Abstract
Combined methylmalonic acidemia and homocystinuria, cblC type, is stated to be the most common inborn error of intracellular cobalamin metabolism. The disorder can display a wide spectrum of clinical manifestations, spanning the prenatal period through late adulthood. While increased homocysteine concentrations and impaired methyl group metabolism may contribute to disease-related complications, the characteristic macular and retinal degeneration seen in many affected patients appears to be unique to cblC disease. The early detection of cblC disease by newborn screening mandates a careful assessment of therapeutic approaches and provides a new opportunity to improve the outcome of affected patients. The following article reviews the current knowledge on the complications, pathophysiology, and outcome of cblC disease in an effort to better guide clinical practice and future therapeutic trials.
Introduction
Combined methylmalonic aciduria and homocystinuria, cblC type (OMIM #277400), is claimed to be the most common intracellular disorder of cobalamin metabolism. It is a rare disorder, but newborn screening studies suggest that its incidence appears to be higher than the previous estimate of 1/200,000 births. One recent study found an approximate incidence of 1/100,000 in New York State (Weisfeld-Adams et al. 2010), while another study found a high estimated prevalence of cblC disease in California of 1 in 60,000 and of 1 in 37,000 in the Hispanic population (Cusmano-Ozog et al. 2007).
Patients with cblC disease can develop severe complications despite treatment and historically have had a poor long-term outcome (Rosenblatt et al. 1997). The pathophysiology of complications seen in the patients is not fully understood, but increased homocysteine concentrations, impaired methyl group metabolism, and oxidative stress (Richard et al. 2009; Mc Guire et al. 2009) may play an important role. A better understanding of these mechanisms and their contribution to disease manifestations should improve the management of patients with cblC.
The long-term outcome in cblC disease is not well-studied, but there is a variable response to treatment and progression of complications among patients. Some disease manifestations, such as pigmentary retinopathy, seem to progress in most patients despite treatment (Enns et al. 1999; Carmel et al. 1980; Cogan et al. 1980; Robb et al. 1984). Other complications such as thrombotic microangiopathy have resolved with appropriate treatment (Van Hove et al. 2002). Neurologic status usually improves with treatment, but the sequelae remain in a large proportion of patients, especially in those where the initiation of treatment was delayed or insufficient. A late-onset phenotype has a more favorable outcome than early-onset cblC disease but is still associated with residual sequelae such as variable learning difficulties, neurobehavioral symptoms, and neurogenic bladder and gait abnormalities (Rosenblatt et al. 1997), and at least one patient that halted treatment died (Thauvin-Robinet et al. 2008).
With the identification of the gene responsible for cblC disease and the detection of cblC disease through expanded newborn screening in recent years, the possibility to improve the outcome in patients with cblC disease has emerged. A thorough understanding of the natural history, pathophysiology, and outcome of the current cohort of patients with cblC disease is critical to guide current and future therapeutic interventions.
The main goals of this study were to summarize the current knowledge on the manifestations and pathophysiology of cblC disease and to systematically review the literature with the specific intention of providing an increased understanding of the factors contributing to the outcome in cblC disease.
Methods
We performed a review of the literature to compile the knowledge on disease progression and outcome of patients with cblC disease and examine the potential relationships with medical management. We conducted a PubMed search from 1969 to 2010 using the following terms: “cblC,” “cobalamin C,” “intracellular disorders of cobalamin,” “outcome,” “complications,” “eye disease,” “vascular disease,” “hemolytic-uremic syndrome,” “chronic microangiopathy.” Additional publications were obtained from references cited in relevant articles. Publications in English describing the following information on patients with cblC were eligible for review: (1) detailed ophthalmological findings (n=22), (2) vascular complications and simultaneous total plasma homocysteine (tHcy) levels (n=8), (3) hemolytic-uremic syndrome and therapeutic interventions (n=8), (4) outcome and management (n=32). Forty-three publications were suitable and included 39 case reports, 1 case series, 2 retrospective studies, and 1 longitudinal study. We abstracted demographic information, relevant findings and metabolites, form of treatment, and the genotype of patients when available. The information is presented under the “Outcome” subsections of “Ophthalmological manifestations,” “Vascular manifestations,” “Renal manifestations,” and “Long-term outcome.”
Eye disease classification
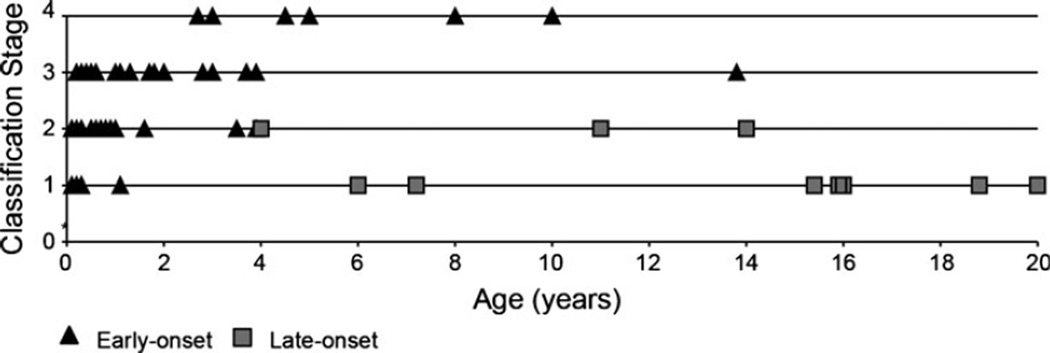
Twenty-seven patients with early-onset and eight with late-onset cblC disease were classified by age and their severity of eye disease as follows: 1 no alterations found; 2 mild maculopathy, peripheral pigmentary retinopathy; 3 progressive maculopathy, retinal extension of pigmentary changes, 4 legally blind (Supplementary Table 1).
Long-term outcome
Twenty-one patients with early-onset and 18 with late-onset cblC disease were classified according to their type of management and outcome. Notably, only 11 of these patients were maintained on daily doses of hydroxocobalamin (OHCbl), only 2 had a dose adjustment for increased body weight, and 18 did not receive therapy with betaine. Consistent monitoring strategies and target metabolite levels to guide treatment were also highly variable among studies (Supplementary Table 2).
Clinical severity classification
Patients were assigned to different clinical stages of disease severity as follows: 1 no clinical alterations; 2 mild manifestations not affecting daily quality of life; 3 moderate manifestations including growth impairment, developmental delay, eye disease; 4 severe disease manifestations either affecting daily quality of life or requiring intensive medical care; 5 death.
Results
Mortality
Early detection and treatment with parenteral OHCbl have decreased the mortality in newborns with cblC. A retrospective analysis of 50 patients with cblC disease reported an overall mortality rate of 30% (13/44) (Rosenblatt et al. 1997). In the 13 patients that perished, 4 had no treatment, 2 received only cyanocobalamin (CNCbl), and 3 were initially treated with CNCbl and then switched to OHCbl (Rosenblatt et al. 1997). A compilation of the reported complications in patients with cblC disease is presented in Table 1.
Table 1.
Complications by system in cblC disease
| System | Complications |
|---|---|
| Growth and habitus | Prenatal growth retardation |
| Postnatal failure to thrive | |
| Marfanoid habitus | |
| Dysmorphic facial features | |
| CNS | Congenital microcephaly |
| Seizures | |
| Developmental delay | |
| Regression | |
| Cognitive impairment ranging from executive dysfunction to severe mental retardation (Rosenblatt et al. 1997) | |
| Neuropsychiatric disturbances | |
| Subacute combined degeneration of the spinal cord | |
| Leukoencephalopathy | |
| Basal ganglia lesions (less frequent)a | |
| Eye | Maculopathy |
| Progressive pigmentary retinopathy | |
| Optic atrophy | |
| Blindness | |
| Blood | Anemia, thrombocytopenia and/or neutropenia, megaloblastosis |
| Vascular | Recurrent venous thrombosis |
| Cor pulmonaleb or subclinical pulmonary thrombosis | |
| Cerebrovascular complicationsc | |
| Renal | Hemolytic-uremic syndrome |
| Chronic thrombotic microangiopathy | |
| Heart | Congenital heart defects |
| Left ventricular noncompaction | |
| Fetal dilated cardiomyopathyd | |
| Other | Hepatic steatosise |
| Dermatitisf |
Growth
Poor growth is one of the most striking manifestations of early-onset cblC disease. Intrauterine growth retardation can be detected in the third trimester as described in a patient homozygous for the c.271dupA mutation who declined from normal growth parameters at 23 weeks to the 4th centile by 35 weeks of gestational age (De Bie et al. 2009).
After birth, untreated patients have very poor weight gain and may remain below birth weight for several weeks. Growth improves after treatment is initiated, and a significant increase in weight gain to 42 from 8 g/day after the initiation of treatment has been reported (Mamlok et al. 1986).
Central nervous system
Patients with early-onset cblC disease display a wide range of neurological manifestations that include microcephaly, hydrocephalus, hypotonia, cognitive deficits, and seizures. Patients with late-onset disease can present with a progressive encephalopathy manifested by regression, deterioration in school or work performance, impaired dexterity and memory, behavioral and personality changes, social withdrawal, speech difficulties, dementia, visual and auditory hallucinations, delirium, psychosis, and episodes of acute mental confusion, lethargy, and seizures that improve with the initiation of therapy (Boxer et al. 2005; Brunelli et al. 2002; Mitchell et al. 1986; Ben-Omran et al. 2007; Thauvin-Robinet et al. 2008; Augoustides-Savvopoulou et al. 1999; Powers et al. 2001; Roze et al. 2003; Tsai et al. 2007; Bodamer et al. 2001; Shinnar and Singer 1984). Subacute combined degeneration of the spinal cord (SCD) is also well described.
Seizures
The most common types of seizures are simple and partial complex seizures. Severely affected patients are difficult to treat and may have recurrent status epilepticus. EEG findings include (1) occurrence of diffuse delta rhythms substituting the normal background activity, (2) focal and multifocal abnormalities associated with slowed background activity, and (3) increased abnormalities during sleep EEG (Biancheri et al. 2002).
Cognitive deficits
Vary considerably depending on the severity of the disease and onset of treatment. Frontal/executive dysfunction appears to be a common cognitive abnormality in cblC disease; it resembles nutritional B12 deficiency (Akdal et al. 2008; Tangney et al. 2009), impacts the acquisition of new skills, and exacerbates further intellectual problems. Neuropsychological testing in two patients with early-onset cblC disease followed longitudinally showed declining attention and executive function, while other skills were relatively spared (Beauchamp et al. 2009). An adult with cblC disease had improvement of executive function when homocysteine levels were lowered (Boxer et al. 2005).
SCD
A recognized complication of late-onset cblC disease. The symptoms are progressive and include numbness of lower extremities, increased frequency of falls, incontinence, gait disturbances, progressive leg weakness, spastic paraparesis, and tetraplegia. There is a significant improvement after treatment is initiated, although a complete recovery of function is not seen frequently (Bodamer et al. 2001; Mitchell et al. 1986; Gold et al. 1996; Tsai et al. 2007; Ben-Omran et al. 2007; Powers et al. 2001; Roze et al. 2003; Thauvin-Robinet et al. 2008). A resolution of spinal cord lesions by MRI has been reported in a patient 6 months after the initiation of daily betaine, folinic acid, and parenteral OHCbl (Thauvin-Robinet et al. 2008). Pathological findings of SCD in patients with cblC disease are comparable to those seen in vitamin B12 deficiency and include multifocal demyelination and vacuolation of the lateral and dorsal columns associated with myelin loss, reactive gliosis, and macrophagocytic infiltration (Smith et al 2006).
Neuroradiological findings
Include hydrocephalus, progressive supratentorial white matter disease, and less commonly lesions in the basal ganglia (Longo et al. 2005). Hydrocephalus can be present in the first months of life, may require shunting, and usually decreases with time (Longo et al. 2005). White matter disease can progress from isolated periventricular white matter hyperintensities to confluent lesions, followed by diffuse white matter loss (Longo et al. 2005; Rossi et al. 2001; Enns et al. 1999). White matter changes have improved in one patient with late-onset disease after the initiation of treatment (Boxer et al. 2005). Magnetic resonance spectroscopic (MRS) imaging may be useful in cblC disease; one study found decreased brain choline concentrations without significant changes in N-acetylaspartate or creatine values (Debray et al. 2008); another found “lactate” peaks in the basal ganglia and the periventricular white matter (Longo et al. 2005).
Post-mortem
Brain findings include diffuse white matter disease characterized by perivascular demyelination, disintegrating myelin sheaths, infiltration of macrophages filled with myelin debris, reactive microglia, and diffuse gliosis (Smith et al. 2006). Deeper structures and the grey matter usually appear normal (Dayan and Ramsey 1974).
Pathophysiology
Elevated cerebrospinal fluid homocysteine levels have been reported in cblC disease (Harding et al. 2003) and have been shown to compromise the blood-brain barrier integrity in mice (Kamath et al. 2006) and cause seizures in a dose-dependent manner when administered to immature rats (Folbergrova 1997; Kubova et al. 1995; Mares et al. 2004). Homocysteic and homocysteine sulphinic acids are excitotoxic in culture and have been postulated to contribute to seizures in patients with hyperhomocysteinemia (Flott-Rahmel et al. 1998). Hyperhomocysteinemia has also been implicated in the development of hydrocephalus (Martinelli et al. 2011; Rossi et al. 2001; Longo et al. 2005).
Deficiency of S-adenosylmethionine (SAM), the main substrate in many methylation reactions, has been found to contribute to demyelination in patients with remethylation defects (Surtees 1998). The same mechanism may apply to patients with cblC, although some have a discordant increase in plasma SAM levels of unknown significance (Bodamer et al. 2005; Debray et al. 2008). Some important reactions that require methyl donors in the brain include the methylation of Arg107 in myelin basic protein, an important step in the formation of myelin (Lee et al. 1992; Horster et al. 2005). The methylation of guanidinoacetate to form creatine consumes approximately 40% of the methyl groups provided by SAM (Brosnan et al. 2011; Longo et al. 2011); methyl donors are also needed in the synthesis of choline and phosphatidylcholine (Bodamer et al. 2005; Debray et al. 2008; Younessi et al. 2009). Myelination and brain growth is significant during the last trimester of pregnancy and during the first year of life. Intensive management during this period, aiming to reduce toxic metabolites and normalize methionine levels, may be helpful in preventing some neurological complications associated with cblC disease (Smith et al. 2006).
The role of hypomethioninemia in the pathophysiology of SCD was suggested by the occurrence of this complication in patients with cblC and MTHFR deficiency, but not in those with cystathionine beta synthase (CBS) deficiency (Smith et al. 2006). Several animal studies have supported these observations (Scott et al. 1981; Weir et al. 1988) and have found that the pharmacologic inhibition of methionine adenosyltransferase causes intramyelinic vacuolation in the white matter of the brain and SCD of the spinal cord in young mice (Lee et al. 1992).
Ophthalmologic manifestations
Maculopathy, progressive retinal dysfunction (Schimel and Mets 2006; Gerth et al. 2008), and less frequently optic atrophy (Patton et al. 2000) are seen in most patients with infantile cblC disease. Some reports also describe ophthalmologic complications in patients with late-onset disease (Gerth et al. 2008; Shinnar and Singer 1984; Van Hove et al. 2002).
Maculopathy
CblC disease is one of the few disorders associated with infantile maculopathy. The first signs of eye disease are frequently noted several weeks after birth and include “wandering eye movements,” inability to fixate, and nystagmus. The fundoscopic exam reveals pigmented macular changes that progress to the characteristic “bull’s eye” maculopathy, a lesion characterized by a hypopigmented perimacular zone surrounded by a hyperpigmented ring (Robb et al. 1984).
Retinopathy
On fundoscopic exam, the salt-and-pepper pigmentary changes usually progress from the periphery to the rest of the retina. In parallel, the electroretinogram shows a progressive decline in scotopic (cones) and/or photopic (rods) responses (Schimel and Mets 2006; Robb et al. 1984; Gerth et al. 2008; Gaillard et al. 2008). In one instance, rod cell sensitivity was reported to improve in a patient with cblC disease following normalization of plasma methionine levels (Tsina et al. 2005). Pathological findings include photoreceptor degeneration in the macula, loss of nerve fibers and ganglion cells, and partial optic atrophy. Electron microscopy showed fine granular inclusions in ganglion and retinal pigment epithelial cells and markedly swollen mitochondria in photoreceptors in one patient (Traboulsi et al. 1992).
Pathophysiology
The pathophysiology of ophthalmologic complications in cblC is unclear. The absence of the characteristic retinopathy and maculopathy in patients with other forms of methylmalonic acidemia and most remethylation defects suggests a distinct pathophysiologic mechanism. Optic atrophy has been reported in isolated MMA (Pinar-Sueiro et al. 2010; Williams et al. 2009) and cblG disease (Poloschek et al. 2005), while retinopathy has been seen in transcobalamin II deficiency (Dharmasena et al. 2008). It is possible that the MMACHC protein may play a specific role in eye tissues. Some studies suggest that MeCbl and SAM have a protective role in retinal cell culture (Kikuchi et al. 1997) and that elevated homocysteine levels may affect eye development (Maestro de las Casas et al. 2003).
Outcome
We reviewed the reports of 27 patients with early-onset and 8 with late-onset cblC disease that described ophthalmological findings in the literature. Some of these patients were monitored longitudinally (Gerth et al. 2008). Several cases of early-onset disease describe the presence of macular pigmentation in the first month of life. This is followed by a bull’s eye maculopathy that appears between 6 and 12 months of life. Several patients develop retinopathy in the first year, and optic atrophy is variably present. The progression of ophthalmologic complications seems to invariably lead to blindness in the first decade of life (Fig. 1; Supplementary Table 1). Patients with late-onset disease do not have vision loss and may only have discrete fundoscopic changes.
Fig. 1.
Ophthalmologic complications in patients with cblC disease. Patients with early and late-onset cblC disease with eye exam performed at different ages (years). Proposed classification stages: 1 no alterations found; 2 mild maculopathy, peripheral pigmentary retinopathy; 3 progressive maculopathy, retinal extension of pigmentary changes; 4 legally blind. See details in Supplementary Table 2
Hematological manifestations
Untreated patients with cblC disease usually have anemia, thrombocytopenia, neutropenia, and a megaloblastic bone marrow. Hypersegmented neutrophils and macrocytes in the peripheral blood smear are sensitive markers of megaloblastosis even when the mean corpuscular volume (MCV) is within normal range (Carmel et al. 1980; Mitchell et al. 1986; Tsai et al. 2007; Ben-Omran et al. 2007; Russo et al. 1992). These findings in association with normal folate and vitamin B12 levels should suggest the diagnosis of a genetic defect of cobalamin metabolism. The initiation of treatment with parenteral OHCbl is associated with a progressive improvement and normalization of the hematological parameters after several weeks (Mamlok et al. 1986).
Pathophysiology
The production of tetrahydrofolate in cblC is decreased because of insufficient MeCbl. Tetrahydrofolate is the main methyl group donor in the thymidylate synthetase reaction, and its deficiency leads to decreased thymine production. The decreased availability of thymine leads to impaired DNA synthesis and eventually to impaired cell division. Hematopoietic cells are dividing constantly, and their reduced DNA synthesis manifests as megaloblastic anemia. The bone marrow of a patient with cblC disease had an abnormal deoxyuridine suppression test in vitro that was corrected with folic acid and OHCbl, but not with methyltetrahydrofolate, CNCbl, adenosylcobalamin, or MeCbl, and was worsened by homocysteine (Carmel et al. 1980).
Vascular manifestations
Thromboembolic complications, including recurrent venous thrombosis (Thauvin-Robinet et al. 2008; Roze et al. 2003; Augoustides-Savvopoulou et al. 1999; Bodamer et al. 2001; Powers et al. 2001; Guigonis et al. 2005), pulmonary thrombosis (Powers et al. 2001; Thauvin-Robinet et al. 2008; McCully 1969; Baumgartner et al. 1979b; Van Hove et al. 2002), cor pulmonale (Brandstetter et al. 1990; Profitlich et al. 2009a), and cerebrovascular complications (Brunelli et al. 2002; Geraghty et al. 1992), are an important cause for morbidity and mortality in patients with cblC disease. The vascular lesions are focal and characterized by intimal and medial proliferation of connective tissue, disruption of the elastica interna, proliferation of perivascular connective tissue, and enlargement of endothelial cells (McCully 1969).
Pathophysiology
The similarities between the vascular pathology seen in patients with CBS deficiency (McCully 1969) and cblC disease suggest that homocysteine or its derivatives play an important role in the pathophysiology of vascular disease (McCully 1996). Several cellular (Upchurch et al. 1997; Heydrick et al. 2004; Wall et al. 1980) and animal studies (Eberhardt et al. 2000; Dayal and Lentz 2007, 2008) have shown evidence of biochemical and physiological dysfunction of vascular endothelial cells (Handy et al. 2005; Weiss et al. 2001; Ungvari et al. 2003; Topal et al. 2004; Vitvitsky et al. 2004) and suggest a contribution to the vascular pathology promoted by hyperhomocysteinemia. Among the consequences of the altered endothelial function secondary to hyperhomocysteinemia are a prothrombotic state (Dayal et al. 2006; Undas et al. 2005) and decreased fibrinolysis (Colucci et al. 2008; Sauls et al. 2006). A potential unifying mechanism was recently proposed to explain the vascular dysfunction associated with hyperhomocysteinemia: homocysteine forms a mixed disulfide bond with the Cys9 residue of annexin A2, decreasing the binding affinity of this receptor to tissue plasminogen activator, reducing plasminogen activation, and impairing fibrinolysis and angiogenesis (Jacovina et al. 2009; Hajjar et al. 1998). Furthermore, murine studies have implicated hyperhomocysteinemia in atherosclerosis (Hofmann et al. 2001) and associated it with hypertriglyceridemia (Mikael et al. 2009).
Hyperhomocysteinemia may also contribute to vascular disease by an elevation of homocysteine thiolactone (HcyTL), a cyclic thioester formed when methionine aminoacyl-tRNA synthetase removes a homocysteine that has been misincorporated in the place of methionine (Jakubowski 2000). HcyTL is thought to be cytotoxic, prothrombotic, and atherogenic (Jakubowski 1997, 1999, 2002) and is elevated in patients with CBS and MTHFR deficiencies (Chwatko et al. 2007).
Numerous large studies examining the influence of lowering homocysteine levels on the risk of common vascular disease have yielded conflicting results. Patients enrolled in these studies had median baseline total plasma homocysteine (tHcy) levels of less than 15 µM, and the interventions caused a decrease of less than 5 µM (Albert et al. 2008; Armitage et al. 2010; Loland et al. 2010). It may not be appropriate to extrapolate the results of these population studies to patients with inborn errors leading to hyperhomocysteinemia.
Outcome
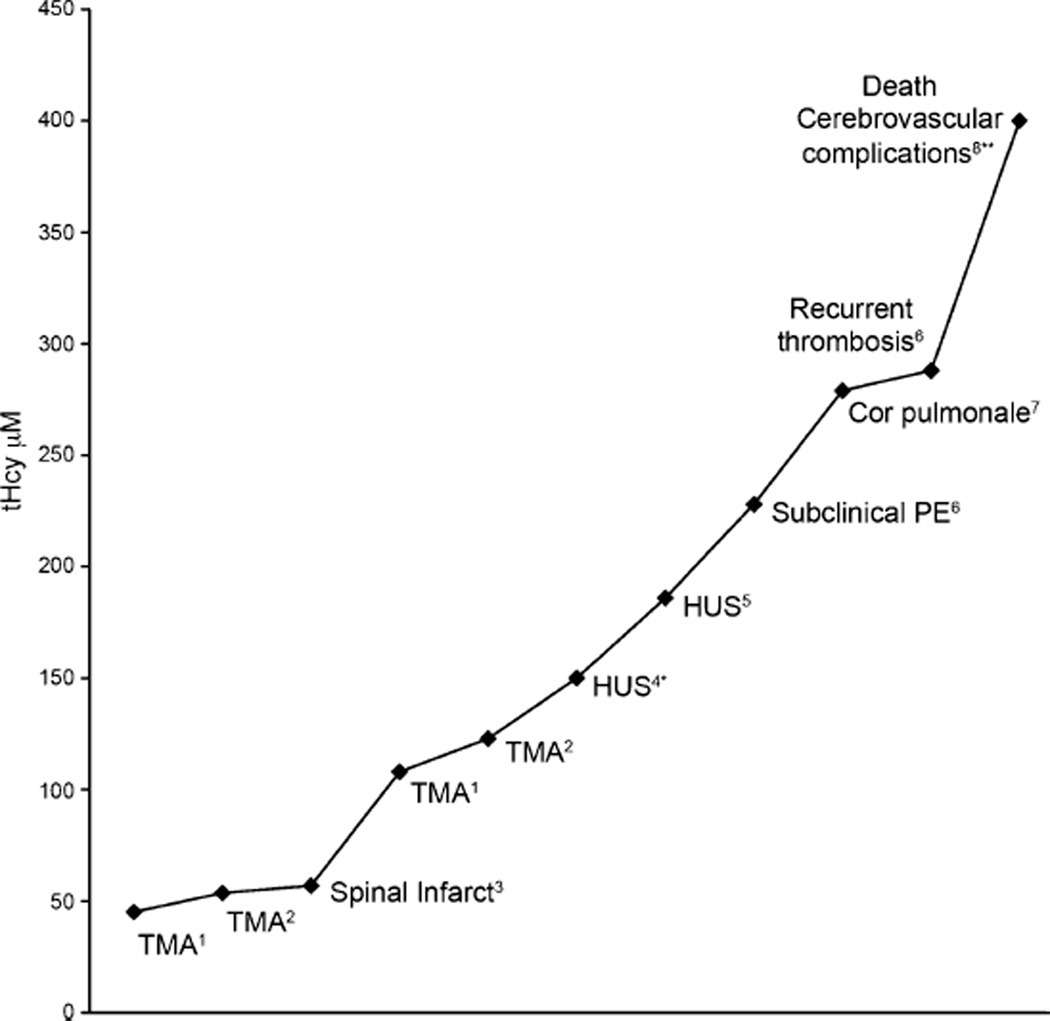
We reviewed eight cases in the literature that reported vascular complications simultaneously with tHcy levels. Our review suggests that vascular complications were seen with tHcy levels above 45 µM, and their severity may correlate with increasing tHcy levels (Fig. 2). More importantly, intensive treatment with OHCbl and betaine led to the resolution of thrombotic microangiopathy in two siblings (Van Hove et al. 2002) and to the complete resolution of cor pulmonale in a 3-year-old patient (Profitlich et al. 2009a). This supports the importance of lowering tHcy in the management of cblC disease.
Fig. 2.
Vascular complications in cblC disease and their relation to plasma total plasma homocysteine (tHcy; normal range 5–15 µM) References: 1Guigonis et al. 2005; 2Van Hove et al. 2002; 3Tsai et al. 2007; 4Kind et al. 2002; 5Sharma et al. 2007; 6Thauvin-Robinet et al. 2008; 7Profitlich et al. 2009a, b; 8Brunelli et al. 2002. TMA Thrombotic microangiopathy, HUS hemolytic uremic syndrome, PE pulmonary embolism. tHcy was approximated from plasma-free homocysteine of *23 and **61 µM
Renal manifestations
Several cases of infantile hemolytic-uremic syndrome manifested by intravascular hemolysis, thrombocytopenia, hypertension, oliguria, and renal failure have been described (Baumgartner et al. 1979b; Geraghty et al. 1992; Russo et al. 1992; Chenel et al. 1993; Kind et al. 2002; Sharma et al. 2007). A chronic form of thrombotic microangiopathy with intravascular hemolysis, microscopic hematuria, proteinuria, hypertension, and intermittent renal function deterioration, occasionally with renal failure, can occur in older patients (Van Hove et al. 2002; Guigonis et al. 2005). Cases of focal segmental glomerulosclerosis (Brandstetter et al. 1990) and atypical glomerulopathy have been reported (Brunelli et al. 2002). Histological findings of the kidneys include widening of the mesangium, swelling of endothelial cells with detachment from the basement membrane, and granular deposits in the subendothelial space (Van Hove et al. 2002; Russo et al. 1992; Brunelli et al. 2002; McCully 1969).
Outcome
Six of the eight patients with cblC and infantile hemolytic-uremic syndrome had a fatal outcome probably associated with insufficient treatment: two patients were not treated and were diagnosed postmortem (Russo et al. 1992); one was treated with CNCbl (Baumgartner et al. 1979a), while another started treatment with IM OHCbl at a late stage of the disease (Russo et al. 1992), and another received vitamin B12, but the form, dose, and frequency were not described (Geraghty et al. 1992). One responded to parenteral OHCbl but died of neurological complications (Chenel et al. 1993). Of the two infants that survived, one developed HUS while on CNCbl, which resolved when daily IM OHCbl was given (Sharma et al. 2007), and the other responded to daily parenteral OHCbl and did not require dialysis (Kind et al. 2002). Neither has reported recurrences (Kind et al. 2002; Sharma et al. 2007), emphasizing the importance of early detection and treatment with daily, appropriate doses of parenteral OHCbl.
Cardiac manifestations
Congenital heart disease (Andersson et al. 1999) and fetal dilated cardiomyopathy (De Bie et al. 2009) have been reported in association with cblC disease. Recently, a retrospective study of 10 patients followed at a single center showed that 50% had structural heart defects (Profitlich et al. 2009b), most commonly left ventricular hypertrabeculation followed by atrial or ventricular septum defects, dysplastic pulmonary valve without stenosis, and mitral valve prolapse with mild regurgitation. Although ventricular hypertrabeculation may be asymptomatic, it can cause progressive impairment of ventricular function and arrythmias (Finsterer 2009). The risk of progressive cardiac deterioration and the high prevalence of heart defects in patients with cblC highlight the importance of cardiac function monitoring as an important part of the management of these patients.
Pathophysiology
The pathophysiology of congenital heart defects and other prenatal manifestations of cblC disease are poorly understood. Elevated MMA or tHcy levels are likely not toxic in utero as fetuses with isolated methylmalonic acidemia and CBS deficiency are not reported to have prenatal manifestations. The activity of methionine synthase is higher in fetal than in mature human tissues (Gaull et al. 1973) suggesting a higher activity may be needed during fetal life. Abnormal methyl group metabolism may affect DNA and histone methylation mechanisms and could potentially modify gene expression during embryogenesis (Geiman and Muegge 2010).
Other manifestations
Hepatic steatosis (Russo et al. 1992; Brandstetter et al. 1990; McCully 1969; Geraghty et al. 1992), severe gastritis with cystic dysplastic mucosal changes (Russo et al. 1992; McCully 1969), and protein-losing enteropathy (Ellaway et al. 1998) have been described in cblC disease. An erosive, desquamating dermatitis associated with cheilitis has been reported in two infants with cblC disease without nutritional deficiencies (Howard et al. 1997). Hemophagocytic lymphohistiocytosis has been described in a 4-month-old patient with cblC disease (Wu et al. 2005).
Long-term outcome
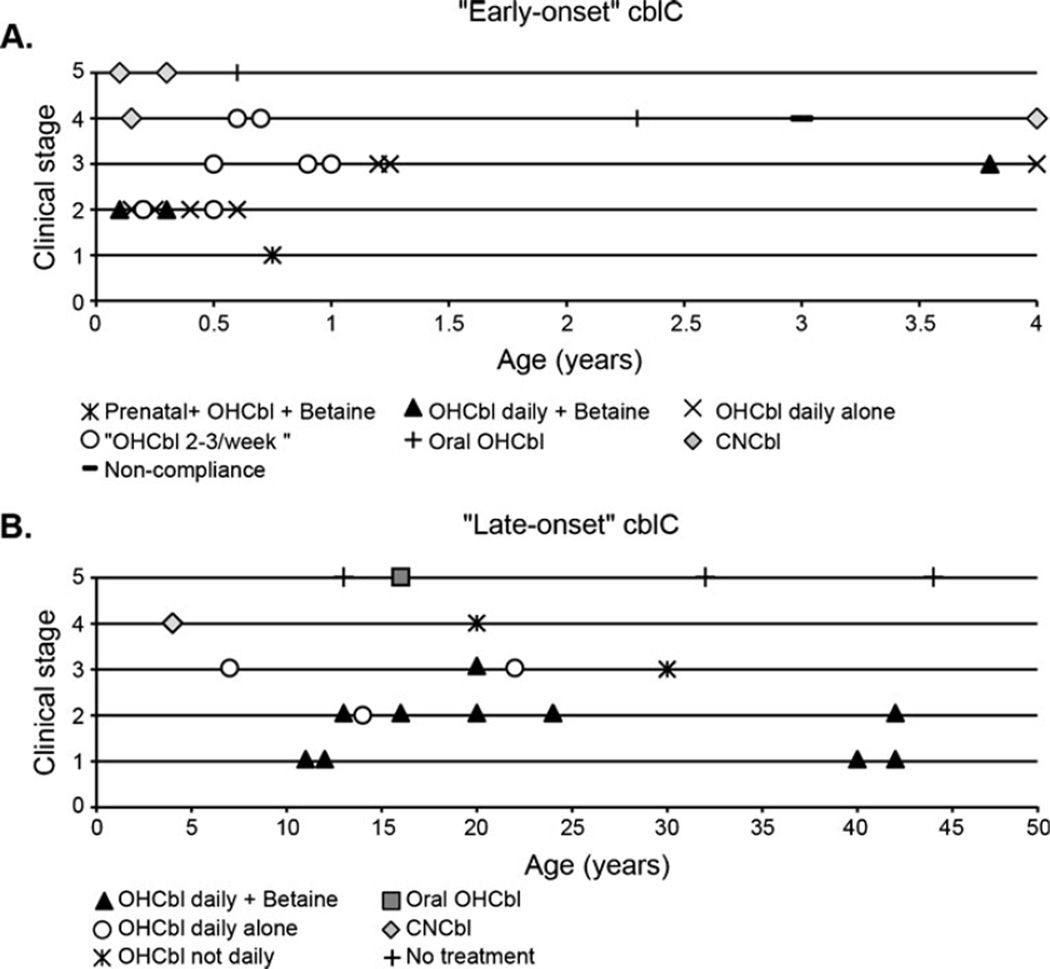
We reviewed the reports of 21 patients with early-onset and 18 with late-onset cblC disease to identify factors associated with the outcome of patients. We confirmed the contribution of the genotype to the age of presentation and outcome of patients with cblC. Besides severity of the disease, we found that the onset and type of management appear to be important contributors to the long-term outcome of patients with both early- and late-onset cblC disease (Fig. 3, Supplementary Table 2). Treatment with daily OHCbl in combination with betaine and initiation of treatment in the prenatal period were associated with a better outcome. Intermediate outcomes were seen in patients receiving daily OHCbl alone (no betaine), followed by those receiving less frequent dosing of OHCbl. A patient in whom there was considerable neurological improvement on daily OHCbl developed seizures when the frequency of injection became weekly (Carmel et al. 1980).
Fig. 3.
Outcome of patients with cblC disease according to their management. Patients with early-onset (a) and late-onset (b) cblC disease evaluated at different ages (years) and classified according to type of management and severity of disease. Proposed clinical staging: 1 no clinical alterations; 2 mild manifestations not affecting daily quality of life; 3 moderate manifestations including growth impairment, developmental delay, eye disease; 4 severe disease manifestations either affecting daily quality of life or requiring intensive medical care; 5 death. See details in Supplementary Table 2
Patients receiving oral OHCbl and CNCbl had a poor outcome (clinical stage 4 or 5), which was comparable to patients not receiving any treatment (Powers et al. 2001). All patients receiving CNCbl in our review (n=4) had a poor outcome, including death (n=1) (Mudd et al. 1969), diffuse pulmonary thromboembolism (n=1) (Brandstetter et al. 1990), hemolytic-uremic syndrome (n=1) (Sharma et al. 2007), and multifocal epilepsy associated with a semicomatose state (n=1) (Mitchell et al. 1986). The last two patients improved dramatically with the initiation of parenteral OHCbl. In our review, two patients receiving oral OHCbl had a fatal outcome; one died at 21 years of age with cerebrovascular complications (Brunelli et al. 2002) and another at 12 months of age with severe neurologic impairment (Frattini et al. 2010).
Discussion
There have been several advances in the understanding of the molecular basis of cblC disease. Diagnostic approaches have improved considerably, and cblC can be diagnosed early in life through expanded newborn screening (Fearing and Marsden 2003). However, the management and outcome of patients with cblC disease have not changed significantly, and there is a need to perform clinical trials. One of the limiting factors in planning such studies is the lack of information regarding the natural history and factors associated with the outcome of patients with cblC disease. The present review attempts to compile the knowledge on the clinical manifestations and pathophysiology since cblC disease was identified in 1969 (Mudd et al. 1969). It also systematically reviews the literature to understand the factors associated with the outcome in cblC disease.
We found that most publications related to cblC disease are case reports; we only found a few case series and small longitudinal studies; no controlled trials were identified. The clinical evaluation, biochemical parameters, monitoring strategies, and management of patients differed significantly among studies, and most did not include genotype data. The heterogeneity among publications and the lack of classification tools complicated the analysis and comparison of the data obtained.
Although cblC disease was identified more than 40 years ago, the management strategies have remained largely unchanged. Parenteral OHCbl remains the treatment of choice, and its use has likely decreased the infantile mortality rate in cblC disease considerably (Rosenblatt et al. 1997). However, the treatment of cblC disease appears to be based on expert opinion rather than clinical trial results, similar to other rare disorders (Vockley and Vockley 2010). Our review of the literature also showed that the treatment of cblC disease varies significantly, and patients receive different formulations, doses, and routes of administration of cobalamin. Not all patients are treated with betaine, and the supplementation of folic acid and carnitine is varied. The use of low-protein diets, which have the possibility to reduce methionine intake, is common. These therapeutic strategies are based on very limited evidence, and there have been no clinical trials to evaluate their efficacy.
When comparing the different therapeutic strategies reported in the literature, we found that the combination of daily doses of parenteral OHCbl with betaine provides a better outcome than other therapeutic strategies. An intermediate response was seen in patients given parenteral OHCbl less frequently or in those not receiving betaine. The review of published case reports discourages the use of CNCbl and oral OHCbl alone in the treatment of cblC, as they were associated consistently with a poor outcome in the cases reviewed (Fig. 3, Supplementary Table 2).
The effectiveness of parenteral OHCbl is supported further by the increased survival of patients with hemolytic-uremic syndrome and the reversal of severe complications in patients receiving other cobalamin formulations (Sharma et al. 2007; Mitchell et al. 1986; Kind et al. 2002). The use of higher doses of parenteral OHCbl has been effective at reducing metabolites (Carrillo-Carrasco et al. 2009) and has led to the resolution of thrombotic microangiopathy in patients with cblC disease (Van Hove et al. 2002).
Although infantile mortality has decreased considerably, there is a growing population of children and young adults with cblC disease who develop complications despite treatment. Patients with early-onset disease have a progressive visual loss leading to blindness in the first decade of life. None of the current interventions seem to slow the progression to blindness, although optimized therapy with OHCbl and betaine and early prenatal therapy could play a role. Patients with late-onset cblC disease have variable visual alterations, suggesting the severity of the disease correlates with the progression of eye disease.
There are few reports describing prenatal treatment in patients with cblC, but this strategy seems to improve the outcome of patients (Patton et al. 2000; Huemer et al. 2005). No cases of prenatal treatment before the third trimester of pregnancy have been reported. Since cblC is a developmental disorder, the initiation of prenatal interventions at the beginning of pregnancy may have a more significant impact.
Vascular complications are a major cause or morbidity and mortality in patients with early- and late-onset cblC disease (Fig. 2). Hyperhomocysteinemia is a recognized risk factor for vascular complications. Although tHcy levels improve with appropriate treatment in cblC disease, they are rarely reduced to safe levels. In our review of the literature, most patients remain in the moderate (26–50 µM) or severe (>50 µM) range, and several have levels above 100 µM. Interestingly, we found tHcy levels above 45 µM have been reported to be associated with the development of vascular complications in several patients. Whether these metabolite levels represent a goal for treatment is uncertain, but it is possible that if tHcy concentrations in cblC could be decreased below this range with optimized OHCbl and betaine doses, outcomes could be affected.
In conclusion, controlled studies are needed to provide appropriate evidence to guide clinical decisions in cblC disease, as has been suggested for other inborn errors of metabolism (Steiner 2005; Vockley and Vockley 2010). These include longitudinal studies to better define the natural history, progression, and factors associated with outcome in cblC disease. The information provided by such studies can be used to create appropriate classification tools to monitor patients and guide the choice of outcomes in controlled studies. Clinical trials are needed to determine whether higher doses of OHCbl, prenatal treatment, or other therapies could improve the management and outcome of patients with cblC disease.
Supplementary Material
Abbreviations
- cblC
Cobalamin C disease
- CBS
Cystathionine beta synthase
- CNCbl
Cyanocobalamin
- IM
Intramuscular
- MeCbl
Methylcobalamin
- MMA
Methylmalonic acid
- MTHFR
Methylenetetrahydrofolate reductase
- OHCbl
Hydroxocobalamin
- SAM
S-Adenosylmethionine
- SCD
Subacute combined degeneration of the spinal cord
- tHcy
Total plasma homocysteine
- HcyTL
Homocysteine thiolactone
Footnotes
References to electronic databases: Methylmalonic aciduria and homocystinuria cblC type: OMIM #277400.
Competing interests: None declared
Electronic supplementary material The online version of this article (doi:10.1007/s10545-011-9365-x) contains supplementary material, which is available to authorized users.
References
- Akdal G, Yener GG, Kurt P. Treatment responsive executive and behavioral dysfunction associated with vitamin B12 deficiency. Neurocase. 2008;14:147–150. doi: 10.1080/13554790802032242. [DOI] [PubMed] [Google Scholar]
- Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson HC, Marble M, Shapira E. Long-term outcome in treated combined methylmalonic acidemia and homocystinemia. Genet Med. 1999;1:146–150. doi: 10.1097/00125817-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto R, Collins R. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303:2486–2494. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- Augoustides-Savvopoulou P, Mylonas I, Sewell AC, Rosenblatt DS. Reversible dementia in an adolescent with cblC disease: clinical heterogeneity within the same family. J Inherit Metab Dis. 1999;22:756–758. doi: 10.1023/a:1005508620919. [DOI] [PubMed] [Google Scholar]
- Baumgartner ER, Wick H, Linnell JC, Gaull GE, Bachmann C, Steinmann B. Congenital defect in intracellular cobalamin metabolism resulting in homocystinuria and methylmalonic aciduria. II. Biochemical investigations. Helv Paediatr Acta. 1979a;34:483–496. [PubMed] [Google Scholar]
- Baumgartner ER, Wick H, Maurer R, Egli N, Steinmann B. Congenital defect in intracellular cobalamin metabolism resulting in homocysteinuria and methylmalonic aciduria. I. Case report and histopathology. Helv Paediatr Acta. 1979b;34:465–482. [PubMed] [Google Scholar]
- Beauchamp MH, Anderson V, Boneh A. Cognitive and social profiles in two patients with cobalamin C disease. J Inherit Metab Dis. 2009 doi: 10.1007/s10545-009-1284-8. [DOI] [PubMed] [Google Scholar]
- Ben-Omran TI, Wong H, Blaser S, Feigenbaum A. Late-onset cobalamin-C disorder: a challenging diagnosis. Am J Med Genet A. 2007;143A:979–984. doi: 10.1002/ajmg.a.31671. [DOI] [PubMed] [Google Scholar]
- Biancheri R, Cerone R, Rossi A, Schiaffino MC, Caruso U, Minniti G, Perrone MV, Tortori-Donati P, Veneselli E. Early-onset cobalamin C/D deficiency: epilepsy and electroencephalographic features. Epilepsia. 2002;43:616–622. doi: 10.1046/j.1528-1157.2002.24001.x. [DOI] [PubMed] [Google Scholar]
- Bodamer OA, Rosenblatt DS, Appel SH, Beaudet AL. Adult-onset combined methylmalonic aciduria and homocystinuria (cblC) Neurology. 2001;56:1113. doi: 10.1212/wnl.56.8.1113. [DOI] [PubMed] [Google Scholar]
- Bodamer OA, Sahoo T, Beaudet AL, O'Brien WE, Bottiglieri T, Stockler-Ipsiroglu S, Wagner C, Scaglia F. Creatine metabolism in combined methylmalonic aciduria and homocystinuria. Ann Neurol. 2005;57:557–560. doi: 10.1002/ana.20419. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Kramer JH, Johnston K, Goldman J, Finley R, Miller BL. Executive dysfunction in hyperhomocystinemia responds to homocysteine-lowering treatment. Neurology. 2005;64:1431–1434. doi: 10.1212/01.WNL.0000158476.74580.A8. [DOI] [PubMed] [Google Scholar]
- Brandstetter Y, Weinhouse E, Splaingard ML, Tang TT. Cor pulmonale as a complication of methylmalonic acidemia and homocystinuria (Cbl-C type) Am J Med Genet. 1990;36:167–171. doi: 10.1002/ajmg.1320360208. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids. 2011;40:1325–1331. doi: 10.1007/s00726-011-0853-y. [DOI] [PubMed] [Google Scholar]
- Brunelli SM, Meyers KE, Guttenberg M, Kaplan P, Kaplan BS. Cobalamin C deficiency complicated by an atypical glomerulopathy. Pediatr Nephrol. 2002;17:800–803. doi: 10.1007/s00467-002-0895-1. [DOI] [PubMed] [Google Scholar]
- Carmel R, Bedros AA, Mace JW, Goodman SI. Congenital methylmalonic aciduria–homocystinuria with megaloblastic anemia: observations on response to hydroxocobalamin and on the effect of homocysteine and methionine on the deoxyuridine suppression test. Blood. 1980;55:570–579. [PubMed] [Google Scholar]
- Carrillo-Carrasco N, Sloan J, Valle D, Hamosh A, Venditti CP. Hydroxocobalamin dose escalation improves metabolic control in cblC. J Inherit Metab Dis. 2009;32:728–731. doi: 10.1007/s10545-009-1257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenel C, Wood C, Gourrier E, Zittoun J, Casadevall I, Ogier H. Neonatal hemolytic-uremic syndrome, methylmalonic aciduria and homocystinuria caused by intracellular vitamin B 12 deficiency. Value of etiological diagnosis. Arch Fr Pediatr. 1993;50:749–754. [PubMed] [Google Scholar]
- Chwatko G, Boers GH, Strauss KA, Shih DM, Jakubowski H. Mutations in methylenetetrahydrofolate reductase or cystathionine beta-synthase gene, or a high-methionine diet, increase homocysteine thiolactone levels in humans and mice. FASEB J. 2007;21:1707–1713. doi: 10.1096/fj.06-7435com. [DOI] [PubMed] [Google Scholar]
- Cogan DG, Schulman J, Porter RJ, Mudd SH. Epileptiform ocular movements with methylmalonic aciduria and homocystinuria. Am J Ophthalmol. 1980;90:251–253. doi: 10.1016/s0002-9394(14)74863-9. [DOI] [PubMed] [Google Scholar]
- Colucci M, Cattaneo M, Martinelli I, Semeraro F, Binetti BM, Semeraro N. Mild hyperhomocysteinemia is associated with increased TAFI levels and reduced plasma fibrinolytic potential. J Thromb Haemost. 2008;6:1571–1577. doi: 10.1111/j.1538-7836.2008.03070.x. [DOI] [PubMed] [Google Scholar]
- Cusmano-Ozog K, Levine S, Martin M, Nicholas E, Packman S, Rosenblatt D, Cederbaum S, Cowan T, Enns G. Cobalamin C disease identified by newborn screening: The California experience. In: Program and abstracts for the SIMD annual meeting. Mol Genet Metab. 2007;90:227–265. [Google Scholar]
- Dayal S, Lentz SR. Role of redox reactions in the vascular phenotype of hyperhomocysteinemic animals. Antioxid Redox Signal. 2007;9:1899–1909. doi: 10.1089/ars.2007.1806. [DOI] [PubMed] [Google Scholar]
- Dayal S, Lentz SR. Murine models of hyperhomocysteinemia and their vascular phenotypes. Arterioscler Thromb Vasc Biol. 2008;28:1596–1605. doi: 10.1161/ATVBAHA.108.166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR. Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood. 2006;108:2237–2243. doi: 10.1182/blood-2006-02-005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan AD, Ramsey RB. An inborn error of vitamin B12 metabolism associated with cellular deficiency of coenzyme forms of the vitamin. Pathological and neurochemical findings in one case. J Neurol Sci. 1974;23:117–128. doi: 10.1016/0022-510x(74)90147-6. [DOI] [PubMed] [Google Scholar]
- De Bie I, Nizard SD, Mitchell GA. Fetal dilated cardiomyopathy: an unsuspected presentation of methylmalonic aciduria and hyperhomocystinuria, cblC type. Prenat Diagn. 2009;29:266–270. doi: 10.1002/pd.2218. [DOI] [PubMed] [Google Scholar]
- Debray FG, Boulanger Y, Khiat A, Decarie JC, Orquin J, Roy MS, Lortie A, Ramos F, Verhoeven NM, Struys E, Blom HJ, Jakobs C, Levy E, Mitchell GA, Lambert M. Reduced brain choline in homocystinuria due to remethylation defects. Neurology. 2008;71:44–49. doi: 10.1212/01.wnl.0000316391.40236.c3. [DOI] [PubMed] [Google Scholar]
- Dharmasena A, Calcagni A, Kerr AR. Retinopathy in inherited transcobalamin II deficiency. Arch Ophthalmol. 2008;126:141–142. doi: 10.1001/archophthalmol.2007.21. [DOI] [PubMed] [Google Scholar]
- Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI, Yaghoubi M, Goldschmidt-Clermont PJ, Farber HW, Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway C, Christodoulou J, Kamath R, Carpenter K, Wilcken B. The association of protein-losing enteropathy with cobalamin C defect. J Inherit Metab Dis. 1998;21:17–22. doi: 10.1023/a:1005303128904. [DOI] [PubMed] [Google Scholar]
- Enns GM, Barkovich AJ, Rosenblatt DS, Fredrick DR, Weisiger K, Ohnstad C, Packman S. Progressive neurological deterioration and MRI changes in cblC methylmalonic acidaemia treated with hydroxocobalamin. J Inherit Metab Dis. 1999;22:599–607. doi: 10.1023/a:1005517727451. [DOI] [PubMed] [Google Scholar]
- Fearing MK, Marsden D. Expanded newborn screening. Pediatr Ann. 2003;32:509–515. doi: 10.3928/0090-4481-20030801-08. [DOI] [PubMed] [Google Scholar]
- Finsterer J. Cardiogenetics, neurogenetics, and pathogenetics of left ventricular hypertrabeculation/noncompaction. Pediatr Cardiol. 2009;30:659–681. doi: 10.1007/s00246-008-9359-0. [DOI] [PubMed] [Google Scholar]
- Flott-Rahmel B, Schurmann M, Schluff P, Fingerhut R, Musshoff U, Fowler B, Ullrich K. Homocysteic and homocysteine sulphinic acid exhibit excitotoxicity in organotypic cultures from rat brain. Eur J Pediatr. 1998;157(Suppl 2):S112–S117. doi: 10.1007/pl00014291. [DOI] [PubMed] [Google Scholar]
- Folbergrova J. Anticonvulsant action of both NMDA and non-NMDA receptor antagonists against seizures induced by homocysteine in immature rats. Exp Neurol. 1997;145:442–450. doi: 10.1006/exnr.1997.6464. [DOI] [PubMed] [Google Scholar]
- Frattini D, Fusco C, Ucchino V, Tavazzi B, Della Giustina E. Early onset methylmalonic aciduria and homocystinuria cblC type with demyelinating neuropathy. Pediatr Neurol. 2010;43:135–138. doi: 10.1016/j.pediatrneurol.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Gaillard MC, Matthieu JM, Borruat FX. Retinal dysfunction in combined methylmalonic aciduria and homocystinuria (Cblc) disease: a spectrum of disorders. Klin Monbl Augenheilkd. 2008;225:491–494. doi: 10.1055/s-2008-1027310. [DOI] [PubMed] [Google Scholar]
- Gaull GE, Von Berg W, Raiha NC, Sturman JA. Development of methyltransferase activities of human fetal tissues. Pediatr Res. 1973;7:527–533. doi: 10.1203/00006450-197305000-00006. [DOI] [PubMed] [Google Scholar]
- Geiman TM, Muegge K. DNA methylation in early development. Mol Reprod Dev. 2010;77:105–113. doi: 10.1002/mrd.21118. [DOI] [PubMed] [Google Scholar]
- Geraghty MT, Perlman EJ, Martin LS, Hayflick SJ, Casella JF, Rosenblatt DS, Valle D. Cobalamin C defect associated with hemolytic-uremic syndrome. J Pediatr. 1992;120:934–937. doi: 10.1016/s0022-3476(05)81967-5. [DOI] [PubMed] [Google Scholar]
- Gerth C, Morel CF, Feigenbaum A, Levin AV. Ocular phenotype in patients with methylmalonic aciduria and homocystinuria, cobalamin C type. J AAPOS. 2008;12:591–596. doi: 10.1016/j.jaapos.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Gold R, Bogdahn U, Kappos L, Toyka KV, Baumgartner ER, Fowler B, Wendel U. Hereditary defect of cobalamin metabolism (homocystinuria and methylmalonic aciduria) of juvenile onset. J Neurol Neurosurg Psychiatry. 1996;60:107–108. doi: 10.1136/jnnp.60.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigonis V, Fremeaux-Bacchi V, Giraudier S, Favier R, Borderie D, Massy Z, Mougenot B, Rosenblatt DS, Deschenes G. Late-onset thrombocytic microangiopathy caused by cblC disease: association with a factor H mutation. Am J Kidney Dis. 2005;45:588–595. doi: 10.1053/j.ajkd.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Hajjar KA, Mauri L, Jacovina AT, Zhong F, Mirza UA, Padovan JC, Chait BT. Tissue plasminogen activator binding to the annexin II tail domain. Direct modulation by homocysteine. J Biol Chem. 1998;273:9987–9993. doi: 10.1074/jbc.273.16.9987. [DOI] [PubMed] [Google Scholar]
- Handy DE, Zhang Y, Loscalzo J. Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J Biol Chem. 2005;280:15518–15525. doi: 10.1074/jbc.M501452200. [DOI] [PubMed] [Google Scholar]
- Harding CO, Pillers DA, Steiner RD, Bottiglieri T, Rosenblatt DS, Debley J, Michael Gibson K. Potential for misdiagnosis due to lack of metabolic derangement in combined methylmalonic aciduria/hyperhomocysteinemia (cblC) in the neonate. J Perinatol. 2003;23:384–386. doi: 10.1038/sj.jp.7210955. [DOI] [PubMed] [Google Scholar]
- Heydrick SJ, Weiss N, Thomas SR, Cap AP, Pimentel DR, Loscalzo J, Keaney JF., Jr L-Homocysteine and L-homocystine stereospecifically induce endothelial nitric oxide synthase-dependent lipid peroxidation in endothelial cells. Free Radic Biol Med. 2004;36:632–640. doi: 10.1016/j.freeradbiomed.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ, Jr, Kohl B, Rao V, Kisiel W, Stern DM, Schmidt AM. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horster F, Surtees R, Hoffmann GF. Disorders of intermediary metabolism: toxic leukoencephalopathies. J Inherit Metab Dis. 2005;28:345–356. doi: 10.1007/s10545-005-2164-5. [DOI] [PubMed] [Google Scholar]
- Howard R, Frieden IJ, Crawford D, McCalmont T, Levy ML, Rosenblatt DS, Sweetman L, Goodman SI, Ohnstad C, Hart K, Berrios M, Packman S. Methylmalonic acidemia, cobalamin C type, presenting with cutaneous manifestations. Arch Dermatol. 1997;133:1563–1566. [PubMed] [Google Scholar]
- Huemer M, Simma B, Fowler B, Suormala T, Bodamer OA, Sass JO. Prenatal and postnatal treatment in cobalamin C defect. J Pediatr. 2005;147:469–472. doi: 10.1016/j.jpeds.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Jacovina AT, Deora AB, Ling Q, Broekman MJ, Almeida D, Greenberg CB, Marcus AJ, Smith JD, Hajjar KA. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2-dependent fibrinolysis. J Clin Invest. 2009;119:3384–3394. doi: 10.1172/JCI39591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski H. Metabolism of homocysteine thiolactone in human cell cultures. Possible mechanism for pathological consequences of elevated homocysteine levels. J Biol Chem. 1997;272:1935–1942. [PubMed] [Google Scholar]
- Jakubowski H. Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J. 1999;13:2277–2283. [PubMed] [Google Scholar]
- Jakubowski H. Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J Nutr. 2000;130:377S–381S. doi: 10.1093/jn/130.2.377S. [DOI] [PubMed] [Google Scholar]
- Jakubowski H. Homocysteine is a protein amino acid in humans. Implications for homocysteine-linked disease. J Biol Chem. 2002;277:30425–30428. doi: 10.1074/jbc.C200267200. [DOI] [PubMed] [Google Scholar]
- Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Kashii S, Honda Y, Tamura Y, Kaneda K, Akaike A. Protective effects of methylcobalamin, a vitamin B12 analog, against glutamate-induced neurotoxicity in retinal cell culture. Invest Ophthalmol Vis Sci. 1997;38:848–854. [PubMed] [Google Scholar]
- Kind T, Levy J, Lee M, Kaicker S, Nicholson JF, Kane SA. Cobalamin C disease presenting as hemolytic-uremic syndrome in the neonatal period. J Pediatr Hematol Oncol. 2002;24:327–329. doi: 10.1097/00043426-200205000-00023. [DOI] [PubMed] [Google Scholar]
- Kubova H, Folbergrova J, Mares P. Seizures induced by homocysteine in rats during ontogenesis. Epilepsia. 1995;36:750–756. doi: 10.1111/j.1528-1157.1995.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Lee CC, Surtees R, Duchen LW. Distal motor axonopathy and central nervous system myelin vacuolation caused by cycloleucine, an inhibitor of methionine adenosyltransferase. Brain. 1992;115(Pt 3):935–955. doi: 10.1093/brain/115.3.935. [DOI] [PubMed] [Google Scholar]
- Loland KH, Bleie O, Blix AJ, Strand E, Ueland PM, Refsum H, Ebbing M, Nordrehaug JE, Nygard O. Effect of homocysteine-lowering B vitamin treatment on angiographic progression of coronary artery disease: a Western Norway B Vitamin Intervention Trial (WENBIT) substudy. Am J Cardiol. 2010;105:1577–1584. doi: 10.1016/j.amjcard.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Longo D, Fariello G, Dionisi-Vici C, Cannata V, Boenzi S, Genovese E, Deodato F. MRI and 1H-MRS findings in early-onset cobalamin C/D defect. Neuropediatrics. 2005;36:366–372. doi: 10.1055/s-2005-873057. [DOI] [PubMed] [Google Scholar]
- Longo N, Ardon O, Vanzo R, Schwartz E, Pasquali M. Disorders of creatine transport and metabolism. Am J Med Genet C Semin Med Genet. 2011;157:72–78. doi: 10.1002/ajmg.c.30292. [DOI] [PubMed] [Google Scholar]
- Maestro de las Casas C, Epeldegui M, Tudela C, Varela-Moreiras G, Perez-Miguelsanz J. High exogenous homocysteine modifies eye development in early chick embryos. Birth Defects Res A Clin Mol Teratol. 2003;67:35–40. doi: 10.1002/bdra.10014. [DOI] [PubMed] [Google Scholar]
- Mamlok RJ, Isenberg JN, Rassin DK, Norcross K, Tallan HH. A cobalamin metabolic defect with homocystinuria, methylmalonic aciduria and macrocytic anemia. Neuropediatrics. 1986;17:94–99. doi: 10.1055/s-2008-1052508. [DOI] [PubMed] [Google Scholar]
- Mares P, Folbergrova J, Kubova H. Excitatory aminoacids and epileptic seizures in immature brain. Physiol Res. 2004;53(Suppl 1):S115–S124. [PubMed] [Google Scholar]
- Martinelli D, Deodato F, Dionisi-Vici C. Cobalamin C defect: natural history, pathophysiology, and treatment. J Inherit Metab Dis. 2011;34:127–135. doi: 10.1007/s10545-010-9161-z. [DOI] [PubMed] [Google Scholar]
- Mc Guire PJ, Parikh A, Diaz GA. Profiling of oxidative stress in patients with inborn errors of metabolism. Mol Genet Metab. 2009;98:173–180. doi: 10.1016/j.ymgme.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–389. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- Mikael LG, Wang XL, Wu Q, Jiang H, Maclean KN, Rozen R. Hyperhomocysteinemia is associated with hypertriglyceridemia in mice with methylenetetrahydrofolate reductase deficiency. Mol Genet Metab. 2009;98:187–194. doi: 10.1016/j.ymgme.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Mitchell GA, Watkins D, Melancon SB, Rosenblatt DS, Geoffroy G, Orquin J, Homsy MB, Dallaire L. Clinical heterogeneity in cobalamin C variant of combined homocystinuria and methylmalonic aciduria. J Pediatr. 1986;108:410–415. doi: 10.1016/s0022-3476(86)80882-4. [DOI] [PubMed] [Google Scholar]
- Mudd SH, Levy HL, Abeles RH, Jennedy JP., Jr A derangement in B12 metabolism leading to homocystinemia, cystathioninemia and methylmalonic aciduria. Biochem Biophys Res Commun. 1969;35:121–126. doi: 10.1016/0006-291x(69)90491-4. [DOI] [PubMed] [Google Scholar]
- Patton N, Beatty S, Lloyd IC, Wraith JE. Optic atrophy in association with cobalamin C (cblC) disease. Ophthalmic Genet. 2000;21:151–154. [PubMed] [Google Scholar]
- Pinar-Sueiro S, Martinez-Fernandez R, Lage-Medina S, Aldamiz-Echevarria L, Vecino E. Optic neuropathy in methylmalonic acidemia: the role of neuroprotection. J Inherit Metab Dis. 2010 doi: 10.1007/s10545-010-9084-8. (in press) [DOI] [PubMed] [Google Scholar]
- Poloschek CM, Fowler B, Unsold R, Lorenz B. Disturbed visual system function in methionine synthase deficiency. Graefes Arch Clin Exp Ophthalmol. 2005;243:497–500. doi: 10.1007/s00417-004-1044-2. [DOI] [PubMed] [Google Scholar]
- Powers JM, Rosenblatt DS, Schmidt RE, Cross AH, Black JT, Moser AB, Moser HW, Morgan DJ. Neurological and neuropathologic heterogeneity in two brothers with cobalamin C deficiency. Ann Neurol. 2001;49:396–400. [PubMed] [Google Scholar]
- Profitlich L, Kirmse B, Wasserstein MP, Diaz G, Srivastava S. Resolution of cor pulmonale after medical management in a patient with cblC-type methylmalonic aciduria and homocystinuria: a case report. Cases J. 2009a;2:8603. doi: 10.4076/1757-1626-2-8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profitlich LE, Kirmse B, Wasserstein MP, Diaz GA, Srivastava S. High prevalence of structural heart disease in children with cblC-type methylmalonic aciduria and homocystinuria. Mol Genet Metab. 2009b;98:344–348. doi: 10.1016/j.ymgme.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Richard E, Jorge-Finnigan A, Garcia-Villoria J, Merinero B, Desviat LR, Gort L, Briones P, Leal F, Perez-Cerda C, Ribes A, Ugarte M, Perez B. Genetic and cellular studies of oxidative stress in methylmalonic aciduria (MMA) cobalamin deficiency type C (cblC) with homocystinuria (MMACHC) Hum Mutat. 2009;30:1558–1566. doi: 10.1002/humu.21107. [DOI] [PubMed] [Google Scholar]
- Robb RM, Dowton SB, Fulton AB, Levy HL. Retinal degeneration in vitamin B12 disorder associated with methylmalonic aciduria and sulfur amino acid abnormalities. Am J Ophthalmol. 1984;97:691–696. doi: 10.1016/0002-9394(84)90499-9. [DOI] [PubMed] [Google Scholar]
- Rosenblatt DS, Aspler AL, Shevell MI, Pletcher BA, Fenton WA, Seashore MR. Clinical heterogeneity and prognosis in combined methylmalonic aciduria and homocystinuria (cblC) J Inherit Metab Dis. 1997;20:528–538. doi: 10.1023/a:1005353530303. [DOI] [PubMed] [Google Scholar]
- Rossi A, Cerone R, Biancheri R, Gatti R, Schiaffino MC, Fonda C, Zammarchi E, Tortori-Donati P. Early-onset combined methylmalonic aciduria and homocystinuria: neuroradiologic findings. AJNR Am J Neuroradiol. 2001;22:554–563. [PMC free article] [PubMed] [Google Scholar]
- Roze E, Gervais D, Demeret S, Ogier de Baulny H, Zittoun J, Benoist JF, Said G, Pierrot-Deseilligny C, Bolgert F. Neuropsychiatric disturbances in presumed late-onset cobalamin C disease. Arch Neurol. 2003;60:1457–1462. doi: 10.1001/archneur.60.10.1457. [DOI] [PubMed] [Google Scholar]
- Russo P, Doyon J, Sonsino E, Ogier H, Saudubray JM. A congenital anomaly of vitamin B12 metabolism: a study of three cases. Hum Pathol. 1992;23:504–512. doi: 10.1016/0046-8177(92)90127-o. [DOI] [PubMed] [Google Scholar]
- Sauls DL, Lockhart E, Warren ME, Lenkowski A, Wilhelm SE, Hoffman M. Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: a potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry. 2006;45:2480–2487. doi: 10.1021/bi052076j. [DOI] [PubMed] [Google Scholar]
- Schimel AM, Mets MB. The natural history of retinal degeneration in association with cobalamin C (cbl C) disease. Ophthalmic Genet. 2006;27:9–14. doi: 10.1080/13816810500481758. [DOI] [PubMed] [Google Scholar]
- Scott JM, Dinn JJ, Wilson P, Weir DG. Pathogenesis of subacute combined degeneration: a result of methyl group deficiency. Lancet. 1981;2:334–337. doi: 10.1016/s0140-6736(81)90649-8. [DOI] [PubMed] [Google Scholar]
- Sharma AP, Greenberg CR, Prasad AN, Prasad C. Hemolytic uremic syndrome (HUS) secondary to cobalamin C (cblC) disorder. Pediatr Nephrol. 2007;22:2097–2103. doi: 10.1007/s00467-007-0604-1. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Singer HS. Cobalamin C mutation (methylmalonic aciduria and homocystinuria) in adolescence. A treatable cause of dementia and myelopathy. N Engl J Med. 1984;311:451–454. doi: 10.1056/NEJM198408163110707. [DOI] [PubMed] [Google Scholar]
- Smith SE, Kinney HC, Swoboda KJ, Levy HL. Subacute combined degeneration of the spinal cord in cblC disorder despite treatment with B12. Mol Genet Metab. 2006;88:138–145. doi: 10.1016/j.ymgme.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Steiner RD. Evidence based medicine in inborn errors of metabolism: is there any and how to find it. Am J Med Genet A. 2005;134A:192–197. doi: 10.1002/ajmg.a.30594. [DOI] [PubMed] [Google Scholar]
- Surtees R. Demyelination and inborn errors of the single carbon transfer pathway. Eur J Pediatr. 1998;157(Suppl 2):S118–S121. doi: 10.1007/pl00014296. [DOI] [PubMed] [Google Scholar]
- Tangney CC, Tang Y, Evans DA, Morris MC. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology. 2009;72:361–367. doi: 10.1212/01.wnl.0000341272.48617.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauvin-Robinet C, Roze E, Couvreur G, Horellou MH, Sedel F, Grabli D, Bruneteau G, Tonneti C, Masurel-Paulet A, Perennou D, Moreau T, Giroud M, de Baulny HO, Giraudier S, Faivre L. The adolescent and adult form of cobalamin C disease: clinical and molecular spectrum. J Neurol Neurosurg Psychiatry. 2008;79:725–728. doi: 10.1136/jnnp.2007.133025. [DOI] [PubMed] [Google Scholar]
- Topal G, Brunet A, Millanvoye E, Boucher JL, Rendu F, Devynck MA, David-Dufilho M. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radic Biol Med. 2004;36:1532–1541. doi: 10.1016/j.freeradbiomed.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Traboulsi EI, Silva JC, Geraghty MT, Maumenee IH, Valle D, Green WR. Ocular histopathologic characteristics of cobalamin C type vitamin B12 defect with methylmalonic aciduria and homocystinuria. Am J Ophthalmol. 1992;113:269–280. doi: 10.1016/s0002-9394(14)71578-8. [DOI] [PubMed] [Google Scholar]
- Tsai AC, Morel CF, Scharer G, Yang M, Lerner-Ellis JP, Rosenblatt DS, Thomas JA. Late-onset combined homocystinuria and methylmalonic aciduria (cblC) and neuropsychiatric disturbance. Am J Med Genet A. 2007;143A:2430–2434. doi: 10.1002/ajmg.a.31932. [DOI] [PubMed] [Google Scholar]
- Tsina EK, Marsden DL, Hansen RM, Fulton AB. Maculopathy and retinal degeneration in cobalamin C methylmalonic aciduria and homocystinuria. Arch Ophthalmol. 2005;123:1143–1146. doi: 10.1001/archopht.123.8.1143. [DOI] [PubMed] [Google Scholar]
- Undas A, Brozek J, Szczeklik A. Homocysteine and thrombosis: from basic science to clinical evidence. Thromb Haemost. 2005;94:907–915. doi: 10.1160/TH05-05-0313. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- Upchurch GR, Jr, Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF, Jr, Loscalzo J. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- Van Hove JL, Van Damme-Lombaerts R, Grunewald S, Peters H, Van Damme B, Fryns JP, Arnout J, Wevers R, Baumgartner ER, Fowler B. Cobalamin disorder Cbl-C presenting with late-onset thrombotic microangiopathy. Am J Med Genet. 2002;111:195–201. doi: 10.1002/ajmg.10499. [DOI] [PubMed] [Google Scholar]
- Vitvitsky V, Dayal S, Stabler S, Zhou Y, Wang H, Lentz SR, Banerjee R. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am J Physiol Regul Integr Comp Physiol. 2004;287:R39–R46. doi: 10.1152/ajpregu.00036.2004. [DOI] [PubMed] [Google Scholar]
- Vockley J, Vockley CM. Clinical trials: curing a critical deficiency in metabolic medicine. Mol Genet Metab. 2010;99:244–245. doi: 10.1016/j.ymgme.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Wall RT, Harlan JM, Harker LA, Striker GE. Homocysteine-induced endothelial cell injury in vitro: a model for the study of vascular injury. Thromb Res. 1980;18:113–121. doi: 10.1016/0049-3848(80)90175-9. [DOI] [PubMed] [Google Scholar]
- Weir DG, Keating S, Molloy A, McPartlin J, Kennedy S, Blanchflower J, Kennedy DG, Rice D, Scott JM. Methylation deficiency causes vitamin B12-associated neuropathy in the pig. J Neurochem. 1988;51:1949–1952. doi: 10.1111/j.1471-4159.1988.tb01184.x. [DOI] [PubMed] [Google Scholar]
- Weisfeld-Adams JD, Morrissey MA, Kirmse BM, Salveson BR, Wasserstein MP, McGuire PJ, Sunny S, Cohen-Pfeffer JL, Yu C, Caggana M, Diaz GA. Newborn screening and early biochemical follow-up in combined methylmalonic aciduria and homocystinuria, cblC type, and utility of methionine as a secondary screening analyte. Mol Genet Metab. 2010;99:116–123. doi: 10.1016/j.ymgme.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Zhang YY, Heydrick S, Bierl C, Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst( e)ine-induced endothelial dysfunction. Proc Natl Acad Sci U S A. 2001;98:12503–12508. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZR, Hurley PE, Altiparmak UE, Feldon SE, Arnold GL, Eggenberger E, Mejico LJ. Late onset optic neuropathy in methylmalonic and propionic acidemia. Am J Ophthalmol. 2009;147:929–933. doi: 10.1016/j.ajo.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Wu S, Gonzalez-Gomez I, Coates T, Yano S. Cobalamin C disease presenting with hemophagocytic lymphohistiocytosis. Pediatr Hematol Oncol. 2005;22:717–721. doi: 10.1080/08880010500278871. [DOI] [PubMed] [Google Scholar]
- Younessi D, Moseley K, Yano S. Creatine metabolism in combined methylmalonic aciduria and homocystinuria disease revisited. Ann Neurol. 2009;65:481–482. doi: 10.1002/ana.21571. author reply 482–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.