Abstract
Using modified oxygen needle microelectrodes and intravital videomicroscopy, measurements were made of tissue oxygen tension (PO2) profiles near cortical arterioles and transmural PO2 gradients in the pial arterioles of the rat. Under control conditions, the transmural PO2 gradient averaged 1.17 ± 0.06 mm Hg/μm (mean ± s.e., n = 40). Local arteriolar dilation resulted in a marked decrease in the transmural PO2 gradient to 0.68 ± 0.04 mm Hg/μm (P < 0.001, n = 38). The major finding of this study is a dependence of the transmural PO2 gradient on the vascular tone of the pial arterioles. Using a model of oxygen transport in an arteriole and experimental PO2 profiles, values of radial perivascular and intravascular O2 fluxes were estimated. Our theoretical estimates show that oxygen flux values at the outer surface of the arteriolar wall are approximately 10−5 mL O2/cm2 per sec, independent of the values of the arteriolar wall O2 consumption within a wide range of consumption values. This also means that PO2 transmural gradients for cerebral arterioles are within the limits of 1 to 2 mm Hg/μm. The data lead to the conclusion that O2 consumption of the arteriolar wall is within the range for the surrounding tissue and O2 consumption of the endothelial layer appears to have no substantial impact on the transmural PO2 gradient.
Keywords: cortical microvessels, O2 transport model, oxygen microelectrodes, tissue PO2 profiles, transmural PO2 gradient
Introduction
Oxygen molecules leaving microvessels diffuse along concentration gradients that are established by the spatial distribution of the vessels and the rate of oxygen consumption in the vascular wall and surrounding tissue. Capillaries are not the only source of oxygen to tissue (Duling and Berne, 1970). Arterioles and venules also supply oxygen to tissue and play a significant role in diffusional O2 exchange between various segments of microvasculature. Extensive experimental studies have shown that arterioles could supply up to 50% (or even above) of all oxygen demand in resting skeletal muscle (Swain and Pittman, 1989; Ellsworth and Pittman, 1990). In cerebral tissue, arterioles supply up to 20% of all consumed oxygen (Vovenko, 1997, 1999).
The role of the wall in microvessels has been the topic of several studies, as it participates in the exchange of gases between blood and tissue. Although some studies point to a very high consumption of O2 by the endothelial and smooth muscle cells of the wall (Shibata et al, 2005; Tsai et al, 1998, 2003), other studies suggest a consumption rate closer to that of the surrounding parenchymal tissue (Golub et al, 2007; Pittman et al, 2005; Vadapalli et al, 2000). Various experimental methods have been developed that make it possible to conduct PO2 measurements in the lumen (intravascularly), or on the outer wall of microvessels and in the surrounding tissue (Wilson et al, 1991; Ivanov et al, 1982; Duling et al, 1979). Using these methods, the transmural gradients of PO2 have been measured on cat pial arterioles (Duling et al, 1979), rat mesenteric arterioles (Tsai et al, 1998), rat cremaster muscle (Shibata et al, 2001, 2005), and other tissues (Santilli et al, 2000; Crawford and Cole, 1985). These data vary significantly, from 1 to 10 mm Hg/μm and above. Such large values of transmural PO2 gradients led to the hypothesis of a very high consumption rate of O2 by endothelium and/or smooth muscle cells (Tsai et al, 1998, 2003). If this hypothesis is validated, it might result in significant revision of the current concept of oxygen transport to tissue and would require taking into consideration additional oxidative metabolism in the neighborhood of the blood–tissue interface.
The main goal of the present study was to test the hypothesis of a large transmural PO2 gradient across the blood–tissue interface. We have studied transmural PO2 gradients on the arterioles of rat brain cortex, a tissue with high oxidative metabolism and, consequently, high blood flow. Using these data, we performed theoretical estimates of O2 consumption in the vascular (arteriolar) wall and estimated transmural PO2 gradients have been assessed. In addition, using oxygen microelectrodes of a special design, we measured transmural PO2 gradients in control and vasodilated pial arterioles. Our experimental results and theoretical estimates show that transmural PO2 gradients for rat cortical arterioles are similar to those in surrounding tissue and are in the range of 1 to 2 mm Hg/μm under control conditions and below 1 mm Hg/μm in dilated vessels.
Materials and methods
Animals and Surgical Procedures
All the experiments were performed in male Wistar rats (Pavlov Institute of Physiology vivarium) weighing 250 to 320 g. The experiments were performed in accordance with ethics regulations of The Pavlov Institute, which are in agreement with the European Communities Council Directive (86/609/EEC) for the care and use of laboratory animals. Animals were initially anesthetized by an intra-peritoneal injection of sodium pentobarbital (60 mg/kg) and then by supplemental doses of 10 to 12 mg/kg per h. They were placed on a Plexiglas platform and the head was fixed using a special teeth rod mounted on a ball-and-socket joint. A tracheostomy was made to ensure proper spontaneous ventilation. A polyethylene catheter (PE-90) was inserted into the femoral artery and vein for collecting blood samples, for gas analysis (ABL-330, Radiometer, Denmark), for measurement of mean arterial pressure, and for blood and solute injections.
A 4 × 8 mm opening was made on the right parietal lobe of the animal’s scull. The dura mater was accurately removed and the exposed brain cortex was superfused with a solution of the following composition (in mmol/L): NaCl 118, KCl 4.5, CaCl2 2.5, KHPO4 1.0, MgSO4 1.0, NaHCO3 25, and glucose 6. The solution was equilibrated with a gas mixture of 5% CO2/5% O2/balance N2 and its pH was adjusted to 7.38±0.05 at 37°C.
Experimental Setup
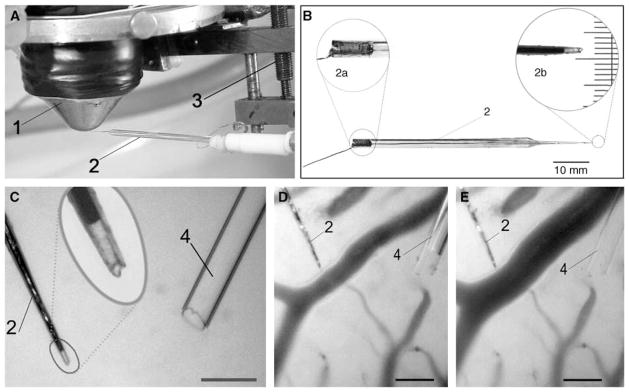
The design and layout of the setup has been described elsewhere (Vovenko, 1999). Briefly, the microscope and 3D-manipulated stage were mounted on a massive metal beam to minimize vibrations and displacements of the microelectrode during PO2 measurements on microvessels. Any displacements of the animal relative to the microscope were performed using the stage under the microscope. A PO2 microelectrode was manipulated by a 3D-micromanipulator that was mounted on the objective of the microscope (Figure 1). The microelectrode tip was positioned at the center of the frontal lens of the objective. A thermostabilizing system maintained a constant temperature (37°C±1°C) of the animal’s body, the objective of the microscope, and the calibration and superfusion solutions.
Figure 1.
Local measurements of oxygen partial pressure using a fine-tip oxygen microelectrode. (A) Tip of the microelectrode (2), mounted on a 3D-micromanipulator (3), is positioned in the center of frontal lens of the contact epiobjective (1) of the microscope. (B) Overview of the polarographic oxygen microelectrode used in the study. 2a—enlarged image of the copper wire fixed relative to a glass body using a small bit of quick-setting glue. 2b—photomicrograph of the microelectrode tip: the tip diameter including glass insulation, 4 μm; recess, 8 μm; scale, 3 μm. (C) Measurements of transmural PO2 gradient in dilated pial arteriole. Oxygen microelectrode (2) with a tip diameter of 4 μm and a recess of 10 μm and a glass micropipette (4) with a tip diameter of 27 μm is shown; scale, 50 μm. (D) The microelectrode tip is positioned on the outer arteriolar wall before applying the vasoactive agent. Arteriolar luminal diameter is 30 μm. (E) The microelectrode tip is positioned on the dilated arteriolar outer wall. Arteriolar luminal diameter is 46 μm; scale, 50 μm.
Visualization
The microvasculature of the brain cortex was visualized using a microscope LUMAM-K1 (LOMO, Russia) equipped with contact lens epiobjectives (×10/0.30, × 20/0.60). For visualization, the frontal lens of the objective was put in immediate contact with the brain tissue (no compression, the gap between lens and brain less than 10 μm). Focusing of the microscope was performed without displacement of the objective relative to tissue using a special lens, moved along the optical axis inside the microscope tube. The microscopic image was viewed on a TV monitor and, at the same time, on a PC monitor using a video camera (PIH-576, Taiwan) and capture card (Pinnacle Deluxe). The linear dimensions (microvascular luminal diameter, thickness of the vascular wall, and distance to the microelectrode tip) were determined during offline analysis using frame capture and a special screen caliper. The error of linear measurements was approximately ±2 μm.
Oxygen microelectrodes
Platinum oxygen microelectrodes with a tip diameter including glass insulation of 3 to 4 μm and a recess depth of 8 to 12 μm were used in the study. The polarographic current in physiologic solution saturated with room air at 37°C ranged from 5 × 10−11 to 9 × 10−11 A. Residual current was negligible. The sensitivity of the microelectrodes to fluid convection was tested by measuring the polarographic current in a stirred and unstirred solution. Convection was produced using a magnetic stirrer (linear velocity of the solution at the microelectrode tip was approximately ~100 mm/s). Oxygen microelectrodes with a recess of l/d = 8 to 10 showed an increase in current during the stirring of less than 2% to 3%, if any. The microelectrodes used in this study revealed a small ‘calibration error’ too, that is, they were not sensitive to variations in O2 diffusion coefficient in the calibration and measurement media (Buerk, 2003; Craw-ford and Cole, 1985; Schneiderman and Goldstick, 1978). The microelectrode was calibrated several times during an experiment. To minimize measurement error before measurements, the electrode tip was inserted into the brain tissue or placed into the venular lumen for several minutes. Then, the electrode was placed into the calibration solution again. This procedure was repeated several times until a stable level of electrode sensitivity to oxygen was attained.
Experimental Protocol
The tissue was allowed to stabilize for a period of at least 10 to 15 mins before the acquisition of PO2 data. In Series 1, measurements of radial tissue PO2 profiles near the walls of rat precortical arterioles were performed. In Series 2, measurements of transmural PO2 gradients were performed on rat pial arterioles. Group 1 of Series 2 consisted of transmural PO2 measurements in control pial arterioles, whereas Group 2 of Series 2 consisted of transmural PO2 measurements on dilated pial arterioles.
Measurements of radial PO2 profiles in the vicinity of precortical arterioles (series 1)
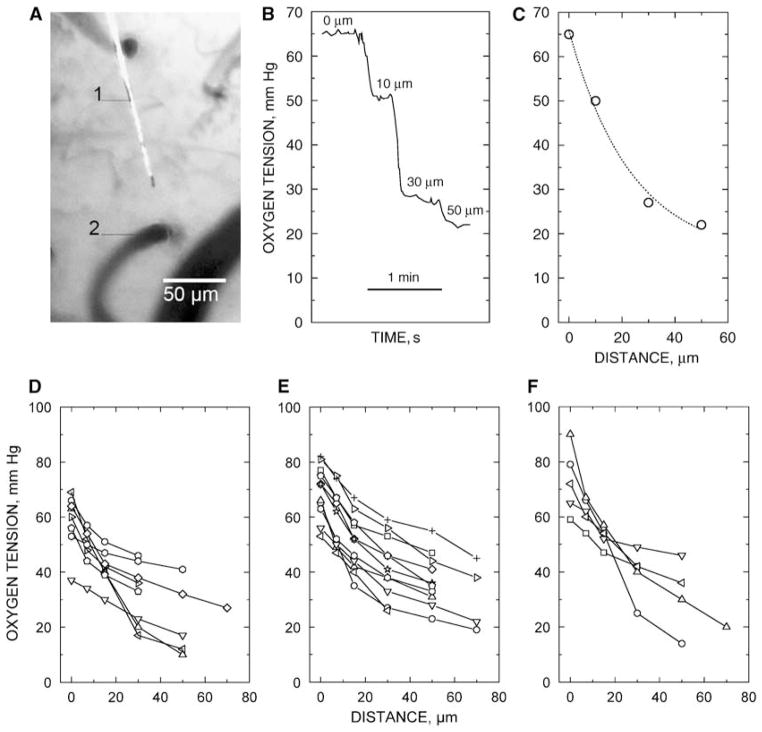
To record a tissue PO2 profile, representing oxygen diffusion across the wall of an arteriole, the PO2 microelectrode was positioned so that the nearest large microvessel (on the course of the electrode track) was at least 70 to 80 μm away. The PO2 microelectrode was positioned perpendicularly to the wall of the selected arteriole. PO2 was then measured at the wall surface and at distances of 10, 20, 30 μm and so on from the vessel wall, sequentially (Figure 2). For reproducible measurements, the PO2 microelectrode was again brought to the surface of the outer wall to recheck the values obtained.
Figure 2.
Measurements of tissue PO2 profiles near precortical arterioles of the rat. (A) The microelectrode tip (1) is 30 μm from the precortical arteriole (2) of luminal diameter 20 μm. Scale, 50 μm. (B) Example of recording of PO2 radial profile near a cortical arteriole; scale, 1 min. (C) Graphical representation of the PO2 profile. (D, E, F) Measurements of radial PO2 profiles on arterioles with a luminal diameter of 10, 20, and 30 μm, respectively. Each symbol represents an individual value. The same symbols on the graphs are independent.
Measurements of PO2 transmural gradients on pial arterioles in control (series 2, group 1)
For assessing the transmural PO2 gradient, it is necessary to know PO2 values on the outer and inner wall of the arteriole and the wall thickness. The transmural PO2 gradient was defined as the difference of intravascular and outer wall PO2 relative to the displacement of the electrode (Δl) defined as the distance from the outer wall surface to the edge of the cell-free plasma layer and red blood cell core in the arteriolar lumen. Δl was measured offline during analysis of the recorded photomicrographs. Δl could be used also as an estimate of the arteriolar wall thickness ΔL (it exceeds wall thickness by the thickness of the cell-free plasma layer) used in the mathematical analysis. Cell-free plasma layer thickness has been measured in arterioles (Kim et al, 2007). To measure the transmural PO2 gradient, the microelectrode tip was positioned on the outer surface of the wall in the midplane of an arteriole. An image of the field was captured and oxygen tension was recorded. The microvessel wall was punctured by quickly moving the microelectrode into the wall using a micromanipulator (Figure S1). Intravascular readings of PO2 were relatively stable during the first 5 to 6 secs after penetration of the vessel. Then, thrombus formation began at the microelectrode tip and a progressive decline of PO2 occurred. Therefore, all intravascular PO2 measurements were obtained during the first 5 to 6 secs after wall puncture, when thrombus size was negligible and it did not disturb blood flow in the arteriole. Then, the microelectrode was withdrawn from the vessel to perform a calibration.
Measurements of transmural PO2 on pial arterioles during local vasodilation (series 2, group 2)
The vasodilator agent (~2 × 10−7 mmol/L sodium nitroprus-side in physiologic solution) was administered into the perivascular zone of the arteriole using a glass micropipette of diameter 25 to 30 μm (Figures 1C–1E). Transmural PO2 measurements on the dilated arteriole were conducted according to the same protocol as above for the control series.
Data Analysis
All data are presented as mean ± s.e. All calculations and graphics were made using the program OriginPro ver. 7.5 (OriginLab Corp.).
Results
Tissue PO2 Profiles (Series 1)
Sixteen male Wistar rats (250 to 280 g body weight) were used in the first series of experiments to measure radial PO2 profiles in the vicinity of precortical arterioles.
Blood gas parameters of systemic blood were as follows: pH = 7.351 ± 0.002; PCO2 = 37.6 ± 1.2 mm Hg; PO2 = 78.7 ± 2.2 mm Hg (n = 19). Mean arterial pressure in the experiments of Series 1 averaged 126 ± 5 mm Hg (n = 57).
Tissue radial PO2 profiles around precortical arterioles of the rat measured using oxygen micro-electrodes are shown in Figures 2D–2F. During measurements, the microelectrode tip was positioned within the brain tissue. The data showed that a marked decrease in tissue PO2 was observed in the region near the arteriolar wall (within a tissue zone of 20 to 30 μm). The PO2 profiles were more shallow farther from the arteriole.
Transmural PO2 Gradients (Series 2)
A total of 31 rats (230 to 250 g body weight) were used in the second series of experiments to explore transmural PO2 gradients in rat pial arterioles under control (Group 1, 17 animals) and vasodilated conditions (Group 2, 14 animals). Blood gas parameters of systemic arterial blood of both groups of animals were as follows: pH = 7.334 ± 0.006, PCO2 = 43.7 ± 0.5 mm Hg, PO2 = 87.3 ± 1.3 mm Hg (n = 34). Mean arterial pressure in Series 2 averaged 136 ± 2 mm Hg (n = 86).
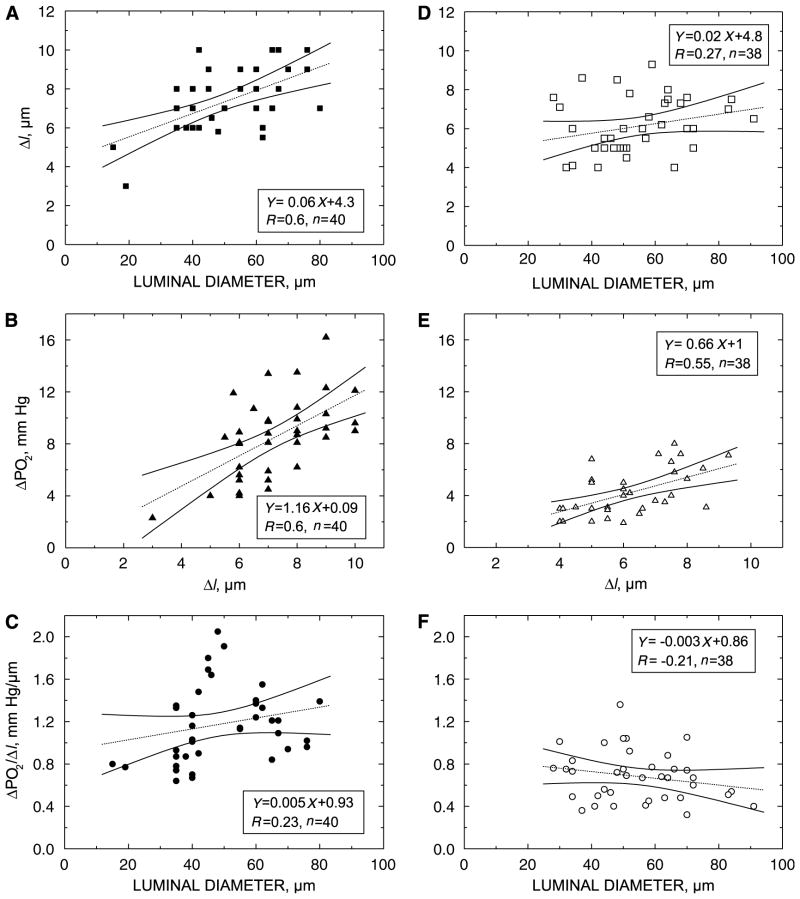
To measure the transmural PO2 gradient, the microelectrode tip was brought close to the outer arteriolar wall and the control level of PO2 was recorded. Then, the microelectrode was moved into the arteriolar lumen and intra-vascular PO2 was recorded. The relationship between Δl and arteriolar luminal diameter is presented in Figure 3A. The relationship between ΔPO2 and the corresponding Δl is presented in Figure 3B. The slope of the regression line in Figure 3B is numerically equal to the transmural PO2 gradient in rat pial arterioles (1.17 ± 0.06 mm Hg/μm, n = 40). Figure 3C shows the relationship of the transmural PO2 gradient and measured arteriolar luminal diameter.
Figure 3.
Results of transmural PO2 measurements in rat pial arterioles during control (A to C) (Series 2, Group 1) and dilation (D to F) conditions (Series 2, Group 2). (A, D) Relationship between Δl and arteriolar luminal diameter. Δl is the distance from the outer wall surface to the edge of cell-free plasma layer and red blood cell core in the arteriolar lumen. Δl could be used as a measure of arteriolar wall thickness (it exceeds the true wall thickness by the depth of cell-free plasma layer). (B, E) Relationship between PO2 increase during penetration of the arteriolar wall (ΔPO2) and the corresponding displacement of the microelectrode (Δl) for control and dilated arterioles, respectively. (C, F) Relationship of the transmural PO2 gradient to the arteriolar luminal diameter for control and dilated arterioles, respectively. R, correlation coefficient; n, number of measurements. 95% confidence intervals for the regression line are shown by solid lines.
The same measurements were performed on dilated arterioles to assess whether vascular tone has an impact on the transmural PO2 gradient in rat pial arterioles. The results of Series 2, Group 2 are presented in Figures 3D–3F. The transmural PO2 gradient in dilated arterioles was 0.68 ± 0.04 mm Hg/μm (P < 0.001, n = 38).
Discussion
We have presented experimental measurements of tissue PO2 profiles around rat cortical arterioles and transmural PO2 measurements in pial arterioles using specially designed fine-tip O2 microcathodes. Our results showed that under control conditions radial and transmural PO2 gradients did not exceed 2 mm Hg/μm. In dilated arterioles, transmural PO2 gradients are ~0.7 mm Hg/μm. Our theoretical estimates showed that oxygen flux values at the outer surface of the arteriolar wall are around 10−5 mL O2/cm2 per sec, independent of the values of the arteriolar wall O2 consumption within a wide range of consumption values. This also means that transmural PO2 gradients for cerebral arterioles are within the limits of 1 to 2 mm Hg/μm.
In this study, attention was focused on O2 diffusion through the arteriolar wall—an interface between blood and tissue. Arterioles do play a significant role in oxygen exchange between these two compartments. Arterioles are powerful sources of O2 to tissue (high PO2, blood flow). Nerve cells located nearby arterioles might favor under the conditions of restricted O2 supply. In addition, arterioles take part in diffusional oxygen exchange of respiratory gases between venules and capillaries. Direct PO2 measurements showed that anatomical capillaries deliver ~60% of all O2 consumed by brain tissue, whereas cortical arterioles of diameter 7 to 20 μm deliver approximately 20% and arterioles of diameter 30 μm and larger deliver less than 10%. The data imply that cerebral microvasculature of caliber ≤20 μm is a primary site of gas transfer between the blood and the tissue (Vovenko, 1997, 1999).
There are a few data in the literature concerning measurements of transmural PO2 gradients in brain arterioles. Duling et al (1979), using Whalen-type oxygen microcathodes in cat pial arterioles, found transmural PO2 gradients of ~0.91 mm Hg/μm. Our measurements of transmural PO2 gradients are smaller than those reported in other tissues using the optical phosphorescence quenching method (Tsai et al, 1998, 2003). This discrepancy might be because of the differences in the tissues studied and differences in the methodology used. Several studies point out that extravascular PO2 measurements using the phosphorescence quenching method might contain a measurement artifact—underestimation of the actual tissue PO2 because of an oxygen consumption artifact (O2 consumption caused by the excitation flash impulse) (Golub and Pittman, 2005).
Shibata et al (2001, 2005), using the phosphorescence quenching method, measured the decrease in PO2 in the arterioles of rat cremaster muscle and found that the PO2 difference was approximately 15 to 20 mm Hg over a distance of 10 to 12 μm, so that the measured transmural PO2 gradient was within 1 to 2 mm Hg/μm. A pronounced PO2 difference (40 to 45 mm Hg) over a distance of 2 μm was found in the rabbit infra-renal aorta using a microelectrode technique (Santilli et al, 2000). This steep decrease in PO2 over such a small distance, however, could be because of tissue compression/distortion during microelectrode penetration of the dense tissue (Crawford and Cole, 1985).
It is worth noting that the oxygen microelectrode technique is probably the only method enabling local PO2 measurements in three different compartments in the brain cortex: tissue (extravascular), vascular wall (perivascular), and vessel lumen (intravascular). The method of phosphorescence quenching is applicable for intravascular (intravascular infusion of phosphor) and extravascular (topical application of phosphor) measurements only, because of the practical impermeability of the blood–brain barrier for extravasation of the albumin-bound phosphorescence probe.
Despite the advantages indicated above, the use of oxygen microelectrodes has a number of limitations. The first limitation is because of the invasive nature of the measurements. The scull is open (no closed cranial window), making the cortical tissue susceptible to the development of edema. However, the duration of the experiments was within 60 to 90 mins, after opening of the skull, and no significant edema was observed during this time.
It is extremely difficult to penetrate the wall of an arteriole of diameter ≤20 to 25 μm, even using thin and specially sharpened microelectrodes (with tip diameter including glass insulation ~3 μm). This is the reason why all our data on transmural gradients were obtained on microvessels of larger caliber (the data in Series 1 on smaller vessels did not involve puncturing the arteriolar wall).
From a methodological point of view, the intra-vascular PO2 measurements represented a serious and difficult task. First of all, the puncture should induce only minimal injury of the arteriolar wall. The microelectrode tip diameter should be small (2 to 4 μm) and the shaft taper of the tip should be ≤3° and be sharpened similarly to a syringe needle (sharpening angle ~15° to 18°). The angle of beveling of the tip plays a critical role in minimizing trauma to the wall (even for microelectrodes with a tip diameter of 3 to 4 μm). In addition, the polaro-graphic current of the microelectrode should not depend on fluid convection (for correct measurements in flowing blood). The microelectrode readings should not be dependent on the O2 permeability coefficient of the measurement and calibration media. Finally, the calibration characteristics of the O2 cathodes should be relatively stable and reproducible. The oxygen microelectrodes used in this study complied with the above requirements.
Dilation of the arteriolar wall resulted in a decrease of the transmural PO2 gradient to 0.68 ± 0.04 mm Hg/μm. This means that the vascular tone and, accordingly, the level of oxidative metabolism of the smooth muscle cells could have an impact on the transmural PO2 gradient. It is possible that endothelial cells also contribute to the observed decrease in oxygen tension in the arteriolar wall. Nevertheless, our data do not provide support for the hypothesis of an important role for the endothelium in the transmural PO2 gradients in arterioles.
Experimental measurements (Vovenko, 1999) yield an intravascular O2 flux Ji of the order of 10−5 mL O2/cm2 per sec (Figure S2). Our theoretical estimates of Ji are consistent with those based on measurements when the PO2 gradients are within 1 to 2 mm Hg/μm. Calculation of Ji in individual vessel segments also supports a value of Ji~10−5 mL O2/cm2 per sec. In addition, a majority of the computed values of Ji in microvessels, compiled by Vadapalli et al (2000), and based on in vivo measurements of longitudinal hemoglobin oxygen saturation or PO2 gradients in arteriolar segments, are ~10−5 mL O2/cm2 per sec, consistent with those based on the observations.
The estimate of O2 flux from the rat cremaster muscle (Shibata et al, 2005) results in Ji values of an order of magnitude higher than that in the present measurements. This estimation is based on morphologic assumptions about vessel length; however, they have reported oxygen gradients between 1 and 2 mm Hg/μm. In their analysis, it was assumed that the entire O2 flux was consumed by the wall and, accordingly, they calculated an O2 consumption in the wall that was 100 times higher than in the tissue.
For the pial arteriolar network in rats, the global estimate also yields a value of Ji = 1.2 × 10−5 mL O2/cm2 per sec (Figure S3). Using morphologic data for the rat mesentery network (Pries et al, 1990), the computed value of Ji is 1.03 × 10−5 mL O2/cm2 per sec. Global estimates of intravascular flux for the pial and mesenteric arteriolar networks in rats support a value of Ji ~ 10−5 mL O2/cm2 per sec. However, global estimates of Ji for the rat spinotrapezius muscle with two sets of morphologic data for that arteriolar network (Engelson et al, 1985) are 3.026 × 10−4 and 9.85 × 10−5 mL O2/cm2 per sec. The foregoing discussion supports theoretical estimates of Ji ~ 10−5 mL O2/cm2 per sec, consistent with the observations when oxygen gradients are within 1 to 2 mm Hg/μm.
In summary, theoretical estimates based on experimental data of tissue PO2 profiles around rat cortical arterioles and direct measurements of transmural PO2 gradients on pial arterioles showed that the decrease in PO2 across the arteriolar wall is within 1 to 2 mm Hg/μm under control conditions and approximately ~0.7 mm Hg/μm under vasodilated conditions. The data presented lead to the conclusion that the O2 consumption of the arteriolar wall is within the range for the surrounding tissue and that the O2 consumption of the endothelial layer, apparently, does not have a substantial impact on the transmural PO2 gradient.
Supplementary Material
Acknowledgments
This study was supported in part by Grants 03-04-49470 and 06-04-49274 from the Russian Basic Research Foundation and by Grant HL18292 from the National Institutes of Health.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
References
- Buerk DG. Recessed oxygen electrodes: getting more than PO2. Adv Exp Med Biol. 2003;510:175–9. doi: 10.1007/978-1-4615-0205-0_29. [DOI] [PubMed] [Google Scholar]
- Crawford DW, Cole MA. Performance evaluation of recessed microcathodes: criteria for tissue PO2 measurement. J Appl Physiol. 1985;58:1400–5. doi: 10.1152/jappl.1985.58.4.1400. [DOI] [PubMed] [Google Scholar]
- Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. Circ Res. 1970;27:669–78. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- Duling BR, Kuschinsky W, Wahl M. Measurements of the perivascular PO2 in the vicinity of the pial vessels of the cat. Pflügers Arch. 1979;383:29–34. doi: 10.1007/BF00584471. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Pittman RN. Arterioles supply oxygen to capillaries by diffusion as well as by convection. Am J Physiol. 1990;258:H1240–3. doi: 10.1152/ajpheart.1990.258.4.H1240. [DOI] [PubMed] [Google Scholar]
- Engelson ET, Skalak TC, Schmid-Schonbein GW. The microvasculature in skeletal muscle I arteriolar network topology. Microvasc Res. 1985;30:29–44. doi: 10.1016/0026-2862(85)90035-4. [DOI] [PubMed] [Google Scholar]
- Golub AS, Barker MC, Pittman RN. PO2 profiles near arterioles and tissue oxygen consumption in rat mesentery. Am J Physiol Heart Circ Physiol. 2007;293:H1097–106. doi: 10.1152/ajpheart.00077.2007. [DOI] [PubMed] [Google Scholar]
- Golub AS, Pittman RN. EATs or not EATs? Letters to the Editor. Am J Physiol Heart Circ Physiol. 2005;289:H1777–79. doi: 10.1152/ajpheart.00503.2005. [DOI] [PubMed] [Google Scholar]
- Ivanov KP, Derry AN, Vovenko EP, Samoilov MO, Semionov DG. Direct measurements of oxygen-tension at the surface of arterioles, capillaries and venules of the cerebral cortex. Pflügers Arch. 1982;393:118–20. doi: 10.1007/BF00582403. [DOI] [PubMed] [Google Scholar]
- Kim S, Kong RL, Popel AS, Intaglietta M, Johnson PC. Temporal and spatial variations of cell-free layer width in arterioles. Am J Physiol Heart Circ Physiol. 2007;293:H1526–35. doi: 10.1152/ajpheart.01090.2006. [DOI] [PubMed] [Google Scholar]
- Pittman RN, Golub AS, Schleicher WF. Rate of decrease of PO2 from an arteriole with arrested flow. Adv Exp Med Biol. 2005;566:257–62. doi: 10.1007/0-387-26206-7_34. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P, Gross JF. Blood flow in microvascular networks—experiments and simulation. Circ Res. 1990;67:826–34. doi: 10.1161/01.res.67.4.826. [DOI] [PubMed] [Google Scholar]
- Santilli SM, Tretinyak AS, Lee ES. Transarterial wall oxygen gradients at the deployment site of an intra-arterial stent in the rabbit. Am J Physiol Heart Circ Physiol. 2000;279:H1518–25. doi: 10.1152/ajpheart.2000.279.4.H1518. [DOI] [PubMed] [Google Scholar]
- Schneiderman G, Goldstick TK. Oxygen electrode design criteria and performance characteristics: recessed cathode. J Appl Physiol. 1978;45:145–54. doi: 10.1152/jappl.1978.45.1.145. [DOI] [PubMed] [Google Scholar]
- Shibata M, Ichioka S, Ando J, Kamiya A. Micro-vascular and interstitial PO2 measurements in rat skeletal muscle by phosphorescence quenching. J Appl Physiol. 2001;91:321–7. doi: 10.1152/jappl.2001.91.1.321. [DOI] [PubMed] [Google Scholar]
- Shibata M, Ichioka S, Kamiya A. Estimating oxygen consumption rates of arteriolar walls under physiological conditions in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2005;289:H295–300. doi: 10.1152/ajpheart.00830.2004. [DOI] [PubMed] [Google Scholar]
- Swain DP, Pittman RN. Oxygen exchange in the microcirculation of hamster retractor muscle. Am J Physiol Heart Circ Physiol. 1989;256:H247–55. doi: 10.1152/ajpheart.1989.256.1.H247. [DOI] [PubMed] [Google Scholar]
- Tsai AG, Friesenecker B, Mazzoni MC, Kerger H, Buerk DG, Johnson PC, Intaglietta M. Microvascular and tissue oxygen gradients in the rat mesentery. Proc Natl Acad Sci USA. 1998;95:6590–5. doi: 10.1073/pnas.95.12.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev. 2003;83:933–63. doi: 10.1152/physrev.00034.2002. [DOI] [PubMed] [Google Scholar]
- Vadapalli A, Pittman RN, Popel AS. Estimating oxygen transport resistance of the microvascular wall. Am J Physiol Heart Circ Physiol. 2000;279:H657–71. doi: 10.1152/ajpheart.2000.279.2.H657. [DOI] [PubMed] [Google Scholar]
- Vovenko EP. Quantitative characteristics of oxygen tension distribution in arterioles, capillaries, and venules of the rat brain cortex in normoxemia. Ross Fiziol Zh Im Sechenova. 1997;83:77–85. (in Russian) [PubMed] [Google Scholar]
- Vovenko EP. Distribution of oxygen tension on the surface of arterioles, capillaries and venules of brain cortex and in tissue in normoxia: an experimental study on rats. Pflügers Arch. 1999;437:617–23. doi: 10.1007/s004240050825. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Pastuszko A, DiGiacomo JE, Pawlowski M, Schneiderman R, Delivoria-Papadopoulos M. Effect of hyperventilation on oxygenation of the brain cortex of newborn piglets. J Appl Physiol. 1991;70:2691–6. doi: 10.1152/jappl.1991.70.6.2691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.