INTRODUCTION
The term cornification (keratinization) is the process by which the epidermal cells undergo terminal differentiation from basal keratinocytes to the highly specialized corneocyte. The large surface area of the skin puts it in constant contact with environmental pollutants, irritants, and allergens; the outermost layer of the epidermis (the stratum corneum) serves as the first line of defense between these environmental hazards and the body itself1. Easily overlooked in histological preparations, the normal corneal layer consists of more than 20 overlapping layers of bland, lightly-staining, polyhedral, anucleate cells, most of which are lost during biopsy sampling, cutting, and processing.
Stratum Corneum Function
The stratum corneum is the key epidermal layer that restricts water movement into and out of the skin. In normal humans, approximately 0.5 L of water vapor is expelled through the stratum corneum per day. Even minor injuries to the corneal layer from tape stripping or applications of solvents will result in increased transepidermal water loss2,3. Like humans, dogs with atopic skin disease have recently been shown to have an increased water loss through the corneal layer4.
Much of the barrier function is attributed to the physical property of continuous desquamation, which allows for the expulsion of pathogens. Furthermore, the stratum corneum is able to absorb UV light to protect the underlying tissue from free radical oxidation. This layer also contains natural antimicrobial peptides such as defensins and cathelicidins such that abnormalities in the corneal layer may predispose the patient (human or animal) to bacterial and yeast infections1.
Diagnostic approach
Disorders of cornification (DOC) are divided into primary and secondary causes. In primary cornification disorders, the excessive scale is due to a direct defect in one or more steps involved in the formation of the stratum corneum. Known defects are related to mutations in genes which encode the structural proteins that form the corneocyte (e.g. transglumatinases), or enzymes involved in lipid formation or lipid transport1. Secondary disorders are those where excessive scaling develops as a result of another condition (e.g. flea bite hypersensitivity, sarcoptic mange, hypothyroidism, epitheliotropic lymphoma, etc.). More than 80% of scaling disorders arise from secondary causes3. Some authors include abnormalities in sebaceous gland function (e.g. sebaceous adenitis, sebaceous gland dysplasia) as primary cornification disorders as well. However, these disorders will not be discussed in this chapter.
Primary disorders of cornification are generally diagnosed by ruling out all secondary causes. The signalment, age of onset and presence or absence of pruritus will aid in the formulation of a differential diagnosis and the diagnostic approach. In a standard veterinary practice, a minimum dermatologic database (e.g. skin scrapings, acetate tape preparations, impression smears, trichograms, dermatophyte culture) along with routine blood work can effectively rule out most secondary disorders. However, skin biopsies are often needed to establish a definitive diagnosis. Primary cornification disorders are generally nonpruritic (when uncomplicated by secondary infections) and arise in young animals, but late onset cases do occasionally occur. Ichthyosiform disorders have strong breed predilections; however, it should be kept in mind that spontaneous mutations can arise in any breed or mixed-breed animals.
Pathophysiology
Briefly, the formation of the stratum corneum is an orchestrated and complex series of steps that occur concomitantly with a modified form of apoptosis. An alteration in any step can lead to disruption of barrier function and derangements in permeability. The scaling phenotype is an adaptive response to repair the injured stratum corneum barrier. When the barrier fails, lipid synthesis is upregulated and the epidermis becomes hyperplastic to deliver more lipid to the stratum corneum. Inflammation may also ensue1.
Several key steps must occur for normal cornification to proceed: (1) bundling of the keratin to establish the corneocyte core, (2) replacement of the cell membrane with a thick cornified envelope, (3) formation of lipid lamellar bilayers and (4) desquamation. As the nucleus and intracellular organelles undergo proteolysis, profillagrin (found in keratohyalin granules) in the granular cell layer is dephosphorylated into filaggrin. Filaggrin serves to bundle the loose keratin filaments in the cytosol to form the core of the corneocyte. Transglutaminases mediate calcium-dependent crosslinking of small peptides to replace the plasma membrane of the keratinocyte with a tough protein layer (corneocyte envelope). Lamellar bodies, small vesicular organelles containing lipid, are formed in the stratum spinosum. At the junction of the stratum granulosum and stratum corneum, the lipid is secreted into the intercellular space and undergoes processing into lamellar bilayers. Enzymes within the lamellar bodies modify polar lipids into nonpolar (hydrophobic) lipids. The resultant lipid in the stratum corneum is an equimolar mixture of cholesterol, long-chain fatty acids and ceramides. Finally, the intercorneocyte adhesions (corneodesmosomes) are cleaved by proteases to allow for desquamation and release of the keratin squames into the environment1.
The final product is a tough hydrophobic but biochemically active layer in a “mortar and bricks” arrangement with the bricks being the corneocytes, which are sandwiched between layers of lipid (the mortar)1.
Ichthyosis-General
In veterinary medicine, the term ichthyosis has been limited to rare congenital or hereditary disorders believed to be due to primary defects in the formation of the stratum corneum5,6. In human medicine, the term “acquired ichthyosis” is also used to denote similar clinical changes related to underlying diseases, but this confusing terminology has not been adopted by veterinary dermatologists. The diagnosis of ichthyosiform disorders can be made via a detailed dermatologic exam (minimum dermatology database), in conjunction with signalment, history and skin biopsy analysis. Genetic testing, when available, along with electron microscopy testing may be needed to further characterize the defect, but is not needed to establish a general diagnosis. To date, ichthyosis is subdivided into epidermolytic and nonepidermolytic forms based on light microscopy6.
Epidermolytic Ichthyosis (EI)
The name “epidermolytic” is based on the light microscopic findings of vacuoles and lysis of keratinocytes within the spinous and granular cell layers, which occur along with hypergranulosis and hyperkeratosis. Unlike nonepidermolytic ichthyosis (see below), this finding uniquely corresponds to a defect in keratin formation (i.e. formation of the corneocyte core). EI in the Norfolk terrier is autosomal recessive and caused by a mutation in epidermal keratin (KRT10)7. EI has been sporadically described in other dogs (Rhodesian ridgeback, Labrador cross)6. The affected dogs have multifocal regions of pigmented scale with alopecia and roughening of the skin. Epidermolytic ichthyosis (aka epidermolytic hyperkeratosis) can be diagnosed on light microscopy by an experienced dermatopathologist.
Nonepidermolytic Ichthyosis (NI)
To date, the nonepidermolytic forms of ichthyosis, which have been characterized in dogs, have been documented or presumed to be autosomal recessive traits. In humans, there are X-linked and dominant forms of NI; however, this has yet to be documented in the dog. Indeed, the veterinary characterization of NI is still in it’s infancy. In humans, autosomal recessive congenital ichthyosis (ARCI) is the official classification for a heterogeneous group of disorders with overlapping phenotypes (e.g. lamellar ichthyosis, congenital ichthyosiform erythroderma)1. A variety of mutations affecting lipids and structural proteins cause similar clinical phenotypes as well as light microscopic changes. The current nomenclature used by pathologists in veterinary medicine is to label these ARCI disorders by the breed predilection; a practice that may change in the future6.
Golden retriever ichthyosis
Although statistics are not known on the prevalence of this disorder, it appears to be common (relative to other forms of ichthyosis) and is unique in its clinical presentation. It is generally considered a “mild” form of scaling, but some owners may argue otherwise. Affected dogs develop large, soft, white to grey adherent scale that is prominent on the trunk and may be associated with ventral hyperpigmentation (Figure 1). Histologically, affected dogs have lesions typical of nonepidermolytic ichthyosis: diffuse lamellar orthokeratotic hyperkeratosis in the absence of epidermal hyperplasia and dermal inflammation8,9. Golden retrievers are typically diagnosed at less than one year of age; however, adult-onset cases are not uncommon8. Some dogs develop secondary bacterial folliculitis, which may lead to pruritus and clinical confusion with allergic skin disease. The disease may wax and wane with periodic bouts of exacerbation and remission.
Figure 1.
Generalized large white scale in a Golden retriever with nonepidermolytic ichthyosis.
A long awaited publication recently documented a mutation in PNPLA1 gene as the cause of golden retriever ichthyosis10. The gene is thought to play a role in lipid organization and metabolism within the outer epidermis. A genetic test is currently offered by a European company [Antagen (http://www.antagene.com)] and maybe useful to assess for a carrier state in breeding dogs. In non-breeding pets suspected of having the disease, a skin biopsy procedure with examination by an experienced dermatopathologist is sufficient for diagnosis.
American bulldog ichthyosis
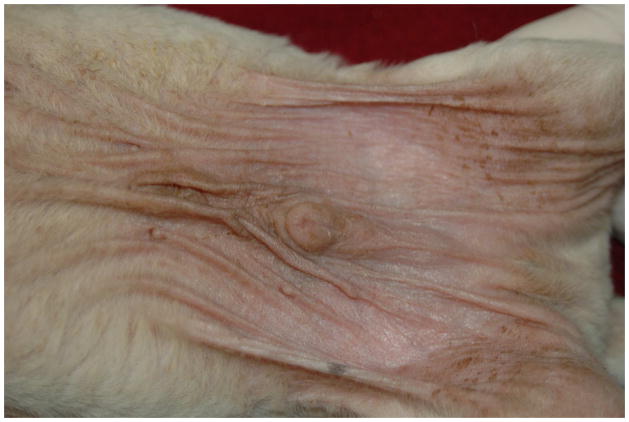
The author, in conjunction with Dr. Margret Casal in the Medical Genetics section at the University of Pennsylvania School of Veterinary Medicine, has characterized a similar but more severe ichthyosiform disorder of American bulldogs11,12. Unlike the golden retriever, the bulldogs consistently develop clinical signs before weaning. Young puppies have a scruffy/disheveled haircoat when compared to the smooth coat of normal littermates. The glabrous skin is erythematous with tightly adherent light brown scale, which gives the abdominal skin a “wrinkled” appearance. In the adult dog, the entire abdomen, axiilla and inguinal regions have a reddish-brown discoloration (Figure 2). Large white to light tan scales are distributed throughout the haircoat. Malassezia yeast overgrowth may be severe. The development of otitis externa, intertigo and pododermatitis corresponds with yeast proliferation and the onset of pruritus. The clinical presentation may be misinterpreted as nonseasonal atopic skin disease. Occasional adult dogs may have footpad hyperkeratosis. Unlike golden retrievers, the skin lesions in bulldogs do not wax or wane and are generally more severe11. The disorder is caused by a mutation in ICHTHYN and similar to the PNPLA-2 mutation in golden retrievers, is likely related to lipid metabolism in the epidermis1,10,12,13. The UPENN School of Veterinary medicine offers a genetic test to assess for carrier dogs (dogderm@vet.upenn.edu).
Figure 2.
Brown-red discoloration of the abdominal skin in 2 week old American bulldog with ichthyosis.
Jack Russell terrier ichthyosis and other breeds
Nonepidermolytic ichthyosis in Jack Russell terriers (JRT) is caused by a loss of function mutation in transglutaminase 1 (TGM1)14. TGM1 mediates calcium dependent cross-linking of peptides (e.g. involucrin, loricrin) to form the cornified envelope- the strong exterior of the corneocyte. The phenotype in the JRT is characterized by large, thick, adherent parchment paper-like scales. This is generally more severe than the previously described disorders. The dogs develop severe Malassezia infections with corresponding inflammation and pruritus.
Ichthyoses not yet characterized
A number of other breeds have been diagnosed on light microscopy and clinical examination (soft-coated Wheaten terriers, and West Highland White terriers) with NI, but further molecular characterizations have not been documented6. Many cases are likely confirmed on skin biopsy and never receive further work up. The author has confirmed cases in English Springer spaniels, Labrador retrievers and West highland white terriers.
A congenital and familal form of keratoconjunctivitis sicca with scaling has been documented in Cavalier King Charles spaniel dogs. The dogs have a syndrome that includes the following features: keraotoconjuntivitis noted from the beginning of eyelid opening, a roughened/curly haircoat, scaling with abdominal hyperpigmentation, footpad hyperkeratosis, and nail dystrophy. A recessive model of inheritance has been proposed; thus far, a candidate gene has not been identified.15
Differential diagnosis for ichthyosiform disorders
The severe scaling of ichthyosiform disorders can be confused with a number of other diseases including sebaceous adenitis, atopy, parasitic disorders (cheyletiellosis, demodicosis, leishmaniasis), as well as hypothyroidism, epitheliotropic cutaneous lymphoma or metabolic disease (e.g. zinc-responsive dermatosis). A skin biopsy is helpful to establish the clinical diagnosis and ensure that other disorders (particularly sebaceous adenitis or sebaceous dysplasia) are not present.
Treatment of Primary Disorders of Cornification
In both human and veterinary medicine, research into the pathogenesis and molecular characterization of primary DOC has out-paced any significant therapeutic achievements. The goal of therapy must be to restore the stratum corneum barrier function to decrease the adaptive responses (hyperplasia, hyperkeratosis, inflammation). The best way to ensure effective therapy is the make sure the diagnosis is solid, as there is no cure for ichthyosiform disorders. However, many of the diseases that can mimic them may be treated successfully. Corticosteroids may temporarily decrease the scale formation associated with ichthyosiform disorders, but unfortunately, steroids will further impede the skin barrier function.
To date, topical therapy remains the treatment of choice for all forms of ichthyosis. The topical therapy may include keratolytic agents to remove excessive scale, moisturizers/emollients to restore the skin barrier (prevent water loss), and topical antimicrobials (e.g. chlorhexidine and miconazole) for secondary bacterial or yeast infections. However, care should be exercised to avoid further damage to the skin barrier with harsh chemicals. The therapy must be tailored to the individual patient and requires good owner compliance. Initially, baths may be required every other day to twice weekly. Shampoos containing 2% sulfur and salicylic acid will help soften the scale and break apart the keratin squames. The shampoo should always be followed by a good moisturizer. Humectants (e.g. Humilac®, Virbac) may be helpful between baths. Avoid the use of harsh topical products that may further harm the skin barrier (e.g. tar-based shampoos) and cause erythema or pruritus. Topical oil based spot-ons such as Duoxo® seborrhea spot-on (Sogeval) which contains 1% phytosphingosine ( a major component of ceramides) and Allerderm® spot-on (Virbac) which contains a combination of ceramides and fatty acids, are helpful between baths and may prolong the bath interval. Oral omega-3 and omega-6 fatty acids may also be beneficial, but the true efficacy is difficult to quantify. The topical regimen is tailored to the degree of scale and then tapered based on the clinical response. Periodic rechecks are warranted to assess scale production, evidence of irritation or skin sensitivity and secondary infection. American bulldogs and Jack Russell terriers may require oral antifungal therapy (e.g. ketoconazole at 8–10 mg/kg q 21days) for secondary Malassezia infections. As research expands our understanding of these disorders, there is more hope for corrective therapy that may be targeted directly at the specific barrier defect.
Unconfirmed Primary Disorders of Cornification
Vitamin A responsive dermatosis
This disorder is most commonly seen in adult Cocker spaniels although cases are reported in Labrador retrievers, miniature schnauzers and Gordon setters. Clinical lesions consist of hyperkeratotic plaques with follicular plugging and follicular casts on the ventral and lateral chest and abdomen. Affected dogs may have greasy haircoats with ceruminous otitis. In the author’s opinion, this disorder has unique histologic features; however, the patients often have other conditions (e.g. superficial spreading pyoderma, atopy, food allergy), which confuses the pathogenesis of the cornification defect (i.e. cause or effect). The diagnosis is achieved by a skin biopsy analysis and response to vitamin A supplementation. The histologic features are marked orthokeratotic hyperkeratosis of the follicular ostea, which is more severe than at the epidermal surface.16 The standard of vitamin A dose for a Cocker spaniel is 10,000 IU/day or 500–800 IU/kg/day5. A clinical response is typically seen in 3 to 8 weeks and dogs may require lifelong therapy.
Generalized Sebaceous Gland Hyperplasia of Terriers
Idiopathic generalized sebaceous gland hyperplasia has been reported in both Border terriers and wire-haired terriers17,18. It is unclear if this disorder is a true cornification defect or a manifestation of another disease process (e.g. atopic dermatitis, demodicosis). This disorder is different from the nodules of sebaceous gland hyperplasia that arise in aging dogs. The terriers present with a greasy haircoat that is most severe on the dorsum. Some dogs, particularly the wire-haired terriers, have been documented with Demodex injai infestation17. These long-bodied demodex mites are often found in sebaceous gland ducts in addition to hair follicles. The relationship of the sebaceous gland hyperplasia to the demodex mites is unknown. Treatment of mite infestation improves the skin but does not reverse the sebaceous gland hyperplasia. It is plausible that the sebaceous gland hyperplasia is the primary lesion and predisposes the dogs to demodex infestation.
Primary seborrhea: A controversy
Use of the term “seborrhea” has been ingrained in the veterinary dermatology literature for at least five decades. Seborrhea literally means, “flow of sebum”, and it has been loosely correlated with abnormal sebaceous gland function. Clinically, “seborrhea” is used to describe excessive scaling, although historically, “seborrhea” has been subdivided into those cases with dry scale (seborrhea sicca) or oily/greasy scale (seborrhea oleosa). The term “seborrheic dermatitis” has been used to describe scaling accompanied by inflammation. In the older literature, the diagnosis of “seborrhea” was based on gross morphology of skin lesions, and in general, histopathology and skin surface cytological assessments were not included in the dermatologic work-up. From a etiopathologic viewpoint, the use of terms such as “seborrhea” and “seborrheic dermatitis” are entirely non-specific and should be used for clinical descriptive purposes only; not to suggest a particular diagnosis.
Use of the term “primary seborrhea” has classically been reserved for cases in which all known causes of scaling have been ruled out (e.g. ectoparasitism, metabolic diseases and endocrinopathies, allergic disease, etc.).5,19 In the 1980’s this designation was potentially useful for treatment purposes, but it antedated the known significance of the role of Malassezia and staphylococcal infections as important promoters of secondary seborrhea and seborrheic dermatitis. Primary seborrhea has been reported to arise in adult springer and Cocker spaniels.5,20,21 Although consistently cited in textbooks, few peer-reviewed publications list scientific criteria for the diagnosis of “primary seborrhea”. Cases vary from early onset to adult onset and from those with mild scale to more severe seborrhea oleosa, which is considered to evolve over time. As dermatologic diagnostic criteria have expanded with time, and our clinical/histopathologic accuracy has improved, many cases that would have been labeled “primary seborrhea” in the past are now readily diagnosed as pyoderma, Malassezia dermatitis, sebaceous adenitis, allergic dermatitis, etc. This is not to deny the presence of mild scale which commonly occurs spontaneously (and often excessively) in canine and feline patients, nor the influence of environment (e.g. humidity, heat, diet) on scale formation; only that the scale formation is not pathognomonic for “primary seborrhea”. In the Cocker spaniel, it is likely that most cases of “primary seborrhea” would now be termed “Vitamin A- responsive dermatosis”.
In brief, it is the author’s opinion that “primary” seborrhea is an adaptive response rather than a constitutive change in the mechanisms of cornification, and that affected dogs most likely suffer from other dermatologic conditions. If primary seborrhea (e.g. spaniel seborrhea) is a true entity unto itself, clear criteria should be established as a first step towards performing reasonable investigations to explore its etiopathogenesis. Until that time, the term “primary seborrhea” should be entirely eliminated from the veterinary dermatology vernacular.
Key points.
The stratum corneum, which is the upper-most layer of the epidermis, serves as a primary barrier between the body and the environment. This layer functions to keep the body hydrated (i.e. retain water) while excluding pathogens and toxins.
Disorders of cornification (DOC) arise when there is an inability to normally form the stratum corneum. Most DOC are secondary to alternations induced by allergic skin diseases, ectoparasitisms, and endocrine/metabolic diseases.
Primary disorders of cornification arise from spontaneous or inherited mutations in genes that regulate structural proteins, lipid metabolism and transport.
Dogs with primary DOC present at a young age with nonpruritic scaling (dandruff) along with variable hyperpigmentation and bacterial or Malassezia yeast infections. Secondary pruritus may lead to misdiagnosis as atopic skin disease.
Few primary cornification disorders have been fully characterized in the dog. Mild forms of ichthyosis (fish scale disease) are not uncommon in the golden retriever and American bulldog.
The step-wise diagnostic approach is fundamental to establishing a correct diagnosis, as there is no cure for ichthysosis. Treatment involves a lifetime regimen of topical therapy as well as medical care to address and prevent secondary bacterial and yeast infections.
Most importantly, primary DOC must be identified in order to the eliminate carrier dogs from the breeding pool.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elias PM, Williams ML, Crumrine D, Schmuth M. Ichthyoses; Clinical, Biochemical and Diagnostic Assessment. Vol. 39. Karger; 2010. Ichthyoses: Current Problems in Dermatology; pp. 1–29. [Google Scholar]
- 2.Marks R. The stratum corneum barrier: the final frontier. Journal of Nutrition. 2004;134:2017S–2021S. doi: 10.1093/jn/134.8.2017S. [DOI] [PubMed] [Google Scholar]
- 3.Shimada K, Yoshihara T, Yamamoto M, et al. Transepidermal water loss (TEWL) reflects skin barrier function of dog. J Vet Med Sci. 2008;70:841–843. doi: 10.1292/jvms.70.841. [DOI] [PubMed] [Google Scholar]
- 4.Shimada K, Yoon J, Yoshihara T, Iwasaki T, Nishifuji Koji. Increased transepidermal water loss and decreased ceramide content in lesional and non-lesional skin of dogs with atopic dermatitis. Vet Dermatol. 2009:541–546. doi: 10.1111/j.1365-3164.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- 5.Scott DW, Miller WH, Griffin CE. Muller and Kirk’s Small Animal Dermatology. 6. W. B. Saunders; Philadelphia: 2001. pp. 913–920. [Google Scholar]
- 6.Credille KM. Primary cornification defects. In: Guaguère Eric., editor. A practical guide to canine dermatology. Pascal Prélaud; Merial: 2008. [Google Scholar]
- 7.Credille KM, Barnhart KF, Minor JS, Dunstan RW. Mild recessive epidermolytic hyperkeratosis with a novel keratin 10 donor splice-site mutation in a family of Norfolk terrier dogs. British Journal of Dermatology. 2005;153(1):51–58. doi: 10.1111/j.1365-2133.2005.06735.x. [DOI] [PubMed] [Google Scholar]
- 8.Mauldin EA, Credille KM, Dunstan RW, Casal ML. The clinical and morphologic features of non-epidermolytic ichthyosis in the golden retriever. Veterinary Pathology. 2008;45:174–180. doi: 10.1354/vp.45-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadiergues MC, Patel A, Shearer DH, Fermor R, Miah S, Hendricks A. Cornification defect in the golden retriever: clinical, histopathological, ultrastructural and genetic characterisation. Vet Dermatol. 2008;19(3):120–9. doi: 10.1111/j.1365-3164.2008.00667.x. [DOI] [PubMed] [Google Scholar]
- 10.Grall S, Guaguère E, Planchais S, et al. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat Genetics. doi: 10.1038/ng.1056. published online 15 January 2012. [DOI] [PubMed] [Google Scholar]
- 11.Mauldin EA, Casal ML. Autosomal recessive ichthyosis in the American bulldog. North American Veterinary Forum Proceedings; April 2008; Denver CO. [Google Scholar]
- 12.Mauldin EA, Casal ML. The molecular characterization of American bulldog ichthyosis. International Society of Veterinary Dermatopathology. Annual Meeting; April 2010; Portland Oregon. [Google Scholar]
- 13.Dahlqvist J, Klar J, Hausser I, et al. Congenital ichthyosis: mutations in ichthyin are associated with specific structural abnormalities in the granular layer of the epidermis. J of Med Genet. 2007;44:615–620. doi: 10.1136/jmg.2007.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Credille K, Minor J, Barnhart K, Lee E, Cox M, Tucker K, Diegel K, Venta P, Hohl D, Huber M, Dunstan R. Transglutaminase 1-deficient recessive lamellar ichthyosis associated with a LINE-1 insertion in Jack Russell terrier dogs. British Journal of Dermatology. 2009;161:265–272. doi: 10.1111/j.1365-2133.2009.09161.x. [DOI] [PubMed] [Google Scholar]
- 15.Hartley C, Donaldson D, Smith KC, et al. Congenital keratoconjunctivitis sicca and ichthyosiform dermatosis in 25 Cavalier King Charles spaniel dogs–part I: clinical signs, histopathology, and inheritance. Veterinary Ophthalmology. 2011:1–12. doi: 10.1111/j.1463-5224.2011.00986.x. [DOI] [PubMed] [Google Scholar]
- 16.Ihrke PJ, Goldschmidt MH. Vitamin A-responsive dermatosis in the dog. J Am Vet Med Assoc. 1983;182:687–90. [PubMed] [Google Scholar]
- 17.Ordeix L, Bardagí M, Scarampella F, Ferrer L, Fondati A. Demodex injai infestation and dorsal greasy skin and hair in eight wirehaired fox terrier dogs. Veterinary Dermatology. 2009;20:267–272. doi: 10.1111/j.1365-3164.2009.00755.x. [DOI] [PubMed] [Google Scholar]
- 18.Dedola C, Ressel L, Hill PB, et al. Idiopathic generalized sebaceous gland hyperplasia of the Border terrier: a morphometric study. Veterinary Dermatology. 2010;21:494–502. doi: 10.1111/j.1365-3164.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 19.Ihrke PJ. Canine seborrheic disease complex. Veterinary Clinics of North America Small Animal Practice. 1979;9:93–106. doi: 10.1016/s0195-5616(79)50009-6. [DOI] [PubMed] [Google Scholar]
- 20.Austin VH. Congenital seborrhea of the springer spaniel. Modern veterinary practice. 1073;54:53–55. [PubMed] [Google Scholar]
- 21.Scott DW, Miller WH. Primary seborrhoea in English springer spaniels: A retrospective study of 14 cases. Journal of Small Animal Practice. 1996;37:173–178. doi: 10.1111/j.1748-5827.1996.tb01955.x. [DOI] [PubMed] [Google Scholar]
- 22.Scott DW. Granulomatous sebaceous adenitis in dogs. J Am Anim Hosp Assoc. 1986;22:631–634. [Google Scholar]