Abstract
A novel multicomponent strategy for the efficient synthesis of tricyclic pyrrolo[1,2-a]quinolines has been described. The bond-forming efficiency, accessibility and generality of this synthesis make it highly attractive to assemble tri-heterocyclic scaffolds.
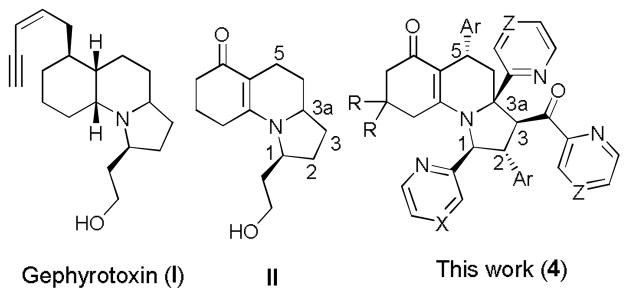
The functional diverse pyrrolo[1,2-a]quinoline skeletons are well-represented in biologically active natural products;1 they have been found in natural alkaloids, such as gephyrotoxin (I) (Figure 1), that was isolated from tropical frogs Dendrobates histrionicus by Daly and coworkers in 1977.2 These compounds exhibited nearly nontoxic activity as a muscarinic antagonist3 and an array of neurological activities.4 In addition, pyrroloquinolines are well-known to possess various biological activities including antitumor,5 antibacterial 6 and antifungal.7 Because of their unique chemical and biological characteristics, the synthesis of I 8 and its analogues 9 has attracted much attention among the synthetic community since the first total synthesis of I was reported by Kishi and coworkers.10 In Kishi’s strategy, one key step was the preparation of the 6-6-5 cyclic framework in the intermediate II (Scheme 1). Although the construction of the 6-6-5 cyclic framework was improved by using shorter synthetic routes, the related approaches to the skeleton of II still suffered from multistep operations, the availability of starting materials and the usage of catalysts.11,12 Therefore, the development of concise approaches to the special tri-heterocyclic skeleton of II for the synthesis of gephyrotoxin analogues of biomedical importance from common starting materials is highly desirable in modern organic and medicinal chemistry. To the best of our knowledge, the utilization of multicomponent domino strategy for the stereospecific construction of tricyclic pyrrolo[1,2-a]quinolin-6(7H)-one skeleton and its poly-functionalization residing in different positions of this unit have not been achieved so far.
Fig. 1.
Scheme 1.
Retrosynthetic analysis of 4
Recently, our group and others have developed various multicomponent domino reactions (MDRs) that led to useful functionalized complex molecules of chemical and pharmaceutical interest from simple substrates.13,14 To continue our study on this topic, herein, we discovered a novel ABC2 type domino reaction 15 of 3-aryl-1- azaaryl-prop-2-en-1-one, 1,3-cyclohexanedione or 5,5-dimethylcyclohexane-1,3-dione and (pyridin-2-yl)methanamine or (pyrizin-2-yl)methanamine. The great features of this chemistry are as indicated below: simultaneously forming rings of pyridine and pyrrole and functionalizations on C1, C2, C3, C3a, and C5 positions of the pyrrolo[1,2-a]quinoline scaffold were readily achieved in domino fashion that involved [2+3+1]/[2+2+1] heterocyclizations; five stereocenters including a quaternary center were controlled well in a one-pot operation from common and inexpensive starting materials (Figure. 1). Since pyridine and pyrazine as heterocycles are prevalent substructures commonly found in natural products and pharmaceuticals,16 we anticipated that the introduction of these functional groups onto the parent ring would largely benefit to the biomedical research, particularly, to numerous chemical entities of bioscreening.
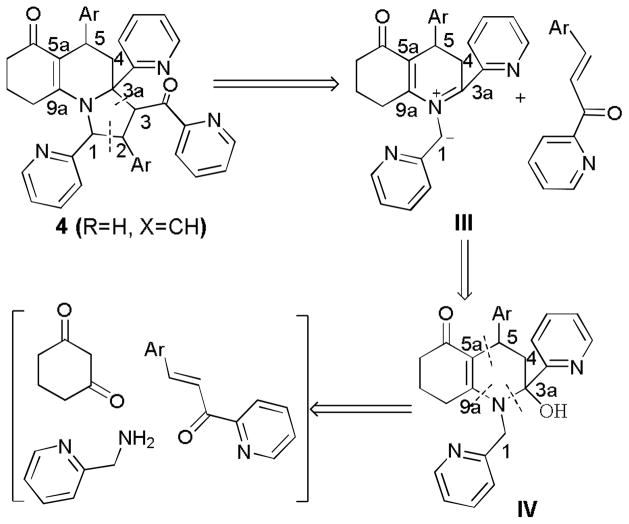
As shown in Scheme 1, the retrosynthetic analysis of 4 (R = H, X = CH) was described in details. We envisaged that the target molecules 4 would be formed via [3+2] cycloaddition of the intermediates III with 2-azaaryl substituted propenones in which III can be generated from IV through dehydration reaction. The cleavage of C3a-N and C5-C5a bonds can give 2-azaaryl substituted propenones and an enaminone intermediate which is generated by the reaction of cyclohexane-1,3-dione with (pyridin-2-yl)methanamine. Based on the above retrosynthetic analysis, we are pleased to realize this new MDRs.
Initially, we chose cyclohexane-1,3-dione (1a), (pyridin-2-yl)methanamine (2a) and 3-(4-chlorophenyl)-1-(pyridin-2-yl)prop-2-en-1-one (3a) as model substrates to optimize reaction conditions (Table 1). This model reaction was carried out under N2 atmosphere in the absence of any catalyst in DMF at 25 °C. The mixture was stirred for a certain time (monitored by TLC). The desired compound 4a was obtained with 40 % chemical yield by chromatographic separation and confirmed by 1H NMR, 13C NMR and HRMS (Table 1, entry 1). In order to further optimize the reaction conditions, the effect of solvents was also investigated. Comparing with EtOH, DMF, CH3CN, CH2Cl2, CHCl3, and THF, the yield of 4a was improved when anhydrous MeOH as a solvent was used (Table 1, entry 3). Next, the influence of reaction temperature was also optimized and indicated that 45 °C let to the best result (Table 1, entry 10).
Table 1.
Optimization of conditions on the model reaction
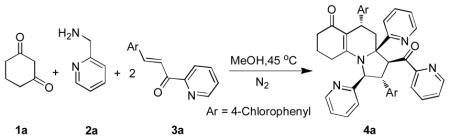
| ||||
|---|---|---|---|---|
| Entry | Solvent | T /°C | Time / h | Yield / %a |
| 1 | DMF | 25 | 18 | 40 |
| 2 | EtOH | 25 | 20 | 38 |
| 3 | MeOH | 25 | 20 | 55 |
| 4 | CH3CN | 25 | 20 | 25 |
| 5 | CHCl3 | 25 | 20 | 25 |
| 6 | CH2Cl2 | 25 | 20 | 26 |
| 7 | THF | 25 | 18 | NR |
| 8 | MeOH | 35 | 16 | 60 |
| 9 | MeOH | 40 | 16 | 70 |
| 10 | MeOH | 45 | 14 | 81 |
| 11 | MeOH | 55 | 14 | 62 |
| 12 | MeOH | 65 | 10 | 40 |
Reported yields were isolated yields
With the optimal reaction conditions in hand, we next turned our attention to the scope of this MDRs with different 2-azaaryl substituted propenones. The reactions proceeded efficiently and the corresponding products (Table 2, entries 2–8) were obtained in moderate to good yields. In view of these results, we next replaced 1,3-cyclohexanedione with 5,5-dimethylcyclohexane-1,3-dione to carry out the reactions under the above conditions. In these cases, the target compounds (Table 2, entries 9–16) were also afforded with good yields. Similarly, (pyrizin-2-yl)methanamine was converted into the corresponding pyrizin-2-yl substituted pyrrolo[1,2-a]quinolines under the same conditions (Table 2, entries 17–18). As shown in Table 2, all the substrates led to the corresponding pyrrolo[1,2-a]quinoline derivatives in 70–81% yields.
Table 2.
Synthesis of compounds 4
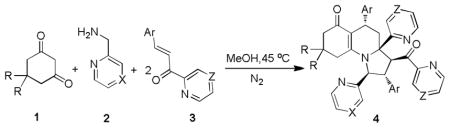
| |||||
|---|---|---|---|---|---|
| Entry | 4 | Ar | R | X/Z | Yield / %a |
| 1 | 4a | 4-ClC6H4 | H | CH/CH | 81 |
| 2 | 4b | 4-BrC6H4 | H | CH/CH | 79 |
| 3 | 4c | 4-FC6H4 | H | CH/CH | 76 |
| 4 | 4d | 4-CH3C6H4 | H | CH/CH | 78 |
| 5 | 4e | 4-CH3OC6H4 | H | CH/CH | 75 |
| 6 | 4f | 2,4-Cl2C6H3 | H | CH/CH | 80 |
| 7 | 4g | 3,4-Cl2C6H3 | H | CH/CH | 78 |
| 8 | 4h | 2,3-Cl2C6H3 | H | CH/N | 70 |
| 9 | 4i | 4-ClC6H4 | CH3 | CH/CH | 80 |
| 10 | 4j | 4-BrC6H4 | CH3 | CH/CH | 78 |
| 11 | 4k | 4-FC6H4 | CH3 | CH/CH | 74 |
| 12 | 4l | 4-CH3C6H4 | CH3 | CH/CH | 78 |
| 13 | 4m | 2,4-Cl2C6H3 | CH3 | CH/CH | 76 |
| 14 | 4n | 3,4-Cl2C6H3 | CH3 | CH/CH | 76 |
| 15 | 4o | 4-ClC6H4 | CH3 | CH/N | 72 |
| 16 | 4p | 4-BrC6H4 | CH3 | CH/N | 70 |
| 17 | 4q | 4-BrC6H4 | H | N/CH | 72 |
| 18 | 4r | 4-ClC6H4 | H | N/N | 70 |
Reported yields were isolated yields.
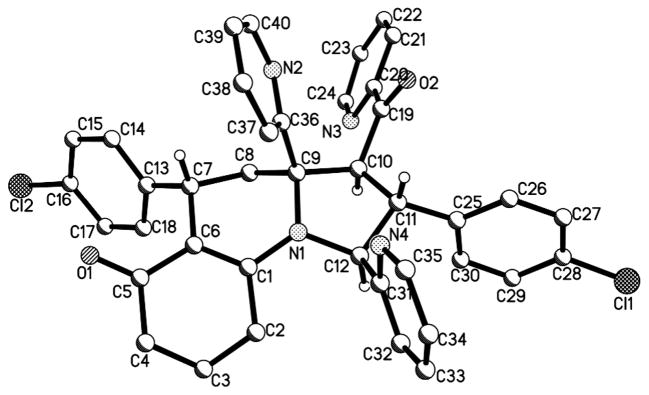
In order to determine the stereochemistry of pyrrolo[1,2-a]quinolines 4, the single crystal of 4a was obtained by slowly evaporating the solvent. Its relative stereo-configuration was established by X- ray diffraction as shown in Figure 2. The perspective diagram shows that there are five strereocenters in the molecular structure and the two aryl groups residing on C2 and C5 positions of the pyrrolo[1,2-a]quinoline scaffold are anti-configuration; this can particularly match stereo pattern of Gephyrotoxin.
Figure 2.
Crystal structure of 4a. All H atoms except H7, H10, H11, H12 were omitted for clarity.17
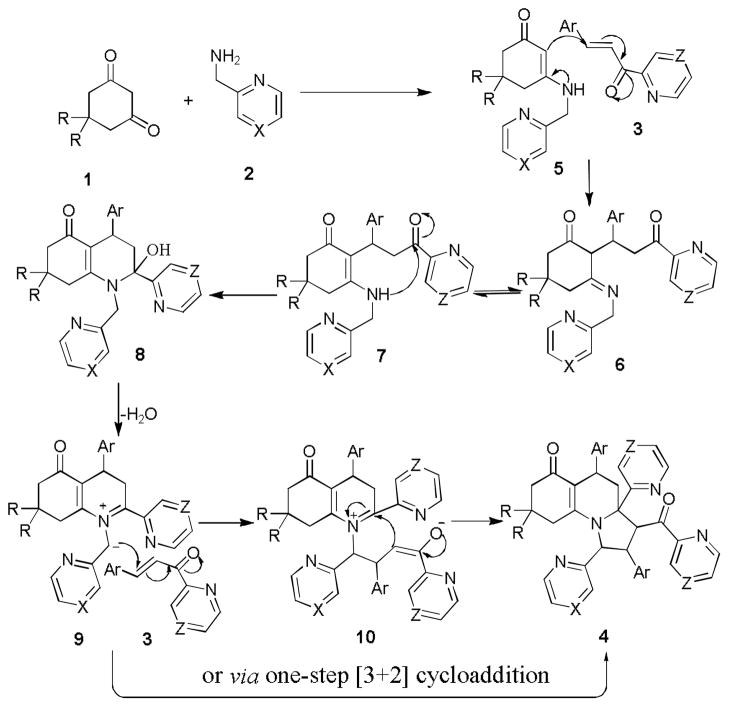
On the basis of the above results, the possible mechanism for this new reaction was proposed and depicted in Scheme 2. The cyclohexanedione was first condensed with (pyridin-2-yl)methanamine or (pyrizin-2-yl)methanamine to generate enaminone 5, followed by intermolecular 1,4-addition with chalones 3 to yield the intermediate 6. Then the isomer 7 of 6 underwent intramolecular cyclization to form 8, which was subsequently converted into the intermediate 9 via dehydration. The intermediate 10 was formed from the Michael addition reaction of 9 with 3. Intramolecular addition led to the formation of the target compounds 4. It should be noted that the pathway for the formation of 4 via [3+2] cycloaddition would not be excluded. The further investigation of the mechanism is to be conducted in due course.
Scheme 2.
Proposed mechanism for the synthesis of 4.
In conclusion, a facile and convenient straightforward one-pot multicomponent strategy for the construction of pyrrolo[1,2-a]quinoline skeleton has been established. This protocol generated five new sigma bonds and five stereocenters by means of simple one-pot operation from commercially available common starting materials. The bond-forming efficiency, accessibility and generality make the present method a highly attractive approach to the tri-heterocyclic scaffolds of chemical and biomedical importance.
Supplementary Material
Acknowledgments
We are grateful to financial support from the NSFC (Nos. 21102124, 21232004 and 21272095), the Priority Academic Program Development of Jiangsu Higher Education Institutions, Jiangsu Science and Technology Support Program (No. BE2011045), Robert A. Welch Foundation (D-1361) and NIH (R21DA031860-01) for partial support.
Notes and references
- 1.(a) Kariba RM, Houghton PJ, Yenesew A. J Nat Prod. 2002;65:566. doi: 10.1021/np010298m. [DOI] [PubMed] [Google Scholar]; (b) Yang SP, Yue JM. J Org Chem. 2003;68:7961. doi: 10.1021/jo034804j. [DOI] [PubMed] [Google Scholar]; (c) Morita H, Kobayashi J. Org Lett. 2003;5(16):2895. doi: 10.1021/ol034969e. [DOI] [PubMed] [Google Scholar]; (d) Shirai O, Miyata O, Tohnai N, Miyata M, Procter DJ, Sucunza D, Naito T. J Org Chem. 2008;73:4464. doi: 10.1021/jo800560p. [DOI] [PubMed] [Google Scholar]; (e) Nicolaou KC, Dalby SM, Li SL, Suzuki T, Chen DYK. Angew Chem, Int Ed. 2009;48(41):7616. doi: 10.1002/anie.200904588. [DOI] [PubMed] [Google Scholar]; (f) Ueda H, Satoh H, Matsumoto K, Sugimoto K, Fukuyama T, Tokuyama H. Angew Chem, Int Ed. 2009;48(41):7600. doi: 10.1002/anie.200902192. [DOI] [PubMed] [Google Scholar]; (g) Sridharan V, Suryavanshi PA, Menéndez JC. Chem Rev. 2011;111:7157. doi: 10.1021/cr100307m. [DOI] [PubMed] [Google Scholar]
- 2.Daly JW, Witkop B, Tokuyama T, Nishikawa T, Karle IL. Helv Chim Acta. 1977;60:1128. doi: 10.1002/hlca.19770600336. [DOI] [PubMed] [Google Scholar]
- 3.Mensah-Dwumah M, Daly JW. Toxicon. 1978;16:189. doi: 10.1016/0041-0101(78)90037-5. [DOI] [PubMed] [Google Scholar]
- 4.Souccar C, Varanda WA, Aronstam RS, Daly JW, Albuquerque EX. Mol Pharmacol. 1984;25:384. [PubMed] [Google Scholar]
- 5.(a) Jossang A, Bitar HE, Pham VC, Sévenet T. J Org Chem. 2002;68:300. doi: 10.1021/jo0203950. [DOI] [PubMed] [Google Scholar]; (b) Ikeda T, Yaegashi T, Matsuzaki T, Hashimoto S, Sawada S. Bioorg Med Chem Lett. 2011;21:342. doi: 10.1016/j.bmcl.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Michael JP, de Koning CB, Hosken GD, Stanbury TV. Tetrahedron. 2001;57:9635. [Google Scholar]
- 7.Hazra A, Mondal S, Maity A, Naskar S, Saha P, Paira R, Sahu KB, Paira P, Ghosh S, Sinha C, Samanta A, Banerjee S, Mondal NB. Eur J Med Chem. 2011;46:2132. doi: 10.1016/j.ejmech.2011.02.066. [DOI] [PubMed] [Google Scholar]
- 8.(a) Fujimoto R, Kishi Y, Blount JF. J Am Chem Soc. 1980;102:7154. [Google Scholar]; (b) Overman LE, Fukaya C. J Am Chem Soc. 1980;102:1454. [Google Scholar]; (c) Hart DJ, Kanai K. J Am Chem Soc. 1983;105:1255. [Google Scholar]; (d) Overman LE, Lesuisse D, Hashimoto M. J Am Chem Soc. 1983;105:5373. [Google Scholar]; (e) Ito Y, Nakajo E, Nakatsuka M, Saegusa T. Tetrahedron Lett. 1983;24:2881. [Google Scholar]; (f) Pearson WH, Fang WK. J Org Chem. 2000;65:7158. doi: 10.1021/jo0011383. [DOI] [PubMed] [Google Scholar]; (g) Miao L, Shu H, Noble AR, Fournet SP, Stevens ED, Trudell ML. Arkivoc. 2010;4:6. [Google Scholar]
- 9.(a) Grigg R, Thornton-Pett M, Yoganathan G. Tetrahedron. 1999;55(26):8129. [Google Scholar]; (b) Li XY, Li C, Zhang WJ, Lu X, Han SQ, Hong R. Org Lett. 2010;12(8):1696. doi: 10.1021/ol100220c. [DOI] [PubMed] [Google Scholar]; (c) Lemen GS, Wolfe JP. Org Lett. 2011;13(12):3218. doi: 10.1021/ol201123b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schultz DM, Wolfe JP. Org Lett. 2010;12(5):1028. doi: 10.1021/ol100033s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Song LY, Liu L, Li CZ. Org Lett. 2011;13(13):3434. doi: 10.1021/ol201180g. [DOI] [PubMed] [Google Scholar]; (g) Kang YK, Kim SM, Kim DY. J Am Chem Soc. 2010;132:11847. doi: 10.1021/ja103786c. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto R, Kishi Y. Tetrahedron Lett. 1981:4197. [Google Scholar]
- 11.Wei LL, Hsung RP, Sklenicka HM, Gerasyuto AI. Angew Chem, Int Ed. 2001;40:1516. [PubMed] [Google Scholar]
- 12.Santarem M, Vanucci-Bacque C, Lhommet G. J Org Chem. 2008;73:6466. doi: 10.1021/jo801150e. [DOI] [PubMed] [Google Scholar]
- 13.(a) Zhu J, Bienayme H. Multicomponent Reactions. Wiley-VCH; Weinheim, Germany: 2005. [Google Scholar]; (b) Bienayme H, Hulme C, Oddon G, Schmitt P. Chem Eur J. 2000;6:3321. doi: 10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]; (c) Dömling A, Wang W, Wang K. Chem Rev. 2012;112:3083. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Pando O, Stark S, Denkert A, Porzel A, Preusentanz R, Wessjohann LA. J Am Chem Soc. 2011;133:7692. doi: 10.1021/ja2022027. [DOI] [PubMed] [Google Scholar]; (e) Hong D, Zhu Y, Li Y, Lin X, Lu P, Wang YG. Org Lett. 2011;13(17):4668. doi: 10.1021/ol201891r. [DOI] [PubMed] [Google Scholar]; (f) Tietze LF, Kinzel T, Brazel CC. Acc Chem Res. 2009;42(2):367. doi: 10.1021/ar800170y. [DOI] [PubMed] [Google Scholar]
- 14.(a) Jiang B, Tu SJ, Kaur P, Wever W, Li G. J Am Chem Soc. 2009;131:11660. doi: 10.1021/ja904011s. [DOI] [PubMed] [Google Scholar]; (b) Jiang B, Wang X, Shi F, Tu SJ, Ai T, Ballew A, Li G. J Org Chem. 2009;74:9486. doi: 10.1021/jo902204s. [DOI] [PubMed] [Google Scholar]; (c) Jiang B, Li C, Shi F, Tu SJ, Kaur P, Wever W, Li G. J Org Chem. 2010;75:2962. doi: 10.1021/jo1002278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ma N, Jiang B, Zhang G, Tu SJ, Wever W, Li G. Green Chem. 2010;12:1357. [Google Scholar]; (e) Yao CS, Wang CH, Jiang B, Feng XD, Yu CX, Li TJ, Tu SJ. Bioorg Med Chem Lett. 2010;20:2884. doi: 10.1016/j.bmcl.2010.03.036. [DOI] [PubMed] [Google Scholar]; (f) Jiang B, Yi MS, Shi F, Tu SJ, Pindi S, McDowell P, Li G. Chem Commun. 2012:808. doi: 10.1039/c1cc15913e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janvier P, Bois-Choussy M, Bienaym H, Zhu JP. Angew Chem, Int Ed. 2003;42(7):811. doi: 10.1002/anie.200390216. [DOI] [PubMed] [Google Scholar]
- 16.(a) David O. Nat Prod Rep. 2000;17:435. doi: 10.1039/a707613d. [DOI] [PubMed] [Google Scholar]; (b) Zhang YC, Li C, Swenson DC, Gloer JB, Wicklow DT, Dowd PF. Org Lett. 2003;5:773. doi: 10.1021/ol0340686. [DOI] [PubMed] [Google Scholar]; (c) Henry GD. Tetrahedron. 2004;60:6043. [Google Scholar]; (d) Niculescu-Duvaz I, Roman E, Whittaker SR, Friedlos F, Kirk R, Scanlon IJ, Davies LC, Niculescu-Duvaz D, Marais R, Springer CJ. J Med Chem. 2006;49:407. doi: 10.1021/jm050983g. [DOI] [PubMed] [Google Scholar]
- 17.The crystal data for 4a see CCDC- 903897
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.