Abstract
The firing of mesolimbic dopamine neurons is important for drug-induced reinforcement, although underlying genetic factors remain poorly understood. In a recent genome-wide association metaanalysis of alcohol intake, we identified a suggestive association of SNP rs26907 in the ras-specific guanine-nucleotide releasing factor 2 (RASGRF2) gene, encoding a protein that mediates Ca2+-dependent activation of the ERK pathway. We performed functional characterization of this gene in relation to alcohol-related phenotypes and mesolimbic dopamine function in both mice and adolescent humans. Ethanol intake and preference were decreased in Rasgrf2−/− mice relative to WT controls. Accordingly, ethanol-induced dopamine release in the ventral striatum was blunted in Rasgrf2−/− mice. Recording of dopamine neurons in the ventral tegmental area revealed reduced excitability in the absence of Ras-GRF2, likely because of lack of inhibition of the IA potassium current by ERK. This deficit provided an explanation for the altered dopamine release, presumably linked to impaired activation of dopamine neurons firing. Functional neuroimaging analysis of a monetary incentive–delay task in 663 adolescent boys revealed significant association of ventral striatal activity during reward anticipation with a RASGRF2 haplotype containing rs26907, the SNP associated with alcohol intake in our previous metaanalysis. This finding suggests a link between the RASGRF2 haplotype and reward sensitivity, a known risk factor for alcohol and drug addiction. Indeed, follow-up of these same boys at age 16 y revealed an association between this haplotype and number of drinking episodes. Together, these combined animal and human data indicate a role for RASGRF2 in the regulation of mesolimbic dopamine neuron activity, reward response, and alcohol use and abuse.
Keywords: IA current, neuroimaging genetic reward-anticipation preference
The reinforcing properties of addictive drugs are dependent on the activity of mesolimbic dopamine (DA) neurons in the ventral tegmental area (VTA) and their projections to the ventral striatum (VS) and prefrontal cortex (PFC) (1). Microdialysis studies using rodent models have shown that acute administration of addictive drugs, including alcohol, results in elevated DA levels in the VS (2). This effect results from local inhibition of DA reuptake, stimulation of its release, or an increase in firing rate of DA neurons in the VTA (3–5). PET studies have shown a similar effect in the VS of humans as evidenced by decreased competitive binding of a DA receptor antagonist [11C]raclopride (6, 7). Illustrating the importance of DA signaling in the regulation of alcohol-induced reinforcement, rats are known to self-administer ethanol in the nucleus accumbens (8) and posterior VTA (9). These studies and other animal studies suggest that midbrain DA neurons are involved in the acquisition of primary alcohol reinforcement (review in ref. 1).
Although the neurobiological and molecular mechanisms controlling DA neuron activity by different drugs are well-established (4), the genetic factors underlying DA neuron firing and responses to addictive drugs in the VTA remain poorly understood. In a recent genome-wide association (GWAS) metaanalysis of alcohol intake, we identified in males a suggestive signal in the ras-specific guanine-nucleotide releasing factor 2 (RASGRF2) gene (10). Ras-GRF2 belongs to a family of calcium/calmodulin-regulated guanine-nucleotide exchange factors that transduces signals from ion channel receptors (11, 12). Ras-GRF2 is an upstream effector of MAPK signaling cascades, including the ERK pathway (12–15). Ethanol administration increases ERK activity in the nucleus accumbens (part of the VS) of rats through a D1 receptor-mediated mechanism (16, 17), and inhibition of ERK activity in mice has been shown to influence ethanol self-administration (18). Together, these findings suggest that the ERK pathway mediates drug-induced reinforcement processes in the mesolimbic system (13).
We, therefore, hypothesized that Ras-GRF2 might influence alcohol drinking behavior by regulating alcohol-induced reinforcement. To test this hypothesis and elucidate the mechanisms of Ras-GRF2 action, we carried out an in-depth functional characterization of the role of Ras-GRF2 in alcohol-related behavior and DA neuron function using animal models, including Rasgrf2−/− mice, as well as a large functional neuroimaging genetics dataset of adolescent boys from the IMAGEN study (19).
Results
Alcohol Preference Is Associated with Rasgrf2 mRNA Expression in Mice.
We first investigated the association of Ras-GRF2 with alcohol preference by comparing Rasgrf2 mRNA whole-brain expression levels between high alcohol-preferring (HAP1) and low alcohol-preferring (LAP1) mouse lines (20). We found a highly significant increase in Rasgrf2 mRNA expression in HAP relative to LAP mice (P = 0.0027) (Table 1). This finding suggests that increased Rasgrf2 gene expression might contribute to alcohol-induced reinforcement.
Table 1.
Increased Rasgrf2 mRNA expression in whole-brain extracts from HAP compared with LAP mice
| Rasgrf2 mRNA expression | |
| HAP1 (n = 6) | 8.092 (0.037)* |
| LAP1 (n = 6) | 7.729 (0.084) |
Mean (SEM) log2-transformed Rasgrf2 mRNA expression values.
*P = 0.0027.
Decreased Ethanol Consumption in Rasgrf2−/− Mice.
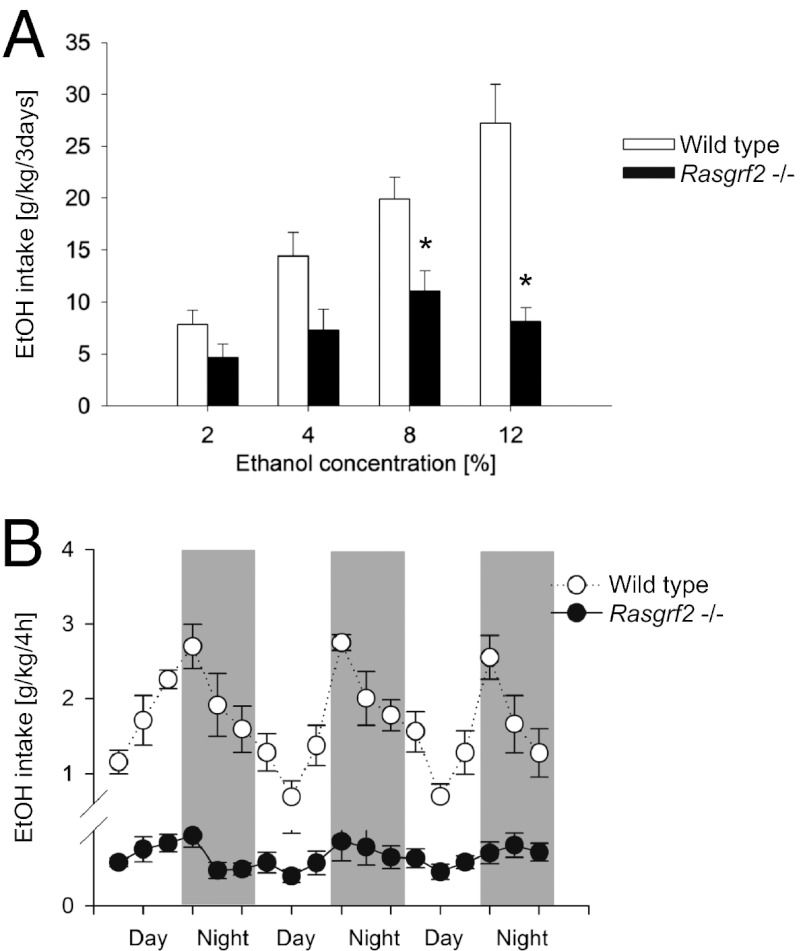
We compared Rasgrf2−/− (21) and WT mice in a two-bottle (ethanol-containing solution vs. water) free choice paradigm with increasing concentrations of ethanol. Rasgrf2−/− mice showed a significant reduction in ethanol intake relative to littermate controls (P < 0.01), with a threefold lower intake at the highest ethanol concentrations (Fig. 1A) and blunted diurnal drinking (Fig. 1B). Rasgrf2−/− mice also showed a significant reduction in ethanol preference, which was measured by the percentage of ethanol intake relative to water intake (Fig. S1). To rule out alternative explanations for changes in alcohol drinking behavior, we assessed if taste sensation and alcohol metabolism were altered in Rasgrf2−/− mice. There was no significant difference in taste sensitivity (for either sweet or bitter solutions) (Fig. S2) or blood ethanol elimination (Fig. S3) in Rasgrf2−/− mice and WT controls. Furthermore, there was no significant difference in the loss of righting reflex (Fig. S4), indicating that sensitivity to the effects of ethanol was not altered in mutant mice.
Fig. 1.
Ethanol intake is reduced in Rasgrf2−/− mice relative to WT controls. (A) Mean (+ SEM) intake of solutions containing increasing concentrations of ethanol in a two-bottle free choice test over a 3-d period (n = 12–13). A two-way ANOVA revealed a significant effect of genotype (F1,23 = 21.5; P < 0.0001) and ethanol concentration (F3,69 = 8.4; P < 0.001) as well as a significant genotype × concentration interaction effect (F3,69 = 8.4; P < 0.0001) on ethanol intake. (B) Mean (± SEM) drinking patterns of a 12% ethanol solution in the home cage over a 3-d period (n = 8–9). A two-way ANOVA revealed a significant genotype (F1,12 = 70.4; P < 0.00001), circadian (F17,204 = 9.3; P < 0.00001), and genotype × circadian (F17,204 = 8.1; P < 0.00001) effect. *P < 0.05.
Ethanol-Induced Dopamine Release Is Impaired in the VS of Rasgrf2−/− Mice.
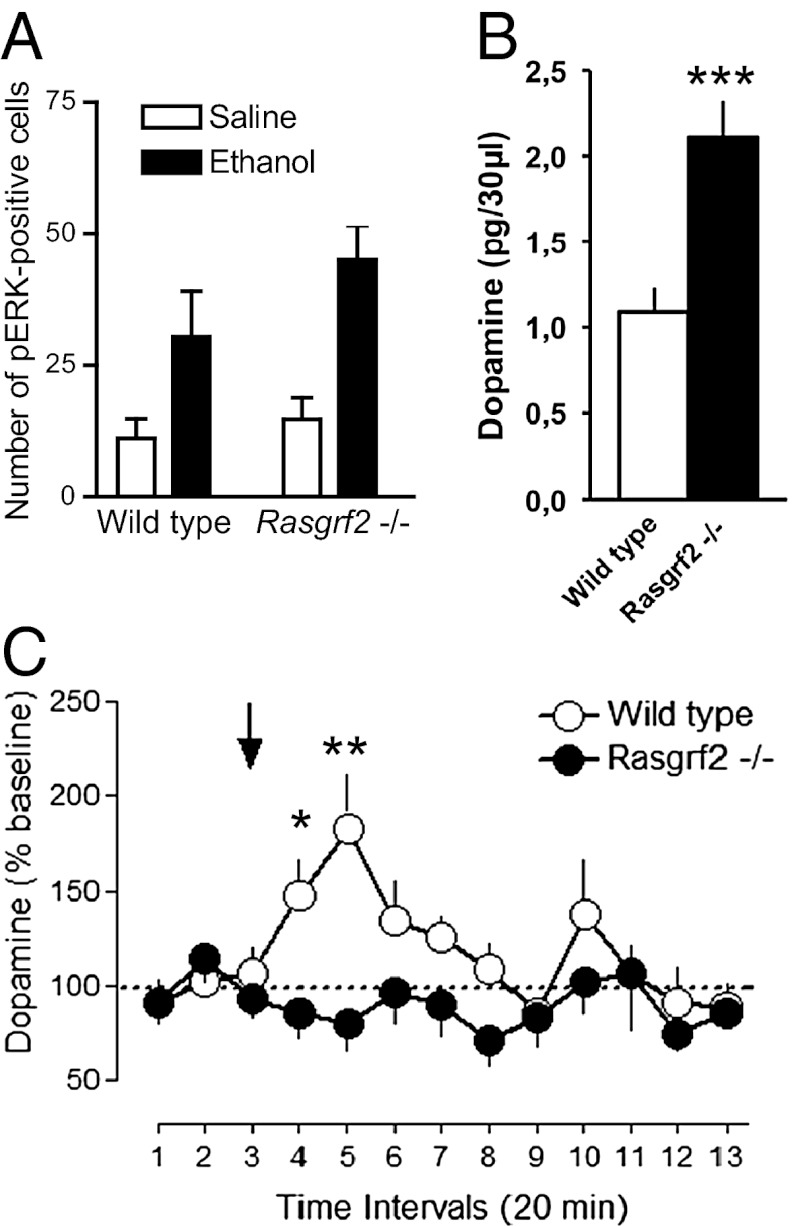
The regulation of ERK pathway by Ras-GRF1 in the striatum is involved in the reinforcing effects of drugs of abuse (12). We, therefore, hypothesized that ERK pathway alteration might be involved in the behavioral phenotype of Ras-GRF2 KO mice. Alcohol significantly induced ERK phosphorylation in the VS (nucleus accumbens), but this induction did not differ between genotypes (Fig. 2A). We then carried out microdialysis to measure extracellular DA levels in the VS of Rasgrf2−/− mice. Basal DA levels of mutant mice were significantly higher (approximately twofold; P < 0.001) than in WT controls (Fig. 2B), whereas directly after an i.p. injection of ethanol, the increase in extracellular DA levels observed in WT mice was completely absent in mutants (Fig. 2C). This apparent lack of a mesolimbic DA response to acute ethanol in Rasgrf2−/− mice suggested a mechanism by which the reinforcing effects of ethanol are diminished relative to WT mice, explaining the observed reduction in ethanol intake and preference.
Fig. 2.
No impairment in acute ethanol-induced ERK activation in the VS of Rasgrf2−/− mice compared with littermate controls. Acute ethanol-evoked dopamine release in the VS is significantly reduced in Rasgrf2−/− mice. (A) Mean (+ SEM) phospho-ERK–positive cells in mouse nucleus accumbens (average between shell and core) after an i.p. injection of either saline or ethanol (1 g/kg; n = 5–7 per group). A two-way ANOVA showed a significant effect of treatment (F1,19 = 14.0, P = 0.0014) but not genotype (F1,19 = 1.9) and no interaction (F1,19 = 0.7). (B) Mean (SEM) basal extracellular dopamine levels (n = 6–7). (C) Mean (+ SEM) extracellular dopamine levels after acute ethanol (2 g/kg i.p.; as indicated by arrow) injection. Data are expressed as percentage relative to basal dopamine levels at 20-min intervals (n = 6–7). A two-way ANOVA revealed a significant effect of genotype (F1,7 = 12.8, P = 0.009) and time (F12,84 = 2.9, P = 0.002) as well as a significant genotype × time interaction (F12,84 = 2.8, P = 0.0029) for extracellular dopamine levels. *P < 0.05; **P < 0.01; ***P < 0.001.
DA Neuron Excitability Is Impaired in the VTA of Rasgrf2−/− Mice.
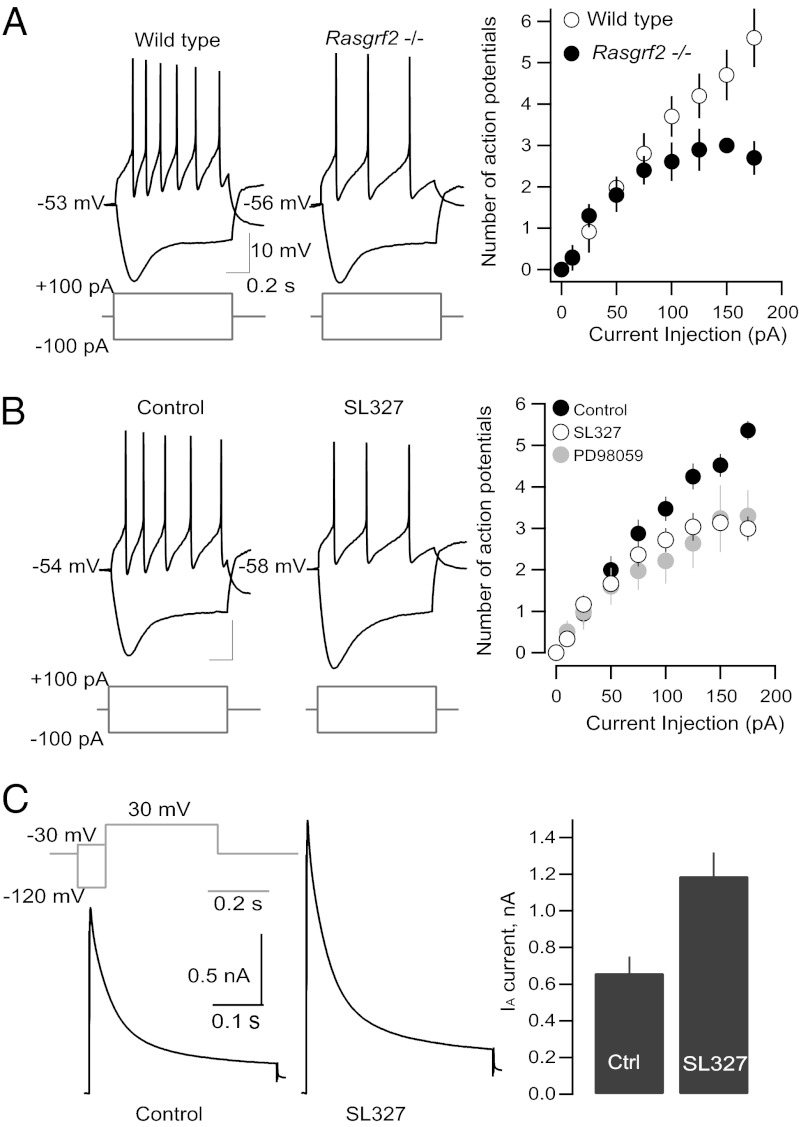
Ras-GRF2 regulates NMDA receptor-dependent long-term potentiation through activation of the ERK pathway (11). To investigate whether the altered extracellular DA levels observed by microdialysis in the VS of Rasgrf2−/− mice were caused by altered synaptic glutamate input to the VTA, we recorded excitatory postsynaptic currents of mesolimbic DA neurons elicited at +40 mV by activation of AMPA and NMDA receptors in the presence of the GABAA receptor blocker bicuculline. We found no significant difference in the AMPA:NMDA ratio between Rasgrf2−/− and WT controls (Fig. S5), indicating no alteration in synaptic input onto DA neurons of the VTA in mutant animals. We then recorded DA neurons in current-clamp mode and measured the action potentials elicited by increasing depolarizing current injections. We found that the number of action potentials elicited in Rasgrf2−/− animals was significantly reduced (P < 0.05) compared with WT controls (Fig. 3A), indicating reduced cell excitability in mutant animals.
Fig. 3.
Reduced excitability of mesolimbic dopaminergic neurons in Rasgrf2−/− mice relies on ERK-dependent modulation of IA currents. (A) Sample traces and pooled data of current injection evoked action potentials in mutant and WT mouse brain slices (n = 5–7). A two-way ANOVA revealed a significant effect of genotype (F1,12 = 5.09, P < 0.05). (B) Sample traces and pooled data of action potentials obtained on current injections in slices pretreated with ERK inhibitors. A two-way ANOVA revealed a significant treatment effects of inhibitors PD098059 (F1,17 = 6.4, P < 0.05) and SL327 (F1,15 = 4.9, P < 0.05; n = 7–8). (C) IA measured in WT brain slices pretreated with the ERK inhibitor or vehicle (t19 = 3.1, P < 0.01; n = 7–10).
DA Neuron Excitability Is Controlled by ERK Regulation of IA Current.
We then examined whether the regulation of DA neurons firing occurred through modification of ERK signaling. We incubated brain slices from WT animals with specific inhibitors of ERK activation. Pretreatment with PD098059 (10 µM) or SL327 (20 µM) led to a significant decrease (P < 0.05) in the number of depolarization-evoked action potentials (Fig. 3B). This recapitulation of the phenotype observed in DA neurons from Rasgrf2−/− animals suggested that the observed effect was ERK-dependent. ERK regulates the A-type K+ channel responsible for the IA current (22, 23). Furthermore, the IA current in cultured DA neurons increases on inhibition of ERK activity (24, 25). We tested whether this finding holds true in brain slices by recording pharmacologically isolated IA currents of DA neurons. Preincubation of slices with SL327 significantly increased the IA amplitude (P < 0.01) (Fig. 3C). Therefore, our data suggest that the decreased excitability of DA neurons in Rasgrf2−/− mice as well as inhibited ERK signaling in WT mice prevent phosphorylation of A-type K+ channels, resulting in an increased IA current and an impaired firing rate.
RASGRF2 Haplotype Is Associated with Blood-Oxygen-Level-Dependent (BOLD) Response in Brain Reward Areas and Drinking Behavior.
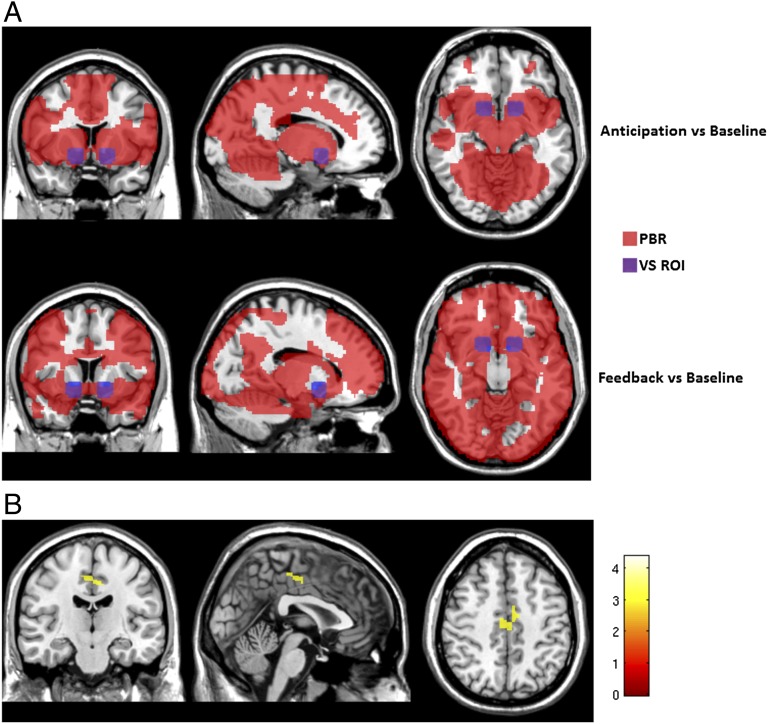
Because impaired firing of DA neurons could be expected to result in dysregulation of reward systems (26, 27), we explored whether variation in the human RASGRF2 gene were associated with reward mechanisms in humans. We used the monetary incentive delay (MID) task (28) as a functional neuroimaging reward paradigm and assessed ventral striatal activation in 14-y-old adolescents recruited by the IMAGEN study (19). Because the original suggestive GWAS signal for alcohol drinking in RASGRF2 was observed in males (10), we analyzed data from boys only. Whole-brain contrast maps of large win vs. baseline during reward anticipation (n = 663) revealed a widespread positive BOLD response, with highest activation in the subcortical regions, including the VS, and extending into the insula, PFC and the parietal cortex (Fig. 4A). Reward feedback (n = 612) induced significant positive BOLD responses predominantly in the cingulate gyrus, the PFC, and the parietal cortex as well as the subcortical regions, including the VS and the thalamus (Fig. 4A).
Fig. 4.
Whole-brain analysis of reward anticipation and reward feedback. (A) Positive BOLD response (PBR) during reward anticipation and reward feedback (FWE P value < 0.05). The location of the VS (±15, 9, −9; 9-mm radius) is depicted in blue. (B) Whole-brain analysis of the association of RASGRF2 haplotype and BOLD response during reward anticipation. This analysis revealed a significant activation in the precentral gyrus that extended into the anterior cingulate cortex. The figure shows activation in the precentral gyrus (FWE-corrected, P = 0.008; 6, 16, 49; t = 4.37; k = 58) with a subpeak in the anterior cingulate gyrus (−6, −1, 43).
For genetic analyses, we performed a haplotype analysis of RASGRF2. Because our previous GWAS finding showed a suggestive association of RASGRF2 rs26907 with alcohol drinking (10), we selected the haplotype block containing rs26907 for an exploratory association analysis with VS BOLD response during reward anticipation and reward feedback. This haplotype block consists of rs26906, rs26907, rs26908, rs252587, rs153227, rs11741062, rs153226, rs190409, and rs10514203 (Fig. S6 and Table S1). In an omnibus test, we found association of the haplotype block with reward anticipation in the left VS (P = 0.00495; Bonferroni-corrected P = 0.0198) but not with reward feedback (P = 0.482). Single haplotype analysis identified one haplotype with a frequency of 22.7%, which was associated with BOLD response during reward anticipation in the left VS (P = 0.00198; Bonferroni-corrected P = 0.01584) (Table 2). Analysis of association of this haplotype with whole-brain BOLD response during reward anticipation revealed significant activation in the anterior cingulate gyrus and the precentral gyrus [P = 0.008, family-wise error (FWE)-corrected] (Fig. 4B). No association was observed in the whole brain analysis during reward feedback. Interestingly, the same haplotype was associated with a high number of lifetime drinking episodes (P = 0.0084) in 16-y-old adolescents (Table 3).
Table 2.
Association analysis of RASGRF2 haplotype block containing rs26907 with adolescent VS activity during the MID task
| Frequency | MID reward anticipation (Con 37) |
||
| L_VS | R_VS | ||
| Hap1 | 0.2443 | 0.00198 | 0.0348 |
| Hap2 | 0.1584 | 0.83 | 0.647 |
| Hap3 | 0.1537 | 0.639 | 0.822 |
| Hap4 | 0.1143 | 0.253 | 0.115 |
| Hap5 | 0.09121 | 0.4 | 0.498 |
| Hap6 | 0.08522 | 0.0195 | 0.13 |
| Hap7 | 0.07633 | 0.74 | 0.926 |
| Hap8 | 0.06225 | 0.0244 | 0.0192 |
| Omnibus test | 0.00495 | 0.0697 | |
P values of omnibus and single haplotype analyses with VS BOLD response during reward anticipation.
Table 3.
Association analysis of RASGRF2 Hap1 allele with lifetime drinking behavior in adolescents
| Frequency | No. of individuals | χ2 statistic (df = 1) | P value with lifetime drinking episodes | |
| Hap1 | 0.2443 | 613 | 6.98 | 0.00844 |
Genotype-Specific Expression Analysis of RASGRF2 Gene.
The haplotype containing rs26907 encompasses a region in RASGRF2 spanning ∼8.5 kb. The SNPs constituting this haplotype are all intronic, although interestingly, SNPs rs26907 and rs26908 reside directly upstream (528 and 42 bp, respectively) of the transcription start site for an alternative, much shorter RASGRF2 isoform (uc003khb.1), which initiates in intron 3 relative to the full-length isoform (uc003kha.1). Therefore, we hypothesized that this haplotype might influence the transcription levels of this short isoform by modulating the efficiency of the corresponding core promoter. To test this hypothesis, we carried out quantitative RT-PCR analyses of postmortem PFC samples [RNA integrity/RIN = 8.49 (SEM = 0.67); postmortem interval/PMI = 3.05 h (SEM = 1.91)] from 41 male control individuals [mean age = 60.78 (SEM = 12.99)] genotyped for rs26907. However, we did not detect a significant association of expression of the short RASGRF2 isoform with rs26907 genotypes in this tissue (linear regression analysis, degrees of freedom (df)1, F = 0.023, P = 0.880).
Discussion
We show that Ras-GRF2 influences alcohol-induced reinforcement presumably by controlling mesolimbic DA release. This effect is likely to result from ERK-dependent regulation of dopamine neuron firing by IA currents. Our human functional neuroimaging data extend these findings, and they indicate that RASGRF2 might influence VS activity and contribute to reward sensitivity, which has been shown to increase risk for alcohol abuse in adolescents (29).
Our data reveal a marked reduction in alcohol-induced reinforcement in Rasgrf2−/− mice compared with WT littermate controls. Ras-GRF2 promotes the dissociation of GDP from ras, thus promoting GTP binding and activation of the downstream ERK pathway (30). The ERK pathway regulates dopamine-controlled synaptic plasticity in striatal medium-size spiny neurons, thereby playing an important role in long-lasting responses to addictive drugs (13); Rasgrf1−/− mice display a partial deficit in ERK activation and rewarding effects of cocaine (12). We, therefore, first hypothesized that a similar defect was central to the blunted ethanol reinforcement in Rasgrf2−/− mice. However, we did not observe such a deficit. We then measured DA release in the nucleus accumbens of the mutant mice by in vivo microdialysis and found that basal DA levels were enhanced in Rasgrf2−/− mice, whereas the response to alcohol was virtually abolished. This finding may suggest a tonically hyperactive DA system, which may not be able to further enhance DA neuron firing on a new salient stimulus in a phasic manner. Salience attribution to a new unconditioned stimulus depends on not only the amplitude of the DA increase in the VS but also, the time scale of the increase (31, 32). Because both are blunted in Rasgrf2−/− mice, the missing DA response may account for the reduced alcohol preference and consumption in the drinking test.
We identified a major defect in the regulation of DA neuron responsiveness that is likely to account for the altered ethanol-induced DA release in the mutant mice. Indeed, ethanol induces an acute increase in DA release, which was detected by fast-cycling voltametry (33), and an increase in DA neuron burst firing was shown to be a major substrate for learning about cues that predict rewarding events (34) and promote drug seeking (32).
Our data also reveal a potential mechanism for the decreased excitability of DA neurons. Using several distinct pharmacological inhibitors of the ERK pathway, we found that inhibition of this pathway decreased the excitability of DA neurons in much the same way as in mutant mice. This inhibition was caused by a lack of inhibition of the IA current, which is known to depend on ERK-mediated phosphorylation of A-type K+ channels (25). Our results are in agreement with previous observations showing ERK-dependent regulation of excitability and IA currents in DA neurons (35). The impaired regulation of DA neuron excitability provides an explanation for the deficient acute release of DA. However, it does not account for the basal increase in extracellular DA in the VS. Functional studies have shown that tonic and phasic activity may be differentially regulated by separate afferent systems in the VTA (36). It is, therefore, possible that compensatory mechanisms may increase basal DA release as an adaptation to the deregulation of DA neuron excitability.
The haplotype containing rs26907 was associated with the left VS BOLD response during reward anticipation but not reward feedback in the MID task. This finding relates directly to our previous GWAS findings (10) and suggests that RASGRF2 may control mechanisms that regulate the incentive salience of reward cues (37, 38). Incentive salience depends on response amplitude and timing of the DA signal initially after an unconditioned reward, which is later shifted to a conditioned reward cue and continuously updated by prediction error signals. A positive prediction error signal is associated with a phasic increase in DA neuronal firing and VS DA release (26, 39). The observed association with reward anticipation but not reward feedback in our study suggests that RASGRF2 controls sensitivity of the prediction error signal by modulating phasic activation of dopaminergic neurons. Functionally, this finding might result from an altered firing rate of VTA DA neurons on depolarizing signals, which was shown in our electrophysiological experiments in Rasgrf2−/− animals. The RASGRF2 haplotype was also associated with BOLD activation of the anterior cingulate gyrus and the precentral gyrus, both of which are known to be activated during reward-based decision-making (40). The enhanced incentive salience of reward predicting cues among 14-y-old children, who have not been exposed to significant amounts of alcohol at that age, is in keeping with a hypersensitive reward system, which renders them more susceptible to pharmacological rewards as well (37). This result is supported by our finding that, during follow-up at 16 y, an age where alcohol-drinking habits are already forming, the same boys showed an association between the RASGRF2 haplotype and number of drinking episodes.
One limitation of this study is the fact that we were not able to identify any allele-specific molecular mechanisms in humans underlying our neuroimaging and behavioral findings. However, brain expression patterns of RASGRF2 gene show distinct temporal variation with pronounced expression differences between adolescence and older age (Fig. S7) as well as significant regional variation (Fig. S8). This finding suggests the presence of temporal- and regional-specific regulatory mechanisms for RASGRF2 gene expression. Given the low availability of postmortem adolescent brains, association analysis of the RASGRF2 haplotype or rs26907 and RASGRF2 gene expression in the VTA of adolescents would be very difficult to carry out.
In summary, our approach of performing functional characterization of a suggestive signal in males from our recent GWAS metaanalysis of alcohol intake (10) has provided evidence for a role for RASGRF2 in alcohol-related phenotypes and mesolimbic DA neuron activity. In this respect, it is worth noting that glial-derived growth factor, which increases DA neurons excitability through ERK-dependent inhibition of IA currents (25), seems to exert profound effects on alcohol dependence in rats (41). Our study identifies the potential role of Ras-GRF2 in controlling reward responses through modulation of DA neuron firing. It suggests that other genetic factors controlling DA neuron excitability may also be important for incentive learning and its deregulation by drugs of abuse. Approaches aiming at correcting the consequences of such genetic alterations [for example, by selectively targeting the Ras-RAF-ERK pathway (42) or DA neuron excitability] may provide therapeutic strategies for addiction treatments.
Materials and Methods
Animals.
All procedures were carried out according to the United Kingdom Animals Scientific Procedures Act 1986. We used 8- to 10-wk-old male WT and Rasgrf2−/− C57BL/6 mice (21) single-housed with food and water ad libitum. Artificial light was provided daily (7:00 AM to 7:00 PM; 12 h of light/12 h of darkness) with room temperature and humidity kept constant (temperature = 22 + 1 °C; humidity = 55 ± 5%).
Free Choice Two-Bottle Drinking Behavior.
After 1 wk of habituation, animals were given continuous free access to aqueous ethanol solution. From days 1–3, animals had access to two bottles containing tap water. At day 4, one of the bottles was changed to 2% ethanol solution. At day 7, one of the bottles was changed to 4% ethanol solution. At day 10, one of the bottles was changed to 8% ethanol solution, and at days 20–48, one of the bottles was changed to 12% ethanol solution. Bottles, animals, and food were weighed every third day, with the position of the bottles swapped to avoid any location preference. Ethanol intake was calculated using alcohol density of 0.8 g/mL, whereas ethanol preference was calculated as percent alcohol intake to total liquid intake. Data were analyzed by two-way ANOVA for factors genotype and ethanol concentration, and posthoc analyses were performed using a Newman Keuls test.
Microdialysis.
Two MAB 6.14-guide cannulas (Microbiotech) with dummies were implanted into the VS (nucleus accumbens); 1 wk after implantation, MAB 6 (Microbiotech) dialysis probes were inserted and perfused at a constant flow rate of 1.5 μL/min with artificial cerebrospinal fluid. Animals were placed into a 20 × 20-cm open field, and experiments were initiated after stable levels of DA transmitter had been obtained. As previously detailed (43), DA levels were determined in dialysate samples taken at 20-min intervals over a period of 3 h by use of HPLC with electrochemical detection. Because no zero-net flux methodology could be used, basal transmitter levels in mutants can only be interpreted in relation to WT levels. An i.p. injection of either ethanol (2 g/kg body weight) or saline was administered 1 h after analyses began. Only male animals were used for microdialysis experiments. Data were analyzed by two-way ANOVA for factors genotype and time.
Electrophysiology.
Animals (20–40 d) were anesthetized with ketamine (90 mg/kg) /xylazine (15 mg/kg) (Sigma-Aldrich) before preparation of horizontal brain slices (250 µm) containing the VTA (SI Materials and Methods). Recordings were made under an Olympus BX51WL. Synaptic currents were evoked by stimuli (0.05–0.1 ms) at 0.1 Hz through bipolar glass electrodes filled with artificial cerebrospinal fluid placed rostrally to VTA. The AMPA receptor to NMDA receptor ratio was calculated by dividing the peak amplitudes at +40 mV after digital subtraction of the AMPA + NMDA component and the AMPA component after amino-5-phosphonovaleric acid (APV) (100 µM). For current-clamp recordings (the internal solution is discussed in SI Materials and Methods), a series of current steps (negative to positive) were injected to induce action potentials. IA currents were obtained by a two-step protocol, including a first depolarization from −30 to +30 mV and a second depolarization from −120 to +30 mV. All recordings were performed at 31–32 °C. Data were collected by a Multiclamp 700B (Molecular Devices) and analyzed by IGOR Wavemetrics (Wavemetrics). Slices were incubated with MAP-kinase/ERK kinase (MEK) inhibitors for 1 h prior recordings. DA neurons in the VTA were identified by the presence of Ih.
Participants.
Six hundred sixty-three males had complete data for the high win vs. baseline anticipation contrast of the MID task with a mean age of 14.45 y (SD = 0.40; range = 12.88–15.70 y); 612 males had complete data for the feedback high win vs. baseline contrast of the MID task with a mean age of 14.44 y (SD = 0.40; range = 12.88–15.70 y). In the association analysis of drinking behavior data, 613 males at age 16 y were included from the ongoing follow-up analysis. Participants were tested in eight IMAGEN assessment centers (London, Nottingham, Dublin, Mannheim, Berlin, Hamburg, Paris, and Dresden). The study was approved by local ethics research committees at each site. A detailed description of recruitment and assessment procedures as well as inclusion/exclusion criteria have previously been published (19). Handedness and study site were controlled for in all analyses.
Genotyping.
DNA purification and genotyping was performed by the Centre National de Génotypage in Paris (SI Materials and Methods).
Neuroimaging Analyses.
MID task.
This version of the MID task has been carried out as previously described (19) (SI Materials and Methods). Details are provided in SI Materials and Methods.
Functional MRI data analysis.
Functional MRI data were analyzed with SPM8 (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm). At the first level of analysis, changes in the BOLD response for each subject were assessed by linear combinations at the individual subject level for each experimental condition, and each trial (i.e., reward anticipation high gain; reward feedback high gain) was convolved with the hemodynamic response function to form regressors that account for potential noise variance associated with the processing of reward anticipation. The normalized and smoothed single-subject contrast images were then taken to a second-level random effects analysis. Using the Marsbar toolbox (http://marsbar.sourceforge.net), the VS region of interest (ROI) was extracted from the anticipation high gain vs. baseline contrast as well as the feedback high gain vs. baseline contrast. The extracted ROI was based on the work by O’Doherty et al. (44) (x, y, z = ±15, 9, −9; radius of 9 mm). More details are provided in SI Materials and Methods.
Behavioral Characterization.
The lifetime drinking phenotype was defined using an adapted version of the 2007 European School Survey Project on Alcohol and Other Drugs (ESPAD) questionnaire (45), which assesses the frequency of drinking of lifetime. The original variable is coded in a seven-point scale ranging from zero (never) to six (40 times or more). In our study, we classified binge drinking behavior into binary categories with (i.e., non- and light alcohol users) categories 0–2 (i.e., less than six times in lifetime) vs. (i.e., frequent and heavy alcohol users) categories 3–6, (i.e., six or more times in lifetime).
Statistical and Genetic Analyses.
Haplotype block of gene RASGRF2 that contains rs26907 was generated using the Four Gamete Rule with threshold 0.01 in Haploview. Haplotype phases are then estimated and tested for association with VS activation and lifetime drinking phenotype in Plink.
Supplementary Material
Acknowledgments
This work was supported by the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology; LSHM-CT-2007-037286), the FP7 projects ADAMS (Genomic variations underlying common neuropsychiatric diseases and disease-related cognitive traits in different human populations; 242257), and the Innovative Medicine Initiative Project EU-AIMS (European Autism Interventions) (115300-2) as well as the Medical Research Council Programme Grant “Developmental pathways into adolescent substance abuse” (93558). Additional support was provided by the Swedish Research Council FORMAS, the German Bundesministerium für Bildung und Forschung (Nationales Genomforschungsnetz Plus; FKZ 01GS08152 and 01EV0711), and Deutsche Forschungsgemeinschaft (DFG) Reinhart–Koselleck Award SP 383/5-1. Funding was also provided by the European Research Council and FP7 SynSys (to J.-A.G.) and Ecole des Neurosciences de Paris-Ile de France (ENP) and City of Paris (to M. Mameli.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211844110/-/DCSupplemental.
References
- 1.Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 1999;22(11):521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 2.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508(1):65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- 4.Lüscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS Med. 2006;3(11):e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11(6):557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 6.Boileau I, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4):226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 7.Schott BH, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28(52):14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samson HH, Hodge CW, Tolliver GA, Haraguchi M. Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: The involvement of the nucleus accumbens. Brain Res Bull. 1993;30(1–2):133–141. doi: 10.1016/0361-9230(93)90049-h. [DOI] [PubMed] [Google Scholar]
- 9.Rodd ZA, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: Evidence for involvement of dopamine neurons. J Neurosci. 2004;24(5):1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumann G, et al. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc Natl Acad Sci USA. 2011;108(17):7119–7124. doi: 10.1073/pnas.1017288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian X, et al. Developmentally regulated role for Ras-GRFs in coupling NMDA glutamate receptors to Ras, Erk and CREB. EMBO J. 2004;23(7):1567–1575. doi: 10.1038/sj.emboj.7600151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasano S, et al. Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol Psychiatry. 2009;66(8):758–768. doi: 10.1016/j.biopsych.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girault J-A, Valjent E, Caboche J, Hervé D. ERK2: A logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7(1):77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Fasano S, Brambilla R. Ras-ERK signaling in behavior: Old questions and new perspectives. Front Behav Neurosci. 2011;5:79. doi: 10.3389/fnbeh.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feig LA. Regulation of neuronal function by Ras-GRF exchange factors. Genes Cancer. 2011;2(3):306–319. doi: 10.1177/1947601911408077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibba F, et al. Ethanol-induced extracellular signal regulated kinase: Role of dopamine D1 receptors. Alcohol Clin Exp Res. 2009;33(5):858–867. doi: 10.1111/j.1530-0277.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- 17.Vinci S, et al. Acetaldehyde elicits ERK phosphorylation in the rat nucleus accumbens and extended amygdala. Synapse. 2010;64(12):916–927. doi: 10.1002/syn.20811. [DOI] [PubMed] [Google Scholar]
- 18.Faccidomo S, Besheer J, Stanford PC, Hodge CW. Increased operant responding for ethanol in male C57BL/6J mice: Specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl) 2009;204(1):135–147. doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumann G, et al. The IMAGEN study: Reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15(12):1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 20.Mulligan MK, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci USA. 2006;103(16):6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Medarde A, et al. Targeted disruption of Ras-Grf2 shows its dispensability for mouse growth and development. Mol Cell Biol. 2002;22(8):2498–2504. doi: 10.1128/MCB.22.8.2498-2504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrader LA, et al. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am J Physiol Cell Physiol. 2006;290(3):C852–C861. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- 23.Adams JP, et al. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75(6):2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- 24.Hu H-J, Gereau RW., 4th ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol. 2003;90(3):1680–1688. doi: 10.1152/jn.00341.2003. [DOI] [PubMed] [Google Scholar]
- 25.Yuan L-L, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22(12):4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 27.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 29.Hommer DW, Bjork JM, Gilman JM. Imaging brain response to reward in addictive disorders. Ann N Y Acad Sci. 2011;1216:50–61. doi: 10.1111/j.1749-6632.2010.05898.x. [DOI] [PubMed] [Google Scholar]
- 30.Fam NP, et al. Cloning and characterization of Ras-GRF2, a novel guanine nucleotide exchange factor for Ras. Mol Cell Biol. 1997;17(3):1396–1406. doi: 10.1128/mcb.17.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samaha A-N, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22(8):3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanat MJ, Willuhn I, Clark JJ, Phillips PEM. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2(2):195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheer JF, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27(4):791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zweifel LS, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA. 2009;106(18):7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang F, et al. GDNF acutely modulates excitability and A-type K(+) channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4(11):1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- 36.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 37.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 38.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1(4):304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 39.Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10(8):1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- 40.Bush G, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barak S, Carnicella S, Yowell QV, Ron D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: Implications for alcohol reward and seeking. J Neurosci. 2011;31(27):9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo HW. Targeting Ras-RAF-ERK and its interactive pathways as a novel therapy for malignant gliomas. Curr Cancer Drug Targets. 2010;10(8):840–848. doi: 10.2174/156800910793357970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pum M, Carey RJ, Huston JP, Müller CP. Dissociating effects of cocaine and d-amphetamine on dopamine and serotonin in the perirhinal, entorhinal, and prefrontal cortex of freely moving rats. Psychopharmacology (Berl) 2007;193(3):375–390. doi: 10.1007/s00213-007-0791-2. [DOI] [PubMed] [Google Scholar]
- 44.O’Doherty J, et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 45.Hibell B, et al. The 2007 ESPAD Report: Substance Use Among Students in 35 European Countries. Stockholm: Swedish Council for Information on Alcohol and Other Drugs; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.