Abstract
The endogenous metabolite of estradiol, 2-Methoxyestradiol (2ME2), is an antimitotic and antiangiogenic cancer drug candidate that also exhibits disease-modifying activity in animal models of rheumatoid arthritis (RA). We found that 2ME2 dramatically suppresses development of mouse experimental autoimmune encephalomyelitis (EAE), a rodent model of multiple sclerosis (MS). 2ME2 inhibits in vitro lymphocyte activation, cytokine production, and proliferation in a dose-dependent fashion. 2ME2 treatment of lymphocytes specifically reduced the nuclear translocation and transcriptional activity of nuclear factor of activated T-cells (NFAT) c1, whereas NF-κB and activator protein 1 (AP-1) activation were not adversely affected. We therefore propose that 2ME2 attenuates EAE through disruption of the NFAT pathway and subsequent lymphocyte activation. By extension, our findings provide a molecular rationale for the use of 2ME2 as a tolerable oral immunomodulatory agent for the treatment of autoimmune disorders such as MS in humans.
Keywords: calcium signaling, autoimmunity
Multiple sclerosis (MS) is among the most common autoimmune disorders in the northern hemisphere, affecting ∼0.1% of the population, primarily young adults (1). The pathological hallmarks of MS include demyelination, inflammation, scarring, and axonal destruction, which result in a variety of clinical symptoms including sensory loss, visual problems, muscle weakness, and speech problems (2). The most extensively used animal model of MS is experimental autoimmune encephalomyelitis (EAE), a T-cell–mediated autoimmune disease of the CNS in which inflammatory demyelination of the CNS induced by CD4+ T lymphocytes specific for CNS autoantigens such as myelin oligodendrocyte glycoprotein (MOG) occurs (3). EAE shares many histopathologic and immunologic characteristics with MS, and as such is considered a valuable model of the human disease (4).
The T helper 17 (Th17) subset of CD4+ lymphocytes is thought to play a critical role in the pathogenesis of many autoimmune disorders, including EAE (5). Pathogenicity of Th17 cells is a direct result of the inflammatory cytokines they produce, which include interleukin (IL)-17A (hereafter called IL-17), IL-17F, IL-21, IL-22, TNF, and GM-CSF (6). IL-17 itself has received the most attention as the driving cytokine underlying the autoimmunity of MS. IL-17 levels are elevated in the circulating leukocytes of MS patients with active disease, and expression of IL-17 correlates with MS disease severity (7, 8). Recent studies have revealed the importance of IL-17 in EAE; mice deficient in either IL-17 or IL-17 receptor (IL-17R) (9–11) or mice in which IL-17 is neutralized (12), exhibit attenuated disease.
2-Methoxyestradiol (2ME2) is an endogenous metabolite of 17β-estradiol (via conversion to hydroxyestradiols by cytochrome p450 (CYP450) and subsequent methylation by catechol-O-methyltransferase) that lacks significant estrogen receptor (ER)-binding ability (13). Orally active and well tolerated, 2ME2 possesses antiproliferative, antiangiogenic, and anti-inflammatory properties (14) and has completed phase I and II clinical trials for the treatment of a broad range of tumor types (15). Mechanistically, 2ME2 is known to bind to tubulin at the colchicine-binding site, resulting in disruption of microtubule (MT) dynamics and depolymerization of tubulin (16–18). As a result, G2–M arrest and apoptosis are induced in rapidly dividing cells, whereas quiescent cells are spared (14). 2ME2 has also been reported to exhibit disease-modifying activity in autoimmune models of rheumatoid arthritis in rodents (19–21). In these models, 2ME2 inhibited angiogenesis, leukocyte infiltration, local inflammation, and bone resorption associated with disease, but the molecular mechanisms underlying these phenomena remain undefined. In humans, plasma levels of 2ME2 may increase up to 1,000-fold to 90 nM during the last months of pregnancy (22, 23), which intriguingly appears to correlate temporally with the remission of clinical symptoms reported in some pregnant MS and RA patients (24, 25).
In this study, we demonstrate that oral administration of 2ME2 inhibits EAE onset and severity in a dose-dependent fashion. T- and B-cell activation and proliferation in vitro are inhibited by 2ME2, as is proinflammatory cytokine production, including IL-17. Furthermore, we propose that these effects are linked to 2ME2-mediated inhibition of NFAT activation and transcriptional activity. Our work thus provides a molecular rationale for the use of 2ME2 as a treatment for MS.
Results
2ME2 Inhibits Development of EAE in Mice.
2ME2 has been reported to display anti-inflammatory properties in collagen-induced arthritis (CIA), an autoimmune model of RA dependent on T-cell activation, and at least partly driven by Th17 cells (26). Given the importance of T-cell activation and the development of encephalitogenic Th17 cells (27) in orchestrating the inflammatory destruction of myelin in EAE, we investigated the therapeutic potential of 2ME2 in this autoimmune disease model.
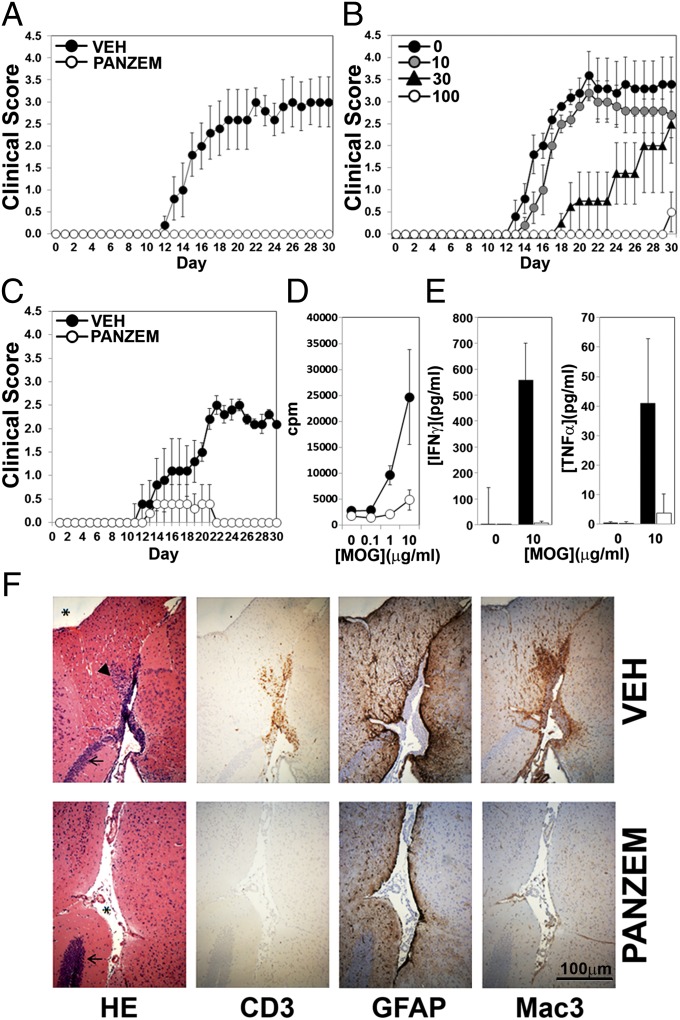
S.c. immunization with MOG35–55 in complete Freund’s adjuvant (CFA), together with injection of petussis toxin (PT), resulted in induction of EAE in all vehicle (water)-treated mice. Disease kinetics in these mice was consistent [mean onset at day (d)14–15], and all mice developed severe disease by d30 (Fig. 1A). In striking contrast, mice dosed orally throughout the course of the experiment (d1 through to d30) with 100 mg/kg Panzem NCD (a NanoCrystal dispersion formulation of 2ME2 that increases bioavailability) (28) were completely resistant to EAE and developed no clinical manifestations of EAE by d30 (Fig. 1A). This inhibition of EAE was dose dependent, with doses of 10, 30, and 100 mg/kg producing progressively greater reductions in clinical score (Fig. 1B). The dramatic nature of EAE inhibition by Panzem NCD when given throughout the disease course prompted us to examine the ability of 2ME2 to inhibit disease development when mice were treated after the induction phase of EAE. Mice were thus dosed with either vehicle or 100 mg/kg Panzem NCD at d12 after MOG peptide immunization, and disease course was monitored daily. Only 1/5 Panzem NCD-treated mice developed very mild, transient disease, whereas 4/4 vehicle-treated mice developed EAE by d30 (Fig. 1C). 2ME2 can therefore inhibit development of disease even when the induction and priming phase of EAE has already occurred. Incidence, onset, mortality rate, and statistical significance for EAE experiments are reported in Table S1.
Fig. 1.
Panzem NCD treatment inhibits EAE. (A) Mice orally dosed with vehicle or 100 mg/kg/d Panzem NCD (d1 to d30) were immunized s.c. with MOG peptide in CFA followed by i.p. injection of PT. Disease severity was scored daily and is representative of two independent experiments. (B) EAE was induced as above, but Panzem NCD was administered at 10, 30, or 100 mg/kg. For A and B, data are shown as mean ± SEM for five mice per group and are representative of two experiments. (C) Vehicle or Panzem NCD was administered orally, starting at d12 after immunization. Data shown are mean clinical scores ± SEM (n = 4–5). (D) CD4+ T cells were isolated from d12 spleens from mice treated as in A with vehicle or Panzem NCD and co-cultured with irradiated naïve splenic antigen presenting cells pulsed with MOG peptide. T-cell–specific proliferation was assessed by [3H]thymidine incorporation. (E) IFNγ and TNFα production from CD4+T-cell cultures stimulated with MOG peptide, assessed by ELISA. Data shown are mean ± SEM for three mice per group. (F) Representative histopathological analyses of CNS cross-sections from mice at 30 d post-MOG injection. Brain sections were stained with H&E (HE) or immunostained to detect CD3 (T cells), GFAP (reactive astrocytes), or Mac3 (activated macrophages). (Scale bar, 100 µm.) Arrow, hippocampus; arrowhead, lymphocytic infiltrates; asterisk, subarachnoid space. Data shown are from one mouse per group and are representative of three mice pergroup in two independent experiments.
EAE is a T-cell–dependent disease. To assay for the presence of MOG-reactive T cells in Panzem NCD-treated mice (dosed from d1), we isolated CD4+ T cells from spleens of vehicle- or drug-treated mice at d12 postimmunization with MOG peptide. Coculture of these CD4+ T cells with irradiated naïve splenic antigen presenting cells pulsed with MOG peptide revealed a severe defect in antigen-specific CD4+ T-cell proliferation in T cells from Panzem NCD-treated mice (Fig. 1D), together with a dramatic reduction in MOG-dependent IFNγ and TNFα production (Fig. 1E). These results show that Panzem NCD effectively curtails the early production and expansion of Ag-specific T cells in this disease model.
Histopathological analyses of brain tissue 30 d after MOG immunization revealed dense immune cell infiltrates in the cerebrum and cerebellum of vehicle-treated mice (Fig. 1F, HE). Immunohistochemical staining indicated a perivascularly centered, diffuse, widespread infiltrate of CD3+ T cells, which was absent in Panzem NCD-treated animals (Fig. 1F, CD3). The recruitment of bone-marrow–derived myeloid cells has been shown to be crucial for development of EAE (29). Accordingly, extensive perivascular macrophage activation was apparent in vehicle-treated mice, whereas activated macrophage staining was dramatically reduced in Panzem-treated mice (Fig. 1F, Mac3). Moreover, whereas vehicle-treated mice showed large numbers of reactive GFAP+ astrocytes within the infiltrated areas (a sign of severe CNS inflammation), a pronounced reduction in the number of reactive astrocytes was detectable in the Panzem NCD-treated group (Fig. 1F, GFAP). Taken together, these results show that 2ME2 inhibits EAE in a dose-dependent manner by blunting the inflammatory response in the CNS.
2ME2 Inhibits T- and B-Cell Proliferation and Activation.
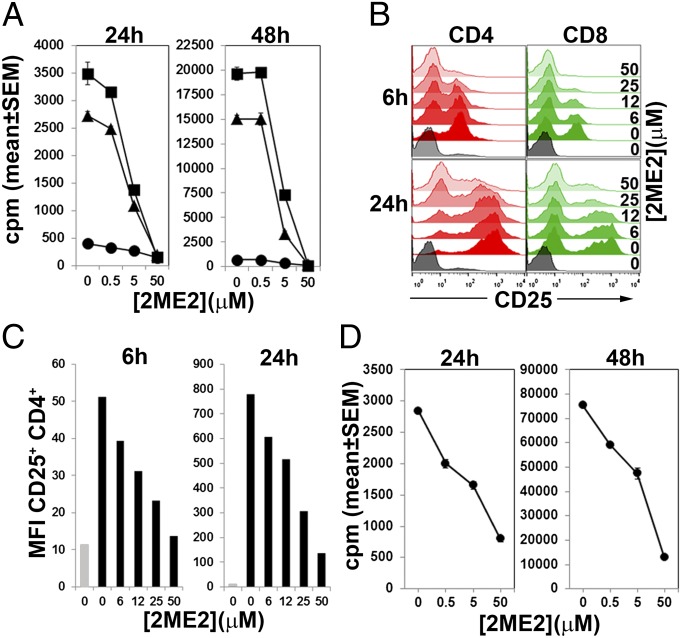
To investigate the effects of 2ME2 on T-cell activation directly, we pretreated purified CD4+ T cells with 2ME2 or vehicle and measured proliferation by [3H]thymidine incorporation at 24 h or 48 h post–T-cell receptor (TCR) stimulation. 2ME2 caused a dose-dependent inhibition of anti-CD3/28 or phorbol myristate acetate (PMA) and calcium ionophore (iono)-dependent proliferation (Fig. 2A). 2ME2 also blocked up-regulation of the early T-cell activation markers CD25 and CD69 in response to TCR ligation (Fig. 2 B and C and Fig. S1A) and pharmacological activation by PMA/iono (Fig. S1B). The inhibition of T-cell activation and proliferation at 24 h by 2ME2 was not due to induction of cell death because only a modest 2ME2-dependent increase in apoptosis was observed in unstimulated 2ME2-treated T cells, whereas none was apparent in activated 2ME2-treated T cells (Fig. S1C). At 48 h poststimulation, however, 2ME2-treated activated T cells showed reduced viability, presumably due to a 2ME2-dependent defect in activation.
Fig. 2.
Proliferation and activation of T cells is inhibited by 2ME2. (A) CD4+ T cells were pretreated with the indicated concentrations of 2ME2 and left untreated (circles), stimulated with plate-bound anti-CD3/28 (triangles), or PMA/iono (squares). Proliferation was assayed 24 h (Left) or 48 h (Right) later by [3H]thymidine incorporation. (B) T cells were left unstimulated (gray histograms) or stimulated with anti-CD3/28 in the presence of 0–50 μM 2ME2. CD25 expression on CD4+ (red histograms) and CD8+ cells (green histograms) was assessed at 6 or 24 h poststimulation by flow cytometry. (C) Quantification of CD25 expression in CD4+ T cells left untreated (gray bars) or pretreated with increasing concentrations of 2ME2 and stimulated with anti CD3/28 for 6 or 24 h (black bars. (D) Human PBMCs were activated with antihuman CD3/28 in the presence of increasing concentrations of 2ME2. Proliferation was determined 24 or 48 h later by [3H]thymidine incorporation. Data are mean ± SD of triplicate determinations and are representative of three separate donors. For A and D, data shown are the mean ± SD of triplicate determinations and are representative of three experiments. For B and C data are representative of three experiments.
2ME2 had a similar inhibitory effect on the proliferation of purified splenic B cells, regardless of whether they were stimulated with soluble anti-IgM, anti-IgM/CD40, or lipopolysaccharide (LPS) (Fig. S1D). The up-regulation of CD69 observed in activated B cells was also prevented by 2ME2 (Fig. S1E).
We also examined the effect of 2ME2 on proliferation of human T cells in vitro. Stimulation of human peripheral blood lymphocytes with anti-CD3/28 antibodies resulted in robust proliferation, which was inhibited in a dose-dependent manner by 2ME2 (Fig. 2D). In summary, we found that 2ME2 inhibits activation of lymphocytes in a dose-dependent manner.
T-Cell Cytokine Expression Is Attenuated by 2ME2.
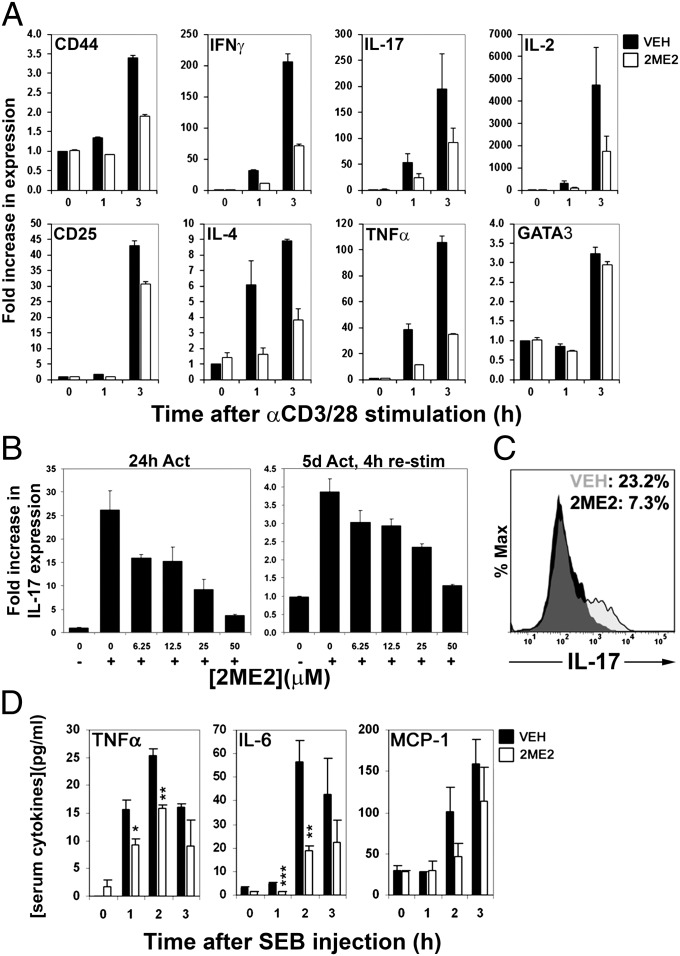
Analysis of mRNA transcripts by quantitative real-time (QRT) PCR revealed that 2ME2 decreased the induction of a range of cytokine and cell surface receptor mRNAs in activated T cells, including IL-2, TNFα, IFNγ, CD25, and CD44 (Fig. 3A). mRNA levels of the transcription factor GATA-binding protein 3, which is up-regulated via signal transducer and activator of transcription 6 (STAT6) activation (30), displayed normal induction 3 h poststimulation. 2ME2’s effects on IL-17 transcription were particularly dramatic, blocking up-regulation of IL-17 mRNA both after 3 h anti-CD3/28 stimulation (Fig. 3A) and 24 h (Fig. 3B, Left). IL-17 production on restimulation after 5 d activation was also blocked by 2ME2 (Fig. 3B, Right).
Fig. 3.
2ME2 treatment impairs cytokine production by activated T cells. (A) T cells were pretreated with vehicle or 2ME2 (50 μM) and stimulated with anti-CD3/28 for the indicated times. Relative expression levels of the indicated mRNAs were determined by real-time PCR. (B) T cells were pretreated with the indicated doses of 2ME2 and left unstimulated (−) or stimulated (+) with anti-CD3/28 for 24 h (Left) or stimulated for 5 d, preincubated with 2ME2, and restimulated for 4 h (Right). Induction of IL-17 mRNA was evaluated by QRT-PCR. For A and B, data are shown as mean fold increase ± SD of triplicate determinations and are representative of two independent experiments. (C) Naïve CD4+ T cells were differentiated into Th17 cells, preincubated with vehicle or 2ME2 for 30 min, and stimulated for 6 h with PMA/iono. IL-17 production in these cells was assessed by flow cytometry. Histograms show IL-17 staining in the absence (gray) and presence (black) of 50 μM 2ME2, whereas data values indicate percentage of IL-17 positive cells. Data are representative of two independent experiments. (D) Mice were dosed with vehicle (black bars) or 100 mg/kg 2ME2 (clear bars) for 2 d and injected with 25 μg SEB. Serum was collected at the indicated times post-SEB and levels of the indicated cytokines were analyzed by CBA. Data shown are the mean ± SD of three mice per group, each individual result being derived from triplicate determinations. *P < 0.05, **P < 0.01, ***P < 0.001.
To address the effect of 2ME2 on Th17 cell function, naïve CD4+ T cells were polarized into Th17 cells, pretreated with vehicle or 2ME2, and subsequently restimulated with PMA/iono for 6 h. Whereas 23.2% of Th17-polarized cells treated with vehicle stained positive for IL-17, only 7.3% of Th17-polarized cells pretreated with 2ME2 were IL-17 positive (Fig. 3C). Production of IFNγ in these Th17-polarized cells was not dramatically affected by 2ME2 treatment (9.1% vs. 7.3%, vehicle vs. control).
To assess whether 2ME2 modulated T-cell cytokine production in vivo, we pretreated mice with vehicle or 2ME2 for 2 d and subsequently injected them with 20 μg Staphylococcus enterotoxin B (SEB), which induces TCR-dependent proinflammatory cytokine production (31). 2ME2-treated mice produced significantly less serum TNFα and IL-6 than controls (Fig. 3D). These results show that 2ME2 reduces the production of inflammatory cytokines in vitro and in vivo.
2ME2 Inhibits NFAT Activation, but Has No Effect on NF-κB or AP-1 Signaling.
Estrogens are known to confer protection against MS and EAE (25). Recent work has implicated the G-protein–coupled estrogen receptor (GPER, also known as GPR30) 1 as a mediator of the protective role of 17-β estradiol in EAE (32–34). Whereas 2ME2 exhibits poor affinity for the ER, exhibiting 500- and 3,200-fold lower affinity than estradiol for ERα and ERβ, respectively (13), its affinity for GPER1 has not been reported. Accordingly, we determined the role of GPER1 signaling in attenuation of T-cell activation by 2ME2, using CD25 and CD69 expression 6 h postactivation as an indicator of inhibitory activity. To this end, we preincubated purified T cells with 2ME2 and G15, a specific GPER1 antagonist, subsequently activating the cells with anti-CD3/28. Blockade of GPER1 with G15 failed to reverse the inhibitory effect of 2ME2 on CD25 or CD69 expression (Fig. S2 A and B), indicating that 2ME2 does not exert its modulatory effects on T cells through GPER1 activation. When we tested the effect of G1, a specific agonist of GPER1, on CD25 and CD69 expression on activated T cells, we found that G1 had no effect on either parameter (Fig. S2C), supporting the fact that the mechanism of action of 2ME2 in T cells does not involve GPER1 signaling.
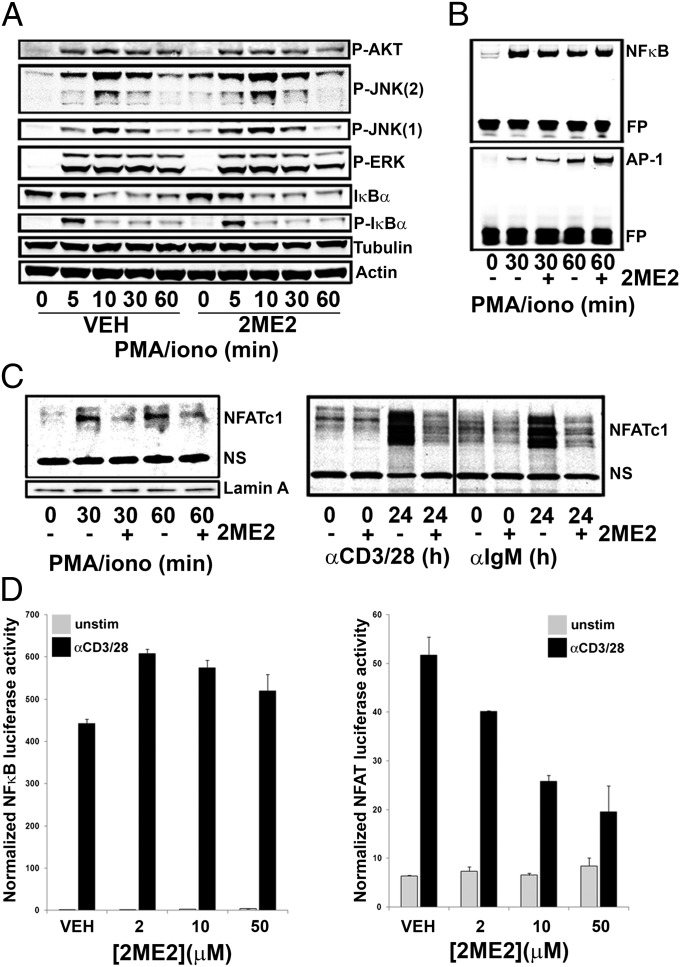
TCR ligation activates the transcription factor NF-κB, the MAPK pathway that triggers the AP-1 transcriptional complex, and the activation of NFAT (35). NFAT acts in concert with NF-κB and AP-1 to modulate the expression of genes critical for cellular activation and proliferation. To determine the effect of 2ME2 on these signaling pathways downstream of the TCR, we pretreated T cells with vehicle or 2ME2 and stimulated them with PMA/iono. IκBα phosphorylation and degradation were normal in 2ME2-treated activated T cells (Fig. 4A), as was the binding of NF-κB to DNA as assessed by EMSA (Fig. 4B). 2ME2 did not inhibit ERK or protein kinase B (AKT) phosphorylation, whereas the modest hyperphosphorylation of JNK 1 and 2 present even in the absence of stimulation (Fig. 4A) presumably reflects the known activation of the stress kinase pathway by 2ME2 (36, 37). These data are consistent with the observation that 2ME2 induced a modest increase in AP-1 binding to DNA (Fig. 4B). In addition, 2ME2 did not inhibit NF-κB p65, JNK, or ERK phosphorylation in T cells activated with anti-CD3/28 (Fig. S3). Thus, 2ME2 has no apparent inhibitory effect on the signaling mediators NF-κB, AP-1, ERK, AKT, or JNK.
Fig. 4.
2ME2 disrupts NFAT signaling while leaving NFκB signaling intact. (A) Purified T cells pretreated with 2ME2 (50 μM) were stimulated with PMA/iono for the indicated times. Whole cell lysates were subjected to immunoblotting to detect the indicated proteins. (B) Nuclear lysates were analyzed by EMSA to detect DNA binding to NF-κB or AP-1. FP, free probe. (C) Purified T cells or B cells were pretreated with vehicle (−) or 50 μM 2ME2 (+) and were stimulated with PMA/iono for 30 or 60 min (Left) or with anti-CD3/28 or anti-IgM/CD40 for 24 h (Right). Nuclear fractions of lymphocytes were analyzed by immunoblotting to detect nuclear translocation of NFATc1. Lamin A, nuclear protein loading control; NS, nonspecific band. For A–C, data are representative of at least two independent experiments. (D) Stably transfected NF-κB and NFAT Jurkat luciferase reporter cell lines were preincubated with 2ME2 for 30 min and stimulated via cross-linking of anti-CD3/28 antibodies with goat antimouse antibodies (GaM). Five hours later, secreted luciferase activity indicates relative transcriptional activity. Data are reported as normalized luciferase activity and are representative of three independent experiments.
Pretreatment with 2ME2 did however reduce the amount of NFATc1 present in the nuclear fraction of T cells at 30–60 min after PMA/iono stimulation (Fig. 4C, Left), as well as in T cells and B cells 24 h after anti-CD3/28 stimulation and anti-IgM/CD40-stimulation, respectively (Fig. 4C, Right). To confirm that 2ME2 caused a specific defect in NFAT activation, we used Jurkat cell lines stably transfected with NF-κB and NFAT luciferase reporters. Luciferase secretion was dependent on NF-κB or NFAT transcriptional activity and was triggered via TCR activation with anti-CD3/28 antibodies (Fig. 4D). Addition of 2ME2 to these cultures before stimulation revealed that 2ME2 had no effect on NF-κB–dependent transcriptional activity; however, transcriptional activation of NFAT via TCR ligation was dramatically inhibited by 2ME2 in a dose-dependent fashion (Fig. 4D). Together, our findings strongly suggest that 2ME2 blocks lymphocyte activation and proliferation by inhibiting NFATc1 nuclear translocation and NFAT-dependent gene transcription.
Discussion
The observation that exogenous 2ME2 induces cell cycle arrest and apoptosis in a variety of rapidly dividing cells (14, 17, 38) has led to the proposal that endogenous 2ME2 is a major mediator of the antimitogenic properties of estradiol (39). In addition to its antiproliferative and antimitogenic effects, 2ME2 also appears to possess anti-inflammatory activity in mice (20, 40), although the mechanism of action remains unclear.
The reported activity of 2ME2 in rodent CIA models led us to investigate the efficacy of 2ME2 in EAE, which shares many key disease drivers with CIA, including Th17 cell development and pathogenic cytokine production. Interestingly, both RA and MS often enter remission during pregnancy, when serum and urine 2ME2 levels become highly elevated. The enzymes that convert estradiol to 2ME2 are ubiquitously expressed, and thus any tissue that is exposed to estradiol can produce 2ME2. It is therefore tempting to speculate that a rise in 2ME2 concentration in immune organs and/or the CNS may occur during pregnancy, and allow 2ME2 to reach concentrations necessary to produce disease modifying effects.
Whereas absorption of 2ME2 is reportedly efficient in mice and humans, low bioavailability is observed (1.5%) (41), largely attributable to poor solubility and high first-pass metabolic transformation. Although it is difficult to achieve high serum 2ME2 concentrations following oral 2ME2 administration (42), the precise nature of local 2ME2 tissue accumulation and metabolism remains unclear, and it is possible that 2ME2 concentrations in local areas are significantly higher than those observed in serum. One approach to address the low solubility of 2ME2 has been the development of a NanoCrystal dispersion formulation of 2ME2 (Panzem NCD), which increases bioavailabililty to 3–4% (43). Clinical trials using 2ME2 have routinely used doses of 1 g/d of 2ME2, which result in a steady state Cmax of ∼30–50 ng/mL (27, 43, 44). These doses appear to be largely benign, with few serious adverse events being recorded. Using allometric scaling (45), a 1-g dose of 2ME2 in humans can be estimated to be approximately equivalent to a 200-mg/kg dose in mice. Translation to humans of the therapeutically effective dose administered to mice in this study, 100 mg/kg orally administered Panzem NCD, would thereby require half of what has been routinely administered to humans in prior trials with this agent. Disease development after T-cell priming was also disrupted, suggesting that 2ME2 suppresses T-cell effector function. These results may imply a potential therapeutic use of Panzem NCD in human MS.
To elucidate the mechanism of action underlying this activity in EAE, we determined whether 2ME2 affected signaling events immediately following antigen receptor TCR ligation in T cells. CD25 up-regulation and production of IL-2 are both prerequisites for efficient T-cell expansion in response to TCR stimulation (35, 46) and are (together with CD69 expression) dependent on NFAT nuclear translocation and subsequent NFAT transcriptional activity (47–49). Whereas NF-κB and MAPK pathways were unaffected by 2ME2 treatment, NFAT nuclear localization and transcriptional activity, together with CD25 and CD69 expression, were dose-dependently inhibited by 2ME2. In addition, we have shown that up-regulation of mRNAs for the NFAT-dependent genes IL-2, IFNγ, IL-4, TNFα, CD25, and IL-17 (50) that occurs in response to TCR ligation was inhibited by 2ME2 treatment. The ability of 2ME2 to inhibit IL-17 production in Th17-polarized cells may be particularly relevant to its disease-modifying role in EAE, IL-17 being a crucial encephalitogenic cytokine in the pathogenesis of EAE (6).
Our hypothesis that 2ME2 exerts its effects by impairing NFAT function in T cells was supported by in vivo experiments. In mice, SEB treatment induces TNFα production by T cells, leading to experimental toxic shock (31). We show that pretreatment of mice with 2ME2 significantly reduced the amount of TNFα and IL-6 produced by mice in response to SEB injection. In contrast, 2ME2-pretreated mice that were injected with LPS, which causes experimental septic shock, showed no reduction in serum TNFα levels (Fig. S4). SEB-induced TNFα production by T cells depends on NFATc2 (another calcium-dependent NFAT family member), whereas LPS-induced TNFα production is attributable largely to macrophages and depends on TLR4 signaling, and is not Ca2+ dependent. Our results are thus consistent with a previous report showing that NFATc2-deficient mice are not susceptible to toxic shock, but retain sensitivity to LPS injection (51). Although it has been reported that 2ME2 interferes with NF-κB transcriptional activity (52), these studies were performed in DAOY cells, an adherent medulloblastoma cell line, which may account for the differences observed between our study and that of Kumar et al. (52). Taken together, these data strongly suggest that disruption of NFAT activity is largely responsible for 2ME2’s inhibitory effects on lymphocytes in vitro and on EAE progression in vivo.
In conclusion, our study shows that 2ME2 reduces NFAT activity specifically. This impairment compromises the activation and proliferation of lymphocytes and may underlie 2ME2’s disease-modifying activity in both RA and EAE. Our promising EAE data in mice, together with favorable preclinical results and safety data obtained from oncology patients, strongly suggest that oral 2ME2 may be a well-tolerated, safe, and convenient alternative to current biologic and small molecule drugs used in the treatment of autoimmune disorders such as MS and RA.
Methods
Mice.
All live animal experiments were approved by the Institutional Animal Care and Use Committee (University Health Network, Toronto) as regulated by the Canadian Council on Animal Care. For all mice experiments C57/BL6 mice were used (Jackson Laboratories).
EAE.
Mice were randomly assigned to cages in groups of five, designated as vehicle or drug treated, and dosed p.o. via a feeding needle with 10–100 mg/kg 2ME2 (Panzem NCD, Entremed) or vehicle (water) at d1 to d30 or from d12 to d30. A dose of 100 mg/kg has been reported to be effective for inhibition of rodent CIA (20, 21, 53) and as such, was chosen as a starting point for EAE studies. For EAE induction, mice were s.c. immunized with 115 μg MOG 35–55 peptide (Washington Biotech) emulsified in CFA (Difco) supplemented with 400 μg/mL Mycobacterium tuberculosis (Difco). On days 0 and 2 postimmunization, mice were i.p. injected with 300 ng pertussis toxin (List Biological). Clinical signs of EAE were monitored daily according to the following criteria: 0, no disease; 1, decreased tail tone; 2, hind limb weakness or partial paralysis; 3, complete hind limb paralysis; 4, front and hind limb paralysis; and 5, moribund state. Mice were killed when they reached an EAE disease score of 4, and these animals were assigned a score of 5 for the rest of the observation period for the purposes of calculating the mean EAE disease score. At the end of the 30-d observation period, any surviving mice were killed and their brains subjected to histological analyses. The study was performed unblinded, but was scored by two independent researchers who obtained identical results.
Lymphocyte Purification.
All lymphocyte subsets were purified to >95% using the I-Mag magnetic bead separation system (BD Biosciences).
Drug Treatment in Vitro.
Cells were preincubated with various concentrations of 2ME2 (Entremed) or vehicle (DMSO) for 30 min at 37 °C.
Lymphocyte Activation, Proliferation, and Viability.
Purified CD4+ T cells were stimulated with plate-bound anti-CD3 (clone 2C11; BD Biosciences; 10 ng/mL) plus anti-CD28 (clone 37.51; BD Biosciences; 100 ng/mL). Purified B220+ B cells were stimulated with anti-IgM (Jackson Laboratories; 5 μg/mL), anti-CD40 (clone 3/20; BD Biosciences; 5 μg/mL), or LPS (Sigma; 20 μg/mL) for the times indicated in Fig. 3. CD25 and CD69 expression was determined by flow cytometry (FACSCanto II; BD Biosciences) and analyzed using FlowJo software (Treestar). Proliferation was assessed by [3H]thymidine incorporation. Viability and up-regulation of cell surface markers on activated T cells were assessed by flow cytometry using annexin V and propidium iodide. Human peripheral blood mononuclear cells (PBMCs) were isolated using Mono-Poly resolving medium (MP Biomedical) according to the manufacturer’s protocols and activated with anti-CD3 (clone UCHT1) and anti-CD28 (clone CD28.2).
Quantitative Real-Time PCR.
Total mRNA and cDNAs were prepared from stimulated T cells and cDNAs added to a Low Density TaqMan array (ABI) according to the manufacturer’s instructions. Fold increase for mRNAs was determined by normalizing against the expression of GAPDH. For IL-17 mRNA, quantitative RT-PCR was performed using Sybr Green (Invitrogen) and fold increase was determined by normalizing against 18S rRNA.
SEB and LPS Injections.
Mice were dosed i.p. for 2 d with 100 mg/kg 2ME2 (Panzem NCD) or vehicle, then injected i.p. with 25 μg SEB (Sigma) or 500 μg LPS (055:B5; Sigma). Serum cytokine levels were assessed with the cytometric bead array (CBA; BD Biosciences) according to manufacturer instructions. Statistical significance was calculated using Student’s t test.
GPER1 Dependence.
Purified C57/BL6 CD4+ T cells were preincubated with the GPER1 antagonist G15 (100 nM; Tocris) and 2ME2 (0–50 µM) or the GPER1 agonist G1 (1–100 nM; Tocris) for 30 min, and activated with plate-bound anti-CD3 plus soluble anti-CD28. Six hours later, cells were harvested and stained with anti-CD4 and anti-CD25. CD25 expression was monitored on a High Throughput Sampler FACSCanto II (BD Biosciences) and analyzed with FlowJo (Treestar).
Biochemical Dissection of Signaling Pathways.
Whole cell lysates of stimulated T cells were immunoblotted with Ab recognizing phospho-AKT (Ser473), phospho-JNK (Thr183/Tyr185, p46 and p54 clone G9, antibody “2”), phospho-ERK (Thr202/Tyr204) or phospho-IκBα (Ser32) (NEB); phospho-JNK (Thr183/Tyr185, p54 pSAPK/JNK, clone G7, antibody “1”) or IκBα (C21) (Santa Cruz), β-tubulin (Millipore), or actin (Sigma). Infrared dye-labeled secondary Ab (antirabbit Alexa Fluor 680; Invitrogen and antimouse IR800; LICOR) were visualized with the Odyssey scanner (LICOR).
EMSA.
Nuclear extracts of T cells stimulated with PMA (10 ng/mL) and calcium ionophore A23187 (iono; Sigma) (50 ng/mL) for 30 or 60 min were prepared according to a standard protocol. These extracts were subjected to EMSA using infrared dye end-labeled DNA oligonucleotides (LICOR) specific for NF-κB and AP-1 and a protocol specified by the manufacturer. Complexes were visualized on the Odyssey scanner (LICOR).
NFATc Analysis.
T or B cells preincubated with vehicle or 2ME2 (50 μM) were stimulated with PMA (10 ng/mL) and iono (50 ng/mL), plate-bound anti-CD3 (30 ng/mL) + anti-CD28 (100 ng/mL), or anti-IgM (1 μg/mL) and anti-CD40 (1 μg/mL). Nuclear extracts (5 μg protein) were immunoblotted with anti-NFATc1 (clone 7A6; Santa Cruz) and anti-lamin A (clone H-102; Santa Cruz).
Gaussia Luciferase Assays.
Luciferase assays were performed using Jurkat cells harboring a stably integrated Gaussia luciferase reporter gene under the control of NF-κB or NFAT binding sites (termed NFAT/NF-κB–GLuc reporter Jurkat). Gaussia luciferase contains a secretion peptide sequence mediating quantitative secretion of active enzyme into the surrounding medium. A total of 1.5 × 105 GLuc reporter cells were stimulated in 96-well plates for 5 h. For each sample, 20 μL of supernatant was subjected to a Gaussia luciferase assay. Luciferase activity was measured in 96-well plates using an Orion L Microplate Luminometer (Berthold Detection Systems) with an integration time of 3.0 s. In parallel, cell viability was determined using the CellTiter-Glo Luminescent Cell Viability Assay (Promega) and normalized GLuc activity was calculated as the ratio between GLuc activity and cell viability counts.
Statistical Methods.
Where appropriate, all differences were evaluated using the two-tailed Student t test as calculated using Excel (Microsoft) software. Data are presented as the mean ± SEM, or mean ± SD. For EAE experiments, the Mann–Whitney test was used.
Supplementary Material
Acknowledgments
The authors thank Trisha Denny, Graham Fletcher, Richard Brokx, Stacy Plum, and William Fogler for reagents and advice; Rakesh Nayyar for flow cytometry advice; and Jörg Thomsen for editing of histology figures. D.B. is supported by a postdoctoral fellowship from the German Research Foundation. T.M. is supported by the José Carreras Leukämie Stiftung e.V.
Footnotes
The authors declare a conflict of interest. T.W.M. is a board member and equity holder of Entremed, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215558110/-/DCSupplemental.
References
- 1.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011;74(1):1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- 2.Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354(9):942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 3.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1(4):1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 4.Batoulis H, Recks MS, Addicks K, Kuerten S. Experimental autoimmune encephalomyelitis—achievements and prospective advances. APMIS. 2011;119(12):819–830. doi: 10.1111/j.1600-0463.2011.02794.x. [DOI] [PubMed] [Google Scholar]
- 5.Pollinger B. IL-17 producing T cells in mouse models of multiple sclerosis and rheumatoid arthritis. J Mol Med. 2012;90(6):613–624. doi: 10.1007/s00109-011-0841-4. [DOI] [PubMed] [Google Scholar]
- 6.Zepp J, Wu L, Li X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol. 2011;32(5):232–239. doi: 10.1016/j.it.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5(2):101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 8.Durelli L, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65(5):499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-García I, et al. IL-17 signaling-independent central nervous system autoimmunity is negatively regulated by TGF-beta. J Immunol. 2009;182(5):2665–2671. doi: 10.4049/jimmunol.0802221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, et al. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 2010;184(8):4307–4316. doi: 10.4049/jimmunol.0903614. [DOI] [PubMed] [Google Scholar]
- 11.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 12.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaVallee TM, et al. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62(13):3691–3697. [PubMed] [Google Scholar]
- 14.Pribluda VS, et al. 2-Methoxyestradiol: An endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 2000;19(1-2):173–179. doi: 10.1023/a:1026543018478. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland TE, et al. 2-Methoxyestradiol—a unique blend of activities generating a new class of anti-tumour/anti-inflammatory agents. Drug Discov Today. 2007;12(13-14):577–584. doi: 10.1016/j.drudis.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Gökmen-Polar Y, et al. beta-Tubulin mutations are associated with resistance to 2-methoxyestradiol in MDA-MB-435 cancer cells. Cancer Res. 2005;65(20):9406–9414. doi: 10.1158/0008-5472.CAN-05-0088. [DOI] [PubMed] [Google Scholar]
- 17.Mabjeesh NJ, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3(4):363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 18.Escuin D, Kline ER, Giannakakou P. Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor-1alpha accumulation and activity by disrupting microtubule function. Cancer Res. 2005;65(19):9021–9028. doi: 10.1158/0008-5472.CAN-04-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josefsson E, Tarkowski A. Suppression of type II collagen-induced arthritis by the endogenous estrogen metabolite 2-methoxyestradiol. Arthritis Rheum. 1997;40(1):154–163. doi: 10.1002/art.1780400120. [DOI] [PubMed] [Google Scholar]
- 20.Plum SM, et al. Disease modifying and antiangiogenic activity of 2-methoxyestradiol in a murine model of rheumatoid arthritis. BMC Musculoskelet Disord. 2009;10:46. doi: 10.1186/1471-2474-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issekutz AC, Sapru K. Modulation of adjuvant arthritis in the rat by 2-methoxyestradiol: An effect independent of an anti-angiogenic action. Int Immunopharmacol. 2008;8(5):708–716. doi: 10.1016/j.intimp.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Berg D, Sonsalla R, Kuss E. Concentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol (Copenh) 1983;103(2):282–288. doi: 10.1530/acta.0.1030282. [DOI] [PubMed] [Google Scholar]
- 23.Huh JI, et al. 2-methoxyestradiol induces mammary gland differentiation through amphiregulin-epithelial growth factor receptor-mediated signaling: Molecular distinctions from the mammary gland of pregnant mice. Endocrinology. 2007;148(3):1266–1277. doi: 10.1210/en.2006-0964. [DOI] [PubMed] [Google Scholar]
- 24.Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol. 2007;29(2):185–191. doi: 10.1007/s00281-007-0072-5. [DOI] [PubMed] [Google Scholar]
- 25.Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2006;1089:343–372. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- 26.Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol. 2010;32(1):43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 28.Matei D, et al. Activity of 2 methoxyestradiol (Panzem NCD) in advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: A Hoosier Oncology Group trial. Gynecol Oncol. 2009;115(1):90–96. doi: 10.1016/j.ygyno.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 29.Becher B, Segal BM. T(H)17 cytokines in autoimmune neuro-inflammation. Curr Opin Immunol. 2011;23(6):707–712. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurata H, Lee HJ, O’Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11(6):677–688. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 31.Miethke T, et al. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: Critical role of tumor necrosis factor. J Exp Med. 1992;175(1):91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blasko E, et al. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214(1-2):67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodhankar S, Offner H. Gpr30 forms an integral part of E2-protective pathway in experimental autoimmune encephalomyelitis. Immunol Endocr Metab Agents Med Chem. 2011;11(4):262–274. doi: 10.2174/1871522211108040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11:20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20(3):250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou H, et al. 2-methoxyestradiol, an endogenous mammalian metabolite, radiosensitizes colon carcinoma cells through c-Jun NH2-terminal kinase activation. Clin Cancer Res. 2006;12(21):6532–6539. doi: 10.1158/1078-0432.CCR-06-0678. [DOI] [PubMed] [Google Scholar]
- 37.Lorin S, et al. c-Jun NH2-terminal kinase activation is essential for DRAM-dependent induction of autophagy and apoptosis in 2-methoxyestradiol-treated Ewing sarcoma cells. Cancer Res. 2009;69(17):6924–6931. doi: 10.1158/0008-5472.CAN-09-1270. [DOI] [PubMed] [Google Scholar]
- 38.D’Amato RJ, Lin CM, Flynn E, Folkman J, Hamel E. 2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc Natl Acad Sci USA. 1994;91(9):3964–3968. doi: 10.1073/pnas.91.9.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zacharia LC, et al. Methoxyestradiols mediate the antimitogenic effects of 17beta-estradiol: Direct evidence from catechol-O-methyltransferase-knockout mice. Circulation. 2003;108(24):2974–2978. doi: 10.1161/01.CIR.0000106900.66354.30. [DOI] [PubMed] [Google Scholar]
- 40.Shand FH, et al. In vitro and in vivo evidence for anti-inflammatory properties of 2-methoxyestradiol. J Pharmacol Exp Ther. 2011;336(3):962–972. doi: 10.1124/jpet.110.174854. [DOI] [PubMed] [Google Scholar]
- 41.Verenich S, Gerk PM. Therapeutic promises of 2-methoxyestradiol and its drug disposition challenges. Mol Pharm. 2010;7(6):2030–2039. doi: 10.1021/mp100190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaVallee TM, et al. Significant antitumor activity in vivo following treatment with the microtubule agent ENMD-1198. Mol Cancer Ther. 2008;7(6):1472–1482. doi: 10.1158/1535-7163.MCT-08-0107. [DOI] [PubMed] [Google Scholar]
- 43.Tevaarwerk AJ, et al. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin Cancer Res. 2009;15(4):1460–1465. doi: 10.1158/1078-0432.CCR-08-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulke MH, et al. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother Pharmacol. 2011;68(2):293–300. doi: 10.1007/s00280-010-1478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 46.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17(18):2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 47.Aussel C, Marhaba R, Pelassy C, Breittmayer JP. Submicromolar La3+ concentrations block the calcium release-activated channel, and impair CD69 and CD25 expression in CD3- or thapsigargin-activated Jurkat cells. Biochem J. 1996;313(Pt 3):909–913. doi: 10.1042/bj3130909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor-Fishwick DA, Siegel JN. Raf-1 provides a dominant but not exclusive signal for the induction of CD69 expression on T cells. Eur J Immunol. 1995;25(12):3215–3221. doi: 10.1002/eji.1830251203. [DOI] [PubMed] [Google Scholar]
- 49.Castellanos MdelC, López-Giral S, López-Cabrera M, de Landázuri MO. Multiple cis-acting elements regulate the expression of the early T cell activation antigen CD69. Eur J Immunol. 2002;32(11):3108–3117. doi: 10.1002/1521-4141(200211)32:11<3108::AID-IMMU3108>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 50.Macián F, López-Rodríguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20(19):2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 51.Tsytsykova AV, Goldfeld AE. Nuclear factor of activated T cells transcription factor NFATp controls superantigen-induced lethal shock. J Exp Med. 2000;192(4):581–586. doi: 10.1084/jem.192.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar AP, Garcia GE, Orsborn J, Levin VA, Slaga TJ. 2-Methoxyestradiol interferes with NF kappa B transcriptional activity in primitive neuroectodermal brain tumors: Implications for management. Carcinogenesis. 2003;24(2):209–216. doi: 10.1093/carcin/24.2.209. [DOI] [PubMed] [Google Scholar]
- 53.Brahn E, et al. An angiogenesis inhibitor, 2-methoxyestradiol, involutes rat collagen-induced arthritis and suppresses gene expression of synovial vascular endothelial growth factor and basic fibroblast growth factor. J Rheumatol. 2008;35(11):2119–2128. doi: 10.3899/jrheum.080302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.