggest that NOD/SCID/IL2Rγ−/− mice are resistant to CLP-induced sepsis and that human cytokines are required to induce organ damage and mortality in humanized mice. These results are also consistent with an earlier report that showed that elevated levels of human cytokines are seen in humanized mice after CLP (23).
Fig. 1.
Human hematopoietic cells are required to induce sepsis in NOD/SCID/IL2Rγ−/− mice. Mice underwent sham or CLP surgery and 24 h post-CLP, sera were tested for HMGB1 by ELISA (A), murine proinflammatory cytokines were tested by cytometric bead array (B), and human proinflammatory cytokines were tested by cytometric bead array (C). Mean ± SD from seven mice per group is shown. (D) Kaplan–Meier survival curves for mice following CLP are shown (n = 14 per group). (E) Representative hematoxylin and eosin (H&E)-stained sections from the liver taken 24 h after CLP from the indicated group of mice are shown. (F) Representative TUNEL-stained liver section (Left) and cumulative percentage of TUNEL+ cells determined by examining 20 high-powered fields from three mice in each group (Right) are shown. (G) Bacterial counts in the blood of indicated groups of mice on indicated days after CLP is shown (n = 5–7 per group). BALB/c, BLT, and neutrophil-depleted NOD/SCID/IL2Rγ−/− mice could not be serially followed because all mice died by day 2–3. (H) Neutrophil depletion was tested in blood 3 d after administration of Ly6G-specific mAb by staining for Ly6G and GR-1. (I) Kaplan–Meier survival curves for NOD/SCID/IL2Rγ−/− mice with intact or depleted neutrophils is shown (n = 5 per group). *P < 0.05.
It was surprising that immunodeficient mice, compared with WT and humanized mice, were resistant to CLP-induced sepsis. Thus, we investigated the mechanism by which NOD/SCID/IL2Rγ−/− mice resist CLP-induced mortality. First, we compared the blood bacterial load in WT, NOD/SCID/IL2Rγ−/−, and humanized mice. Although all three groups of mice had equivalent bacterial titres 1 d after CLP, in NOD/SCID/IL2Rγ−/− mice, bacterial load steadily declined and was undetectable between days 7–9 (Fig. 1G). WT and humanized mice could not be serially followed because they all died by day 3. Although NOD/SCID/IL2Rγ−/− mice lack immune cells, they have intact neutrophils, and, thus, we tested whether neutrophil depletion would render them susceptible to sepsis. We depleted neutrophils (Fig. 1H) by treatment with Ly6G-specific mAb, clone 1A8 [which is known to specifically deplete neutrophils but not monocytes/macrophages (24)] and then induced CLP. Indeed, in neutrophil-depleted mice, bacterial count did not decrease by day 3, and most of the mice died between days 3–4, probably because of overwhelming bacterial burden (Fig. 1I). Collectively, these results suggest that neutrophils can effectively control sepsis-induced bacteremia in the immunodeficient NOD/SCID/IL2Rγ−/− mice that lack macrophages and DCs. Therefore, the acute death observed in WT and humanized mice is most likely attributable to the cytokine storm induced by innate immune cells, such as macrophages and DCs. These results imply that if the aberrant cytokine response can be neutralized, sepsis-induced mortality can be effectively reduced.
RVG-9R Delivers siRNA to Primary Human Macrophages and Dendritic Cells in Vitro and in Vivo.
HMGB1 secreted from macrophages and DCs after induction of sepsis in animal models acts as a master regulator of late and sustained cytokine storm, up-regulating many cytokines, including TNFα, IL-6, IL-1β, and IL-8 (reviewed in refs. 10, 11). However, although HMGB1 is also secreted in human sepsis (12), its role in sepsis pathogenesis or the impact of its neutralization on human cells remains unclear. Thus, to clarify the role of HMGB1 in human sepsis, we attempted to silence HMGB1 in human macrophages and DCs in vivo. For this, first we needed to develop a reagent that enables siRNA delivery to human macrophages and DCs in vivo.
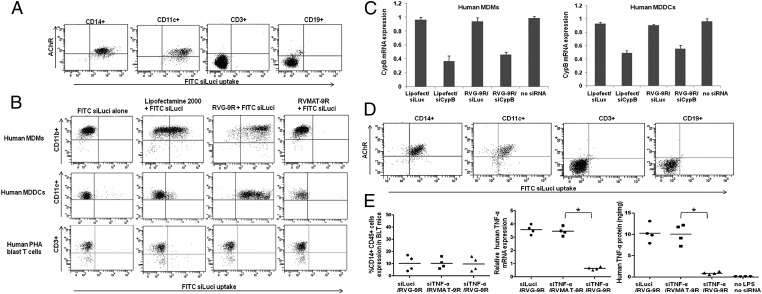
We reported previously that a short 29-aa peptide derived from the RVG, fused to 9R residues (RVG-9R), can deliver siRNA to murine macrophages and neuronal cells by specific binding to its ligand, AchR (19, 20). Because AchR is also expressed in human macrophages and DCs (21), we first tested whether RVG-9R can deliver siRNA to human macrophages and DCs. Freshly isolated peripheral blood mononuclear cells (PBMCs) were incubated with a FITC-labeled siRNA complexed with RVG-9R, and after 4 h, the cells were stained with AchR antibody and lineage-specific markers. Human CD14+ monocyte/macrophages and CD11c+ DCs, but not T cells or B cells, expressed AchR, and, correspondingly, FITC siRNA uptake was seen only in these cell types (Fig. 2A). Next, we tested whether RVG-9R can also deliver siRNA to cultured human monocyte-derived macrophages (MDMs) and monocyte-derived DCs (MDDCs). Human PBMCs were cultured with macrophage colony-stimulating factor (M-CSF) or granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 for 6 d, followed by treatment with FITC siRNA complexed with RVG-9R or a control peptide derived from the rabies virus matrix protein (RV-MAT-9R). Both MDMs and MDDCs, but not phytohaemagglutinin (PHA)-activated T-cell blasts, were amenable for transduction with siRNA selectively by RVG-9R but not the control peptide RV-MAT-9R (Fig. 2B). Next, we tested gene silencing using siRNA to cyclophilin B (siCyPB). RVG-9R–mediated siCyPB delivery resulted in specific gene silencing, as revealed by quantitative (q)RT-PCR in both MDMs and MDDCs (Fig. 2C). We also tested whether macrophages and DCs generated by transplantation of human HSCs in NOD/SCID/IL2Rγ−/− mice also retain AchR expression, and can be transduced by RVG-9R. Both macrophages and DCs from ex vivo–isolated spleen cells could be transduced by treatment with siRNA/RVG-9R (Fig. 2D). Collectively, these results suggest that RVG-9R delivers siRNA to primary, as well as to cultured, human macrophages and DCs in vitro.
Fig. 2.
RVG-9R delivers siRNA to primary human macrophages and DCs. (A) Ex vivo–isolated human PBMCs were incubated with FITC siLuci complexed with RVG-9R for 4 h before staining with antibodies to AchR, CD14, CD11c, CD3, and CD19. CD14-, CD11c-, CD3-, and CD19-gated cell populations were examined for FITC uptake by flow cytometry. The experiment was repeated two times with similar results. (B) In vitro–cultured human macrophages, DCs, and T-cell blasts were treated with FITC siLuci alone, transfected with FITC siLuci using Lipofectamine 2000 or transduced with FITC siLuci complexed with RVG-9R or a control RV-MAT-9R peptide, and cells were examined for FITC siLuci uptake by flow cytometry. The experiment was repeated two times with similar results. (C) Cultured macrophages and DCs were transfected/transduced as in B with siRNA targeting cyclophilin B and, after 24 h, tested for cyclophilin B mRNA levels by qRT-PCR. Mean ± SD of triplicates is shown. (D) Splenocytes from humanized mice were examined for FITC siRNA transduction as in A. (E) BLT mice were injected (i.v.) with either control Luci siRNA complexed with RVG-9R or TNF-α siRNA complexed with RVG-9R or a control RV-MAT-9R peptide 18 and 6 h before LPS injection (i.p.). One hour after LPS injection, human TNF-α mRNA levels in PBMCs were tested by qRT-PCR, and serum human TNF-α protein levels were tested by ELISA (Center and Right). Human CD14+ cells in PBMCs of the three groups of mice before the start of the experiment are shown in the left panel. Each symbol represents an individual mouse. *P < 0.05.
To test whether RVG-9R can be used for in vivo delivery of siRNA to human macrophages and DCs, we injected humanized mice with siRNA specific for human TNF-α or a control luciferase (Luci), complexed with RVG-9R, i.v. 18 and 6 h before injection of (5 mg/kg) of lipopolysaccharide (LPS) intraperitoneally. One hour after LPS treatment, PBMCs were tested for TNF-α mRNA levels by qRT-PCR, and sera were tested for human TNF-α protein levels by ELISA. Both TNF-α mRNA and protein levels were significantly reduced in mice treated with RVG-9R/siTNF compared with those treated with RVG-9R/siLuci or siTNF/RV-MAT-9R (Fig. 2E). These results confirmed that RVG-9R could deliver siRNA to human macrophages and DCs in vivo.
RVG-9R–Mediated HMGB1 siRNA Delivery Ameliorates Sepsis in Humanized Mice.
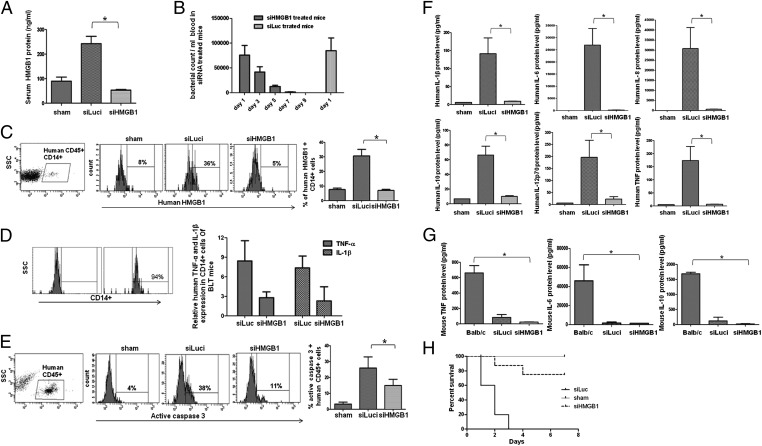
As mentioned above, although HMGB1 levels are raised in human sepsis (12), whether it is directly involved in the pathogenesis of human sepsis remains unclear. Therefore, having established that RVG-9R can deliver siRNA to human macrophages and DCs, we wished to test the effect of silencing HMGB1 in the CLP model of sepsis in humanized mice. Before this, we first tested if human macrophages and/or DCs secrete HMGB1. Cultured macrophages and DCs stimulated with LPS, but not T cells, secreted HMGB1 in equivalent amounts peaking at 12 h after activation and slowly declining over 36 h (Fig. S1). We also confirmed that HMGB1 could be suppressed by siRNA treatment effective as early as 12 h (Fig. S1). Next, we tested whether siRNA treatment would suppress CLP in humanized mice. Because variability in human immune cell reconstitution can affect results, we selected humanized mice that had similar levels of different human immune cell types for these experiments (Fig. S2). Moreover, we included a large number of animals in control and siRNA treatment group (14 mice per group) for survival analysis to rule out differences in reconstitution as a possible reason for our observation. Because the needle size/puncture site and numbers can influence the outcome of CLP, these parameters were kept identical between the different groups of mice (see SI Materials and Methods for details). Mice were injected i.v. with siRNAs specific to human HMGB1 or control Luci, complexed with RVG-9R, 15 h before CLP, with sham CLP animals serving as controls. The siRNA treatment was repeated 2 and 24 h after surgery, and mice were followed up for CLP-induced bacteremia, increase in human HMGB1 levels, human lymphocyte apoptosis, serum human cytokine levels, and illness. Compared with sham CLP control animals, serum HMGB1 levels in CLP animals treated with Luci siRNA were greatly elevated 20 h after surgery. In striking contrast, in HMGB1 siRNA–treated CLP animals, serum HMGB1 levels were dramatically reduced (Fig. 3A). Although the blood bacterial load was similar in the control Luci siRNA and HMGB1 siRNA–treated mice on day 1, the bacterial load steadily declined over time and was undetectable by day 9 in the HMGB1 siRNA–treated mice (Fig. 3B). Moreover, HMGB1 levels within human CD14+ cells in the spleen were also reduced, as revealed by flow-cytometric analysis (Fig. 3C). Concomitant with reduction of HMGB1, the mRNA levels of other cytokines such as TNF-α and IL-1β determined by qRT-PCR were also reduced in CD14+ macrophages isolated from HMGB1siRNA–treated mice compared with siLuc-treated mice (Fig. 3D). Apoptosis of human lymphocytes was tested by activated caspase-3 staining of PBMCs gated for human CD45 expression 20 h after surgery. As shown in Fig. 3E, a significant number of human lymphocytes were active caspase-3+ in Luci siRNA treated animals, whereas this was significantly reduced in HMGB1 siRNA–treated animals. Moreover, the reduced levels of caspase-3+ cells seen on day 1 became undetectable in HMGB1 siRNA–treated mice 8 d after surgery (Fig. S3). Because RVG-9R delivers siRNA to macrophages and DCs, but not T cells or B cells (Fig. 2), we interpret these results to mean that silencing HMGB1 in macrophages and DCs and resultant reduced serum levels of HMGB1 led to protection against lymphocyte apoptosis. We also tested for human cytokine levels in the serum 20 h after surgery by BD cytometric bead array. Again, as shown in Fig. 3F, the cytokine levels, including those of human TNFα, IL1β, IL-8, IL-10, IL-12p70, and IL-6, were all highly elevated in control siRNA–treated mice but greatly reduced in HMGB1 siRNA–treated mice. We also confirmed that mouse cytokines were not up-regulated in either group of mice (Fig. 3G). Finally, we tested the survival of siRNA treated mice. Mice treated with the control Luci siRNA after CLP all died by day 3 after surgery. In contrast, 60% of HMGB1 siRNA–treated mice were completely protected and lived symptom-free for at least an 8-d period of observation (Fig. 3H). Upon TUNEL staining of liver sections, whereas high numbers of TUNEL+ cells were seen in control siRNA–treated mice, apoptotic cells were greatly reduced in the livers of siRNA-treated mice (Fig. S4). We also confirmed that RVG-9R/HMGB1 siRNA treatment does not induce IFN or IFN-stimulated genes in human PBMCs (Fig. S5). Taken together, our results show that silencing HMGB1 in human macrophages and DCs effectively reduces serum HMGB1 levels, leading to diminished cytokine production, reduced lymphocyte apoptosis, and improved survival of humanized mice after CLP.
Fig. 3.
Treatment of humanized mice with HMGB1 siRNA/RVG-9R suppresses CLP-induced sepsis. Humanized mice were treated i.v. with either a control Luci siRNA or HMGB1 siRNA complexed with RVG-9R, as detailed in SI Materials and Methods, before and after CLP. Sham indicates sham surgery without CLP or siRNA treatment. (A) Sera obtained 20 h post-CLP were tested for HMGB1 protein levels by ELISA. Mean ± SD from seven mice per group is shown. (B) Bacterial counts in the blood of control and siRNA treated mice at indicated times after CLP are shown. Control mice could not be serially followed because they all died by day 3. (C) Twenty hours post-CLP, splenocytes were tested for HMGB1 expression in human CD45+, CD14+ gated cells by flow cytometry. (Left and Right) Representative flow-cytometric results (Left) and cumulative data from seven mice per group ± SD (Right) are shown. (D) CD14+ cells isolated from control and siRNA-treated mice were tested for TNF-α and IL-1β levels by qRT-PCR (n = 5 per group). (Left) Purity of isolated cells. (E) PBMCs obtained 20 h post-CLP were tested for active caspase-3+ cells within human CD45+ cells by flow cytometry. (Left and Right) Representative flow-cytometric result (Left) and cumulative data from seven mice per group (Right) are shown. (F) Sera obtained 20 h post-CLP were tested for indicated human cytokines by cytometric bead array. Mean ± SD from seven mice per group is shown. (G) Sera obtained 20 h post-CLP were tested for indicated murine cytokines by cytometric bead array. Mean ± SD from seven mice per group is shown. (H) Kaplan–Meier survival curves for mice following CLP is shown (n = 14 per group).
Silencing HMGB1 After the Development of Symptoms Can Reverse Sepsis in Humanized Mice.
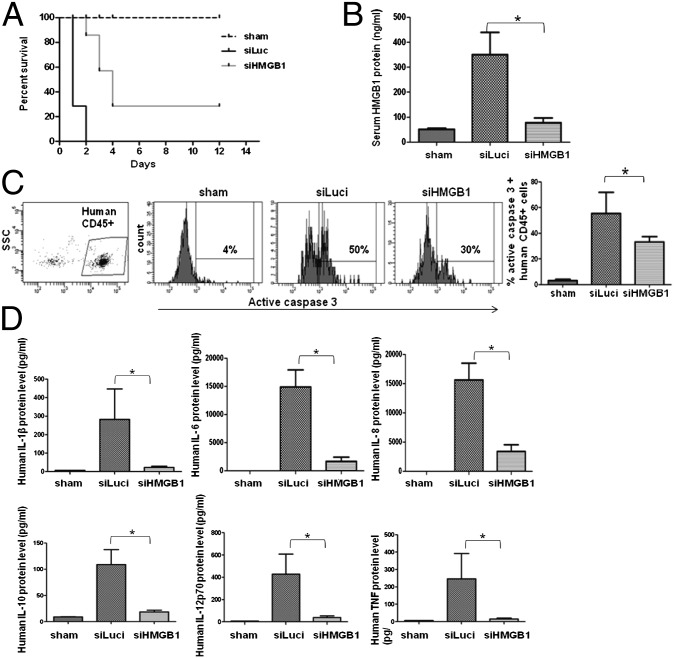
To be therapeutically useful, siRNA treatment must be effective when administered after the onset of symptoms. Thus, we tested whether siRNA treatment is also effective when begun after the development of CLP-induced sepsis symptoms. CLP in humanized mice causes a hyperacute illness, and 10 h after CLP, 2 of 16 mice had already died from the disease, and all mice showed signs and symptoms of sepsis (e.g., ruffling of hair, hunchback, and lethargy). Thus, we began siRNA/RVG-9R treatment beginning 10 h after CLP and repeated the siRNA treatment after another 24 and 48 h. Compared with control siRNA treatment where all animals died between 16–36 h after CLP, treatment with HMGB1 siRNA was able to rescue ∼30% mice from lethality and delay the onset of death in others by 1–2 d (Fig. 4A). Correspondingly, serum HMGB1 levels rapidly reduced following siRNA treatment (Fig. 4B). Human lymphocyte apoptosis was also reversed, accompanied by reduced serum cytokine levels (Fig. 4 C and D). Thus, HMGB1 siRNA treatment can reverse the disease severity even when administered after the onset of symptoms.
Fig. 4.
Post-CLP treatment with HMGB1 siRNA/RVG-9R also affords protection. (A) CLP was performed, and siRNA treatment, as detailed in the legend of Fig. 3, was started 10 h later, and mice were followed for survival. (B–D) Twenty-four hours after CLP, mice were tested for serum HMGB1 levels (B), active caspase-3 positivity in PBMCs (C), and serum human cytokine levels (D), as described in the legend of Fig. 3 (n = 7 mice per group).
Discussion
Here, we showed that HMGB1 plays a critical role in inducing sepsis in humanized mice, most likely by inducing a human cytokine storm that is not seen in nonhumanized counterparts. We also showed that RVG-9R provides a reagent to deliver siRNA to human macrophages and DCs, and RVG-9R–mediated HMGB1 siRNA treatment effectively rescued mice from sepsis-induced mortality. It is, thus, likely that neutralizing HMGB1 has potential to be therapeutically beneficial in human sepsis.
Recently, several reagents have been developed to deliver siRNA to murine macrophages. d-glucan–encapsulated siRNA particles were reported as efficient oral-delivery vehicles that silenced genes in mouse macrophages in vitro and in vivo (25). An ionizable cationic lipid called DLinKC2DMA, which was originally shown to deliver siRNA to liver cells (26), was also reported to deliver siRNA to mouse macrophages and DCs (27). Similarly, a lipidoid nanoparticle called C12-200, also developed originally for liver-specific siRNA delivery (28), was recently shown to target monocytes, and silencing CCR2 with this reagent was shown to reduce a number of inflammatory conditions in mice (18). siRNAs synthetically linked to C-phosphate-G (CpG) oligonucleotide has also been used to deliver siRNA to toll-like receptor (TLR)-9+ murine macrophages and B cells (29). However, the utility of any of these reagents to specifically silence genes in human macrophages and DCs in vitro or in vivo remains to be determined. On the other hand, our study addresses siRNA delivery to human macrophages and DCs in vivo. Also, compared with the complexity of generating the previously mentioned reagents, RVG-9R provides a simple peptide that can be readily synthesized and siRNA binding is achieved by simple mixing of siRNA with the peptide. Moreover, RVG-9R is nontoxic and nonimmunogenic, and peptide binding protects siRNA against serum nuclease cleavage (19), thus enabling the use of regular (not stabilized) siRNA that would make siRNA therapy relatively inexpensive.
Antibodies to suppress inflammatory mediators such as TNF-α and IL-1β have failed in clinical trails for sepsis (4–6). However, from studies in animal models of sepsis, it has now become clear that HMGB1 secreted from macrophages and DCs acts as a key cytokine to stimulate a sustained inflammation and organ damage (10, 11). Serum HMGB1 is also elevated in human sepsis (12), although its role in sepsis pathogenesis remained unclear. Our results suggest that HMGB1 may also play a major role in human sepsis, in that silencing HMGB1 was enough to suppress both the cytokine storm and lymphocyte apoptosis, resulting in reduced mortality in the humanized mice. However, it must be noted that although human hematopoietic system is reconstituted in humanized mice, the other key organs, particularly endothelial cells, are of mouse origin, and, thus, this model does not exactly represent human sepsis. Even so, our results at least indicate that humanized mice provide a model closest to human sepsis, RVG-9R can deliver siRNA to human cells in vivo, and silencing HMGB1 might be useful to reduce sepsis. Although our results strongly suggest that silencing HMGB1, by suppressing the secreted HMGB1 and the concomitant human cytokine storm, reduces CLP-induced mortality, it remains possible that silencing HMGB1 might also suppress some unknown events in the initiation of sepsis, and reduction in cytokines may be just an associated event. Another possibility is that silencing HMGB1 may kill or render macrophages and DCs functionally anergic, thereby reducing the cytokine storm. However, macrophages and DCs remained viable after HMGB1 silencing in vivo (Fig. 3C and Figs. S1 and S3), making this possibility unlikely.
It has been reported that cholinergic agonists can suppress HMGB1 release. Administration of nicotine was enough to suppress endotoxemia in mice, presumably by inhibition of NF-κB pathway via signaling through AchR (30). However, mere RVG binding to AchR appears not to be sufficient for suppression of HMGB1 in our experiments because the control Luci siRNA was also complexed to RVG-9R and yet failed to suppress HMGB1 or reduce mortality (Fig. 3). However, to definitively rule out RVG binding to AchR as a plausible reason for the protection observed in the siRNA-treated animals, we directly compared treatment with RVG peptide alone versus RVG-9R/siRNA. RVG treatment by itself did not show any protection in contrast to RVG-9R siRNA treatment (Fig. S6). These results also rule out the possibility that RVG binding to neuronal cells could activate nonspecific vagal cholinergic anti-inflammatory reflex, accounting for our results. Moreover, we also confirmed the physical presence of HMGB1 siRNA in splenic macrophages from siRNA-treated mice by RT-PCR (Fig. S7). Thus, RVG-9R–mediated siRNA delivery to macrophages and DCs appears to be needed to efficiently silence HMGB1 levels and to reduce mortality in this model.
Collectively, our results show that sepsis in the humanized mice is dependent on HMGB1 and, likely, its ability to induce human cytokine storm, because silencing HMGB1 effectively rescued mice from mortality. Our results also suggest that RVG-9R may provide a clinically viable tool for gene silencing in human macrophages and DCs that could be useful in the treatment of sepsis and other inflammatory conditions.
Materials and Methods
Details of peptides, siRNAs, flow cytometry, neutrophil depletion, and qPCR are provided in SI Materials and Methods. Humanized BLT mice were generated as described previously (31). CLP was performed as described in ref. 32. For all siRNA delivery experiments in vivo, peptide (RVG-9R or RVGMAT-9R)/siRNA complexes were prepared in 200 µL of 5% (wt/vol) glucose, incubated at RT for 10 min, and then injected i.v. at 50 µg of siRNA per mouse per injection.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases Grant U01AI075419 (to M.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.J.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216195109/-/DCSupplemental.
References
- 1.Antonopoulou A, Giamarellos-Bourboulis EJ. Immunomodulation in sepsis: State of the art and future perspective. Immunotherapy. 2011;3(1):117–128. doi: 10.2217/imt.10.82. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Skirecki T, Borkowska-Zielinska U, Zlotorowicz M, Hoser G. Sepsis immunopathology: Perspectives of monitoring and modulation of the immune disturbances. Arch Immunol Ther Exp (Warsz) 2012;60(2):123–135. doi: 10.1007/s00005-012-0166-1. [DOI] [PubMed] [Google Scholar]
- 4.Abraham E, et al. Lenercept Study Group Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: A randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med. 2001;29(3):503–510. doi: 10.1097/00003246-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Abraham E, et al. NORASEPT II Study Group Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet. 1998;351(9107):929–933. [PubMed] [Google Scholar]
- 6.Fisher CJ, Jr, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271(23):1836–1843. [PubMed] [Google Scholar]
- 7.Yang H, Wang H, Tracey KJ. HMG-1 rediscovered as a cytokine. Shock. 2001;15(4):247–253. doi: 10.1097/00024382-200115040-00001. [DOI] [PubMed] [Google Scholar]
- 8.Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34(6):1503–1512. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- 9.Fink MP. Bench-to-bedside review: High-mobility group box 1 and critical illness. Crit Care. 2007;11(5):229. doi: 10.1186/cc6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai B, Deitch EA, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators Inflamm. 2010;2010:642462. doi: 10.1155/2010/642462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51(2):119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 13.Suda K, et al. Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg. 2006;30(9):1755–1762. doi: 10.1007/s00268-005-0369-2. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peer D, Lieberman J. Special delivery: Targeted therapy with small RNAs. Gene Ther. 2011;18(12):1127–1133. doi: 10.1038/gt.2011.56. [DOI] [PubMed] [Google Scholar]
- 16.Manjunath N, Dykxhoorn DM. Advances in synthetic siRNA delivery. Discov Med. 2010;9(48):418–430. [PubMed] [Google Scholar]
- 17.Novobrantseva TI, Akinc A, Borodovsky A, de Fougerolles A. Delivering silence: Advancements in developing siRNA therapeutics. Curr Opin Drug Discov Devel. 2008;11(2):217–224. [PubMed] [Google Scholar]
- 18.Leuschner F, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29(11):1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 20.Kim SS, et al. Targeted delivery of siRNA to macrophages for anti-inflammatory treatment. Mol Ther. 2010;18(5):993–1001. doi: 10.1038/mt.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol Med. 2003;9(5-8):125–134. [PMC free article] [PubMed] [Google Scholar]
- 22.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 23.Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J Leukoc Biol. 2009;86(2):219–227. doi: 10.1189/jlb.1008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 25.Aouadi M, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458(7242):1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semple SC, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 27.Basha G, et al. Influence of cationic lipid composition on gene silencing properties of lipid nanoparticle formulations of siRNA in antigen-presenting cells. Mol Ther. 2011;19(12):2186–2200. doi: 10.1038/mt.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Love KT, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107(5):1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kortylewski M, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27(10):925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 31.Melkus MW, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 32.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.