Abstract
Despite the fact that halogenation of alkenes has been known for centuries, enantioselective variants of this reaction have only recently been developed. In the past three years, catalytic enantioselective versions of halofunctionalizations with the four common halogens have appeared and although important breakthroughs, they represent just the very beginnings of a nascent field. This Minireview provides a critical analysis of the challenges that accompany the development of general and highly enantioselective halofunctionalization reactions. Moreover, the focus herein, diverges from previous reviews of the field by identifying the various modes of catalysis and the different strategies implemented for asymmetric induction.
Keywords: alkenes, halogenation, homogeneous catalysis, Lewis base, synthetic methods
1. Introduction
Electrophilic functionalization of alkenes by halogens (coined in 1842 by Berzelius from the Greek hals “salt” and -gen “to make or produce”) was already a classic textbook reaction in the 1930s.[1] Countless studies established the factors that influence substitution versus addition and radical (homolytic) versus polar (heterolytic) reactions. Detailed kinetic and stereochemical investigations placed these reactions on solid mechanistic foundations.[2] Not surprisingly then, as synthetic organic chemists turned their attention to more and more challenging targets in the second half of the 20th century, the predictability and synthetic utility of alkene halogenation were put to good use. For example, steroid halogenation played a major role in the beginnings of conformational analysis of six-membered rings.[3, 4] In addition, diastereoselective halofunctionalization evolved as a critical strategy-level reaction in the era of acyclic stereoselection beginning in the mid 1970s.[5] Surprisingly however, as the field evolved to introduce catalytic, enantioselective variants of the major families of synthetic reactions, the electrophilic halofunctionalization of alkenes appeared to reach an evolutionary dead end. Perhaps it was the spectacular success of enantioselective epoxidation, dihydroxylation, and later aziridination that caused the asymmetric introduction of other heteroatoms, particularly halogens, to be overlooked. Or, perhaps it was just too difficult.
Possible answers to this conundrum are just beginning to emerge. The past few years have witnessed a dramatic upsurge of interest in catalytic, enantioselective halofunctionalization reactions for the construction of a wide array of structural motifs common to natural products, drug targets, and intermediates. Interestingly, the recent flurry of publications on this topic has also generated a considerable number of reviews that chronicle the rapidly advancing state of the art. Some of these reviews are comprehensive and include both stoichiometric and catalytic enantioselective methods,[6, 7] whereas others are narrow and focus on catalytic[8] or purely organocatalytic[9] methods. Regardless of scope, these reviews are all organized either chronologically or by the halogen and the nucleophile. Although such presentations are beneficial to catalogue these transformations, they are less instructive for understanding why catalytic asymmetric halofunctionalization has lagged so far behind other methods and for identifying unifying concepts, common strategies, and new opportunities for catalysis and stereocontrol. This review endeavors to present a different structure by classifying catalytic, asymmetric halofunctionalization under a conceptual rubric based upon modes of catalysis and strategies for asymmetric induction.
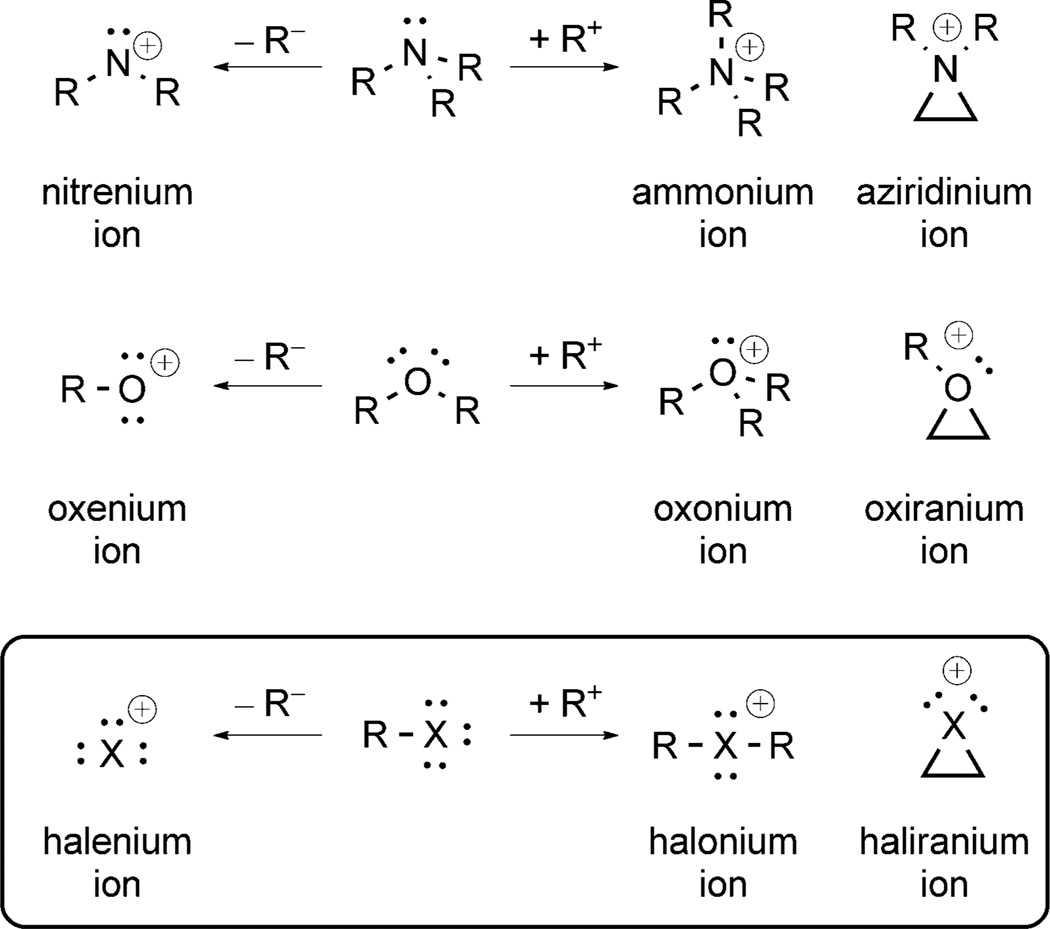
At the outset, a brief presentation of the nomenclature used throughout the review is warranted (Figure 1). By analogy to the terms used for charged and electron-deficient species in Groups 15 and 16, hypercoordinate (10-X-2)+ Group 17[10] cations are called halonium ions; the special case of cyclic halonium ions fall into the standard nomenclature of ring compounds, for example, -iranium (3), -etidium (4), -olidinium (5) ions. Hypocoordinate species (6-X-0)+ are called halenium ions.
Figure 1.
Nomenclature of halogen cations.
2. Historical Background and Development of Mechanistic Understanding
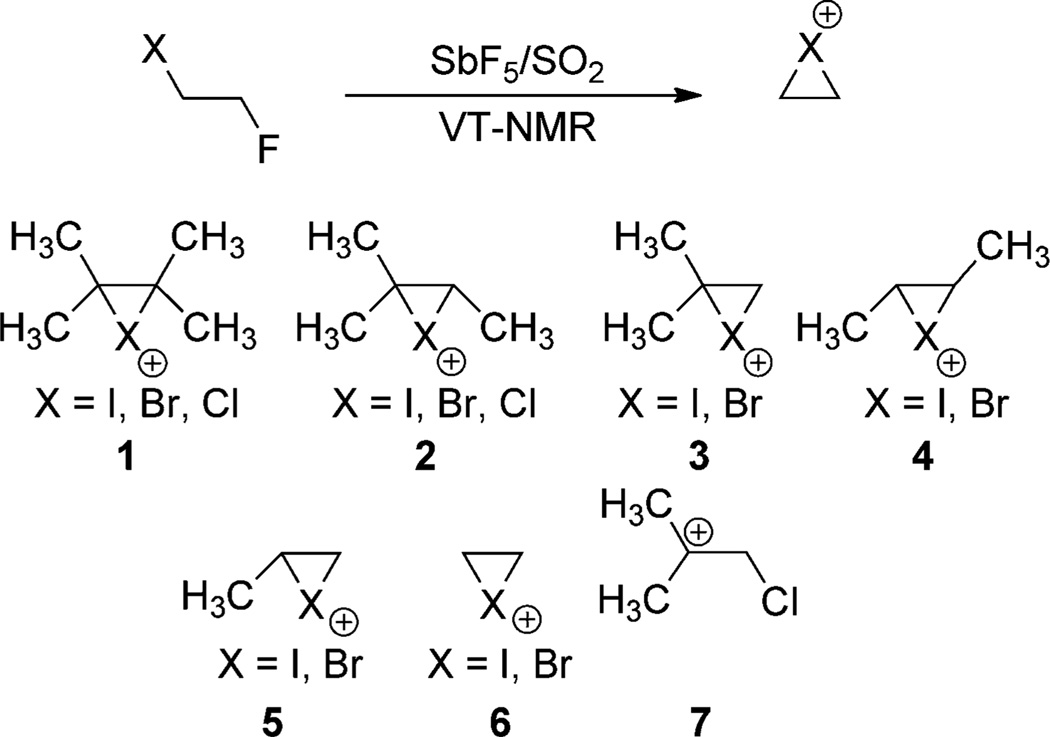
Halofunctionalizations of olefins are stereospecific processes that result in a 1,2-anti arrangement of the halogen and nucleophile.[11] Such processes typically proceed by the formation of an olefin/dihalogen π complex with subsequent ionization to form a cyclic halonium (haliranium) ion, which can be captured by nucleophiles.[12] The intermediacy of haliranium ions was first proposed by Roberts and Kimball[13] to explain the stereospecificity observed in the bromination and chlorination of disubstituted alkenes. Haliranium ions were first directly observed in liquid SO2 by Olah et al.,[14] who found that all iodonium and bromonium ions (1–6) as well as many chloronium ions (1 and 2, but not 7) are cyclic on the 1H NMR time scale between −60 and −80 °C (Scheme 1), but the fluorine analogues existed only as acyclic β-fluorocarbenium ions. Consequently, the intermediacy of acyclic cations in fluorofunctionalization reactions makes such processes non-stereospecific. Several chloriranium ions could not be observed because of their low chemical stability.
Scheme 1.
Spectroscopically observable haliranium ions.
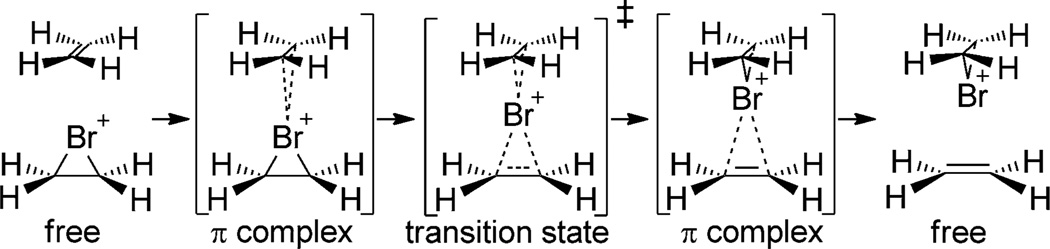
Variable-temperature 1H NMR spectroscopic studies by Brown and co-workers demonstrated that a rapid, degenerate, olefin-to-olefin transfer process occurs between adamantylidene adamantane and the isolable bromiranium and iodiranium ions derived from it.[15] Computational studies support the hypothesis that this process occurs through a low-barrier associative displacement at the halogen (Scheme 2).[15a]
Scheme 2.
Mechanism of olefin-to-olefin transfer of bromenium ions.
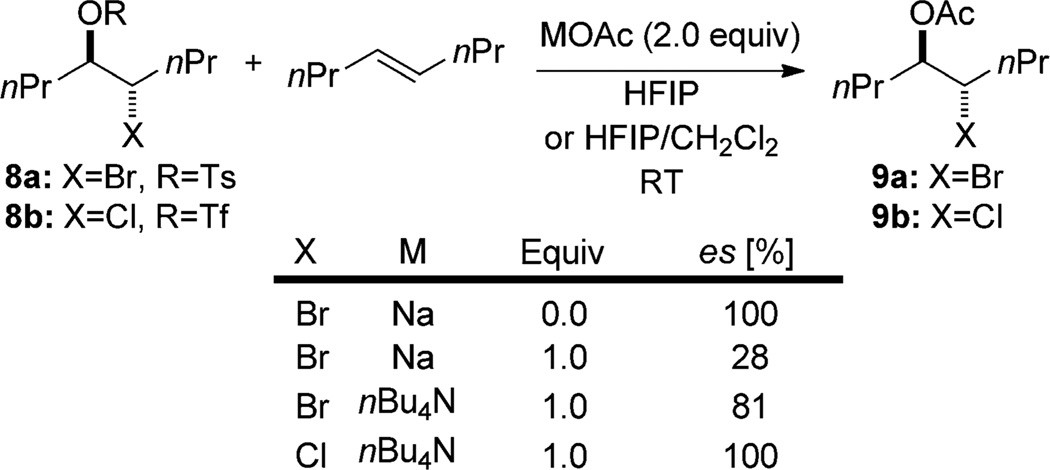
In the context of enantioselective halofunctionalization, olefin-to-olefin transfer must be suppressed because it has the potential to racemize enantiomerically enriched haliranium ions, provided that it is faster than nucleophilic capture. In an achiral environment this equilibration will result in racemic products even if the initial halogen delivery is perfectly selective. Acetolysis experiments carried out by Denmark and co-workers demonstrate that the bromiranium ion derived from the bromotosylate 8a can be captured enantiospecifically in the absence of olefins, however the addition of 4-octene to the reaction mixture results in substantial erosion of the enantiospecificity (Scheme 3).[16] The degree of erosion depends on the concentrations of the olefin and the nucleophile as well as the identity of the counterion. Under similar reaction conditions, the analogous chloriranium ion from 8b is trapped with complete enantiospecificity even in the presence of (E)-4-octene.
Scheme 3.
Erosion of enantiospecificity in acetolysis from olefin-to-olefin transfer. HFIP = hexafluoroisopropyl alcohol, Tf = trifluoromethanesulfonyl, Ts = 4-toluenesulfonyl. es = (eeproduct/eestarting material) × 100%.
3. Approaches to Catalytic Enantioselective Halofunctionalization: Catalysis versus Enantioselectivity
Many different strategies for catalytic, enantioselective halofunctionalization reactions have been reported in recent years. A thorough analysis requires that a distinction be made between the strategies for catalysis and the strategies for obtaining enantioselectivity, although considerable overlap exists between these components.
Catalysis of halofunctionalization reactions can be effected by Brønsted acids, Lewis acids, Lewis bases, and phase-transfer catalysts (Scheme 4). Brønsted and Lewis acids increase the electrophilicity of halogen sources by binding and withdrawing electron density. Moreover, protonation of N-haloimides may lead to halogen transfer to the conjugate base of the Brønsted acid, thus generating to a more electrophilic reagent (10).[17] Lewis bases are presumed to activate halogen sources by forming a polarized complex (11) in equilibrium with a highly reactive cationic halogen species (12), thus following the general mechanism for Lewis base activation of Lewis acids.[18]
Scheme 4.
General strategies for catalysis of halofunctionalization.
The aforementioned strategies for catalysis share a common feature: uncatalyzed, and hence unselective, halofunctionalization is suppressed by choosing a halogen source of sufficiently low reactivity such that it does not react with the substrate until activated by the catalyst. In contrast, phase-transfer catalysis does not require the catalyst to activate the halogen source, instead the halogen source and substrate are physically separated into different phases until brought together by the catalyst (Scheme 4).
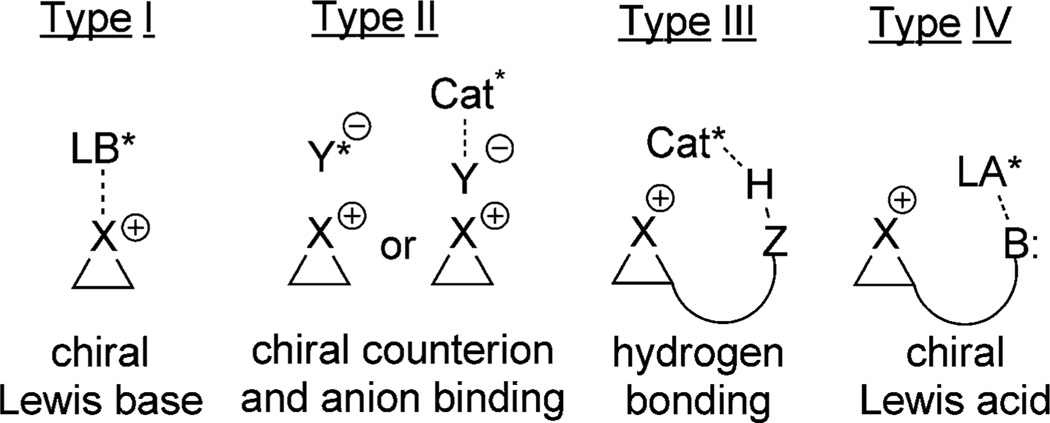
Engineering enantioselectivity in halofunctionalization reactions is more challenging than catalysis, and consequently this review is organized according to the strategies by which enantioselectivity is obtained. For bromo- and iodofunctionalization, any successful strategy must maintain an association between the chiral catalyst and the haliranium ion intermediate. In this scenario, racemization of the haliranium ion leads to diastereomeric ion pairs which can have different stabilities and reactivities. Existing efforts in this area may be divided into four categories according to the tactics applied to maintain the catalyst/haliranium ion association (Types I–IV, Figure 2).[19] This classification scheme leads to ambiguities because many successful examples employ more than one of these tactics and frequently multiple mechanisms are consistent with the available data. Nevertheless, this scheme provides a valuable framework for analyzing what is known, what is unknown, and what may be possible in this rapidly moving field.
Figure 2.
Classification of tactics for maintaining catalyst/haliranium ion association. LA = Lewis acid, LB = Lewis base.
The first tactic for maintaining a chiral environment is by association of the haliranium ion (Lewis acidic heteroatom) intermediate with a chiral Lewis base (Type I; Figure 2), provided that the Lewis base does not dissociate prior to nucleophilic capture of the haliranium ion. However, the paucity of catalytic enantioselective methods that operate purely by Lewis base complexation suggests that this strategy is most effective in combination with other control elements.
The second tactic is a variant of the first wherein the nature of the interaction between the catalyst and the Lewis acidic haliranium ion is Coulombic rather than dative (Type IIa; Figure 2). The formation of an ion pair with a chiral anion represents a distinct advantage because the principle of electroneutrality and the strength of Coulombic interactions guarantee the continuous association of such ion pairs in nonpolar media. Anion binding, wherein a Lewis or Brønsted acidic chiral catalyst binds to the achiral counter anion derived from the halogen source may be classified as a subset of the chiral ion-pair strategy (Type IIb).
The next two tactics differ from the first two in that the locus of association of the chiral catalyst is at a secondary site on the substrate, not at the haliranium ion. The majority of asymmetric halofunctionalizations reported to date make use of hydrogen-bonding interactions between the catalyst and the substrate to maintain proximity to the haliranium ion intermediate (Type III; Figure 2). This tactic is often accomplished by the incorporation of hydrogen-bond donors such as alcohols, carboxylic acids, and sulfonamides into the substrate, and hydrogen-bond acceptors such as phosphoryl groups and tertiary or heteroaromatic amines into the catalyst (Type IIIa). Because amines are also strong Brønsted and Lewis bases, an additional ambiguity as to their role in some systems is introduced. Alternatively, the catalyst may incorporate hydrogen-bond-donating functionality in the form of ureas, sulfonamides and thiocarbamates (Type IIIb).
Finally, the interaction of a Lewis basic site on the substrate with a chiral Lewis acid can provide the necessary chiral environment for activated capture of the haliranium ion (Type IV; Figure 2). The Lewis acid can activate either the nucleophile (Type IVa) or the electrophile (Type IVb).
4. Type I: Chiral Lewis Base Catalysis
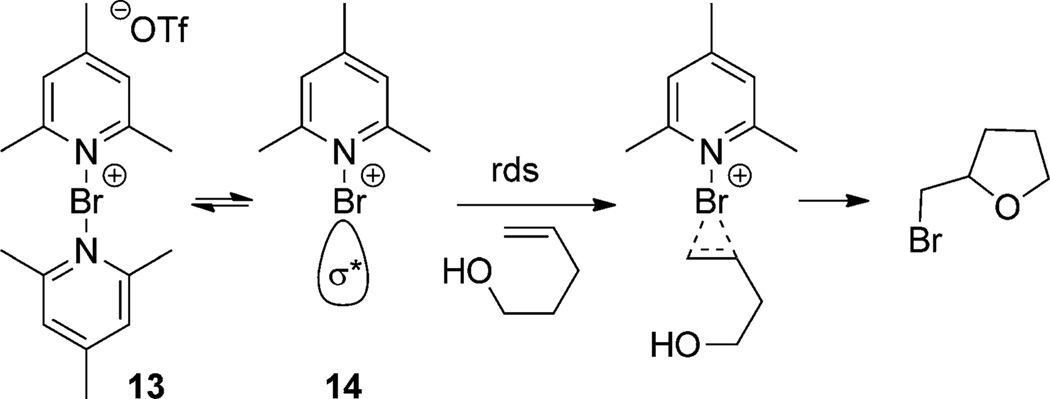
Some of the earliest work on enantioselective halocyclization by the groups of Grossman,[20] Brown,[21] and Wirth[22] focused on the use of preformed halogen/amine complexes (Scheme 5).[22a] None of these systems provided more than 75:25 e.r. in any case, however valuable insight into the mechanism of halocyclization was obtained. In particular, the rate-determining step in the reaction of bis(pyridinohalonium) triflates (Scheme 6) with alkenols is preceded by the reversible dissociation of one amine ligand to form the monoligated intermediate 14 which possesses an accessible N–Br σ* orbital.[23] The doubly ligated reagent 13 is coordinatively saturated and unreactive with alkenes. Simple consideration of the lack of available orbitals suggests that this predissociation equilibrium is likely common to all doubly ligated halogen(I) sources, as is the end-on approach dictated by the N–Br σ* orbital geometry.[24] These considerations have obvious implications for the depiction of reasonable reaction mechanisms.
Scheme 5.
Iodolactonization using a stoichiometric amount of an amine/ICl complex.
Scheme 6.
Mechanism of (collidinium)2Br+-promoted bromoetherification.
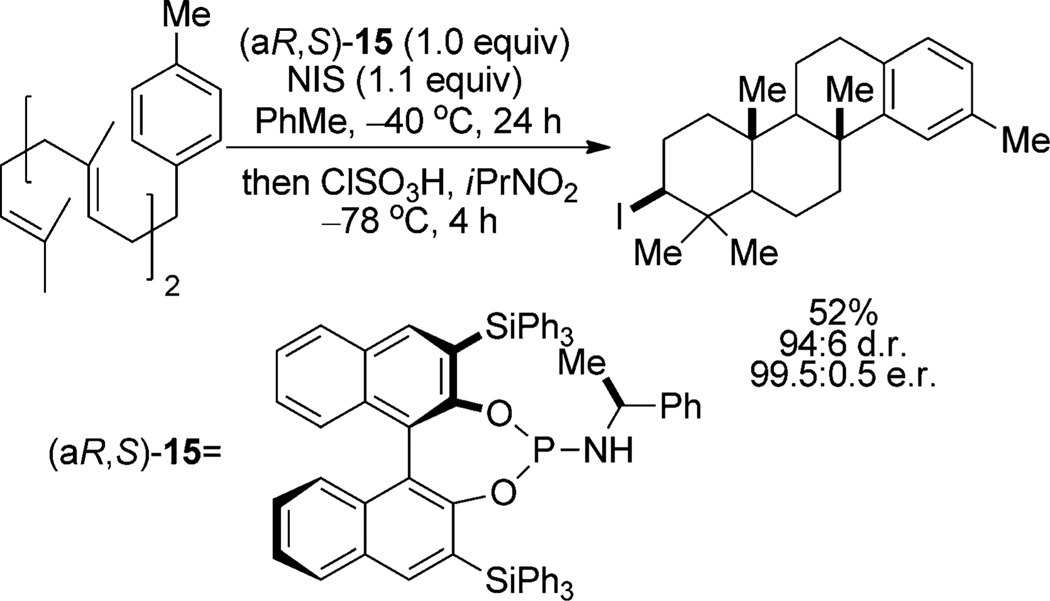
The first and only highly enantioselective halofunctionalization method using a stoichiometric amount of a chiral Lewis base is the polyene carbocyclization with N-iodosuccinimide (NIS) developed by Ishihara and co-workers (Scheme 7).[25] The chiral phosphoramidite, (aR,S)-15, is used in stoichiometric quantities, although the authors do not clarify whether this is required for reactivity or selectivity. Substoichiometric quantities of similar phosphoramidites are effective catalysts in dichloromethane, although only racemic products are obtained.
Scheme 7.
Phosphoramidite-promoted iodocarbocyclization.
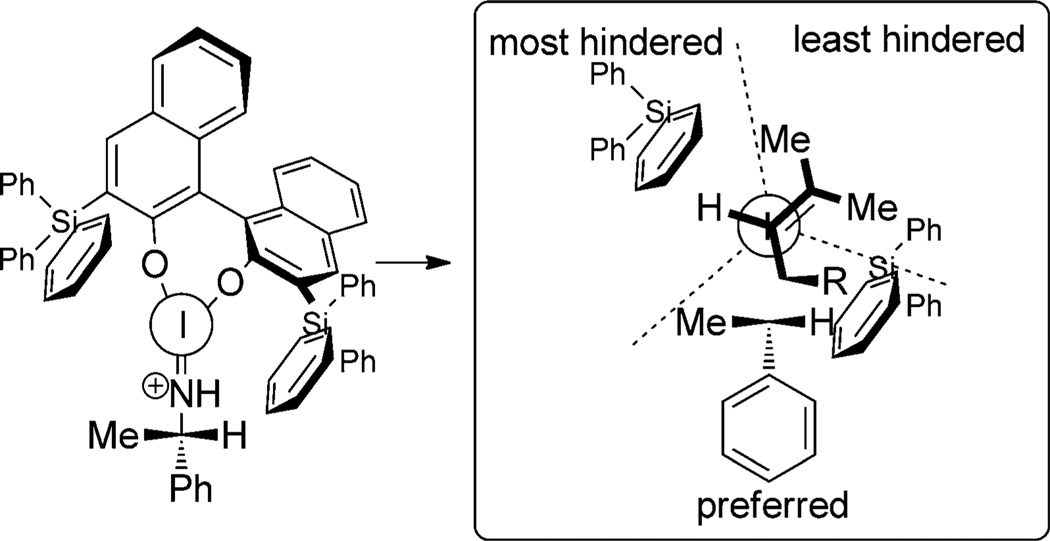
The authors hypothesize that in toluene a tighter ion pair is formed wherein hydrogen bonding between the succinimide counterion and the phosphoramide NH impose a hindered rotation around the P–N bond, thus leading to higher enantioselectivity at the cost of lower reactivity. A stereochemical model is advanced to rationalize the observed enantioselectivity wherein the reactive site is divided into three sectors (Figure 3). The preferred conformation places the bulky gem-dimethyl group in the least hindered upper-right sector and the hydrogen in the most hindered upper-left sector.
Figure 3.
Ishihara’s stereochemical model for phosphoramidite promoted iodocarbocyclization.
This model is predicated on the assumption that the origin of enantioselectivity is kinetically controlled which, given the weak nucleophile used to capture the iodiranium ion is highly unlikely.[26] Moreover, the formation of a tetracyclic product from a triene strongly suggests that iodiranium ion formation is reversible. Thus, the high selectivity and the need for a stoichiometric amount of the phosphoramidite are best explained by a Type I mechanism in which the more-reactive iodiranium catalyst complex is captured in the stereodetermining step.
5. Type II: Chiral Ion Pairing Catalysis
The formation of chiral ion pairs is one of the most successful strategies for enantioselective halofunctionalization reactions. Several recent advances have demonstrated the special utility of binol-derived phosphates as chiral counter anions in processes representing a variety of catalytic manifolds.[27]
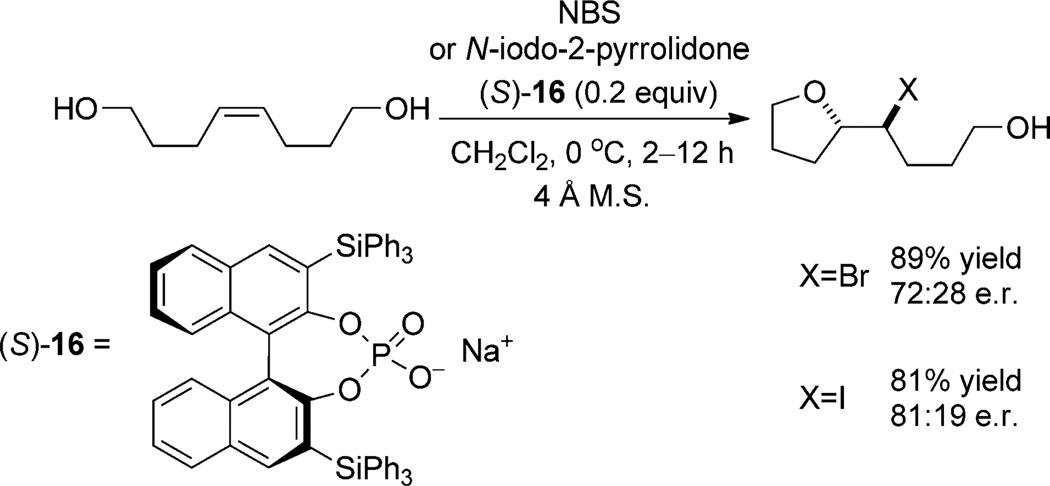
The utility of chiral phosphate counter anions in halofunctionalization reactions was first demonstrated by Hennecke et al. in the desymmetrization of meso haliranium ions formed from 1,8-oct-4-ene diols in the presence of the chiral sodium phosphate (S)-16 (Scheme 8).[28] Although the enantioselectivities achieved are moderate and the scope is limited by the symmetry requirements of the meso intermediate, these reactions serve as an excellent proof of concept. Because the haliranium ion is achiral (meso), the observation of enantioselectivity demonstrates that phosphate (S)-16 is associated with the haliranium intermediate during nucleophilic capture as it must distinguish enantiotopic, electrophilic sites. The authors propose that (S)-16 catalyzes the reactions by acting as an anionic Lewis base, which could potentially involve a phosphate hypoiodite intermediate.
Scheme 8.
Enantioselective desymmetrization of meso haliranium ions by pairing with a chiral phosphate counterion. M.S. = molecular sieves.
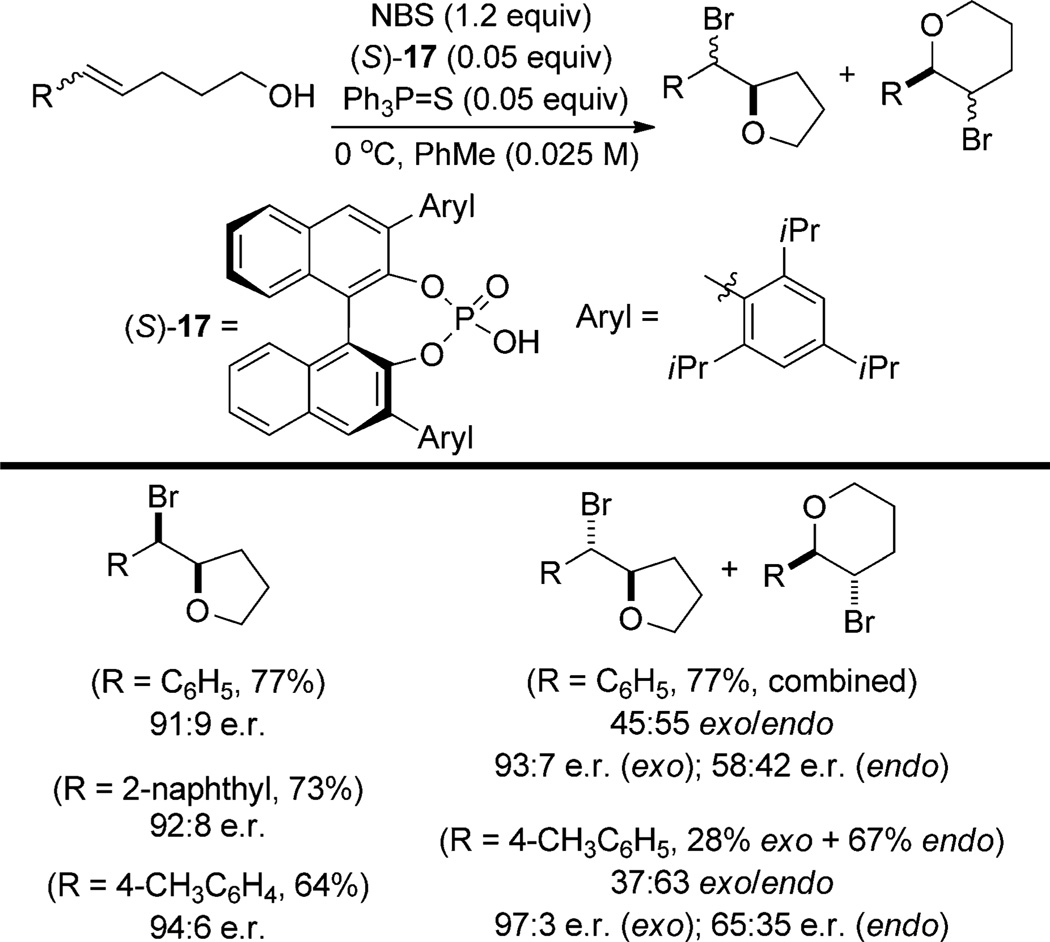
Enantioselective bromocycloetherification reactions using N-bromosuccinimide (NBS) and catalyzed by (S)-17 (TRIP[29] ; see Scheme 9) have been simultaneously developed by the groups of Denmark[30] and Shi.[31] Despite using the same chiral acid, several key differences between these studies should be noted.
Scheme 9.
Scope of chiral phosphoric acid/achiral Lewis base cocatalyzed bromocycloetherification.
In the Denmark system, (S)-17 is used together with a cocatalytic amount of triphenylphosphine sulfide (Scheme 9) and the range of substrates is limited to 5-aryl-4-pentenols.[30] Alcohols with Z-configured double bonds yield almost exclusively the product of exo cyclization in good to high enantioselectivity, however alcohols with E-configured double bonds result in mixtures of enantioenriched exo cyclization products and nearly racemic endo cyclization products.
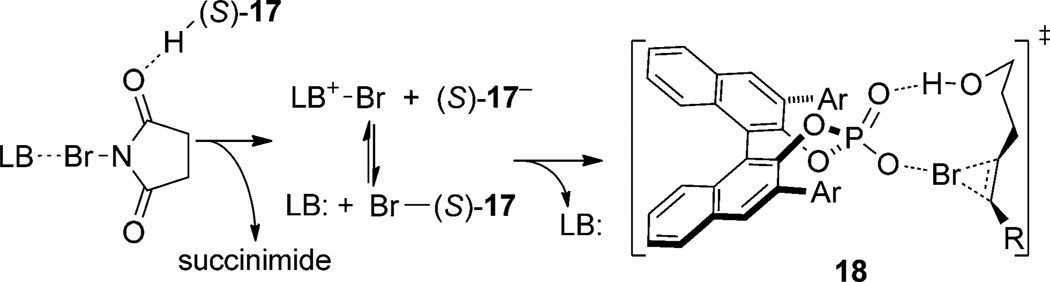
Cooperative activation of NBS by the Brønsted acid (S)- 17 and the Lewis base Ph3P = S is believed to form a phosphate hypobromite which transfers bromine to the substrate via the hydrogen-bonded transition state 18, thus involving a chiral bromonium·phosphate ion pair (Scheme 10). Thus, this system illustrates the ambiguity in identifying the controlling features at it possesses characteristics of Type I, Type II, and Type IIIa interactions.
Scheme 10.
Proposed mechanism of halogen activation and delivery in chiral phosphoric acid/achiral Lewis base cocatalyzed bromocycloetherifications.
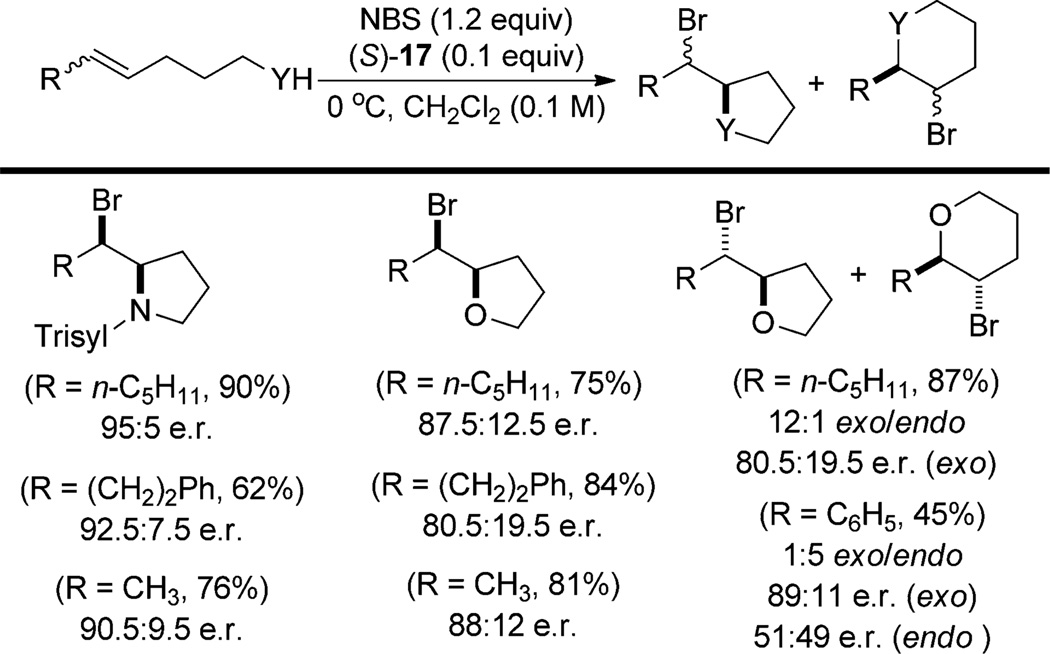
In contrast, the Shi system employs (S)-17 as the sole catalyst (Scheme 11).[31] Moderate to good enantioselectivities are obtained in the bromocycloetherification of a series of unconjugated alkenols, thus providing largely or exclusively the product of exo cyclization (bromofurans). The sole example of a conjugated alkene affords predominately the product of endo cyclization with negligible enantioselectivity although the minor product of exo cyclization was enantioenriched much like in the Denmark system. Bromocyclization of analogous sulfonamides proceeded with higher site- and enantioselectivity. The authors rationalize the observed selectivity on the basis of a model wherein the substrate acts as a hydrogen-bond donor and NBS accepts a hydrogen bond from the catalyst, thus classifying this as a Type III process (Figure 4).
Scheme 11.
Scope of chiral phosphoric acid catalyzed bromocyclofunctionalizations. Trisyl = 2,4,6-triisopropylbenzenesulfonyl.
Figure 4.
Proposed model of enantioselectivity in chiral phosphoric acid catalyzed bromocyclofunctionalizations.
Given the similarities between the Denmark and Shi systems it is unlikely that they have two different mechanisms of enantioselection; ergo, one (or both) of these proposals is probably incorrect. A key difference between these hypotheses is the presence or absence of succinimide in the transition-state structure. Thus experiments analogous to those performed by Borhan and co-workers[32] (see Scheme 22) might be able to distinguish these hypotheses.
Scheme 22.
Enantioselective chlorolactonizations employing chiral dichlorohydantoin 45.
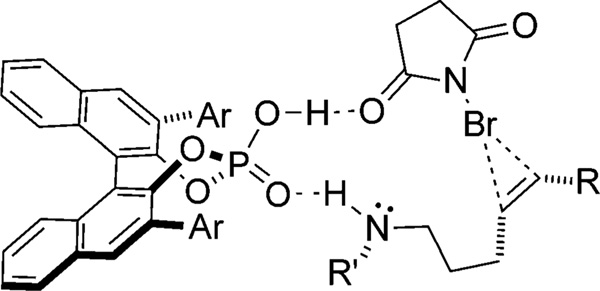
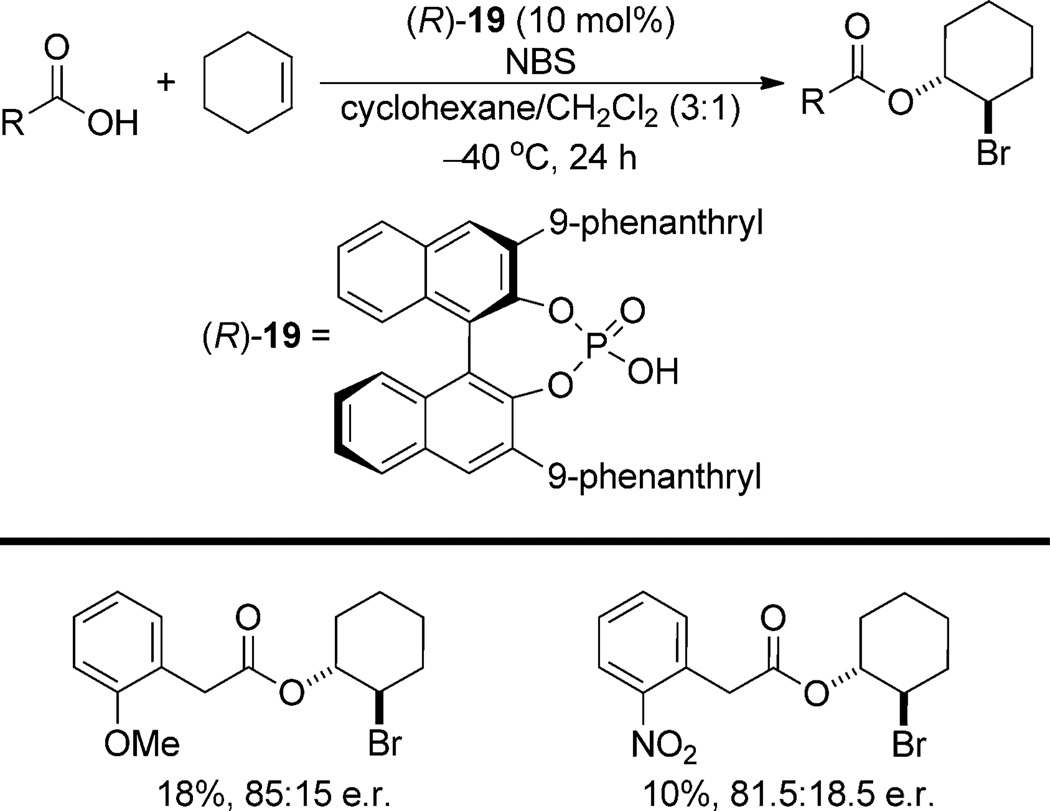
In principle, the nondissociating chiral environment provided by ion pairing with a chiral phosphate should allow the development of enantioselective halofunctionalization reactions even in cases where nucleophilic capture is relatively slow. However, efforts aimed at the development of an intermolecular bromoesterification reaction using NBS and the catalyst (R)-19 have uncovered a serious difficulty which likely results from slow capture of the bromonium ion (Scheme 12). The catalyst (R)-19 promotes the bromoesterification of cyclohexene with moderate but promising enantioselectivity in favorable cases.[33] Unfortunately, the products are obtained in low yield because capture of the bromonium ion by the chiral phosphate anion is competitive with capture by the carboxylic acid, thus resulting in catalyst deactivation.
Scheme 12.
Intermolecular bromoesterification.
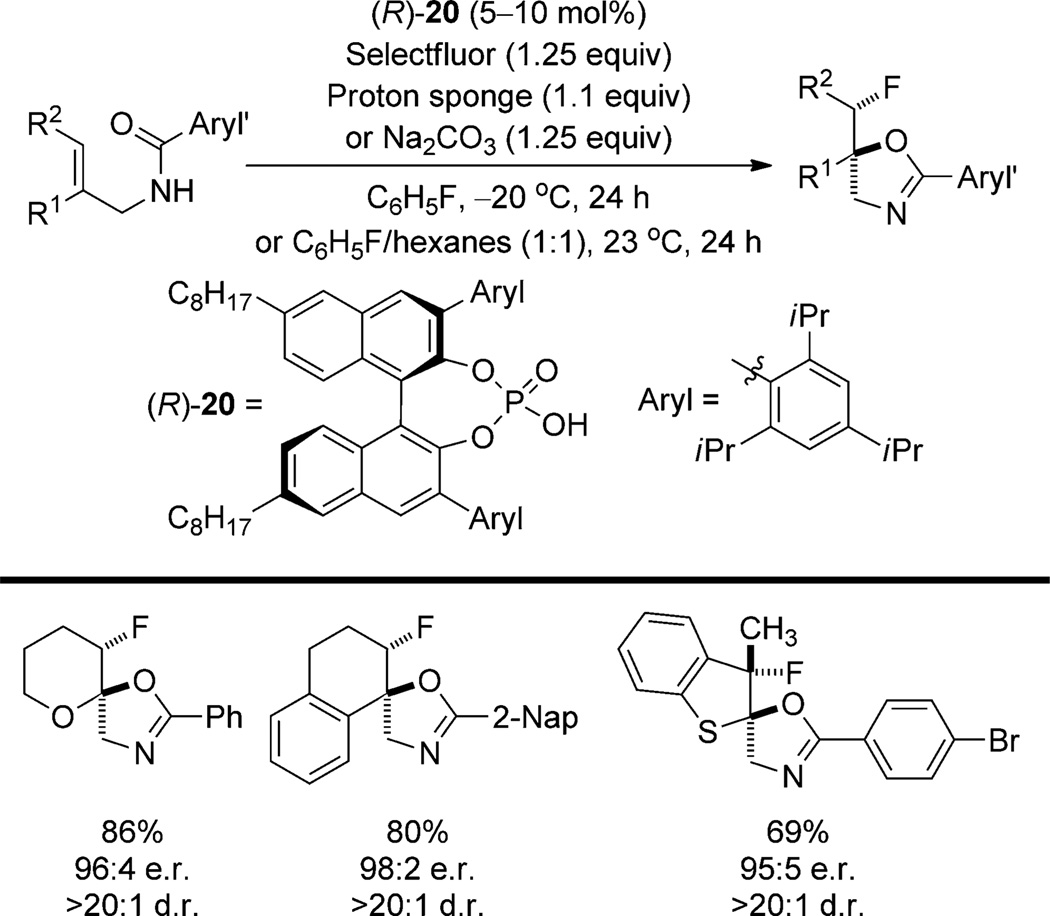
An unambiguous example of Type II stereocontrol is found in the enantioselective fluorocyclization reported by Toste et al. (Scheme 13).[34] This tactic takes advantage of the insolubility of dicationic Selectfluor in nonpolar solvents. Solid/liquid phase-transfer catalysis is provided by the solubilizing effect of ion exchange with the catalyst (R)-20, which is a more soluble analogue of (R)-17. This method is applicable to a variety of chromenes and dihydronaphthalenes. One example of an unconjugated, unactivated olefin is reported with an alternative catalyst. A nonlinear effect study[35] demonstrated that the active species has at least two chiral phosphate counterions in the stereodetermining transition structure. The substrates all contain cation-stabilizing groups to assist in the formation of the short-lived β-fluorocarbenium ion intermediate.
Scheme 13.
Enantioselective fluorocyclization by chiral phosphate, phase-transfer catalysis.
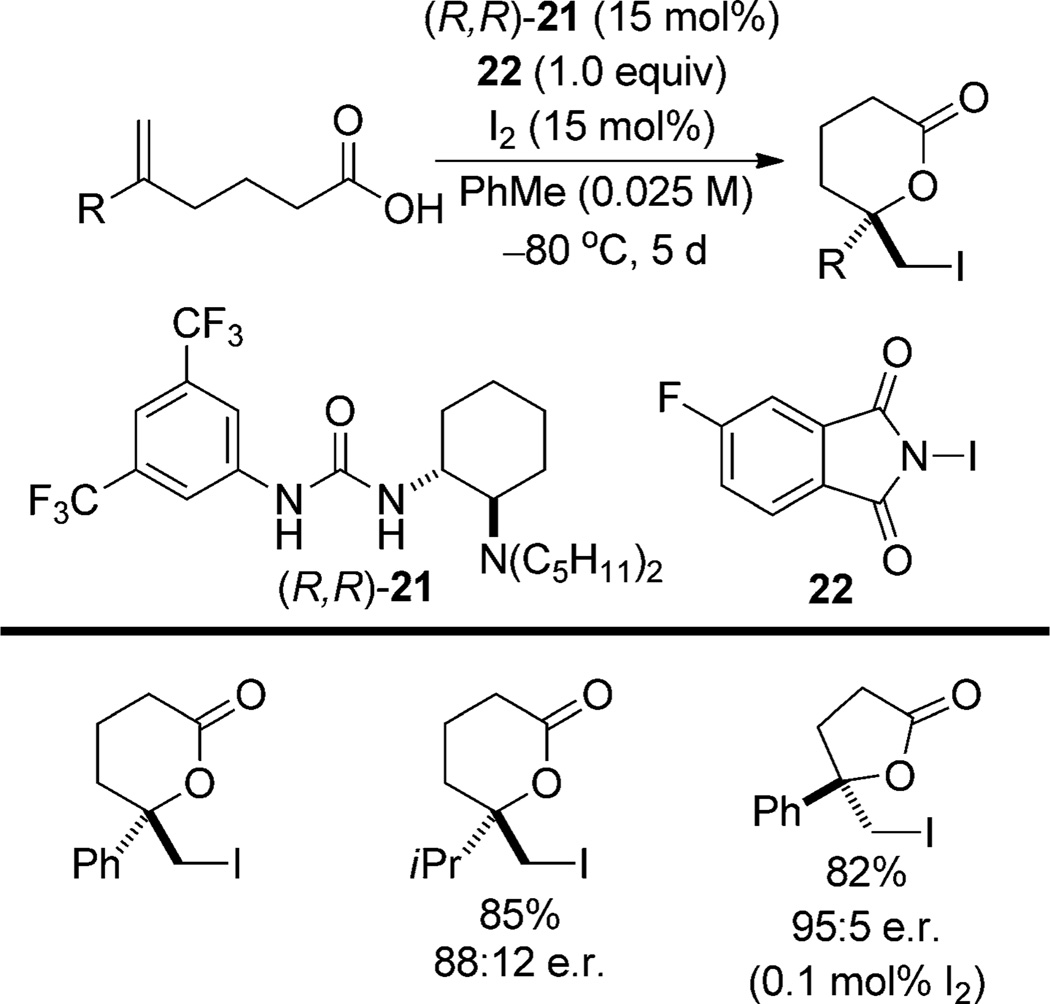
In a unique example of Type IIb stereoselection, Veitch and Jacobsen have applied their elegant anion-binding strategy for iodolactonization of δ,ε-unsaturated carboxylic acids with the amino urea catalyst (R,R)-21 and N-iodofluoro phthalimide (22; Scheme 14).[36] Both conjugated and unconjugated alkenes are suitable substrates for this system, although conjugation to electron-rich arenes results in low enantioselectivity. High enantioselectivity is observed also in iodolactonization of δ,ε-unsaturated carboxylic acids (with 0.1 mol% I2), although inexplicably, the absolute configuration of the butyrolactone is inverted compared to the valerolactones. The iodine additive may play multiple roles, but its primary function is believed to be formation of an activated complex with N-iodofluorophthalimide.
Scheme 14.
Scope of iodolactonization using the amino/urea catalyst (R,R)-21.
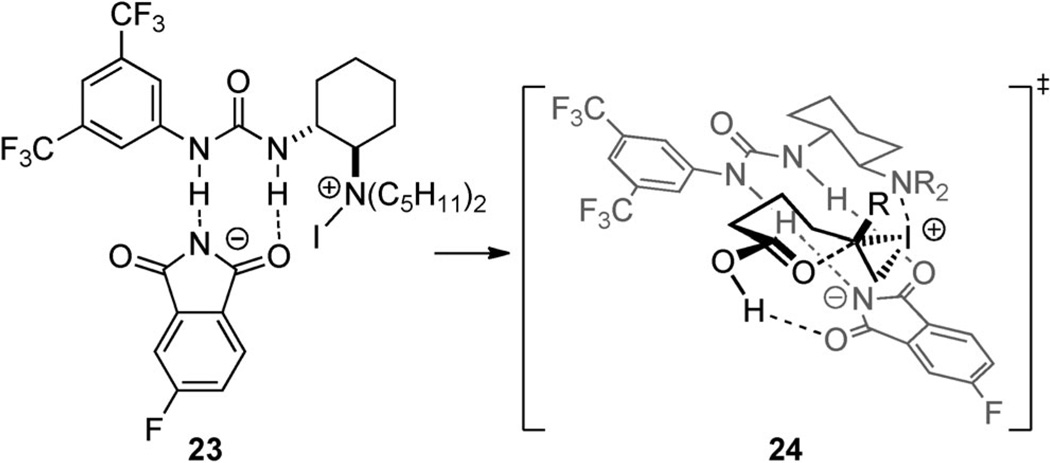
On the basis of NMR observations of the catalyst/reagent complex and re-isolation of catalyst degradation products, the authors propose that the urea binds 22 and the tertiary amine acts as a Lewis base to form 23 (Scheme 15). The resulting iodoammonium ion delivers the iodenium ion to the olefin while the fluorophthalimide, still hydrogen bonded to the urea, is poised to assist in proton transfer from the carboxylic acid (transition-structure 24). Thus the mechanism of stereoinduction involves aspects of Type I as well as Type IIIa behavior.
Scheme 15.
Proposed key intermediate and cyclization transition state for amino/urea-catalyzed iodolactonization.
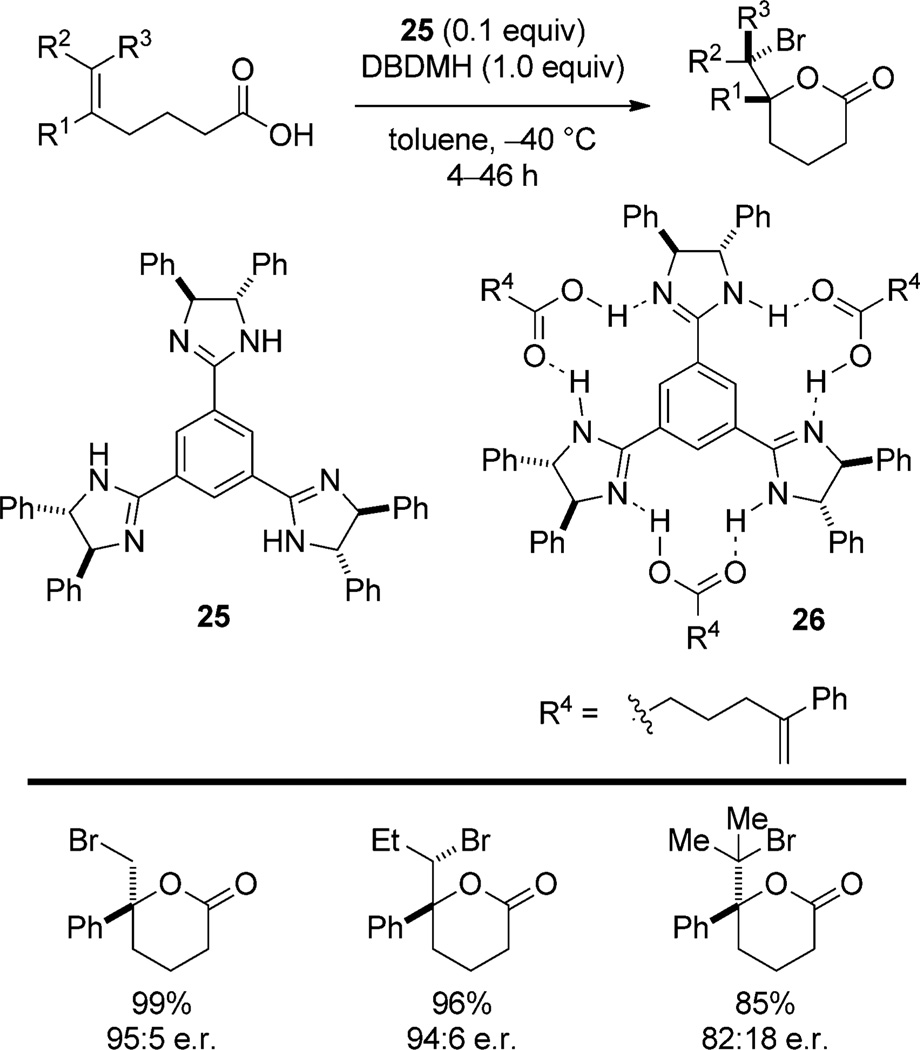
The C3-symmetric trisimidazoline 25 catalyzes the bromolactonization of 1,1-disubstituted,[37] trisubstituted, and tetrasubstituted[38] olefins in the presence of 1,3-dibromo-5,5- dimethylhydantoin (DBDMH; Scheme 16). All of the examples are 5-aryl-substituted hexenoic acids (R1) but both aromatic and aliphatic substituents can be incorporated at the terminus (R2/R3). Interestingly, the di- and monoimidazolinyl-substituted arene catalysts afford successively lower enantioselectivities, thus indicating that the C3-symmetry is necessary for selectivity. Fujioka and co-workers posit the intermediacy of the complex 26, which is believed to enforce a conformational bias in the substrate. To explain the origin of catalysis, one could propose a formal deprotonation of the carboxylic acid residue which results in the formation of a chiral imidazolinium/carboxylate ion pair. The carboxylate anion is directed by the chiral cation to trap the rapidly interconverting bromiranium ion via diastereomorphic transition structures. Therefore, this system falls in the Type IIa classification because ion pairing is invoked to justify both catalysis and stereoinduction. However, although 26 has been observed by 1H NMR spectroscopic analysis of a 3:1 mixture of acid and catalyst, it is not certain that this complex is kinetically competent. As a result, the authors could not exclude the possibility that one imidazoline activates the bromiranium electrophile through Lewis base activation (Type I association) while another imidazoline activates and directs the nucleophile through hydrogen bonding (Type IIIa) or deprotonation/chiral ion pairing (Type IIa).
Scheme 16.
C3-Symmetric trisimidazolinylbenzene-catalyzed bromolactonization.
6. Type III: Hydrogen Bonding—Catalysis or Stereoinduction?
In all of the examples of the Type III category, a Lewis-basic heteroatom (N, O, S) is present, and in many cases is hypothesized to be involved in catalysis by formation of a kinetically active halenium ion complex (8-X-1)+. However, the failure of such species to provide high levels of enantioselectivity by themselves[30] requires the incorporation of secondary organizing interactions to: 1) maintain association of the chiral haliranium ion with the catalyst or 2) activate/align the nucleophilic group for selective capture of the haliranium ion. The examples below all invoke some form of hydrogen-bonding interaction for stereoinduction (Type IIIa or IIIb) and are organized by the Lewis-basic atom involved in catalysis.
6.1. Lewis Base Catalysis with Sulfur
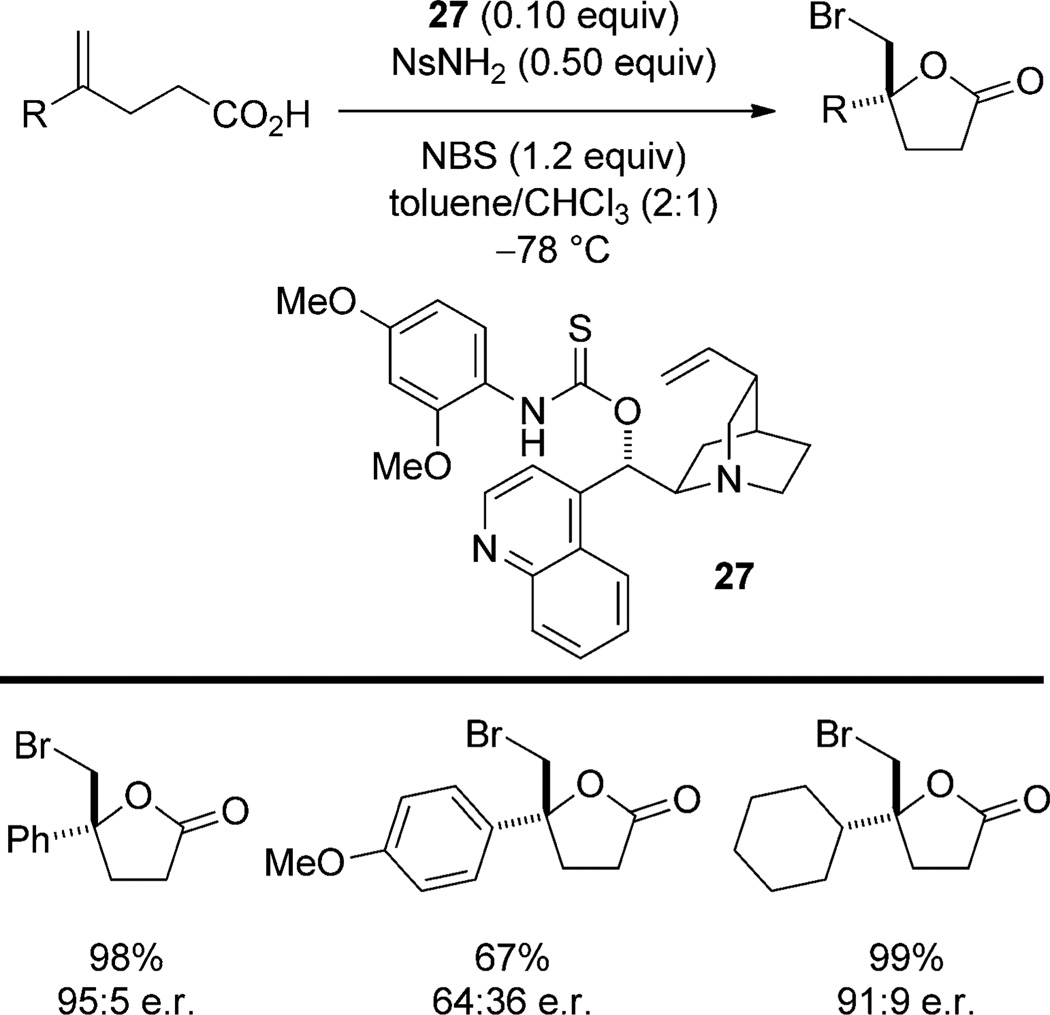
Sulfur-containing functional groups are among the most effective Lewis base catalysts for the activation of bromine and iodine sources.[39] The catalytic activity of sulfur is parlayed with the hydrogen-bonding ability of Cinchona-derived O-thiocarbamate catalysts 27 for enantioselective bromocyclization reactions. This class of catalysts has been employed in the cyclization of 1,1-disubstituted[40] (Scheme 17) and 1,2-disubstituted[41, 42] alkenes to generate halolactones, the cyclization of unsaturated amides for the preparation of 3-bromopyrrolidines,[43] and the cyclization of 2-vinyl-benzoic acids for the synthesis of 3,4-dihydroisocoumarins.[44] In all of these transformations, the enantioselectivities are generally high.
Scheme 17.
Enantioselective bromolactonization catalyzed by cinchonine-derived thiocarbamate 27.
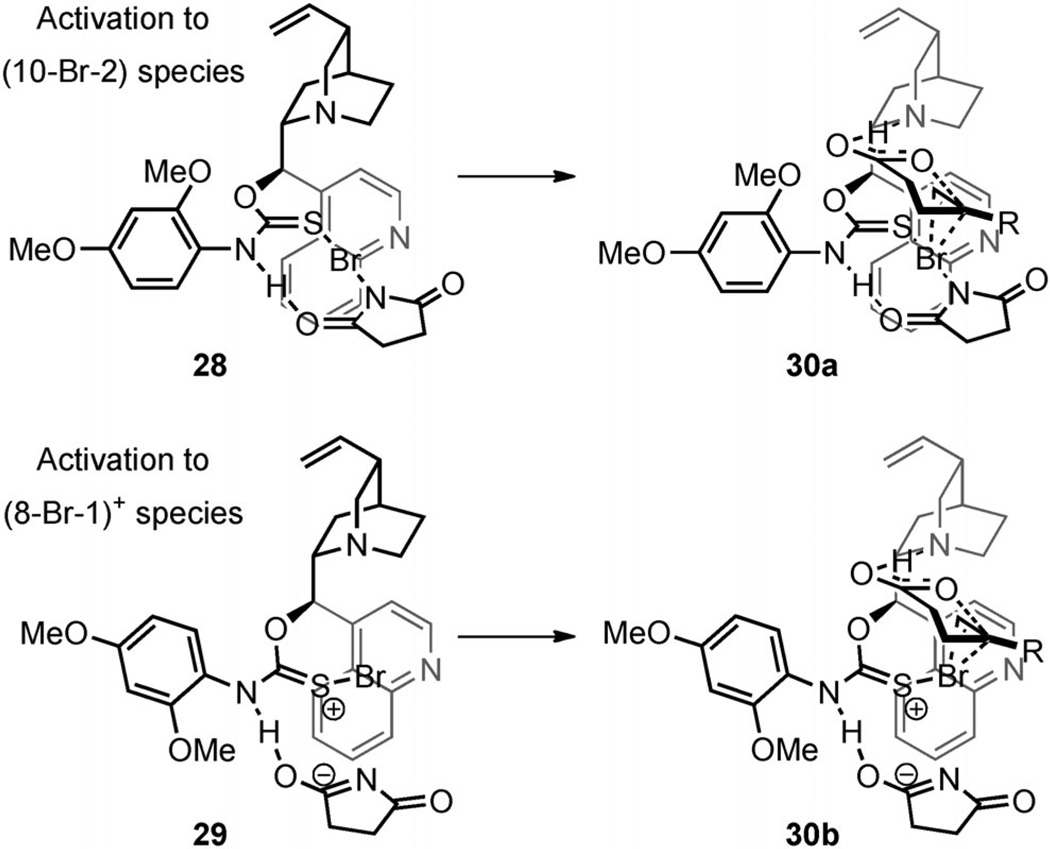
On the basis of the known reactivity of sulfur-containing catalysts,[39] Yeung and co-workers propose that the sulfur of the thiocarbamate acts as a Lewis base to activate NBS through binding of the bromine atom. The detailed nature of this interaction is not specified, that is, hypercoordinate bromine (10-Br-2) as in 28 or an ionized complex such as 29 (Figure 5). In addition, the authors propose a hydrogen-bonding interaction between the thiocarbamate NH and the succinimide oxygen atom (30). This complex is then believed to undergo electrophilic attack on the alkene and the pendant nucleophile is then directed by the quinuclidine nitrogen atom to capture the bromiranium ion.
Figure 5.
Possible models of activation and asymmetric induction by catalyst 27.
Although it is very likely that activation involves one or more of these interactions, no mechanistic studies have been conducted to establish the composition of the transition state. To reconcile this model with the findings of Neverov and Brown (Scheme 6), however,[23] the thiobromonium complex 28 must dissociate from the succinimidate by ionization prior to attack by the alkene to expose the relevant S–Br σ* orbital as in 30b. In this case, the succinimidate likely remains coordinated to the catalyst–substrate complex through hydrogen bonding similar to models proposed by Ishihara and co-workers[25] (Figure 3) and Veitch and Jacobsen[36] (Scheme 15).
6.2. Lewis Base Catalysis with Nitrogen
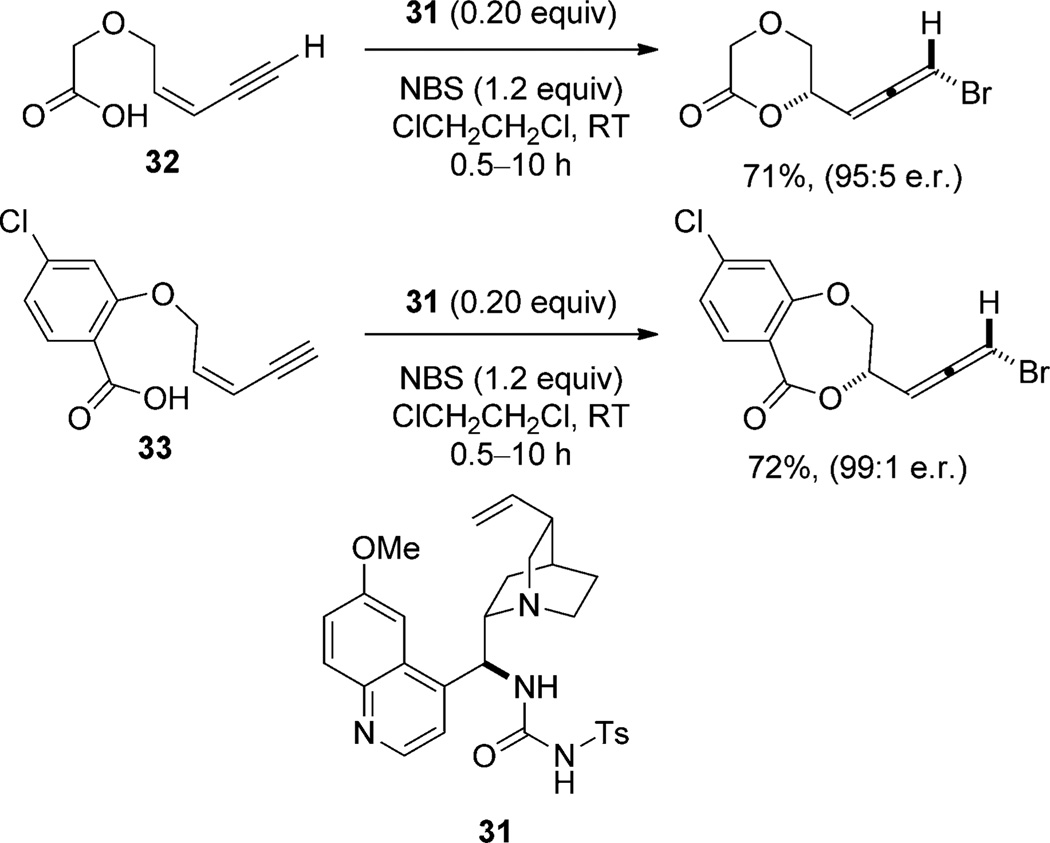
Lewis base catalysis of the bromolactonization (with NBS) of conjugated Z enynes using 1,4-diazabicyclo-[2.2.2]octane (DABCO) affords racemic bromoallenes in good yield.[45] Subsequently, an enantioselective variant was developed by Tang and co-workers using the Cinchonaderived tosyl urea 31 (Scheme 18). Highly selective bromolactonization of the 1,3-enynes 32 and 1,3-enynyl benzoic acids 33 are achieved.[46] This same class of catalysts, modified with a linker, are adapted for the chlorolactonization of simple olefins with moderate to high enantioselectivities.[47]
Scheme 18.
Bromolactonization of Z-1,3-enynes catalyzed by quinine-derived urea 31.
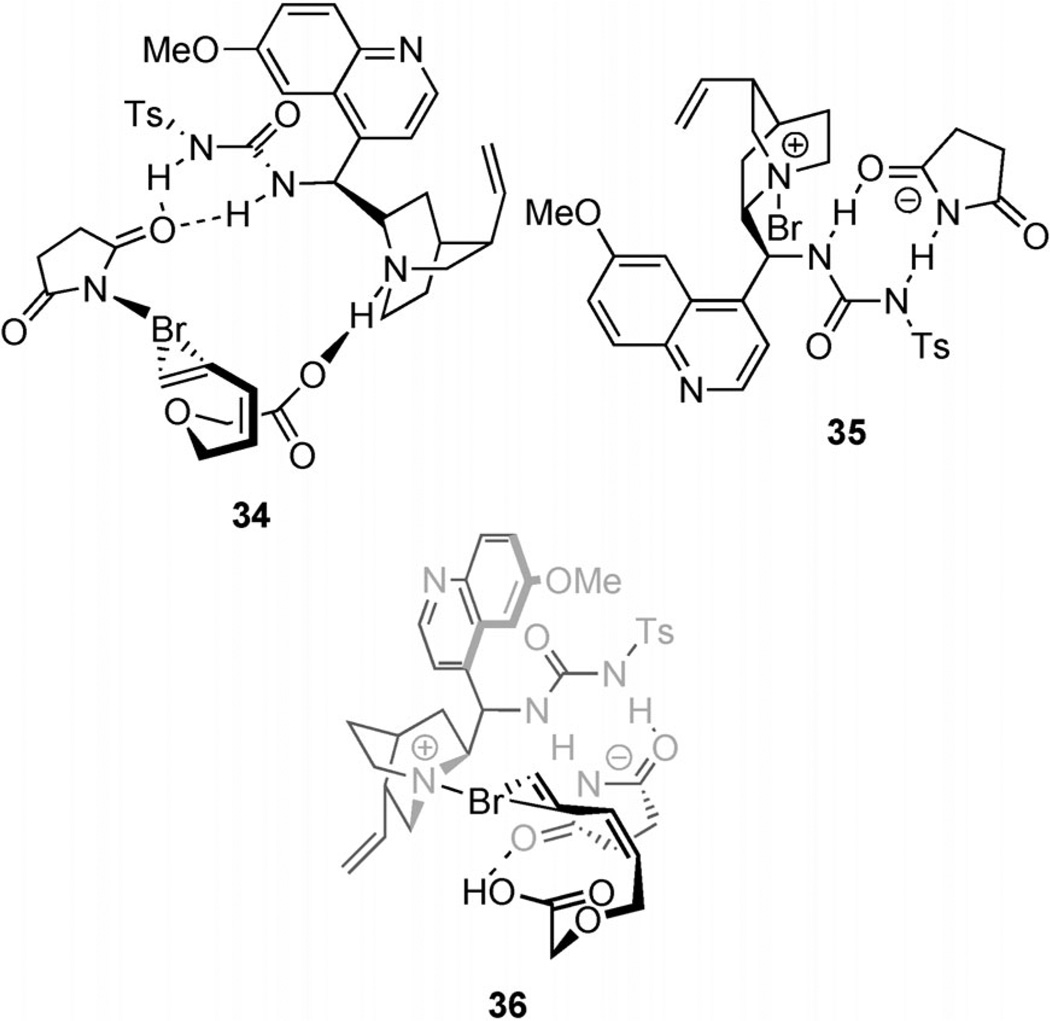
Despite their own observations concerning catalysis by DABCO, the authors propose that the catalyst 31 activates NBS toward electrophilic attack on an alkene through hydrogen bonding between the NH groups of the urea and the succinimide oxygen atom. The nucleophile, they hypothesize, is directed by Brønsted acid/base interactions with the quinuclidine nitrogen atom (34; Figure 6).[47] This hypothesis arises from two observations. The first is the dependence of enantioselectivity on the halogen source which implies an associative pathway. Furthermore, when 31 (0.2 equiv) and NBS (1.2 equiv) are incubated for 20 minutes prior to addition of 32, the enantioselectivity of cyclization is reduced from 95:5 to 77:23 with no change in yield. With 4 hours of incubation, the enantiomeric composition drops to 63:37. On the basis of these experiments, the authors conclude that the reaction between the catalyst and NBS is not part of the enantioselective pathway.
Figure 6.
Possible intermediates in the bromolactonization of (Z)-1,3-enynes.
The first observation is consistent with several other examples of halocyclization[36, 48] which describe similar dependence on an interaction with an anion. These reports invoke a halogen ionization pathway in which an achiral anion, typically the conjugate anion of the halenium source, is associated with the catalyst through ion-pairing interactions and thereby modulates the stereocontrolling ability of the catalyst.
However, another likely interpretation, given the similarity of 31 to the tertiary amino urea catalyst employed by Veitch and Jacobsen[36] (see Scheme 15), is that the catalyst serves as a Lewis base activator of NBS by nucleophilic attack on the bromine by the quinuclidine nitrogen atom. The catalyst then binds the succinimidate by hydrogen bonding at the urea (35; Figure 6). This intermediate would then electrophilically attack an alkene while the nucleophile is directed by hydrogen bonding with succinimidate (36). This hypothesis is reconciled with the second observation if in the absence of substrate, the active N-bromenium intermediate is converted into a species that remains active but is less selective (e.g. bromination of the vinyl group in the catalyst). In the presence of substrate, the self-reaction of the N-bromenium intermediate is suppressed, and thus selectivity is maintained. This interpretation would illustrate another example of combined Type I and Type IIIa behavior.
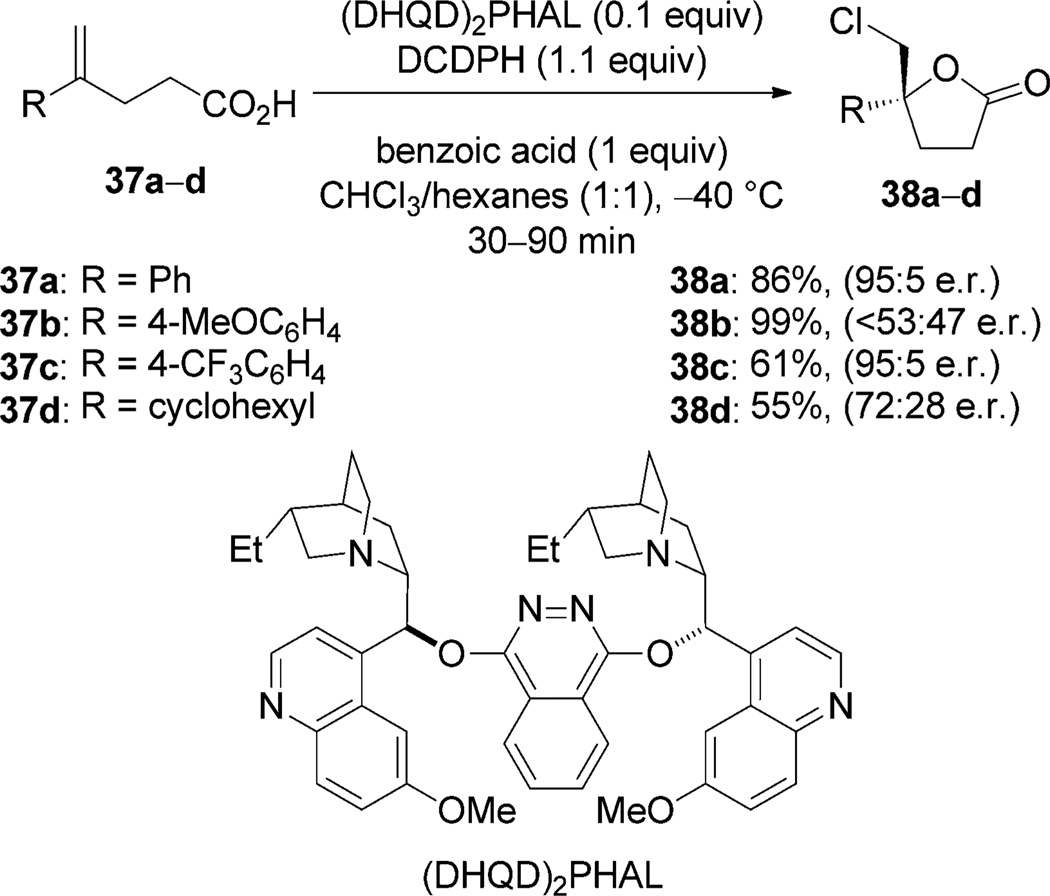
The commercially available, dimeric Cinchona-alkaloid derivatives (DHQD)2PHAL and (DHQD)2PYR have been employed to catalyze fluoro-, chloro-, and bromofunctionalization reactions. These catalysts likely operate through a typical Type IIIa mechanism as they are primarily hydrogen- bond acceptors and work with substrates bearing hydrogen-bond donor groups (carboxylic acids, amides, alcohols, and/or sulfonamides). For example, (DHQD)2PHAL catalyzes the chlorolactonization reactions of the 1,1-disubstituted alkenes 37 with 1,3-dichloro-5,5-diphenylhydantoin (DCDPH) as the stoichiometric chlorenium source in the presence of benzoic acid. (Scheme 19).[49] Butyrolactones 38 are generated with high enantioselectivity from electron-poor and electron-neutral aryl-substituted olefins; lower selectivity is observed when electron-rich styrenes and aliphatic olefins are used as substrates.
Scheme 19.
Catalytic asymmetric chlorolactonization with (DHQD)2PHAL.
(DHQD)2PHAL also effects the catalytic, enantioselective formation of the 1,3-oxazolines 41 a–c and 1,3-oxazines 42a–d from unsaturated amides (Scheme 20).[50] The arylsubstituted allylic benzamides 39 a–c and 40 a,b cyclize with high enantioselectivity in 2,2,2-trifuoroethanol.With a change of solvent to 1-nitropropane and the addition of molecular sieves, the alkyl-substituted 42c and 42d are also obtained with high enantiomeric purity. Finally, upon acidic hydrolysis of the imidate moiety of 41 and 42, amino alcohols are produced without erosion of enantiomeric composition.
Scheme 20.
Catalytic asymmetric formation of chiral oxazolines and 4,5-dihydrooxazines.
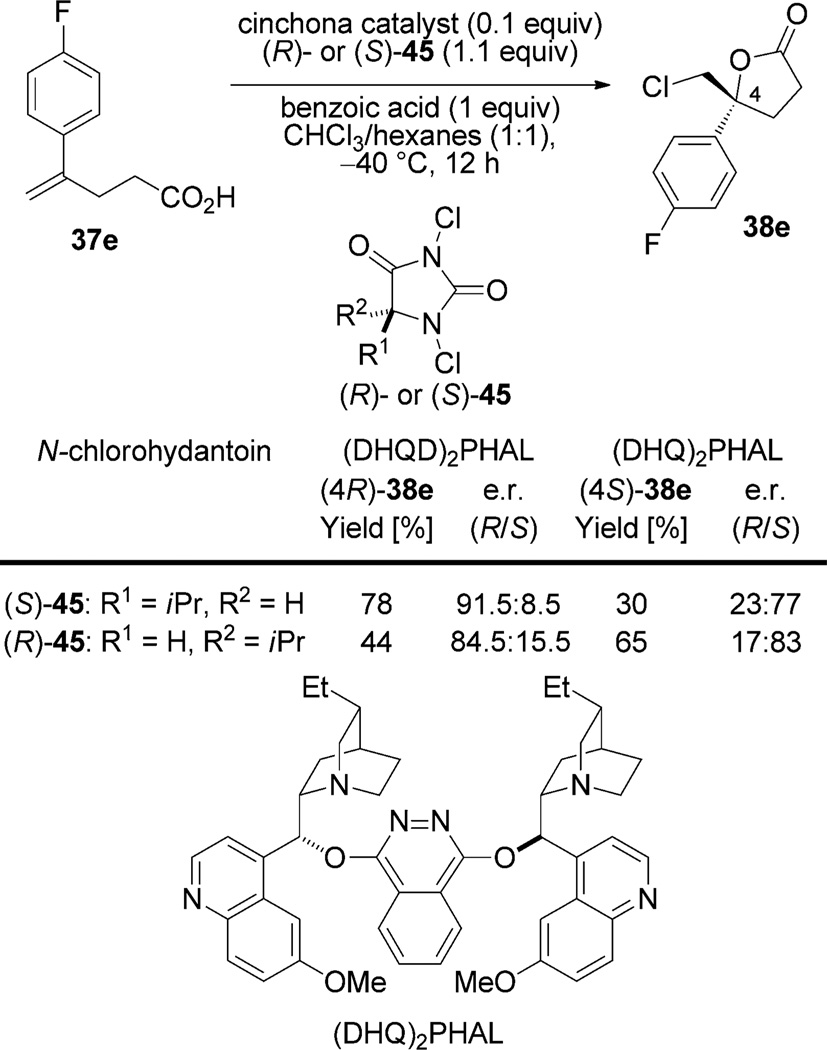
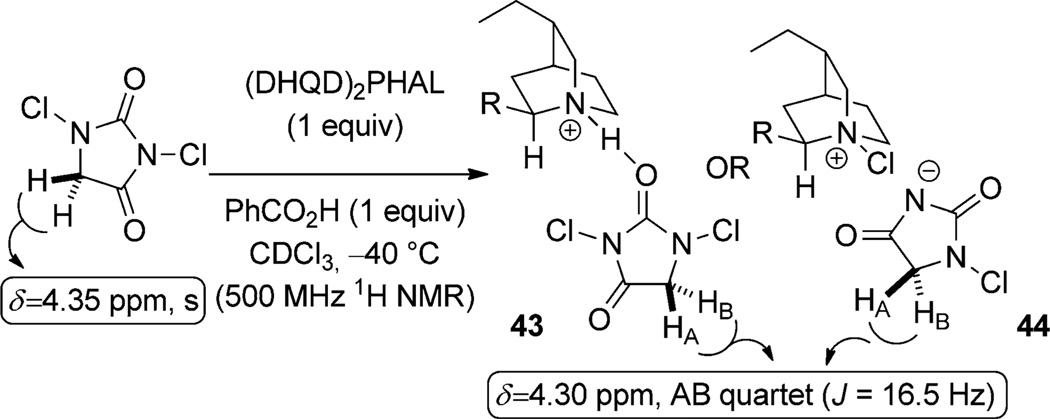
A number of experiments have shed light on the enantiodetermining interactions in these cyclizations. The first of these experiments demonstrates that a close association of the hydantoin to the quinuclidine residue of the dihydroquinidine dimer contributes to the high selectivity of these reactions. Increasing the steric demand of the C5-position of the hydantoin leads to an increase in reaction selectivity.[49] This observation suggests that the hydantoin must remain associated with the catalyst during the formation of the chloriranium ion. Furthermore, a complex of 1,3-dichlorohydantoin (DCH) and (DHQD)2PHAL is observed by 1H NMR spectroscopy upon combination of DCH with 1.0 equivalent of the catalyst and 2.0 equivalents of the benzoic acid in CDCl3 at −40 °C (Scheme 21). The enantiotopic protons of DCH (δ= 4.35 ppm, s) are rendered diastereotopic and appear as an AB quartet at higher field (δ =4.30 ppm, JAB = 16.5 Hz) in the complex (43 or 44).[49]
Scheme 21.
Observation of the hydantoin/catalyst complex and proposed modes of binding.
The most recent evidence for the intermediacy of this complex derives from a study in which each enantiomer of 5-isopropyl-1,3-dichlorohydantoin (45) is employed in the reaction with both (DHQD)2PHAL and pseudoenantiomeric (DHQ)2PHAL (Scheme 22).[32] If (DHQD)2PHAL is employed as the catalyst, (S)-45 affords the lactone (4R)-38 e in 78% yield and 91.5:8.5 e.r. However, when (R)-45 is used as the chlorenium source, the yield and e.r. value are reduced to 44% and 84.5:15.5, respectively, thus demonstrating a matched/mismatched relationship. The observed trends are reversed with (DHQ)2PHAL, and the magnitudes of the differences are also reduced because (DHQ)2PHAL is a less selective and efficient catalyst.
Two different mechanistic models are proposed from this data (Scheme 21). The first proposal involves the hydrogen-bonded complex 43 between the protonated quinuclidine and the hydantoin. The second posits the formation of an N-chloroquinuclidinium species ion paired with the conjugate base of the hydantoin (44). Given the necessity of a stoichiometric amount of a Brønsted acid in the lactonization reaction, these authors conclude that the first complex, 43, is the relevant intermediate. However since the cyclization of unsaturated amides occurs without Brønsted acid (Scheme 20), it is likely that both transformations proceed via complex 44.
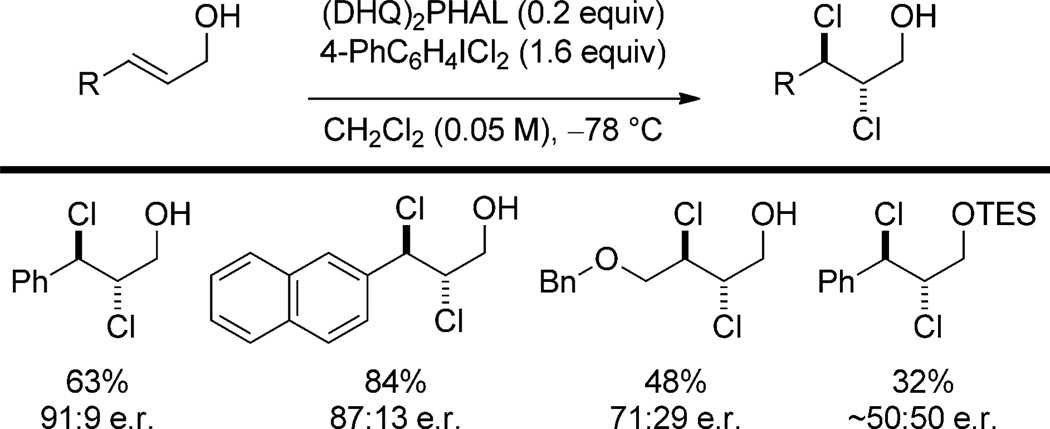
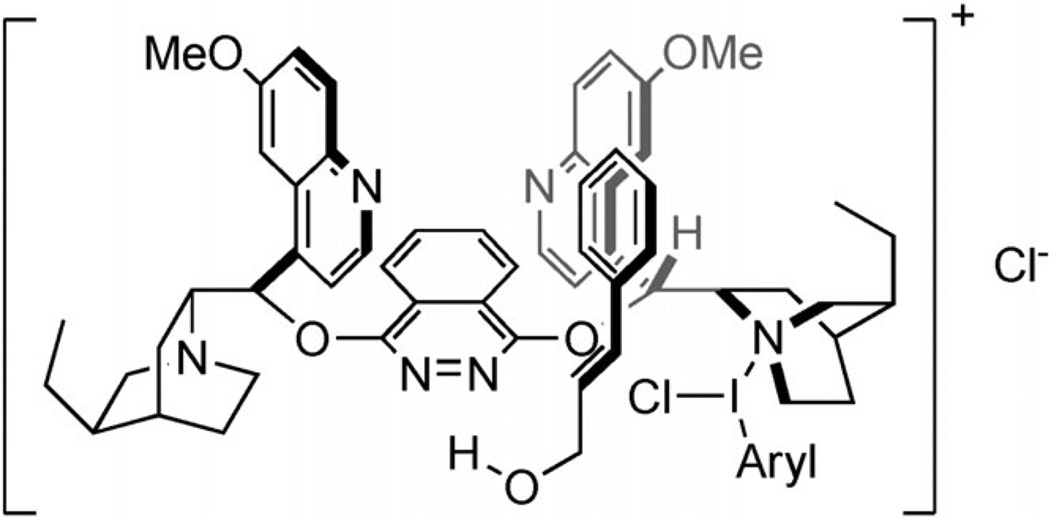
These same Cinchona alkaloids are also employed as Lewis-basic catalysts in dichlorination reactions. Nicolaou and co-workers have reported that (DHQ)2PHAL catalyzes the 1,2-dichlorination of allylic alcohols with aryl iododichlorides in moderate to good enantioselectivities.[51] (Scheme 23) The observed facial selectivity is the same as that observed in the Sharpless asymmetric dihydroxylation in which (DHQ)2PHAL is used as a ligand. Therefore, the authors propose a model similar to those proposed for the Sharpless dihydroxylation,[52] in which an activated chlorenium ion is generated by nucleophilic attack by a quinuclidine nitrogen atom on the aryl iododichloride. Because protection of the allylic alcohol is detrimental to enantioselectivity, the authors invoke hydrogen bonding with the phthalazine moiety to determine facial selectivity of attack on the chlorenium ion (Figure 7).
Scheme 23.
Catalytic dichlorination of allylic alcohols. TES = triethylsilyl.
Figure 7.
Proposed model of selectivity for dichlorination.
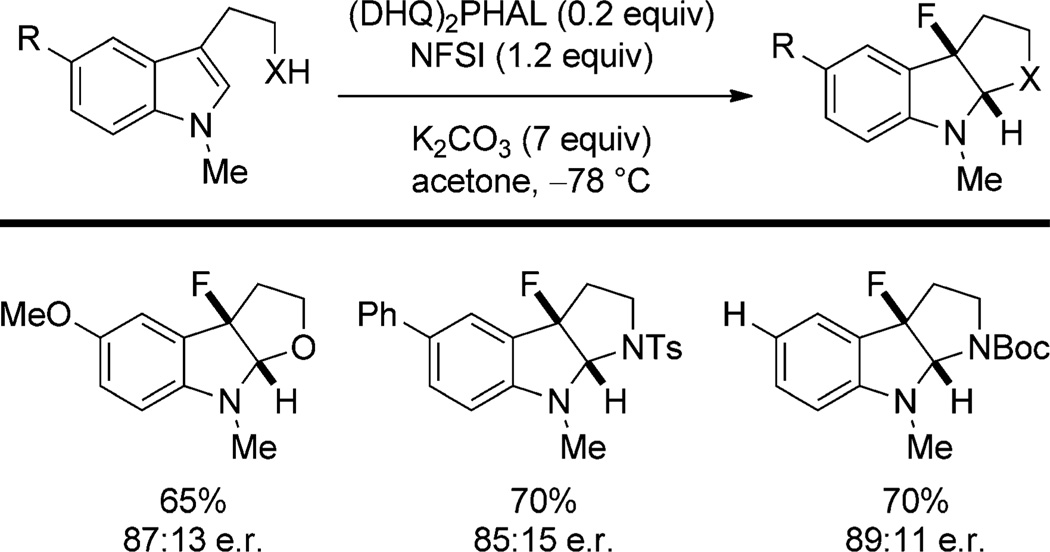
Dimeric Cinchona alkaloids have also found utility in enantioselective fluorocyclizations and halenium-ion-induced semipinacol rearrangements. Gouverneur and co-workers[53] have developed an enantioselective fluorocyclization of indoles with Selectfluor and a stoichiometric amount of (DHQ)2PHAL. The same transformation may also be performed catalytically with (DHQ)2PHAL (0.2 equiv) and N-fluorophenylsulfonimide (NFSI) with similar enantioselectivities (Scheme 24).
Scheme 24.
(DHQ)2PHAL-catalyzed fluorocyclization of indoles. Boc = tert-butoxycarbonyl.
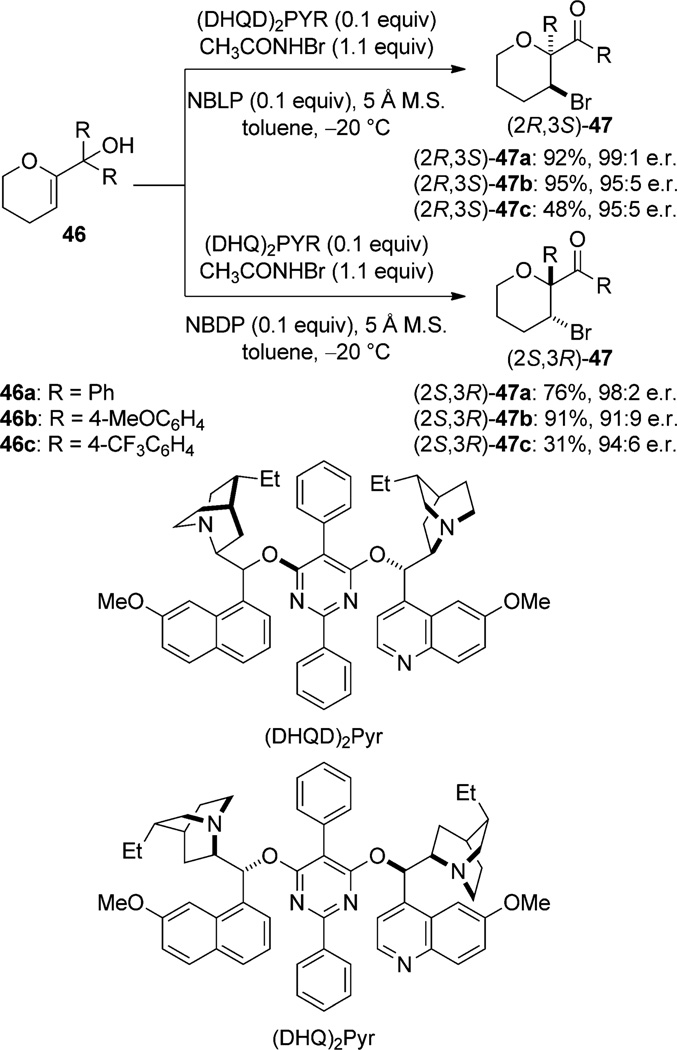
In the bromine-induced semipinacol rearrangement of the tertiary allylic alcohols 46 to bromoketones 47, both enantiomeric products of the rearrangement are accessible with either (DHQD)2PYR (0.1 equiv) and N-Boc-l-phenylglycine (NBLP, 0.1 equiv) or (DHQ)2PYR (0.1 equiv) and N-Boc-d-phenylglycine (NBDP, 0.1 equiv) with N-bromoacetamide (Scheme 25).[54] The use of 1,3-dichloro-5,5-dimethylhydantoin (DCDMH) as the halogen source requires that the reaction be carried out at −50 °C, and the result is a slightly less enantioselective and considerably lower yielding transformation.
Scheme 25.
Enantioselective bromine-induced semipinacol rearrangement.
Neither the mechanism nor the source of asymmetric induction of either of these transformations is understood. As in the previous examples of catalysis by dimeric Cinchona alkaloids, it is possible, if not likely, that a coordinated halenium ion is generated by nucleophilic attack of one of the quinuclidine nitrogen atoms of the catalyst on the halogen source, and that this complex transfers the halenium ion to the alkene. In the absence of mechanistic data, however, little else can be speculated, such as the origin of enantiotopic facial selectivity, or in the case of fluorocyclization, diastereoselectivity.
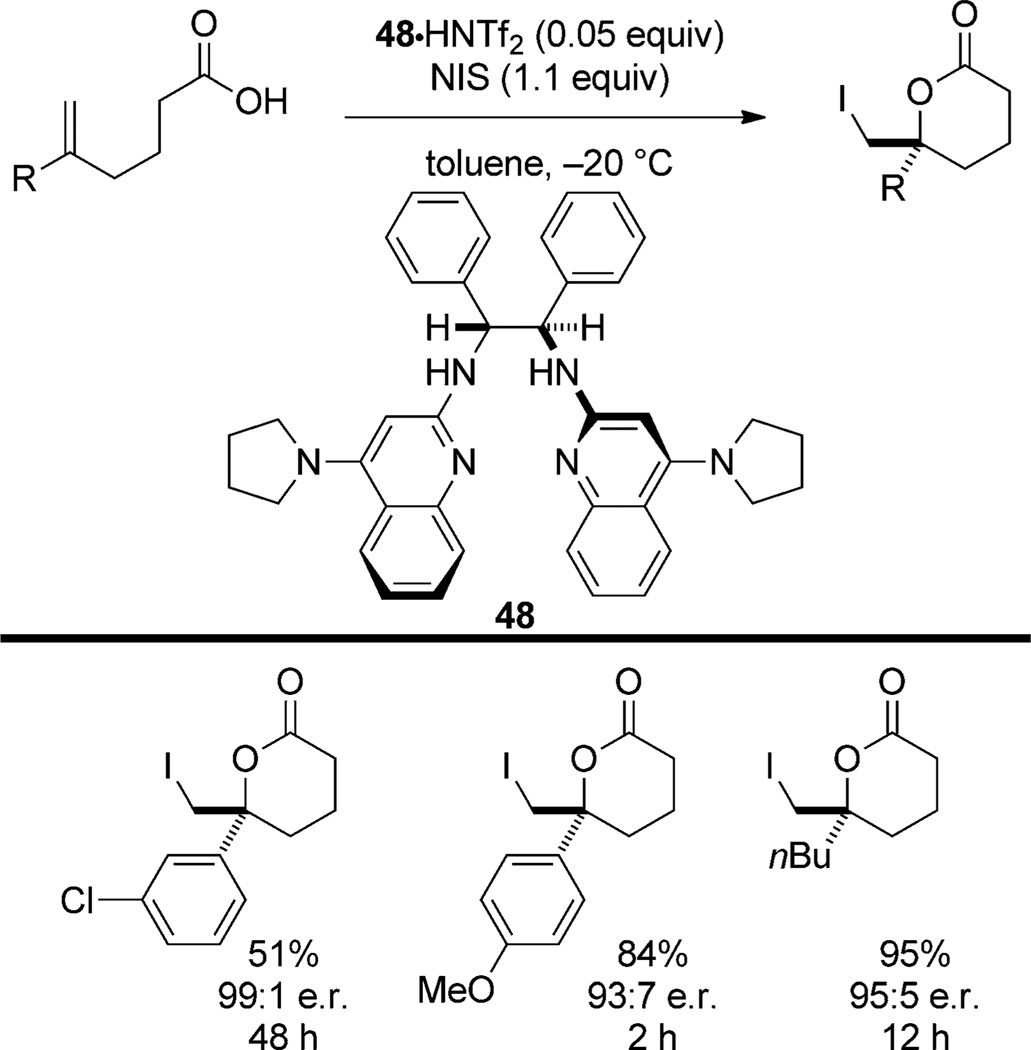
Finally, the iodolactonization of 5-substituted-5-hexenoic acids with NIS is catalyzed by stilbene bis(4-pyrrolidinylquinolinyl) bisamidine hydrogen triflimide 48·HNTf2 (Stilb-PBAM·HNTf2) to afford iodolactones in high enantioselectivity and moderate to high yield[48] (Scheme 26). Interestingly, under the same reaction conditions, cyclization of the analogous pentenoic acid affords the butyrolactone with considerably reduced selectivity. The scope of the cyclization is narrow (1,1-disubstituted alkenes only), but the reactions conditions demonstrate little sensitivity of selectivity to the electronic properties of the styryl substrates. The reaction rate, in contrast, exhibits a high dependence on the electronic properties of alkene substituents. Moreover, the authors note a dependence of enantioselectivity and efficiency upon the conjugate base of the Brønsted acid. The authors hypothesize that NIS is activated by a Brønsted acid and that the nucleophile is activated and directed by a Brønsted base. There are, however, several basic sites on the catalyst, any of which may be operative in the stereodetermining step.
Scheme 26.
Asymmetric iodolactonization catalyzed by 48·HNTf2.
7. Type IV: Lewis Acid Catalyzed or Directed Halocyclization
Lewis acid catalyzed, asymmetric halofunctionalizations comprise two distinct strategies depending on whether the Lewis acid activates the nucleophile (Type IVa) or the electrophile (Type IVb). For enantioselective cyclizations, activation of the nucleophile has been exclusively the domain of titanium complexes, whereas activation of the halogen source is typically performed by CoII/salen or CrIIICl/salen complexes.
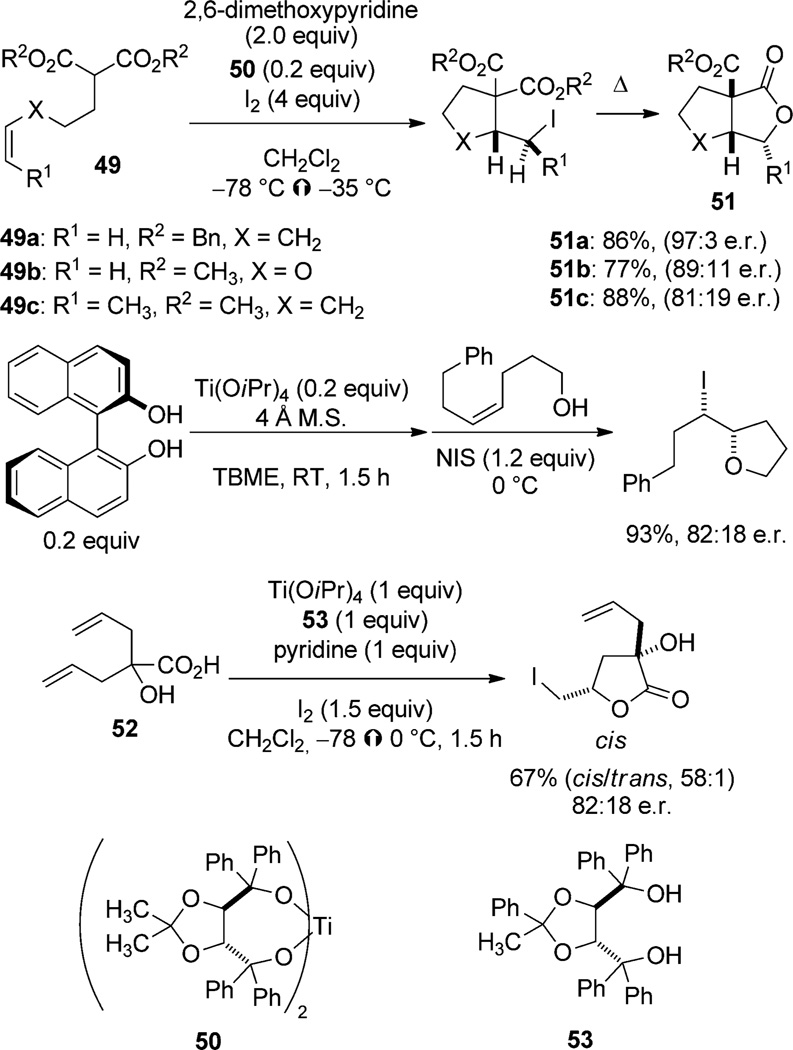
The use of titanium-based Lewis acids to activate stabilized carbon nucleophiles for attack on any number of electrophiles is a well-established tactic,[55] and the use of chiral ligands to effect these transformations enantioselectively is similarly well documented.[56] It is not surprising, then, that when the electrophile is an iodiranium ion, the reaction would proceed with high enantioselectivity. This transformation is precisely what Taguchi and co-workers demonstrate in reports of iodocarbocyclization of unsaturated malonate 49 using the preformed titanium/taddol complex 50.[57, 58] After enantioselective trapping of the iodonium ion, nucleophilic displacement of iodide by one of the diastereotopic ester groups forms the fused butyrolactone 51 (Scheme 27). Unfortunately, if a carboxyl group is employed as the nucleophile (such as in 52), the enantioselectivity is greatly reduced, even in the presence of a stoichiometric amount of a chiral titanium complex (formed in situ from 53).[59] In this approach, the iodiranium ions are likely interconverting, but the stereodetermining event is the capture by a chirally modified nucleophile which can select the faster-acting ion (diastereomorphic group selectivity).[60] An in situ formed titanium binol complex is also capable of promoting iodoetherification with modest selectivity.[61]
Scheme 27.
Enantioselective titanium-promoted iodocyclizations.
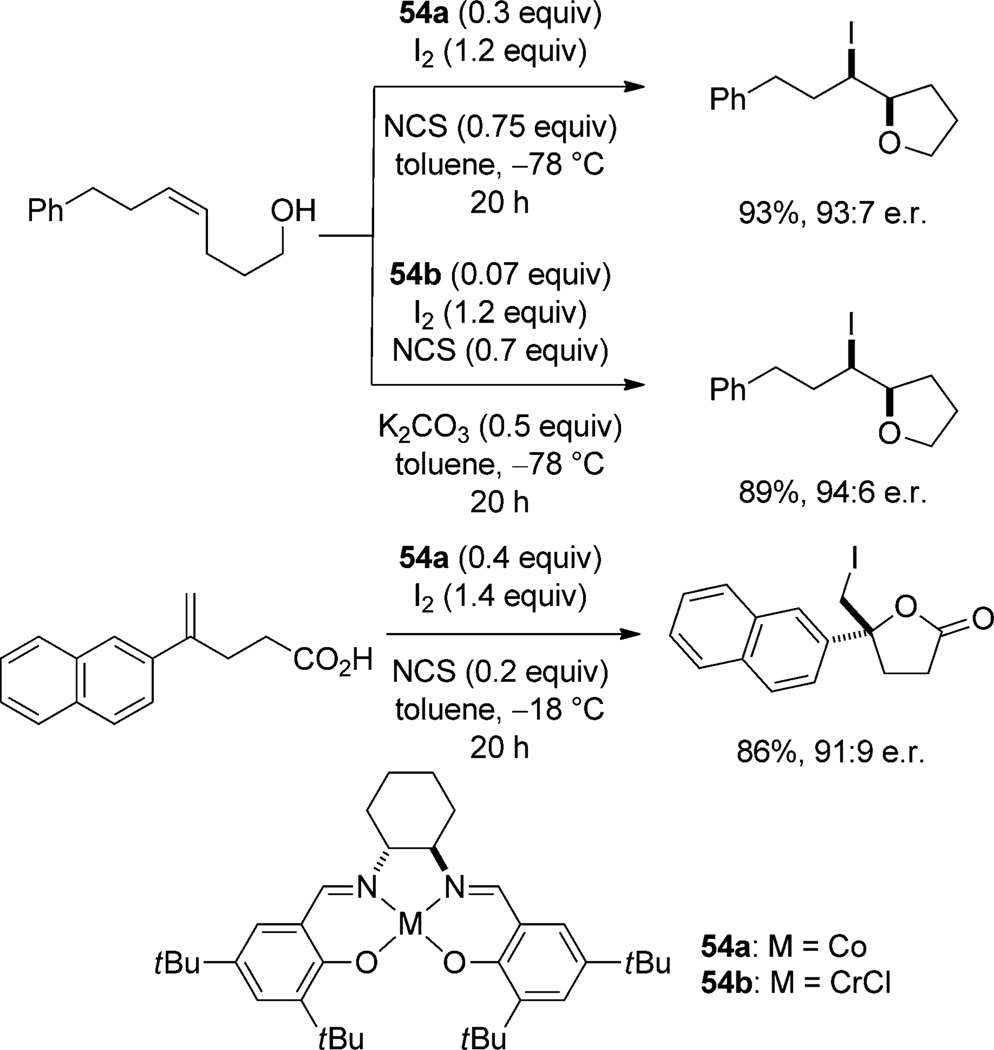
In Type IVb processes, the Lewis acid activates a halogen source for attack by an alkene. This type of catalysis has been performed enantioselectively with salen complexes of cobalt(II) and chromium(III). Kang and co-workers have reported that the CoII/salen complex 54a catalyzes the iodoetherification of (Z)-5-substituted-5-pentenols, in very high yield and good enantioselectivity (Scheme 28).[62] When the CrIIICl/salen complex 54b is employed as the catalyst, the same substrates are cyclized with equal or greater enantioselectivity at one quarter of the loading of 54a.[63] Gao and co-workers have extended the utility of 54a to the iodolactonization of 4-substituted-5-pentenoic acids in high yield and moderate to good enantioselectivity.[64]
Scheme 28.
CoII/salen- and CrIIICl/salen-catalyzed halocyclizations.
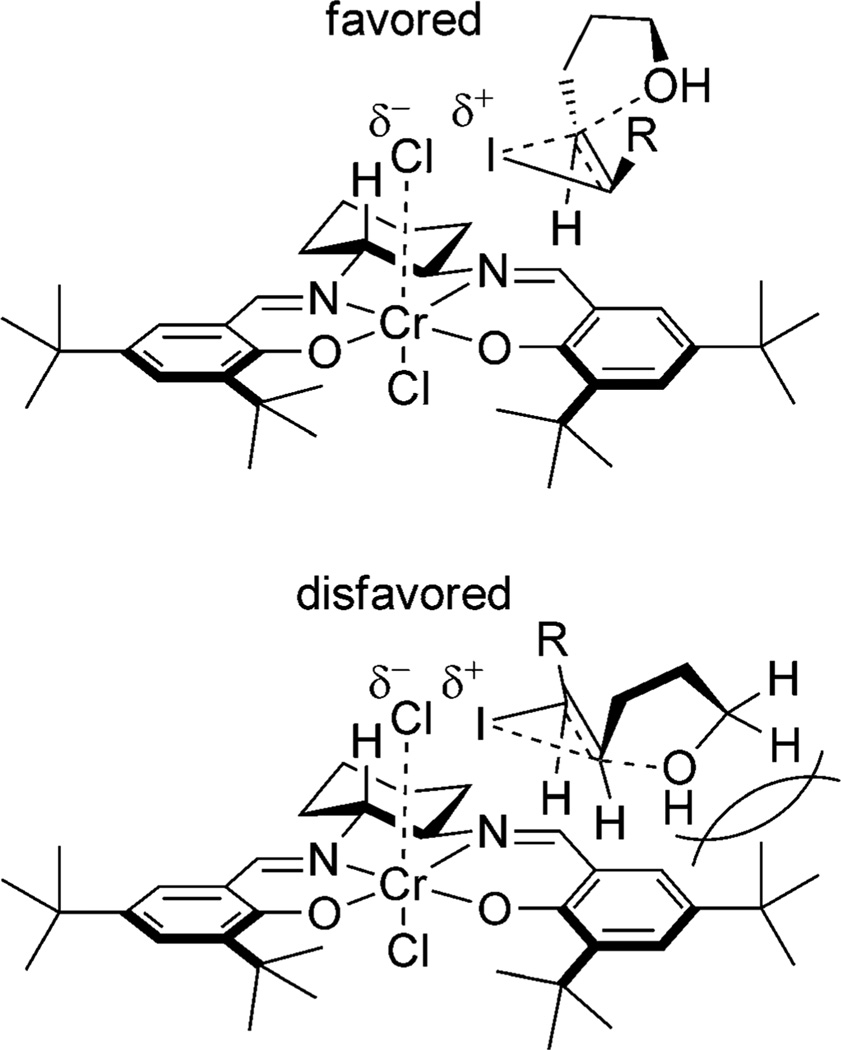
Two pathways for the activation of iodine are proposed. The first posits that N-chlorosuccinimide (NCS) reacts with I2 to slowly release ICl, which is further activated by complexation with the Lewis-acidic catalyst. In this pathway, the slow formation of ICl may be necessary to minimize the uncatalyzed racemic pathway. In the alternative hypothesis, NCS oxidizes CrIII to a higher oxidation state(s), which enhances its Lewis acidity. The oxidized chromium complex then activates iodine directly to effect the iodocyclization reaction. Both of these proposals explain why the reaction is efficient with less than a full equivalent of NCS, and either may lead to an activated complex of [I][salen-CrCln]. The proposed model for enantioselectivity involves a skewed side-on approach from the right (Figure 8) to avoid the axial proton of the cyclohexane ring. Facial selectivity of trapping is prejudiced by an unfavorable interaction between the hydroxypropyl group of the pentenol and the tert-butyl group of the salen ligand.
Figure 8.
Proposed model for enantioselectivity of CrIII/salen-catalyzed iodocyclization.
Another example of Type IVb stereoselection is the scandium-catalyzed, enantioselective haloamination of chalcones and α,β-unsaturated-γ-keto esters reported by Feng and co-workers.[65] The transformation affords β-sulfonamido-α-halo carbonyl products in high yields and generally very high enantioselectivities with very low loadings of ligand 55 (Scheme 29).
Scheme 29.
Scandium-catalyzed asymmetric haloamination of chalcones.
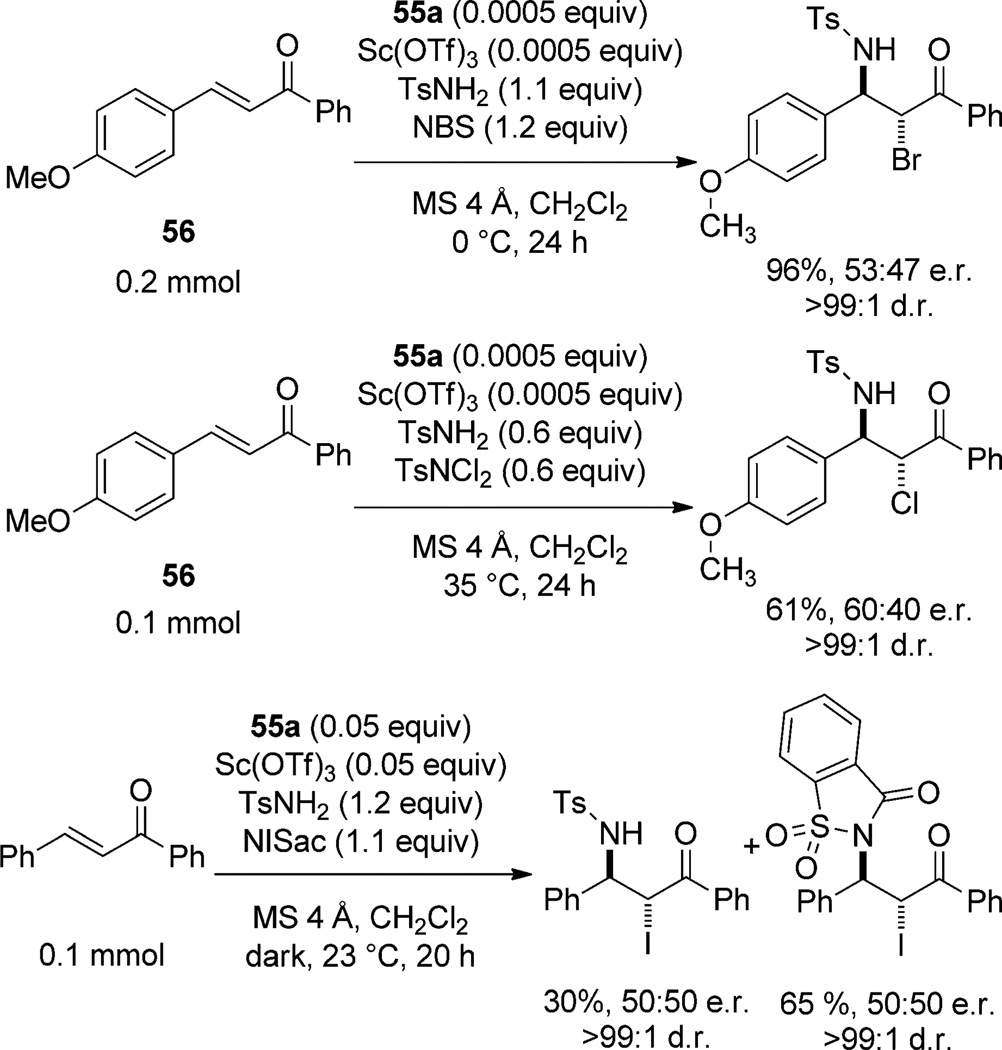
The invertive opening of a putative haliranium ion arising from electrophilic attack of NBS on the electron-poor enone is invoked to describe the anti relationship of the substituents. The authors advance an organized transition-state model in which an enone bound to scandium through the carbonyl oxygen atom suffers electrophilic attack by a similarly coordinated NBS. This assertion is based upon a control experiment that afforded no product in the absence of the halogen electrophile. This result, however, is also consistent with a reversible conjugate addition of TsNH2 or in situ generated TsNHBr[65a] to the activated chalcone, with a subsequent electrophilic quench of the enolate with NBS. Furthermore, given the normal mode of Lewis acid activation of α,β-unsaturated carbonyls (LUMO lowering), this pathway is a more likely mechanism for this transformation. The formation of a haliranium ion is favored under certain reaction conditions. Cursory molecular orbital analysis suggests that the HOMO–LUMO overlap should increase with increasing electron density on the alkene and increasing dipole moment of the X–R bond of the halogen source. When the 4-methoxy-substituted chalcone 56 is subjected to the bromo-[65a] and chloroamination[65b] conditions, enantioselectivity is markedly lower than all other substrates (Scheme 30). Additionally, when N-iodosaccharin (NISac), a strongly electrophilic iodinating reagent, is used as the halenium source, the resulting products are racemic.[65c] This data strongly suggests that the catalyzed pathway does not involve a haliranium ion intermediate. Without more compelling control experiments and an interpretation of the data that is rooted in established theoretical and empirical chemical knowledge, the conjugate addition pathway cannot be ruled out as a possible mechanism, and given the Lewis acidic nature of the catalyst, it is the most likely pathway.
Scheme 30.
Selected cyclizations that contradict the proposed haliranium ion pathway.
8. Summary and Outlook
Significant progress has been recorded in the past three years in the development of catalytic, enantioselective versions of alkene halofunctionalizations with the four common halogens. However, by any yardstick, the field has only just started to take shape. A number of factors conspire to stimulate continued interest: the importance of stereodefined, halogenated compounds in natural and unnatural products, the bounty of well-established reactivity of the carbon–halogen bonds, and the opportunities to discover new mechanisms of catalysis and stereoinduction.
Many important challenges remain. Enantioselective variants of simple dichlorination and dibromination in numerous settings are clearly needed. Moreover, for halofunctionalization reactions to achieve the strategy-level generality and predictability characteristic of for example, asymmetric dihydroxylation, new combinations of existing catalytic and stereocontrolling strategies must be developed and new mechanisms for these components must be discovered or invented. The challenge of configurational lability of bromiranium and iodiranium ions needs to be addressed in new ways. The configurational stability of chloriranium ions offers great opportunities for new modes of stereocontrol, but at the same time, strategies to tame the hyperreactivity of these species must be introduced. Fluorofunctionalization reactions are even more poorly understood, yet the products are of significant importance.
As in all newly emergent endeavors, this field is passing through the typical period of vibrant activity, as rich with discoveries and mysteries as it is deficient in guiding principles and understanding. It is an exciting time when new results accumulate rapidly while the foundations only slowly take shape. For the field to develop successfully, (at least) two kinds of activities and investigators are essential; the explorers whose talent for discovery takes them to uncharted territories unfettered by restrictions of existing paradigms and the cartographer who brings clarity to disorder and integrates these discoveries into roadmaps that guide the merger of this field into the greater edifice of synthetic organic chemistry.[66]
Acknowledgments
We are grateful to the National Institutes of Health for generous financial support (GM085235).
Biographies

Scott E. Denmark obtained an S.B. degree from MIT in 1975 and his D.Sc.Tech. (under the direction of Albert Eschenmoser) from the ETH Zürich in 1980. That same year he began his career at the University of Illinois and since 1991 he has been the Reynold C. Fuson Professor of Chemistry. His research interests include the invention of new synthetic reactions, exploratory organoelement chemistry, and the origin of stereocontrol in fundamental carbon–carbon bond-forming processes.

William Kuester obtained his M.S. in 2008 from Southern Illinois University Edwardsville under the guidance of Dr. Yun Lu. He joined the Denmark research group in the fall of 2010 where his research pursuits have included the configurational stability of iodiranium ions and catalytic, asymmetric halocyclization.

Matthew T. Burk obtained a BS in chemistry from Indiana University in 2006, where he carried out undergraduate research with Prof. P. Andrew Evans. He continued on to graduate study at the University of Illinois under Prof. Scott E. Denmark, where his research focused on Lewis base catalysis of enantioselective, halocyclization reactions. He obtained his Ph.D. in 2012 and is currently carrying out post-doctoral research with Prof. Seth Herzon at Yale University.
References
- 1.Gilman H. Organic Chemistry: An Advanced Treatise. Vol. 1. New York: Wiley; 1938. pp. 36–43. [Google Scholar]
- 2.Ingold CK. Structure and Mechanism in Organic Chemistry. Ithaca: Cornell University Press; 1953. pp. 658–670. [Google Scholar]
- 3.a) Barton DHR. Experientia. 1950;6:316–320. doi: 10.1007/BF02170915. [DOI] [PubMed] [Google Scholar]; b) Fieser LF, Fieser M. Steroids. New York: Reinhold; 1959. pp. 26–41. [Google Scholar]
- 4.In passing, it is interesting to note that dibromocholesterol was already reported in 1868! Wislicenus J, Moldenhauer W. Justus Liebigs Ann. Chem. 1868;146:175–180.
- 5.a) French AN, Bissmire S, Wirth T. Chem. Soc. Rev. 2004;33:354–362. doi: 10.1039/b310389g. [DOI] [PubMed] [Google Scholar]; b) Bartlett PA. In: Asymmetric Synthesis. Morrison J, editor. Vol. 3. Orlando: Academic Press; 1983. pp. 411–454. [Google Scholar]; c) Bartlett PA, Richardson DP, Myerson J. Tetrahedron. 1984;40:2317–2327. [Google Scholar]
- 6.Castellanos A, Fletcher SP. Chem. Eur. J. 2011;17:5766–5776. doi: 10.1002/chem.201100105. [DOI] [PubMed] [Google Scholar]
- 7. Snyder SA, Treitler DS, Brucks AP. Aldrichimica Acta. 2011;44:27–40. Includes examples of racemic catalysis.
- 8.Hennecke U. Chem. Asian J. 2012;7:456–465. doi: 10.1002/asia.201100856. [DOI] [PubMed] [Google Scholar]
- 9.Tan CK, Ling Z, Yeung Y-Y. Synlett. 2011:1335–1339. [Google Scholar]
- 10.Perkins CW, Martin JC, Arduengo AJ, Lau W, Alegria A, Kochi JK. J. Am. Chem. Soc. 1980;102:7753–7759. [Google Scholar]
- 11.a) Schmid GH, Garratt DG. In: The Chemistry of Double-Bonded Functional Groups. Part 2. Patai S, editor. New York: Wiley; 1977. pp. 725–912. [Google Scholar]; b) Schmid GH. In: The Chemistry of Double-Bonded Functional Groups. Part 1. Patai S, editor. Vol. 2. New York: Wiley; 1989. pp. 679–731. [Google Scholar]
- 12.a) Lenoir D, Chiappe C. Chem. Eur. J. 2003;9:1036–1044. doi: 10.1002/chem.200390097. [DOI] [PubMed] [Google Scholar]; b) Bellucci G, Chiappe C, Bianchini R, Lenoir D, Herges R. J. Am. Chem. Soc. 1995;117:12001–12002. [Google Scholar]
- 13.Roberts I, Kimball GE. J. Am. Chem. Soc. 1937;59:947–948. [Google Scholar]
- 14.a) Olah GA, Bollinger JM. J. Am. Chem. Soc. 1967;89:4744–4752. doi: 10.1021/ja00988a034. [DOI] [PubMed] [Google Scholar]; b) Olah GA, Bollinger JM. J. Am. Chem. Soc. 1968;90:947–953. [Google Scholar]; c) Olah GA, Bollinger JM, Brinich J. J. Am. Chem. Soc. 1968;90:2587–2594. [Google Scholar]
- 15.a) Brown RS, Nagorski RW, Bennet AJ, McClung RED, Aarts GHM, Klobukowski M, McDonald R, Santarsiero BD. J. Am. Chem. Soc. 1994;116:2448–2456. [Google Scholar]; b) Neverov AA, Brown RS. J. Org. Chem. 1996;61:962–968. [Google Scholar]; c) Brown RS. Acc. Chem. Res. 1997;30:131–137. [Google Scholar]
- 16.Denmark SE, Burk MT, Hoover AJ. J. Am. Chem. Soc. 2010;132:1232–1233. doi: 10.1021/ja909965h. [DOI] [PubMed] [Google Scholar]
- 17.a) Olah GA, Wang Q, Sandford G, Prakash GKS. J. Org. Chem. 1993;58:3194–3195. [Google Scholar]; b) Prakash GKS, Mathew T, Hoole D, Esteves PM, Wang Q, Rasul G, Olah GA. J. Am. Chem. Soc. 2004;126:15770–15776. doi: 10.1021/ja0465247. [DOI] [PubMed] [Google Scholar]
- 18.a) Gutmann V. Coord. Chem. Rev. 1975;15:207–237. [Google Scholar]; b) Gutmann V. The Donor-Acceptor Approach to Molecular Interactions. New York: Plenum; 1978. [Google Scholar]; c) Denmark SE, Beutner GL. Angew. Chem. 2008;120:1584–1663. Angew. Chem. Int. Ed.2008, 47, 1560 – 1638. [Google Scholar]
- 19.Chloro- and fluorofunctionalization reactions do not suffer from rapid olefin-to-olefin transfer and therefore do not require that the catalyst/haliranium ion interaction be maintained. However, they can be placed in the same classification scheme because some of the same tactics have been applied to controlling the initial halenium ion delivery.
- 20.Grossman RB, Trupp RJ. Can. J. Chem. 1998;76:1233–1237. [Google Scholar]
- 21.a) Cui X-L, Brown RS. J. Org. Chem. 2000;65:5653–5658. doi: 10.1021/jo000449a. [DOI] [PubMed] [Google Scholar]; b) Brown RS, Neverov AA, Liu CT, Maxwell CI. In: Recent Developments in Carbocation and Onium Ion Chemistry. Laali K, editor. Washington: American Chemical Society; 2007. pp. 458–476. [Google Scholar]
- 22.a) Haas J, Piguel S, Wirth T. Org. Lett. 2002;4:297–300. doi: 10.1021/ol0171113. [DOI] [PubMed] [Google Scholar]; b) Haas J, Bissmire S, Wirth T. Chem. Eur. J. 2005;11:5777–5785. doi: 10.1002/chem.200500507. [DOI] [PubMed] [Google Scholar]
- 23.Neverov AA, Brown RS. J. Org. Chem. 1998;63:5977–5982. doi: 10.1021/jo980627o. [DOI] [PubMed] [Google Scholar]
- 24.This may not be true for nucleophiles stronger than olefins, however such reactions are beyond the scope of this review. See Neverov AA, Feng HX, Hamilton K, Brown RS. J. Org. Chem. 2003;68:3802–3810. doi: 10.1021/jo020750m.
- 25.Sakakura A, Ukai A, Ishihara K. Nature. 2007;445:900–903. doi: 10.1038/nature05553. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlin AR, Mulholland RL, Kahn SD, Hehre WJ. J. Am. Chem. Soc. 1987;109:672–677. [Google Scholar]
- 27.For general reviews on chiral Brønsted acid catalysis and chiral ion pairs, see Taylor MS, Jacobsen EN. Angew. Chem. 2006;118:1550–1573. Angew. Chem. Int. Ed.2006, 45, 1520 – 1543; Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713–5743. doi: 10.1021/cr068373r. Pihko PM, editor. Hydrogen Bonding in Organic Synthesis. Weinheim: Wiley-Interscience; 2009. Rueping M, Koenigs RM, Atodiresei I, editors. Chem. Eur. J. 2010;16:9350–9365. doi: 10.1002/chem.201001140. Terada M. Synthesis. 2010:1929–1982. Rueping M, Nachtsheim BJ, Ieawsuwan W, Atodiresei I. Angew. Chem. 2011;123:6838–6853. doi: 10.1002/anie.201100169. Angew. Chem. Int. Ed.2011, 50, 6706 – 6720; Schenker S, Zamfir A, Freund M, Tsogoeva SB. Eur. J. Org. Chem. 2011:2209–2222.
- 28.Hennecke U, Müller CH, Fröhlich R. Org. Lett. 2011;13:860–863. doi: 10.1021/ol1028805. [DOI] [PubMed] [Google Scholar]
- 29.a) Hoffmann S, Seayad AM, List B. Angew. Chem. 2005;117:7590–7593. doi: 10.1002/anie.200503062. Angew. Chem. Int. Ed.2005, 44, 7424 – 7427; [DOI] [PubMed] [Google Scholar]; b) Adair G, Mukherjee S, List B. Aldrichimica Acta. 2008;41:31–39. [Google Scholar]; c) Klussmann M, Ratjen L, Hoffmann S, Wakchaure V, Goddard R, List B. Synlett. 2010:2189–2192. [Google Scholar]
- 30.Denmark SE, Burk MT. Org. Lett. 2012;14:256–259. doi: 10.1021/ol203033k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang D, Wang H, Xue F, Guan H, Li L, Peng X, Shi Y. Org. Lett. 2011;13:6350–6353. doi: 10.1021/ol202527g. [DOI] [PubMed] [Google Scholar]
- 32.Yousefi R, Whitehead DC, Mueller JM, Staples RJ, Borhan B. Org. Lett. 2011;13:608–611. doi: 10.1021/ol102850m. [DOI] [PubMed] [Google Scholar]
- 33.Li G-x, Fu Q-q, Zhang X-m, Jiang J, Tang Z. Tetrahedron: Asymmetry. 2012;23:245–251. [Google Scholar]
- 34.Rauniyar V, Lackner AD, Hamilton GL, Toste FD. Science. 2011;334:1681–1684. doi: 10.1126/science.1213918. [DOI] [PubMed] [Google Scholar]
- 35.a) Girard C, Kagan HB. Angew. Chem. 1998;110:3088–3127. doi: 10.1002/(SICI)1521-3773(19981116)37:21<2922::AID-ANIE2922>3.0.CO;2-1. Angew. Chem. Int. Ed.1998, 37, 2922 – 2959; [DOI] [PubMed] [Google Scholar]; b) Fenwick DR, Kagan HB. Top. Stereochem. 1999;22:257–296. [Google Scholar]; c) Satyanarayana T, Abraham S, Kagan HB. Angew. Chem. 2009;121:464–503. doi: 10.1002/anie.200705241. Angew. Chem. Int. Ed.2009, 48, 456 – 494. [DOI] [PubMed] [Google Scholar]
- 36.Veitch GE, Jacobsen EN. Angew. Chem. 2010;122:7490–7493. doi: 10.1002/anie.201003681. Angew. Chem. Int. Ed.2010, 49, 7332 – 7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murai K, Matsushita T, Nakamura A, Fukushima S, Shimura M, Fujioka H. Angew. Chem. 2010;122:9360–9363. doi: 10.1002/anie.201005409. Angew. Chem. Int. Ed.2010, 49, 9174 – 9177. [DOI] [PubMed] [Google Scholar]
- 38. Murai K, Nakamura A, Matsushita T, Shimura M, Fujioka H. Chem. Eur. J. 2012;18:8448–8453. doi: 10.1002/chem.201200647. This constitutes the only reported method for the enantioselective halofunctionalization of a tetrasubstituted alkene.
- 39.Denmark SE, Burk MT. Proc. Natl. Acad. Sci. USA. 2010;107:20655–20660. doi: 10.1073/pnas.1005296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou L, Tan CK, Jiang X, Chen F, Yeung Y-Y. J. Am. Chem. Soc. 2010;132:15474–15476. doi: 10.1021/ja1048972. [DOI] [PubMed] [Google Scholar]
- 41.Tan CK, Zhou L, Yeung Y-Y. Org. Lett. 2011;13:2738–2741. doi: 10.1021/ol200840e. [DOI] [PubMed] [Google Scholar]
- 42.Tan CK, Le C, Yeung Y-Y. Chem. Commun. 2012;48:5793–5795. doi: 10.1039/c2cc31148h. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Zhou L, Yeung Y-Y. Org. Biomol. Chem. 2012;10:3808–3811. doi: 10.1039/c2ob25327e. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Zhou L, Tan CK, Yeung Y-Y. J. Org. Chem. 2012;77:999–1009. doi: 10.1021/jo202221c. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Xu H, Xu H, Tang W. J. Am. Chem. Soc. 2009;131:3832–3833. doi: 10.1021/ja8099008. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Zheng S, Liu N, Werness JB, Guzei IA, Tang W. J. Am. Chem. Soc. 2010;132:3664–3665. doi: 10.1021/ja100173w. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Liu N, Schienebeck CM, Decloux K, Zheng S, Werness JB, Tang W. Chem. Eur. J. 2012;18:7296–7305. doi: 10.1002/chem.201103809. [DOI] [PubMed] [Google Scholar]
- 48.Dobish MC, Johnston JN. J. Am. Chem. Soc. 2012;134:6068–6071. doi: 10.1021/ja301858r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitehead DC, Yousefi R, Jaganathan A, Borhan B. J. Am. Chem. Soc. 2010;132:3298–3300. doi: 10.1021/ja100502f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaganathan A, Garzan A, Whitehead DC, Staples RJ, Borhan B. Angew. Chem. 2011;123:2641–2644. doi: 10.1002/anie.201006910. Angew. Chem. Int. Ed.2011, 50, 2593 – 2596. [DOI] [PubMed] [Google Scholar]
- 51.Nicolaou KC, Simmons NL, Ying Y, Heretsch PM, Chen JS. J. Am. Chem. Soc. 2011;133:8134–8137. doi: 10.1021/ja202555m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.a) Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547. [Google Scholar]; b) Corey EJ, Noe MC. J. Am. Chem. Soc. 1996;118:11038–11053. [Google Scholar]
- 53.Lozano O, Blessley G, Martinez del Campo T, Thompson AL, Giuffredi GT, Bettati M, Walker M, Borman R, Gouverneur V. Angew. Chem. 2011;123:8255–8259. doi: 10.1002/anie.201103151. Angew. Chem. Int. Ed.2011, 50, 8105 – 8109. [DOI] [PubMed] [Google Scholar]
- 54.Chen Z-M, Zhang Q-W, Chen Z-H, Li H, Tu Y-Q, Zhang F-M, Tian J-M. J. Am. Chem. Soc. 2011;133:8818–8821. doi: 10.1021/ja201794v. [DOI] [PubMed] [Google Scholar]
- 55.Urabe H, Sato F. In: Lewis Acids in Organic Synthesis. Yamamoto H, editor. Vol. 2. Weinheim: Wiley-VCH; 2000. pp. 653–798. [Google Scholar]
- 56.Mikami K, Terada M. In: Lewis Acids in Organic Synthesis. Yamamoto H, editor. Vol. 2. Weinheim: Wiley-VCH; 2000. pp. 799–848. [Google Scholar]
- 57.Inoue T, Kitagawa O, Ochiai O, Shiro M, Taguchi T. Tetrahedron Lett. 1995;36:9333–9336. [Google Scholar]
- 58.Inoue T, Kitagawa O, Saito A, Taguchi T. J. Org. Chem. 1997;62:7384–7389. doi: 10.1021/jo970970d. [DOI] [PubMed] [Google Scholar]
- 59.Kitagawa O, Hanano T, Tanabe K, Shiro M, Taguchi T. J. Chem. Soc. Chem. Commun. 1992:1005–1007. [Google Scholar]
- 60.Izumi Y, Tai A. Stereo-Differentiating Reactions—The Nature of Asymmetric Reactions. chapt. 2. New York: Academic Press; 1977. [Google Scholar]
- 61.Kang SH, Park CM, Lee SB, Kim M. Synlett. 2004:1279–1281. [Google Scholar]
- 62.Kang SH, Lee SB, Park CM. J. Am. Chem. Soc. 2003;125:15748–15749. doi: 10.1021/ja0369921. [DOI] [PubMed] [Google Scholar]
- 63.Kwon HY, Park CM, Lee SB, Youn J-H, Kang SH. Chem. Eur. J. 2008;14:1023–1028. doi: 10.1002/chem.200701199. [DOI] [PubMed] [Google Scholar]
- 64.Ning Z, Jin R, Ding J, Gao L. Synlett. 2009:2291–2294. [Google Scholar]
- 65.a) Cai Y, Liu X, Hui Y, Jiang J, Wang W, Chen W, Lin L, Feng X. Angew. Chem. 2010;122:6296–6300. Angew. Chem. Int. Ed.2010, 49, 6160 – 6164; [Google Scholar]; b) Cai Y, Liu X, Jiang J, Chen W, Lin L, Feng X. J. Am. Chem. Soc. 2011;133:5636–5639. doi: 10.1021/ja110668c. [DOI] [PubMed] [Google Scholar]; c) Cai Y, Liu X, Li J, Chen W, Wang W, Lin L, Feng X. Chem. Eur. J. 2011;17:14916–14921. doi: 10.1002/chem.201102453. [DOI] [PubMed] [Google Scholar]
- 66.Note added in proof. The following papers on enantioselective halofunctionalization appeared after submission of this manuscript: Wang Y-M, Wu J, Hoong C, Rauniyar V, Toste FD. J. Am. Chem. Soc. 2012;134:12928–12931. doi: 10.1021/ja305795x. Jiang X, Tan CK, Zhou L, Yeung Y-Y. Angew. Chem. 2012;124:7891–7895. Angew. Chem. Int. Ed.2012, 51, 7771 – 7775; Paull DH, Fang C, Donald JR, Pansick AD, Martin SF. J. Am. Chem. Soc. 2012;134:11128–11131. doi: 10.1021/ja305117m.