Abstract
The teleost swimbladder is assumed a homolog of the tetrapod lung. Both swimbladder and lung are developmental targets of persistent aryl hydrocarbon receptor (AHR1) agonists; in zebrafish (Danio rerio) the swimbladder fails to inflate with exposure to 3,3’,4,4’,5-pentachlorobiphenyl (PCB126). The mechanism for this effect is unknown, but studies have suggested roles of cytochrome P4501 (CYP1) and cyclooxygenase 2 (Cox-2) in some Ahr-mediated developmental effects in zebrafish. We determined relationships between swimbladder inflation and CYP1 and Cox-2 mRNA expression in PCB126-exposed zebrafish embryos. We also examined effects on β-catenin dependent transcription, histological effects, and Ahr2 dependance of the effect of PCB126 on swimbladder using morpholinos targeting ahr2. One-day-old embryos were exposed to waterborne PCB126 or carrier (DMSO) for 24 h and then held in clean water until day 4, a normal time for swimbladder inflation. The effects of PCB126 were concentration-dependent with EC50 values of 1.4 to 2.0 nM for induction of the CYP1s, 3.7 and 5.1 nM (or higher) for cox-2a and cox-2b induction, and 2.5 nM for inhibition of swimbladder inflation. Histological defects included a compaction of the developing bladder. Ahr2-morpholino treatment rescued the effect of PCB126 (5 nM) on swimbladder inflation and blocked induction of CYP1A, cox-2a, and cox-2b. With 2 nM PCB126 approximately 30% of eleutheroembryos2 failed to inflate the swimbladder, but there was no difference in CYP1 or cox-2 mRNA expression between those embryos and embryos showing inflated swimbladder. Our results indicate that PCB126 blocks swimbladder inflation via an Ahr2-mediated mechanism. This mechanism seems independent of CYP1 or cox-2 mRNA induction but may involve abnormal development of swimbladder cells.
Keywords: zebrafish; swimbladder; aryl hydrocarbon receptor (Ahr); 3,3’,4,4’,5-pentachlorobiphenyl (PCB126); cytochrome P450 1 (CYP1); cyclooxygenase 2 (Cox-2); embryonic development
Introduction
In developing zebrafish the swimbladder is one of the most sensitive targets for Ahr-mediated toxicity (Jönsson et al., 2007a; King Heiden et al., 2009). The swimbladder is an air-filled sac located dorsally in the abdominal cavity, which helps fish balance hydrostatic pressure and reduce energetic cost of swimming. Morphological and molecular evidence suggest that the swimbladder is evolutionarily homologous to the lung (Perry, 1998; Winata et al., 2009; Zheng et al., 2011). Three phases of swimbladder development have been defined in zebrafish (Danio rerio): i) at 36–48 hours post fertilization (hpf) an epithelial bud forms dorsally; ii) the following 2–3 days involve differentiation and growth during which two additional mesodermal layers form; iii) inflation of the swimbladder posterior and anterior chambers occurs at approximately 4.5 and 21 days post fertilization (dpf), respectively (Winata et al., 2009). Endothelia and blood circulation play important roles in organization and differentiation of swimbladder structures and in swimbladder inflation (Winata et al., 2010).
Normal swimbladder development also requires proper Wnt/β-catenin signaling (Yin et al., 2011; Yin et al., 2012). Recent studies show that there is crosstalk between Wnt signaling and the aryl hydrocarbon receptor (AHR), and that exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) causes AHR dependent misregulation of Wnt/β-catenin target genes (Prochazkova et al., 2011; Yoshioka et al., 2011). TCDD also blocks fin regeneration in fin-amputated zebrafish via an Ahr2-mediated mechanism which leads to increased levels of R-spondin1 and activation of β-catenin dependent Wnt signaling (Mathew et al., 2008; Mathew et al., 2009)
Early life stages of fish are highly sensitive to the toxicity of TCDD, 3,3’,4,4’,5-pentachlorobiphenyl (PCB126), and other planar halogenated aromatic hydrocarbons (HAHs) that are AHR agonists. In addition to disrupted swimbladder development effects of HAHs in embryonic fish include craniofacial and cardiovascular malformations, circulatory failure, edemas, and hemorrhages (Henry et al., 1997; Handley-Goldstone et al., 2005; Carney et al., 2006a; Jönsson et al., 2007a). Most toxic effects of HAHs are mediated via the AHR, but the downstream molecular mechanisms leading to toxicity remain largely unknown. In zebrafish, many developmental effects of HAHs depend on Ahr2 as demonstrated by morpholino knockdown (Prasch et al., 2003; Dong et al., 2004; Antkiewicz et al., 2006). However, prior morpholino studies have not shown whether the swimbladder effect is also Ahr2-dependent.
Binding of TCDD and PCB126 to Ahr2 induces expression of cytochrome P450 1 (CYP1) family genes. Four inducible CYP1 genes have been characterized in zebrafish, CYP1A, CYP1B1, CYP1C1, and CYP1C2 (Yamazaki et al., 2002; Jönsson et al., 2007b; Yin et al., 2008). A fifth CYP1 gene, CYP1D1, is not inducible by Ahr agonists (Goldstone et al., 2009). Induction of the CYP1A, CYP1B, and CYP1C genes precedes malformations caused by HAHs (Andreasen et al., 2002; Jönsson et al., 2007a). Knockdown studies have shown inconsistent results regarding the role of CYP1A in TCDD toxicities (Teraoka et al., 2003b; Carney et al., 2004), which suggests the importance of CYP1A could be endpoint specific. CYP1B1 knockdown did not prevent PAH or TCDD induced craniofacial malformations and pericardial edema (Timme-Laragy et al., 2008; Yin et al., 2008). However, a recent study showed that blocked translation of either CYP1C1 or CYP1C2 transcript protected zebrafish embryos from TCDD-induced circulation failure in the dorsal midbrain, implying that the CYP1Cs play roles in this effect (Kubota et al., 2011).
Cyclooxygenase-2 (Cox-2) enzymes (or prostaglandin endoperoxide G/H synthases), have been proposed to be involved in some AHR-mediated toxicities (Puga et al., 1997; Vogel et al., 2007; Teraoka et al., 2009; Dong et al., 2010). Zebrafish have two cox-2 genes, cox-2a and cox-2b, which are constitutively expressed in various tissues (Grosser et al., 2002; Ishikawa et al., 2007). In adult zebrafish TCDD induced cox-2b (but not cox-2a) in liver, while in mesenteric artery expression of neither cox-2a nor cox-2b was affected by TCDD (Bugiak and Weber, 2009). The Cox-2 specific inhibitor NS-398 provided protection from TCDD-induced circulation failure in the dorsal midbrain, and knockdown of Cox-2a also rescued embryos from this effect (Teraoka et al., 2009). A similar protection from TCDD-induced pericardial edema by knockdown of cox-2a was observed in medaka (Dong et al., 2010). Dong et al. (2010) also showed that the prevalence of pericardial edema correlates with increased cox-2 expression in TCDD-exposed medaka embryos. Whether swimbladder inflation or other endpoints of dioxin toxicity correlate with cox-2 or CYP1 expression remains unclear.
The objectives of this study were to 1) examine whether the impaired swimbladder inflation resulting from PCB126 exposure is Ahr2 dependent, and 2) determine the relationships between expression of CYP1, cox-2 and β-catenin regulated genes, and disrupted swimbladder development in developing zebrafish.
Material and Methods
Animals
Zebrafish of the Tup/Long fin (TL) type were used in the experiments. Fertilized eggs were obtained by breeding multiple groups of 30 female and 15 male fish as previously described (Jönsson et al., 2007a). The day after fertilization, unfertilized eggs and dead embryos were removed. Generally, no mortality was observed subsequent to this. Embryos used in the experiments were held in 0.3×Danieau’s solution at 28.5 °C and at a 14 h light/10 h dark diurnal cycle. The experimental procedures were approved by the Animal Care and Use Committee of the Woods Hole Oceanographic Institution. All exposures were performed in glass petri dishes.
Exposure to various PCB126 concentrations
In a concentration response experiment, groups of 1-day post-fertilization (dpf) embryos (222±8 embryos per dish) were exposed to various concentrations of PCB126 (0.5–10 nM) or carrier (0.02% DMSO, v/v) in 150 mL of 0.3×Danieau’s solution. After 24 hours the exposure solutions were replaced with fresh 0.3×Danieau’s solution and the embryos were held, with daily changes of the 0.3×Danieau’s solution, until 4 dpf. At this time, the swimbladder is inflated in normally developing embryos (Jönsson et al., 2007a). During sampling, embryos were scored based on whether they exhibited an inflated swimbladder or not. From each treatment group replicates composed of 18–32 pooled embryos were collected; embryos with and without inflated swimbladder were collected in separate samples. The samples were flash frozen in liquid nitrogen and stored at −80 °C until used for quantitative real time PCR.
Exposure to Cox-2 inhibitor
In another experiment, groups of 180 embryos (1-dpf) were treated with 5 nM PCB126 or carrier (0.01% DMSO) in combination with 0, 2, 5 or 10 µM of the Cox-2 inhibitor N-[2-(cyclohexyloxy)-4-nitrophenyl] methanesulfonamide (NS-398) (Cayman Chemical, Ann Arbor, MI) in 150 ml of 0.3×Danieau’s solution. Other conditions were as described above.
Histology
Embryos (1-dpf) to be used for histology were exposed to 2 nM PCB126 or to the carrier (0.01% DMSO) as described above. At 4 dpf the embryos were sorted based on swimbladder inflation, fixed in 4% formaldehyde in phosphate buffer, and stored in 70% ethanol until embedding. Fixed embryos were dehydrated, embedded in Technovit 7100 (Heraeus Kulzer, Hanau, Germany), sectioned (2 µm), mounted on superfrost glass slides, and stained with hematoxylin and eosin. Sections were examined for histopathology by light microscopy (Leica DMRXE, Leica Microsystems GmbH, Wetzlar, Germany), and photographed. Cell death was indicated by fragmented nuclei.
Ahr2 knockdown by morpholinos
To examine the role of Ahr2 in the effect of PCB126 on swimbladder inflation, we treated zebrafish embryos with a morpholino antisense oligonucleotide blocking ahr2 translation, as previously described (Jönsson et al., 2009). Morpholinos targeting the transcriptional start site of ahr2 (Ahr2-MO; 5-TGTACCGATACCCGCCGACATGGTT-3) (Prasch et al., 2003; Dong et al., 2004) and negative control morpholinos (control-MO; 5-CCTCTTACCTCAGTTACAATTTATA-3) were obtained from Gene Tools (Philomath, OR, USA). The morpholinos were fluorescein-tagged to enable selection of successfully injected embryos for the experiments. Both morpholinos were diluted in deionized water. A Narishige IM-300 microinjector (Tokyo, Japan) with a fine glass needle was used to inject 2 nL (0.36 pmoles) of morpholinos into the yolk of 1-to 4-cell stage embryos. Embryos were screened at 3 hours postfertilization (hpf) and 24 hpf by fluorescence microscopy to verify incorporation of morpholinos. Any damaged embryos or those not displaying homogenous fluorescence were removed. Half of each embryo group was exposed to 5 nM PCB126 and the other half was exposed to DMSO (0.01%). In addition to the control-MO, groups of uninjected embryos were also exposed to PCB126 or DMSO. Groups of 50 embryos were exposed in glass petri dishes containing 100 mL 0.3×Danieau’s solution. After 24 hours the exposure solutions were replaced with fresh 0.3×Danieau’s solution and the embryos were held with daily changes of 0.3×Danieau’s solution. At 4 dpf, embryos with inflated and uninflated swimbladders were counted. Pools of 15–18 embryos were flash frozen in liquid nitrogen and stored at −80 °C for analysis by quantitative PCR.
Quantitative real-time RT-PCR
RNA was isolated using RNA STAT-60™ (Tel-Test Inc. Friendswood, TX, USA) and the isolates were DNase treated by the TURBO DNA-free™ kit (Applied Biosystems, Austin TX, USA). The quantity of RNA was determined spectrophotometrically (NanoDrop ND-1000; NanoDrop Technologies, Wilmington, DE, USA). Total RNA (1 µg per sample) was reverse transcribed using the iScript cDNA Synthesis kit (Bio-Rad Hercules, CA, USA). Gene-specific primers for real time PCR were synthesized by Eurofins MWG Operon (Huntsville, AL, USA). Primer sequences for CYP1A, CYP1B1, CYP1C1, CYP1C2, CYP1D1, and ef1a have been published previously (Goldstone et al., 2009; Jönsson et al., 2009; Goldstone et al., 2010). The primer sequences for axin2 (GenBank ID: NM_131561.1): F-GGACACTTCAAGGAACAACTAC and R-CCTCATACATTGGCAGAACTG and myca (GenBank ID: NM_131412.1): F-TAACAGCTCCAGCAGCAGTG and R-GCTTCAAAACTAGGGGACTG were from Yin et al. (2011). New primers were designed for cox-2a (GenBank ID: NM_153657.1): F-ACTACCCCTGAGCTTCTCACA and R-GATGCTGTTGATGATATCCCAGATTG; and cox-2b (GenBank ID: NM_001025504.2): F-GGCTCATCCTTATTGGTGAGACTAT and R-TCGGGATCAAACTTGAGCTTAAAATA (5’ to 3’ sequences). Real time PCR was performed with 25 µl reactions using 50 ng cDNA and 5 pmoles of each primer (forward and reverse) with the iQ SYBR Green Supermix (Bio-Rad) as previously described (Jönsson et al., 2007b). To ensure that a single product was amplified, melt curve analysis was performed on the PCR products at the end of each PCR run. Relative mRNA expression of the target genes was calculated for each reaction by E−ΔΔCt (Livak and Schmittgen, 2001) using ef1a as the reference gene (McCurley and Callard, 2008). PCR efficiencies (E) for within-experiment amplicon groups were determined by the LinRegPCR program (Ramakers et al., 2003; Ruijter et al., 2009).
Promoter analysis
The zebrafish cox-2a, cox-2b, myca, and axin2 genes were localized in Zv9 in Ensembl, and the regions 0–5000 bp upstream of the untranslated region (UTR) and the UTRs (including any intron upstream of the start codon) of these genes were downloaded. Putative dioxin response elements (DREs) were searched for using the DRE consensus sequence identified by (Fujisawa-Sehara et al., 1987) 5’-T/GNGCGTG-3’ (and the reverse complement of this). For comparison we also searched for putative DREs in the corresponding regions of the CYP1A gene.
Statistics
Outliers were excluded based on the Grubbs test (1969). The statistical analyses were performed using Prism 5 by GraphPad Software Inc. (San Diego, CA, USA). Data were log-transformed when the variation differed between groups. In the figures data are shown as mean + standard deviation of the mean (SD); n = 4 in the dose response experiment and n = 2 in the morpholino experiment. EC50 values, i.e., the PCB126 concentrations causing half maximal effect, were determined by the curve-fitting routine of Prism for nonlinear regression using sigmoidal dose response with variable slope.
Results
Effect of PCB126 on swimbladder inflation and histology
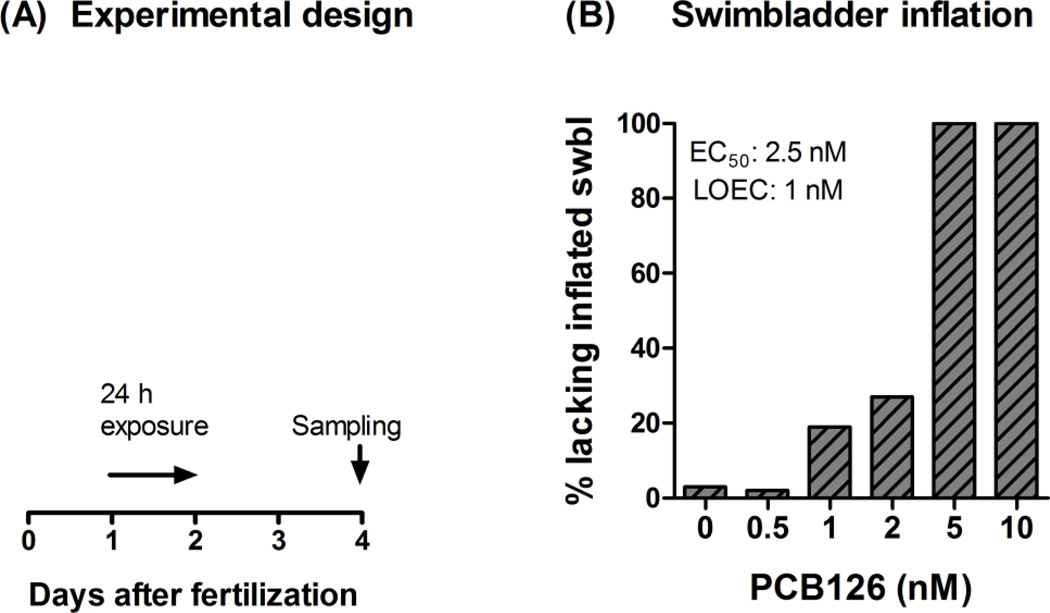
We first examined the nature of the effect of PCB126 on swimbladder inflation, and asked whether this was a dose-dependent effect. Figure 1A shows the design of the PCB126 concentration-response experiment. Phenotypic effects of PCB126 on swimbladder development were screened in 4-dpf zebrafish embryos after exposure to nominal concentrations varying between 0.5 and 10 nM. The results showed a PCB126 concentration-dependent reduction in the number of individuals exhibiting inflated swimbladders at 4 dpf, with EC50 and LOEC values of 2.5 nM and 1 nM, respectively (Fig. 1B). In this experiment, embryos exposed to 0.5 nM PCB126 showed no phenotypic difference compared with the controls, while all those exposed to 5 or 10 nM PCB126 lacked inflated swimbladders (Fig. 1B and Figs. 2A–F). Among the embryos exposed to 2 nM PCB126 (454 embryos in total), only 1.3% showed pericardial edema while 27% lacked inflated swimbladder at 4 dpf. Many embryos in the 5-nM PCB126 exposure group (not quantified) and all embryos in the 10-nM PCB126 group exhibited pericardial edema. Qualitative observations noted a higher degree of severity of pericardial edema among fish exposed to 10 nM PCB126 than among those exposed to 5 nM PCB126. In a previous study in developing zebrafish (monitored at 80 hpf) the frequencies of pericardial edema were about 25% and 90% after exposure to 3 and 10 nM PCB126, respectively (Jönsson et al., 2007a).
Figure 1.
Exposure regime (A) and effect of PCB126 on swimbladder inflation (B) in developing zebrafish. A) At 1 dpf embryos were exposed to PCB126 (in DMSO) mixed in 0.3× Danieau’s solution at nominal concentrations ranging from 0.5 to 10 nM (0.02% DMSO), or 0.02% DMSO only. After 24 hours of exposure, the embryos were transferred to clean 0.3× Danieau’s solution. The 0.3× Danieau’s solution was also refreshed on day three. The experiment was ended on day four at which numbers of embryos exhibiting disrupted swimbladder inflation in the different exposure groups were counted. B) The bar representing the 2-nM PCB126 exposure group shows data from 454 embryos, while all other bars show data from 214–232 embryos.
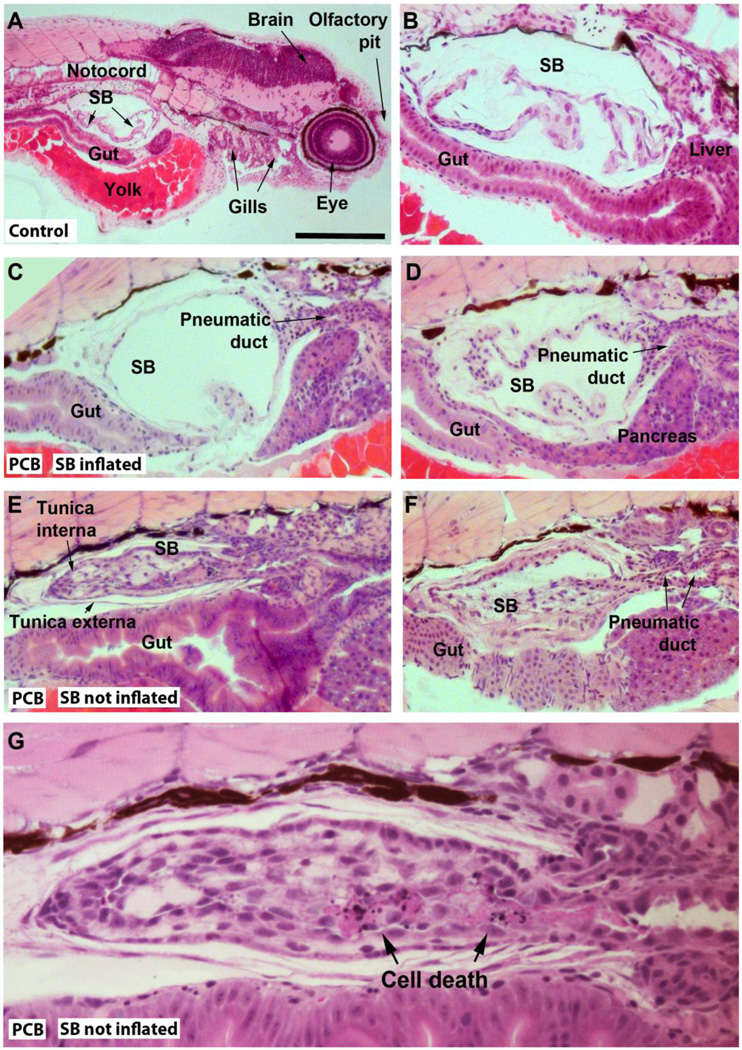
Figure 2.
Representations of histological sections of 4-day-old zebrafish embryos exposed to the carrier (0.01 % DMSO; A–B), or to 2 nM PCB126 (C–G). Exposure conditions are described in “Histology” in Materials and methods. Slides A–D show sections of embryos with a normal swimbladder phenotype (A–B: controls; C–D: PCB126-exposed) and E–F show swimbladders of embryos that failed to inflate their swimbladders (PCB126-exposed). SB=swimbladder. In G an area with cell death in the swimbladder tissue is indicated (another section from the fish represented in E). The scale bar shown in A represents approximately 400 µm (A), 100 µm (B–F), and 40 µm (G).
Histological examination of the 4-dpf zebrafish lacking inflated swimbladder revealed the presence of swimbladder membranes and a pneumatic duct, but instead of an open bladder with a thin epithelial wall, the cells formed a compact structure (Fig. 2E–G). In the swimbladder tissue of one of the 11 embryos examined, clusters of dying cells with fragmented dark nuclei surrounded by eosin staining were observed (Fig. 2G), and are interpreted as cell necrosis.
Dose-dependent expression of CYP1 and cox-2
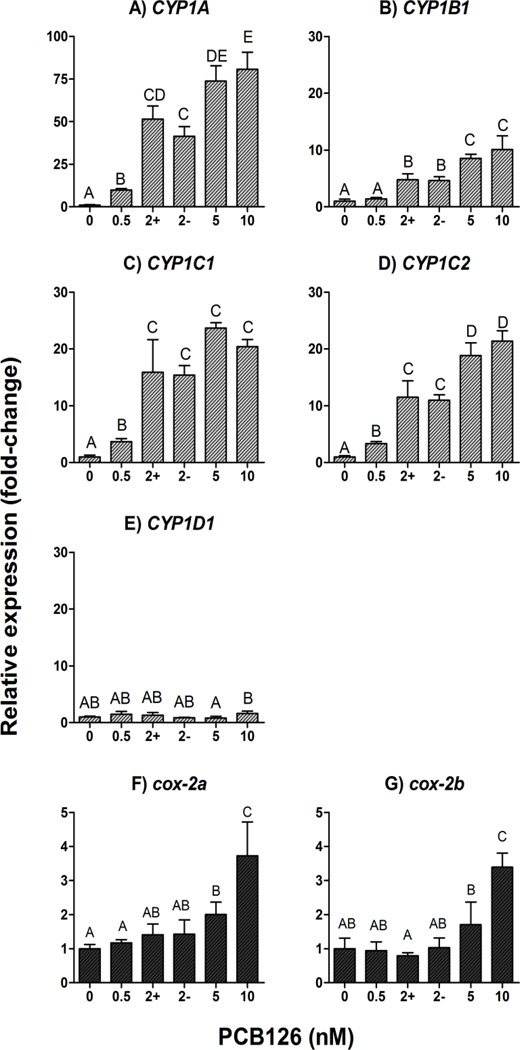
PCB126 induced the mRNAs of the four inducible CYP1s and the two cox-2 genes in a concentration-dependent manner, with EC50 values of 1.7, 2.0, 1.4, and 2.0 nM, respectively for CYP1A, CYP1B1, CYP1C1, and CYP1C2 (Fig. 3A–D) and EC50 values higher than 3.7 and 5.4 nM for the cox-2a and cox-2b (Fig. 3F–G). LOEC values for induction by PCB126 were 0.5 nM for CYP1A, CYP1C1, and CYP1C2, 2 nM for CYP1B1, 5 nM for cox-2a, and 10 nM for cox-2b. As expected, expression of CYP1D1 was not significantly changed compared with the control by PCB126 exposure (Fig. 3E). Day-4 zebrafish exposed to 2 nM PCB126 were sorted based on swimbladder phenotype for comparison of mRNA expression levels (i.e., in groups showing inflated and not inflated swimbladder). However, the two groups showed no statistical difference in CYP1 or cox-2 mRNA expression levels (Fig. 3A–G).
Figure 3.
Concentration-response relationship for PCB126 induced mRNA expression of CYP1 (A–D) and cox-2 (E–F) in developing zebrafish (determined at 4 dpf). Detailed exposure regimen is given in Figure 1A. Embryos were exposed to carrier (0.02% DMSO) or various concentrations of PCB126 (0.5, 2, 5, or 10 nM) for 24 hours starting at 1 dpf. At 4 dpf, embryos were sorted based on whether they exhibited inflated swimbladder or not, and sampled for quantitative real time PCR analysis. In the figures, “+” and “−“ in horizontal axis represent the groups of embryos that exhibited inflated and uninflated swimbladder, respectively. Relative expression (fold-control) was calculated by E−ΔΔCt (Livak and Schmittgen, 2001), using ef1a as a reference gene. Statistical differences among groups were determined by one-way ANOVA followed by Tukey’s multiple comparisons test and are shown by different letters (p<0.05, n=4).
We also treated zebrafish embryos with the Cox-2 inhibitor NS-398 (2–10 µM) in combination with the carrier (0.01% DMSO) or 5 nM PCB126. Treatment with the highest concentration of NS-398 (10 µM) and the carrier caused pericardial edema, that was partially reversible after removal of the inhibitor. However, the effect of PCB126 on swimbladder inflation was not influenced by NS-398 at any concentration.
The effect of PCB126 on swimbladder inflation is Ahr2-dependent
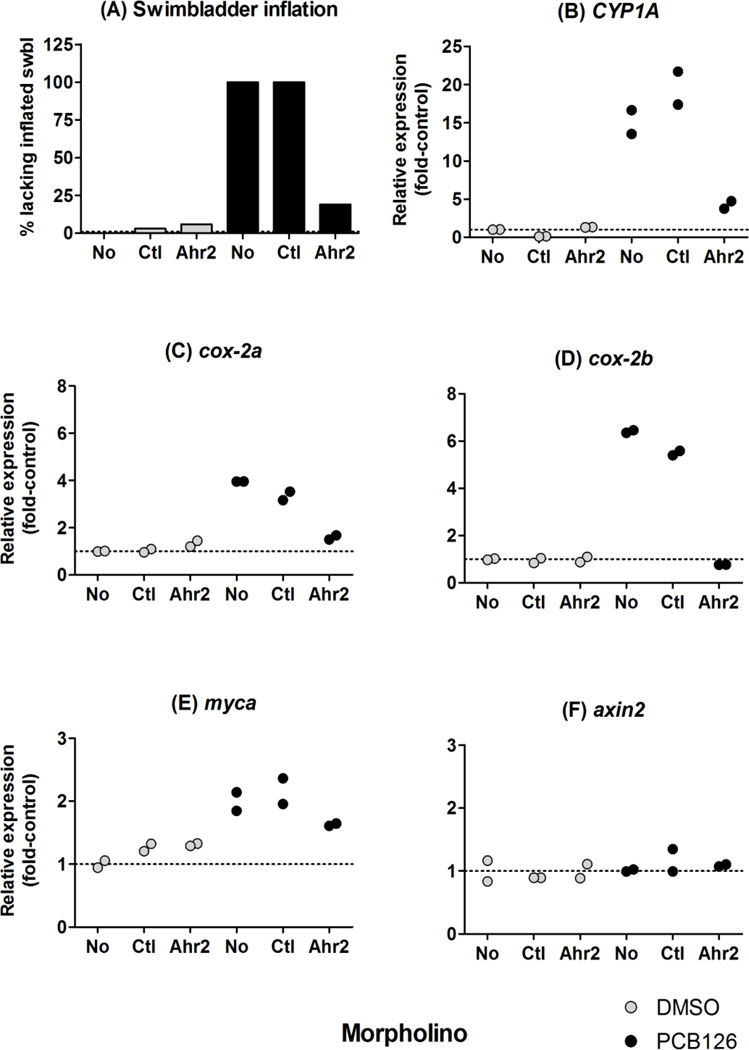
Jönsson et al. (2007a) demonstrated that morpholino-knockdown of Ahr2 significantly decreased the PCB126-induced expression of CYP1 transcripts in 2-dpf zebrafish embryos. Here we examined the effect of the Ahr2-MO on disruption of swimbladder inflation and on induction of CYP1A, cox-2a and cox-2b mRNA triggered by PCB126 (5 nM), in 4-dpf embryos. As shown in Fig. 4A, treatment with the Ahr2-MO but not with the negative control-MO rescued the PCB126 effect on the swimbladder, i.e., the swimbladder was inflated in most embryos treated with both the Ahr2-MO and PCB126. Repeated experiments with the same MO yielded similar results. We confirmed that the morpholino knockdown was still effective at 4 dpf, by measuring expression of the most responsive Ahr2-regulated gene, CYP1A. Induction of CYP1A by PCB126 was significantly attenuated by Ahr2-MO treatment in these embryos (Fig. 4B). Furthermore, knockdown of Ahr2 almost abolished the PCB126-induced expression of cox-2a and cox-2b transcripts at 4 dpf (Fig. 4C–D). A third experiment showed that the Ahr2 MO was still effective in blocking 5 nM PCB126 effects on swimbladder inflation (and on pericardial edema) at 5–7 dpf.
Figure 4.
Effect of Ahr2-MO treatment on the swimbladder inflation (A) and mRNA expression of CYP1A (B), cox-2a (C), cox-2b (D), myca (E) and axin2 (F) in embryos exposed to PCB126 (black bar or bullet) or DMSO (grey bar or bullet). Embryos injected with a control morpholino, “Ctl-MO”, or a morpholino against Ahr2, “Ahr2-MO”, and embryos not injected with any morpholino, “No”, were exposed to carrier (0.01% of DMSO) or 5 nM PCB126 for 24 hours starting at 1 dpf. At 2 dpf the exposure solution was replaced with fresh 0.3× Danieau’s solution. At 4 dpf numbers of embryos exhibiting disrupted swimbladder inflation in the different groups were counted. The embryos then were harvested for quantitative real time PCR analysis. In A each bar represents data from 30–36 embryos. In B, C and D, individual data were plotted to show the difference in the expression between two biological replicates, each composed of 15–18 embryos (n=2).
In order to examine if PCB126 had any effect on Wnt signaling we analyzed the mRNA expression of two β-catenin regulated genes, axin2 and myca, in samples from the morpholino experiment. There was an upregulation of myca in all PCB126 exposed groups compared with the controls, but because of the small magnitude of the change (a doubling) an effect by the Ahr2-MO could not be discriminated with certainty (Fig. 4E). The mRNA expression of axin2 seemed not to vary between the control and the PCB126-exposed groups (Fig. 4F).
Numbers of putative DREs in the gene promoters
We also searched the zebrafish genome for putative DREs in the cox-2a, cox-2b, myca, and axin2 genes. As previously reported there are a large number of putative DREs in the zebrafish CYP1A gene promoter (Jönsson et al., 2007b; Zeruth and Pollenz, 2007). Data for putative DREs in the CYP1B1, CYP1C1, CYP1C2, and CYP1D1 promoters have also been published (Jönsson et al., 2007a; Goldstone et al., 2009). We found two putative DRE sequences in the cox-2b gene, one within the UTR and one within 1000 bp upstream of the UTR; in axin2 one putative DRE was located within 1000 bp upstream of the UTR, while no such sequence occurred within 5000 bp upsteam of cox-2a or myca, or in the UTRs of these genes (Table 1).
Table 1.
Putative dioxin response elements (DREs) upstream of untranslated region (UTR) and within UTR1)
| Gene | −5000 bp | −4000 bp | −3000 bp | −2000 bp | −1000 bp | UTR |
|---|---|---|---|---|---|---|
| CYP1A | 18 | 14 | 13 | 5 | 5 | 2 |
| cox-2a | 0 | 0 | 0 | 0 | 0 | 0 |
| cox-2b | 2 | 2 | 2 | 2 | 2 | 1 |
| myca | 0 | 0 | 0 | 0 | 0 | 0 |
| axin2 | 1 | 1 | 1 | 1 | 1 | 0 |
UTR including the 1st intron if present upstream of the start codon (i.e., in CYP1A, myca, and axin2)
Discussion
Swimbladder effect of PCB126 is Ahr2-dependent
The studies here demonstrate that planar HAH effects on swimbladder inflation are dependent on the Ahr2. Thus, we found that treatment with a morpholino knocking down Ahr2 rescued the effect of PCB126 (5 nM) on the developing swimbladder in 4-dpf zebrafish. This is in contrast to what has been observed in this system using other concentrations, chemicals, and later developmental endpoints (Prasch et al., 2003; Jönsson et al., 2007a). It has been hypothesized that the efficacy of the Ahr2-MO in zebrafish is transient, with dilution of the morpholino by growth resulting in amounts of Ahr2-MO too low to have an effect in older larvae. However, recent studies in killifish and medaka (Matson et al., 2008; Clark et al., 2010; Dong et al., 2010) showed that the amount of morpholino used is sufficient to have an effect on target cells/organs in older fish. This suggests that the amount of Ahr2-MO may not be a critical issue in explaining differences in what was observed for the swimbladder endpoint between the present study and the earlier studies (Prasch et al., 2003; Jönsson et al., 2007a).
In an earlier study (Jönsson et al., 2007a), treatment with the Ahr2-MO failed to rescue swimbladder inflation in zebrafish embryos exposed to 30 nM PCB126, i.e., a concentration 6-fold greater than used in the present study. Dong et al. (2002) found that TCDD decreases blood flow in the mesencephalic vein of 2-dpf zebrafish embryos. This effect was rescued by treatment with Ahr2-MO in embryos exposed to 0.3 ppb of TCDD (ca. 1 nM), but not in those exposed to higher concentrations of TCDD (0.5 or 1 ppb) (Dong et al., 2004). Thus, it appears that the different results seen for the ability of Ahr2-MO to block the effects of PCB126 and TCDD might be attributed to differences in the concentrations of AHR agonists used.
Ahr2-dependent expression of cox-2
The present study revealed that the transcription of both cox-2a and cox-2b was increased by PCB126 in a dose-dependent manner in 4-dpf zebrafish (whole body homogenates). A similar dose-dependent increase in the cox-2 transcript level was observed in medaka embryos exposed to TCDD (Dong et al., 2010). We also observed that Ahr2 is required for the increase in expression of cox-2a and cox-2b by PCB126. Thus, our results, together with the study by Dong et al. (2010), indicate that cox-2 genes are downstream targets of the Ahr signaling pathway in some bony fish. AHR dependent regulation of COX-2 has been observed in rodent cells (Wolfle et al., 2000; Yang and Bleich, 2004). On the other hand, TCDD did not induce cox-2a in 2-dpf zebrafish embryos (whole body homogenates), although knocking down cox-2a rescued the embryos from the TCDD effect on mesencephalic circulation (Teraoka et al., 2009). This could occur if there was highly localized induction of cox-2a at the developmental stage at which TCDD caused mesencephalic circulation failure (Teraoka et al. 2009).
In addition to Ahr2 binding to DREs, there may be other mechanisms for upregulation of the zebrafish cox-2s. Yang and Bleich (2004) found that COX-2 expression has three transcriptional regulators in mouse (C/EBP, AHR, and CREB) and single mutations to each of the three types of response elements significantly reduced COX-2 expression. In human cells (HaCaT cells) COX-2 mRNA expression apparently can be upregulated by an AHR dependent mechanism which does not involve AHR binding to DREs (Vogel et al., 2000; Fritsche et al., 2007). Instead, C-SRC, a component of the cytosolic AHR complex which is released upon AHR agonist binding, has been suggested to interact with epidermal growth factor receptor (EGFR), leading to upregulation of COX-2 expression (Vogel et al., 2000; Fritsche et al., 2007). Our promoter search revealed two putative DREs within 5 kb upstream in the cox-2b promoter region, while cox-2a does not have any DRE consensus sequence in 5 kb of the 5’-UTR. Further studies are needed to determine the regulatory mechanisms underlying Ahr2 dependent increases in the two cox-2 genes in zebrafish.
Correlations of the PCB126 effects on cox-2 expression and swimbladder inflation
Our results indicate that Cox-2s may not be direct mediators of an adverse effect by PCB126 on the swimbladder in developing zebrafish. We found no significant difference in the cox-2 mRNA expression between 4-days old embryos with and without inflated swimbladders. The present dose-response study also showed that while failure to inflate the swimbladder occurred in all embryos exposed to 5 nM PCB126, that dose induced cox-2 expression only 2-fold, showing only a weak relationship between these two endpoints. However, the use of whole body homogenate for extraction of RNA does have limitations in not revealing the cell specificity of expression. Thus, whether PCB126 changes the transcript levels of cox-2a and cox-2b in cells of the developing swimbladder or in cells important for this process remains to be determined.
It has been reported that Cox-2 is involved in circulation failure caused by dioxin in fish. In zebrafish, TCDD caused reduction in mesencephalic vein blood flow and this inhibitory effect of TCDD was blocked by knockdown of Cox-2a, as well as by a Cox-2 specific inhibitor, NS-398 (Teraoka et al., 2009). Dong et al. (2010) showed that TCDD-exposed medaka embryos that exhibited pericardial edema had a cox-2 gene expression significantly greater than their non-edematous group. This may be a species-specific difference (zebrafish versus medaka), or perhaps a chemical-specific one (PCB126 versus TCDD). A study by Prasch et al. (2003) showed that Ahr2 morphants exposed to TCDD failed to inflate swimbladder as larvae (10 dpf), while at the same time they had no pericardial and yolk sac edema. Furthermore, our previous study revealed that the PCB126-EC50 value for disruption of swimbladder inflation was lower than that for pericardial edema (Jönsson et al., 2007a). Hence, there may be a dose-dependent explanation for why others have observed differences in cox-2 expression associated with HAH-induced deformities, as the dose required for pericardial edema is higher than that required for the swimbladder effect.
We treated zebrafish embryos with the Cox-2 inhibitor NS-398 (2–10 µM) in combination with the vehicle (DMSO) or 5 nM PCB126. Contrary to the rescue of TCDD-induced circulation failure in mesencephalic vein at 50 hpf (Teraoka et al., 2009), we found that 10 µM NS-398 by itself was sufficient to cause pericardial edema at 2 dpf. Furthermore, the effect of PCB126 on swimbladder inflation at 4 dpf was not influenced by NS-398 at any concentration. This can be interpreted as further support that Cox-2 does not play a role in the Ahr2-mediated PCB126 toxicity in the developing swimbladder. Further studies with inhibitors or gene knockdown specific for zebrafish Cox-2 are required for further understanding the roles of zebrafish Cox-2 gene(s) in the various endpoints related to dioxin toxicity.
AHR and Wnt signaling crosstalk
Histological analysis in PCB126-exposed 4-dpf embryos revealed pathological changes at the cellular level of the swimbladder tissue similar to those reported by Henry et al. (1997) in TCDD-exposed zebrafish embryos. Although the mechanisms are not known, AHR-mediated effects of TCDD and PCB126 are often associated with disturbances of cell proliferation, apoptosis, and differentiation (Carney et al., 2006b; Puga et al., 2009). An increasing number of studies indicate that the AHR interacts with Wnt/β-catenin signaling (Jackson et al., 2005; Kawajiri et al., 2009; Abel and Haarmann-Stemmann, 2010; Prochazkova et al., 2011). This pathway organizes cell differentiation and proliferation in growing tissues and is central for the establishment of cell patterns in developing structures, such as the swimbladder (Nusse, 2008; Yin et al., 2011; Yin et al., 2012). In zebrafish, both blockage and overstimulation of the Wnt/β-catenin pathway perturb swimbladder development (Yin et al., 2011; Yin et al., 2012). Blocked Wnt/β-catenin signaling is associated with cell cycle arrest in G1 phase and reduced expression of β-catenin regulated genes, including axin2 and myca (Tang et al., 2009; Yin et al., 2011).
Our results could mean that disruption of the Wnt/β-catenin pathway occurs with PCB126, as indicated by the 2-fold induction of myca expression, a gene downstream of Wnt; however, dependence of this increase on Ahr2 was not verified by Ahr2-knockdown. C-Myc is an important oncoprotein which can stimulate cell cycle progression, but which also can induce apoptosis (Amati et al., 1998). Expression of myca is regulated by several pathways including by β-catenin and E2F (Hiebert et al., 1989). Since expression of myca is closely correlated to cell proliferation (Obaya et al., 1999) the present results could mean that PCB126 has stimulated cell proliferation. This is seemingly in contrast to our previous finding in 3-dpf zebrafish embryos that PCB126 dose-dependently suppresses expression of proliferating cell nuclear antigen (pcna) (Jönsson et al., 2007a). Indeed, PCB-126 has been shown to cause reduced proliferation, but not apoptosis, in the developing heart of 3-dpf zebrafish exposed to PCB-126 (Grimes et al., 2008). The myca induction observed in the 4-day fish of the present study could be a compensatory mechanism in response to earlier suppression of cell proliferation. It is also plausible that some cell populations respond to PCB126 with proliferation and some with suppression of proliferation and that the numbers of cells with these two different types of response changes during the developmental process. While it is thus possible that PCB126 interferes with Wnt/β-catenin signaling in developing zebrafish tissues (such as the swimbladder), the elucidation of this relationship requires further study.
PCB126 effects on swimbladder inflation in association with reduction in blood flow
Chemicals that are Ahr2 agonists are known to reduce blood flow in trunk vessels of zebrafish embryos at 72 hpf or later (Teraoka et al., 2003a; Carney et al., 2004). Ahr2 dependence of the reduction in blood flow has also been confirmed (Dong et al., 2004). A recent study demonstrated important roles of a functional blood circulation in inflation of the swimbladder as well as its normal growth (Winata et al., 2010). In zebrafish troponin T type 2 (Tnnt2) morphants, which lack blood flow due to defective cardiac contractility, the main chamber primordium appears not to be present in the swimbladder epithelium at 72 hpf, resulting in failure to inflate the swimbladder at 5 dpf (Winata et al., 2010). Thus, failure to inflate the swimbladder in zebrafish embryos exposed to PCB126 possibly could be secondary to the Ahr2-dependent reduction in blood flow in the swimbladder tissues.
Studies in developing rats have shown that gestational exposure to TCDD causes changes in lung morphology and function (Kransler et al., 2009). Thus, potent AHR agonists can perturb morphogenesis of the swimbladder and lung, which are homologous organs and related also on the molecular level (Zheng et al., 2011; Yin et al., 2012). Notably, the Wnt signaling pathway also is involved in development and differentiation in lung, as it is in swimbladder (Goss et al., 2009; Hashimoto et al., 2012). Zebrafish are physostomous, retaining a pneumatic duct from the digestive tract to the swimbladder past the larval stage. It will be interesting to determine whether there are similar effects of TCDD on swimbladder inflation and histology in physoclistous fish, in which the pneumatic duct is resorbed or absent, and in which inflation can occur by a different path. Problems with swimbladder inflation have been noted in physoclist fish (Perlberg et al., 2008) and further defining mechanisms of dioxin effect in zebrafish could yield insights that may be tested in those fish. The swimbladder in developing zebrafish may model the pathological changes and molecular basis for those changes occurring in lung, as well as in swimbladder of other fish. Further identification of mechanisms of dioxin toxicity in zebrafish could yield insights that may be applicable to other models.
Conclusion
This study shows that PCB126 causes morphological changes at the cellular level in the swimbladder tissue that could lead to loss of swimbladder inflation. Further, this failure of the swimbladder to inflate is mediated via a yet unknown, but Ahr2-dependent mechanism. Although PCB126-EC50 values for loss of swimbladder inflation and induction of CYP1s were close to one another (approximately 2 nM), we detected no significant difference in CYP1 mRNA expression levels between larvae with and without inflated swimbladders in the 2-nM PCB126 exposure group. Similarly, the effect on the swimbladder could not be linked to the induction of cox-2a and cox-2b. This suggests the molecular mechanism triggered by PCB126 and leading to disrupted swimbladder inflation involves other factors than the CYP1s and Cox-2s, at least at low PCB126 concentrations. Using quantitative PCR we found upregulation of the oncogene myca in PCB126 exposed 4-day zebrafish, which supports the idea that PCB126 perturbs regulation of cell proliferation. Localization of changes in expression of myca and other mRNAs related to Wnt signaling by in situ hybridization over the course of development may show if PCB126 interferes with Wnt signaling in the developing swimbladder. It is conceivable as well that the effect on swimbladder is secondary to effects on blood flow.
Highlights.
PCB126 caused cellular changes in the developing swimbladder.
Swimbladder inflation was not related to expression of CYP1 or cox-2.
Failure of swimbladder inflation is mediated via an Ahr2-dependent mechanism.
PCB126-exposed zebrafish larvae showed upregulation of the oncogene myca.
Acknowledgements
We thank Dr. Roxanna Smolowicz for expert advice on histopathology and Margareta Mattsson for technical assistance. Economic support was granted by The Swedish Research Council Formas, and Carl Trygger’s Stiftelse to M.E.J. This study was also supported by Grant-in-Aid for JSPS Postdoctoral Fellows to A. K. from the Japan Society for the Promotion of Science. The awards of the Postdoctoral Fellowships and of the Postdoctoral Fellowships for Research Abroad to A. K. are acknowledged (nos. 4313 and 820), and NIH: F32ES017585 (A. T.-L.). Funding for BRW and JJS was from the United States National Institutes of Health (National Institute of Environmental Health Sciences), grants R01ES015912 and P42ES007381 to J.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Zebrafish cytochrome P450 family 1 genes/mRNAs and proteins are referred to as CYP1 and CYP1 according to Nelson et al. (1996). For other genes/mRNAs and proteins in zebrafish we have followed the approved guidelines for zebrafish, e.g., ahr2 and Ahr2 (https://wiki.zfin.org/display/general/ZFIN+Zebrafish+Nomenclature+Guidelines). The aryl hydrocarbon receptor is denoted AHR when not referring to a particular species.
Fish embryos that have hatched but are still dependent on yolk as a nutrition source are technically “eleutheroembryos”, but for simplicity we will refer to them generically as “embryos" for the remainder of this paper. Once independent feeding begins (day 6–7 post fertilization in zebrafish), the fish are then called larvae
Conflict of interest statements for authors
None of the authors has any conflict of interest regarding the research described in this article.
Contributor Information
Maria E. Jönsson, Email: maria.jonsson@ebc.uu.se.
Akira Kubota, Email: akubota@whoi.edu.
Alicia Timme-Laragy, Email: atimmelaragy@whoi.edu.
Bruce Woodin, Email: bwoodin@whoi.edu.
John J. Stegeman, Email: jstegeman@whoi.edu.
References
- Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol. Chem. 2010;391:1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front. Biosci. 1998;3:d250–d268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol. Sci. 2002;68:403–419. doi: 10.1093/toxsci/68.2.403. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Peterson RE, Heideman W. Blocking Expression of AHR2 and ARNT1 in Zebrafish Larvae Protects Against Cardiac Toxicity of 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Bugiak B, Weber LP. Hepatic and vascular mRNA expression in adult zebrafish (Danio rerio) following exposure to benzo-a-pyrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin. Aquat. Toxicol. 2009;95:299–306. doi: 10.1016/j.aquatox.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 2006a;70:549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol. Pharmacol. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Carney SA, Prasch AL, Heideman W, Peterson RE. Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res. A. 2006b;76:7–18. doi: 10.1002/bdra.20216. [DOI] [PubMed] [Google Scholar]
- Dong W, Matsumura F, Kullman SW. TCDD induced pericardial edema and relative COX-2 expression in medaka (Oryzias Latipes) embryos. Toxicol. Sci. 2010;118:213–223. doi: 10.1093/toxsci/kfq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Tsujimoto Y, Stegeman JJ, Hiraga T. Role of aryl hydrocarbon receptor in mesencephalic circulation failure and apoptosis in zebrafish embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2004;77:109–116. doi: 10.1093/toxsci/kfh023. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Yamazaki K, Tsukiyama S, Imani S, Imagawa T, Stegeman JJ, Peterson RE, Hiraga T. 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicol. Sci. 2002;69:191–201. doi: 10.1093/toxsci/69.1.191. [DOI] [PubMed] [Google Scholar]
- Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, Klotz LO, Rannug A, Furst P, Hanenberg H, Abel J, Krutmann J. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A, Sogawa K, Yamane M, Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987;15:4179. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone JV, Jönsson ME, Behrendt L, Woodin BR, Jenny MJ, Nelson DR, Stegeman JJ. Cytochrome P450 1D1: a novel CYP1A-related gene that is not transcriptionally activated by PCB126 or TCDD. Arch. Biochem. Biophys. 2009;482:7–16. doi: 10.1016/j.abb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jonsson ME, Nelson DR, Stegeman JJ. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 2010;11:643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai HJ, Kirby ML. PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol. Sci. 2008;106:193–205. doi: 10.1093/toxsci/kfn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser T, Yusuff S, Cheskis E, Pack MA, FitzGerald GA. Developmental expression of functional cyclooxygenases in zebrafish. Proc. Natl. Acad. Sci. USA. 2002;99:8418–8423. doi: 10.1073/pnas.112217799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Handley-Goldstone HM, Grow MW, Stegeman JJ. Cardiovascular gene expression profiles of dioxin exposure in zebrafish embryos. Toxicol. Sci. 2005;85:683–693. doi: 10.1093/toxsci/kfi116. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Chen H, Que J, Brockway BL, Drake JA, Snyder JC, Randell SH, Stripp BR. beta-Catenin-SOX2 signaling regulates the fate of developing airway epithelium. J. Cell Sci. 2012;125:932–942. doi: 10.1242/jcs.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol. Appl. Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Hiebert SW, Lipp M, Nevins JR. E1A-dependent trans-activation of the human MYC promoter is mediated by the E2F factor. Proc. Natl. Acad. Sci. USA. 1989;86:3594–3598. doi: 10.1073/pnas.86.10.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa TO, Griffin KJ, Banerjee U, Herschman HR. The zebrafish genome contains two inducible, functional cyclooxygenase-2 genes. Biochem. Biophys. Res. Commun. 2007;352:181–187. doi: 10.1016/j.bbrc.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Vayssiere B, Garcia T, Newell W, Baron R, Roman-Roman S, Rawadi G. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36:585–598. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Jönsson ME, Franks DG, Woodin BR, Jenny MJ, Garrick RA, Behrendt L, Hahn ME, Stegeman JJ. The tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) binds multiple AHRs and induces multiple CYP1 genes via AHR2 in zebrafish. Chem.-Biol. Interact. 2009;181:447–454. doi: 10.1016/j.cbi.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson ME, Jenny MJ, Woodin BR, Hahn ME, Stegeman JJ. Role of AHR2 in the expression of novel cytochrome P450 1 family genes, cell cycle genes, and morphological defects in developing zebrafish exposed to 3,3’,4,4’,5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2007a;100:180–193. doi: 10.1093/toxsci/kfm207. [DOI] [PubMed] [Google Scholar]
- Jönsson ME, Orrego R, Woodin BR, Goldstone JV, Stegeman JJ. Basal and 3,3,'4,4',5-Pentachlorobiphenyl-induced expression of Cytochrome P450 1A, 1B and 1C Genes in Zebrafish. Toxicol. Appl. Pharmacol. 2007b;221:29–41. doi: 10.1016/j.taap.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, Hirokawa T, Akiyama T, Kurosumi M, Poellinger L, Kato S, Fujii-Kuriyama Y. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl. Acad. Sci. USA. 2009;106:13481–13486. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Heiden TC, Spitsbergen J, Heideman W, Peterson RE. Persistent adverse effects on health and reproduction caused by exposure of zebrafish to 2,3,7,8-tetrachlorodibenzo-p-dioxin during early development and gonad differentiation. Toxicol. Sci. 2009;109:75–87. doi: 10.1093/toxsci/kfp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kransler KM, McGarrigle BP, Swartz DD, Olson JR. Lung development in the Holtzman rat is adversely affected by gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2009;107:498–511. doi: 10.1093/toxsci/kfn235. [DOI] [PubMed] [Google Scholar]
- Kubota A, Stegeman JJ, Woodin BR, Iwanaga T, Harano R, Peterson RE, Hiraga T, Teraoka H. Role of zebrafish cytochrome P450 CYP1C genes in the reduced mesencephalic vein blood flow caused by activation of AHR2. Toxicol. Appl. Pharmacol. 2011;253:244–252. doi: 10.1016/j.taap.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mathew LK, Sengupta SS, Ladu J, Andreasen EA, Tanguay RL. Crosstalk between AHR and Wnt signaling through R-Spondin1 impairs tissue regeneration in zebrafish. FASEB. J. 2008;22:3087–3096. doi: 10.1096/fj.08-109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew LK, Simonich MT, Tanguay RL. AHR-dependent misregulation of Wnt signaling disrupts tissue regeneration. Biochem. Pharmacol. 2009;77:498–507. doi: 10.1016/j.bcp.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley AT, Callard GV. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008;9:102. doi: 10.1186/1471-2199-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling and stem cell control. Cell research. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- Obaya AJ, Mateyak MK, Sedivy JM. Mysterious liaisons: the relationship between c-Myc and the cell cycle. Oncogene. 1999;18:2934–2941. doi: 10.1038/sj.onc.1202749. [DOI] [PubMed] [Google Scholar]
- Perlberg ST, Diamant A, Ofir R, Zilberg D. Characterization of swim bladder non-inflation (SBN) in angelfish, Pterophyllum scalare (Schultz), and the effect of exposure to methylene blue. J. Fish Dis. 2008;31:215–228. doi: 10.1111/j.1365-2761.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- Perry SF. Relationships between branchial chloride cells and gas transfer in freshwater fish. Comp. Biochem. Physiol. A. 1998;119:9–16. doi: 10.1016/s1095-6433(97)00411-x. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Prochazkova J, Kabatkova M, Bryja V, Umannova L, Bernatik O, Kozubik A, Machala M, Vondracek J. The interplay of the aryl hydrocarbon receptor and beta-catenin alters both AhR-dependent transcription and Wnt/beta-catenin signaling in liver progenitors. Toxicol. Sci. 2011;122:349–360. doi: 10.1093/toxsci/kfr129. [DOI] [PubMed] [Google Scholar]
- Puga A, Hoffer A, Zhou S, Bohm JM, Leikauf GD, Shertzer HG. Sustained increase in intracellular free calcium and activation of cyclooxygenase-2 expression in mouse hepatoma cells treated with dioxin. Biochem. Pharmacol. 1997;54:1287–1296. doi: 10.1016/s0006-2952(97)00417-6. [DOI] [PubMed] [Google Scholar]
- Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem. Pharmacol. 2009;77:713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman AFM. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Simoneau AR, Liao WX, Yi G, Hope C, Liu F, Li S, Xie J, Holcombe RF, Jurnak FA, Mercola D, Hoang BH, Zi X. WIF1, a Wnt pathway inhibitor, regulates SKP2 and c-myc expression leading to G1 arrest and growth inhibition of human invasive urinary bladder cancer cells. Molecular cancer therapeutics. 2009;8:458–468. doi: 10.1158/1535-7163.MCT-08-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenit. Anom. 2003a;43:123–132. doi: 10.1111/j.1741-4520.2003.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem. Biophys. Res. Commun. 2003b;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Kubota A, Dong W, Kawai Y, Yamazaki K, Mori C, Harada Y, Peterson RE, Hiraga T. Role of the cyclooxygenase 2-thromboxane pathway in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced decrease in mesencephalic vein blood flow in the zebrafish embryo. Toxicol. Appl. Pharmacol. 2009;234:33–40. doi: 10.1016/j.taap.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy AR, Noyes PD, Buhler DR, Di Giulio RT. CYP1B1 knockdown does not alter synergistic developmental toxicity of polycyclic aromatic hydrocarbons in zebrafish (Danio rerio) Mar. Environ. Res. 2008;66:85–87. doi: 10.1016/j.marenvres.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winata CL, Korzh S, Kondrychyn I, Korzh V, Gong Z. The role of vasculature and blood circulation in zebrafish swimbladder development. BMC Dev. Biol. 2010;10:3. doi: 10.1186/1471-213X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winata CL, Korzh S, Kondrychyn I, Zheng W, Korzh V, Gong Z. Development of zebrafish swimbladder: The requirement of Hedgehog signaling in specification and organization of the three tissue layers. Dev. Biol. 2009;331:222–236. doi: 10.1016/j.ydbio.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Vogel C, Boerboom AM, Baechle C, El-Bahay C, Kahl R, Degen GH, Abel J. Regulation of prostaglandin endoperoxide H synthase-2 induction by dioxin in rat hepatocytes: possible c-Src-mediated pathway. Carcinogenesis. 2000;21:2267–2274. doi: 10.1093/carcin/21.12.2267. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Li W, Sciullo E, Newman J, Hammock B, Reader JR, Tuscano J, Matsumura F. Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am. J. Pathol. 2007;171:1538–1548. doi: 10.2353/ajpath.2007.070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfle D, Marotzki S, Dartsch D, Schafer W, Marquardt H. Induction of cyclooxygenase expression and enhancement of malignant cell transformation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Carcinogenesis. 2000;21:15–21. doi: 10.1093/carcin/21.1.15. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Teraoka H, Dong W, Stegeman JJ, Hiraga T. cDNA cloning and expressions of cytochrome P450 1A in zebrafish embryos. J. Vet. Med. Sci. 2002;64:829–833. doi: 10.1292/jvms.64.829. [DOI] [PubMed] [Google Scholar]
- Yang F, Bleich D. Transcriptional regulation of cyclooxygenase-2 gene in pancreatic beta-cells. J. Biol. Chem. 2004;279:35403–35411. doi: 10.1074/jbc.M404055200. [DOI] [PubMed] [Google Scholar]
- Yin A, Korzh S, Winata CL, Korzh V, Gong Z. Wnt signaling is required for early development of zebrafish swimbladder. PloS one. 2011;6:e18431. doi: 10.1371/journal.pone.0018431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin A, Korzh V, Gong Z. Perturbation of zebrafish swimbladder development by enhancing Wnt signaling in Wif1 morphants. Biochim. Biophys. Acta. 2012;1823:236–244. doi: 10.1016/j.bbamcr.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Yin HC, Tseng HP, Chung HY, Ko CY, Tzou WS, Buhler DR, Hu CH. Influence of TCDD on zebrafish CYP1B1 transcription during development. Toxicol. Sci. 2008;103:158–168. doi: 10.1093/toxsci/kfn035. [DOI] [PubMed] [Google Scholar]
- Yoshioka W, Peterson RE, Tohyama C. Molecular targets that link dioxin exposure to toxicity phenotypes. J. Steroid Biochem. Mol. Biol. 2011;127:96–101. doi: 10.1016/j.jsbmb.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeruth G, Pollenz RS. Functional analysis of cis-regulatory regions within the dioxin-inducible CYP1A promoter/enhancer region from zebrafish (Danio rerio) Chem.-Biol. Interact. 2007;170:100–113. doi: 10.1016/j.cbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang Z, Collins JE, Andrews RM, Stemple D, Gong Z. Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung. PloS one. 2011;6:e24019. doi: 10.1371/journal.pone.0024019. [DOI] [PMC free article] [PubMed] [Google Scholar]