Abstract
BACKGROUND
Many significant microsurgical series of patients with giant aneurysms predate changes in practice during the endovascular era.
OBJECTIVE
A contemporary surgical experience is presented to examine changes in management relative to earlier reports, to establish the role of open microsurgery in the management strategy, and to quantify results for comparison with evolving endovascular therapies.
METHODS
During a 13-year period, 140 patients with 141 giant aneurysms were treated surgically. 100 aneurysms (71%) were located in the anterior circulation, and 41 aneurysms were located in the posterior circulation.
RESULTS
108 aneurysms (77%) were completely occluded, 14 aneurysms (10%) had minimal residual aneurysm, and 16 aneurysms (11%) were incompletely occluded with reversed or diminished flow. 3 patients with calcified aneurysms were coiled after unsuccessful clipping attempts. 18 patients died in the perioperative period (surgical mortality, 13%). Bypass-related complications resulted from bypass occlusion (7 patients), aneurysm hemorrhage due to incomplete aneurysm occlusion (4 patients), or aneurysm thrombosis with perforator or branch artery occlusion (4 patients). 13 patients were worse at late follow-up (permanent neurological morbidity, 9%; mean length of follow-up, 23±1.9 months). Overall, good outcomes (GOS 5 or 4) were observed in 114 patients (81%) and 109 patients (78%) were improved or unchanged after therapy.
CONCLUSION
A heavy reliance on bypass techniques plus indirect giant aneurysm occlusion distinguishes this contemporary surgical experience from earlier ones, and obviates the need for hypothermic circulatory arrest. Experienced neurosurgeons can achieve excellent results with surgery as the “first-line” management approach and endovascular techniques as adjuncts to surgery.
Keywords: bypass, direct clipping, giant aneurysm, indirect aneurysm occlusion, microsurgery
INTRODUCTION
Giant intracranial aneurysms have always been, and remain, among the most difficult cerebrovascular lesions to treat. Surgical therapy has evolved with refinement of microsurgical technique, improvements in instrumentation, application of skull base surgical techniques, and application of anesthetic techniques like hypothermic circulatory arrest and cerebroprotection. Despite these advances, combined surgical morbidity and mortality have remained in the 20% - 30% range for many years, partly due to inherent treatment risks and partly due to merciless pathological anatomy, like wide aneurysm necks, complex arterial branches, intraluminal thrombus, atherosclerotic degeneration of arterial tissues, and adherent perforating arteries. Persistent morbidity with surgical therapy and steady advances in endovascular therapy have encouraged attempts at coiling of giant aneurysms 1, with or without adjunctive techniques like stents or balloon assistance. In addition to coiling techniques, flow diversion and endoluminal reconstruction with devices like Pipeline 2 have been utilized with promising early results, particularly with giant aneurysms located along petrocavernous and paraclinoid segments of the internal carotid artery (ICA), and along the basilar trunk.
Rational decision-making with giant aneurysms requires comparison of safety and efficacy of newer endovascular therapies with published surgical experiences. Many of the most significant series of surgically treated giant aneurysms are decades old and predate changes in surgical practices since the introduction of Guglielmi detachable coils in 1990. Endovascular alternatives have changed indications for open microsurgery and curtailed the use of extreme techniques like hypothermic circulatory arrest 3. Fewer giant aneurysms come to surgical management due to improved radiological imaging and earlier diagnosis of large aneurysms, before they reach giant sizes. Consequently, surgical results from older publications do not reflect the current practice environment. In this report, we reviewed a contemporary surgical experience with giant aneurysms with the following objectives: to examine specific changes in surgical management relative to early reports; to better establish the role of open microsurgery in the management strategy; and to quantify surgical results for comparison with evolving endovascular therapies.
METHODS
Patients
This study was approved by the University of California, San Francisco Committee on Human Research and conducted in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations. Giant aneurysms were defined as having a diameter ≥ 25 mm. With thrombotic aneurysms whose intraluminal diameter on angiography was less than overall aneurysm diameter, axial computed tomography and/or magnetic resonance imaging were used to measure aneurysm size. Patients with giant intracranial aneurysms who were treated microsurgically were identified from the prospectively maintained Vascular Neurosurgery database. Operative reports, inpatient charts, angiographic studies, magnetic resonance imaging, computed tomographic imaging, and outpatient clinic data were analyzed retrospectively.
During a 13-year period from September 1997 to March 2010, 2455 aneurysms were treated microsurgically in 1913 patients by the senior author (MTL). Of these patients, 140 patients had 141 giant aneurysms (5.7% of all aneurysms). There were 90 women (64%) and 50 men (36%), with a mean age of 54 years (range, 1 – 85 years).
Twenty-three patients presented with subarachnoid hemorrhage (16%). Hunt-Hess grades were: grade I, 5 patients; grade II, 5 patients; grade III, 6 patients; and grade IV, 7 patients. All 19 patients with cavernous ICA aneurysms presented with cranial neuropathies in the form of cavernous sinus syndrome. Six patients presented with recurrent aneurysms after previous endovascular coiling, and one patient presented with recurrent aneurysm after previous microsurgical clipping.
Aneurysms
141 giant aneurysms were identified in 140 patients. 100 aneurysms (71%) were located in the anterior circulation, the most common sites being the middle cerebral artery (MCA, 23 aneurysms, 16.3%), cavernous ICA (19 aneurysms, 13.5%), and supraclinoid ICA (18 aneurysms, 12.8%) (Table 1). 41 aneurysms were located in the posterior circulation, the most common sites being the basilar bifurcation (14 aneurysms, 9.9%) and basilar trunk (9 aneurysms, 6.4%) (Table 1). Mean aneurysm diameter was 29 mm (range, 25 – 86 mm). 21 patients had additional aneurysms, 28 in total. One of these patients had two giant aneurysms.
Table 1.
Location giant aneurysms. Abbreviations: ICA = internal carotid artery; PCoA = posterior communicating artery; MCA = middle cerebral artery; ACoA = anterior communicating artery; PCA = posterior cerebral artery; SCA = superior cerebellar artery; PICA = posterior inferior cerebellar artery.
| Anterior Circulation | N | % |
|---|---|---|
| Cavernous ICA | 19 | 13.5% |
| Supraclinoid ICA | 18 | 12.8% |
| Ophthalmic | 15 | 10.6% |
| Superior Hypophyseal | 5 | 3.5% |
| PCoA | 5 | 3.5% |
| MCA | 23 | 16.3% |
| ACoA | 13 | 9.2% |
| Pericallosal | 2 | 1.4% |
|
| ||
| Posterior Circulation | N | % |
|
| ||
| Basilar Bifurcation | 14 | 9.9% |
| PCA | 7 | 5.0% |
| SCA | 2 | 1.4% |
| Basilar Trunk | 9 | 6.4% |
| PICA | 4 | 2.8% |
| Vertebral Artery | 5 | 3.5% |
| Total | 141 | |
Surgical Management
Decisions to treat microsurgically or endovascularly were made on an individual basis in conjunction with our multidisciplinary team, which included neurointerventional radiologists and neurovascular neurologists. Aneurysm location, morphology, presenting neurological condition, medical co-morbidities, predicted treatment risks, preferences of the treating team, and ultimately, preferences of the patient and family were considered in this decision. Exclusion criteria included Hunt-Hess grade V presentation, aneurysm calcification, location on the basilar trunk or vertebrobasilar junction, advanced age, significant anesthetic or surgical risks, and strong patient or family preferences against open surgery.
Giant aneurysms were exposed using standard site-appropriate surgical approaches, including 70 pterional craniotomies, 54 orbitozygomatic-pterional craniotomies, 13 far lateral-suboccipital craniotomies, 3 bifrontal craniotomies, and 1 torcular craniotomy. Direct aneurysm occlusion with conventional clipping of the neck was the primary treatment strategy, sometimes requiring temporary trapping, thrombectomy, and clip reconstruction. Indirect aneurysm occlusion was used as the alternative treatment strategy when direct neck clipping was not possible or considered too risky. Indirect aneurysm occlusion consisted of clipping the parent artery (proximal or distal occlusion), bypass with clipping the parent artery (proximal or distal occlusion, or trapping), or bypass with endovascular occlusion (proximal parent artery occlusion or aneurysm occlusion).
Balloon test occlusion (BTO) was used to select 26 patients for aneurysm management with a bypass, all of them with cavernous or supraclinoid ICA aneurysms. Ten patients failed the test with balloon inflation alone, and 16 patients failed with additional hypotensive challenge (lowering mean arterial pressure with nitroprusside drip by 20 mmHg, or 25% of mean arterial pressure, whichever was greater). Failed BTO was used as an indication for bypass. High-flow bypass was used in patients who failed BTO immediately, and low-flow bypass was used in patients who failed BTO after hypotensive challenge. The decision to perform a bypass with aneurysms in other locations was based on the aneurysm’s unclippability and patients’ angiographic anatomy (presence or absence of collateral circulation from the circle of Willis or leptomeningeal connections).
Adequacy of treatment and patency of parent vessels was analyzed intraoperatively using intraoperative angiography, indocyanine green (ICG) fluorescence videoangiography, and/or Doppler flow measurements.
Outcomes
Aneurysm occlusion was classified using postoperative angiography as: complete (no residual aneurysm), minimal residual aneurysm (small neck remnant or dog-ear), or incomplete (>5% of the original aneurysm lumen remaining). Bypass patency was also assessed angiographically. Aneurysm treatment failure was defined as post-treatment growth of residual aneurysm documented by angiography, or post-treatment aneurysm rupture.
Neurological outcomes were assessed using the Glasgow Outcome Score (GOS). A clinical nurse or clinician not directly involved in the care of these patients performed all outcome assessments preoperatively, early postoperatively (6 weeks), and at late follow-up. Preoperative neurologic condition was used as a reference point, and patient outcomes were expressed in terms of changes from this baseline (improved, unchanged, worse, or dead). Thus, asymptomatic patients who remain without neurologic deficits were classified as unchanged.
Statistical Analysis
Survival analyses for durability of surgical aneurysm repair were performed using the Kaplan-Meier method with group comparisons using the log-rank test. Binary variables were compared using Pearson’s χ2 test. Continuous variables were compared using an independent samples t-test. Continuous variables are presented as mean±standard error. Statistical tests were considered significant when p<0.05 after correcting for multiple comparisons using the Bonferroni method.
Given the potential confounding effect of fusiform morphology on the outcomes of posterior circulation aneurysms, we performed logistic regression to further assess for the impact of posterior circulation aneurysm location on the rate of ischemic injuries. Univariate analysis was used to identify covariates which might affect the rate of ischemic neurologic deficits in these patients. Binary and categorical variables were compared using Pearson’s χ2 test, or the χ 2 test for trend, respectively. Odds ratios on multivariate analysis reflect the risk of developing ischemic neurologic at the time of surgery. The goodness of fit of the regression model was confirmed by demonstrating a non-significant p-value on the Hosmer-Lemeshow test 4, 5.
RESULTS
Surgical Treatment
Overall, 141 giant aneurysms were treated in 140 patients (Table 2). Less than half (66 aneurysms, 46%) were treated directly with neck clipping and more than half (72 aneurysms, 51%) were treated indirectly with parent artery occlusion with or without bypass. Thrombectomy was performed with 34 aneurysms (24%) to facilitate direct clipping or to decompress brain or cranial nerves after direct clipping or trapping. Parent artery occlusion was performed surgically by clipping the proximal afferent artery (19 aneurysms), clipping the distal efferent artery or arteries (6 aneurysms), or complete trapping (24 aneurysms). Parent artery occlusion was performed endovascularly by coiling the proximal artery or aneurysm (23 aneurysms).
Table 2.
Surgical management of giant aneurysms
| Surgical Treatment | N | % |
|---|---|---|
| Direct Aneurysm Occlusion | ||
| Neck Clipping | 64 | 45% |
| Neck Clipping + Bypass | 2 | 1% |
| Indirect Aneurysm Occlusion | ||
| Parent Artery Clipping | 20 | 14% |
| Parent Artery Clipping + Bypass | 29 | 21% |
| Endovascular Occlusion + Bypass | 23 | 16% |
| Exploration (calcified) → Coiling | 3 | 2% |
| Thrombectomy | 34 | 24% |
| Parent Artery Clipping | ||
| Proximal Occlusion | 19 | |
| Distal Occlusion | 6 | |
| Trapping | 24 | |
| Hypothermic Circulatory Arrest | 4 | 3% |
Bypass was part of the treatment with 54 aneurysms (38%). The external carotid artery-to-MCA bypass was the most common high-flow bypass (14 aneurysms, 26% of all bypasses), and the superficial temporal artery-to-MCA bypass was the most common low-flow bypass (11 aneurysms, 20%). Intracranial-intracranial bypasses were used with 15 aneurysms (28% of all bypasses), and included in situ bypasses (2), reimplantation of efferent arteries (2), aneurysm excision with re-anastomosis of parent artery (3), and intracranial bypass grafts (8). Radial artery grafts were used in 10 patients and saphenous veins were used in 21 patients.
Three aneurysms were densely calcified at the base and could not be treated surgically. These patients were treated subsequently with coiling.
Aneurysm Outcomes
108 of 141 aneurysms (77%) were completely occluded with no residual aneurysm or neck remnant. 14 aneurysms (10%) had minimal residual aneurysm after clipping (small neck remnant or dog-ear) and further treatment was deemed unnecessary. 16 aneurysms (11%) were incompletely occluded after parent artery clipping with or without bypass (>5% of the original aneurysm lumen remaining). Postoperative angiography demonstrated reduced or reversed flow through the aneurysm, new intraluminal thrombosis, and decreased luminal size. The 3 calcified aneurysms were coiled incompletely or with minimal residual.
Complete aneurysm obliteration was achieved in 79% of cases (52/66) in which direct clipping was performed, and in 72% of cases (56/72) in which indirect aneurysm occlusion was performed. Bypass patency was confirmed in 47 of 54 bypasses (87%), with 7 bypasses occluding acutely (13%). Rates of aneurysm occlusion varied with location, with the lower rates with giant basilar trunk, posterior cerebral artery (PCA), basilar bifurcation, and MCA aneurysms. Kaplan-Meier analysis demonstrated 30 month aneurysm control rates of 96%, 93%, and 84% for completely occluded, minimally residual, and incompletely occluded giant aneurysms, respectively (log rank p=0.05) (Figure 1).
Figure 1.
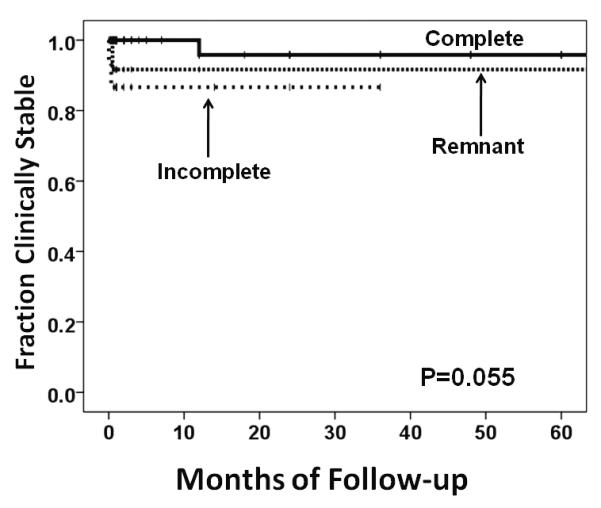
Kaplan-Meier curves depicting the post-operative durability of aneurysm control of complete occluded, minimally residual, and incompletely occluded giant aneurysms.
Five patients required additional treatment, 3 for recurrent aneurysms and 2 for incompletely occluded aneurysms (retreatment rate, 3.5%). One thrombotic P1-P2 junction aneurysm recurred 8 months after thrombectomy and clip reconstruction; the initially preserved posterior cerebral artery (PCA) required trapping with MCA-PCA bypass. A similar PCA aneurysm in another patient was clip occluded proximally, preserving PCoA to supply distal PCA flow; PCoA recanalized the aneurysm, which was treated with endovascular coiling. A dysplastic basilar bifurcation aneurysm was clipped directly with no residual aneurysm postoperatively; 2-year follow-up angiography demonstrated recurrent thrombotic aneurysm at the right P1 origin that was coiled. Large PCoA collaterals enabled one dolichoectatic basilar trunk aneurysm to be treated with proximal clip occlusion; although luminal filling was markedly reduced post-operatively, persistent aneurysm filling at 6-month follow-up required coiling. Another dolichoectatic vertebrobasilar aneurysm underwent VA clip occlusion and VA-SCA bypass with intra-aneurysmal thrombosis; she underwent contralateral endovascular VA occlusion when the aneurysm enlarged two years later.
Patient Outcomes
18 patients died in the perioperative period (surgical mortality, 13%) (Table 3). Eight patients died of ischemic complications, which included perforator infarctions associated with basilar artery aneurysms (6 patients), an intraoperative ICA tear that required occlusion (1 patient), and multiple embolic infarctions that were probably cardiogenic (1 patient). Six patients died of hemorrhagic complications, which included 3 postoperative hemorrhages, one hemorrhagic infarction resulting from bypass occlusion, one preoperative aneurysm re-rupture, and one intraoperative aneurysm rupture. Two patients died of medical complications, including one myocardial infarction with cardiac arrest and one coumadin-related subdural hematoma. Two patients failed to improve despite uncomplicated treatment and support was withdrawn.
Table 3.
Patient outcomes after surgical intervention. Abbreviations: GOS = Glasgow outcome scale; NA = not applicable.
| Preoperative | Early Postoperative | Late Follow-up | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| GOS 5 | 90 | 64% | 83 | 59% | 93 | 66% |
| GOS 4 | 28 | 20% | 14 | 10% | 21 | 15% |
| GOS 3 | 22 | 16% | 22 | 16% | 6 | 4% |
| GOS 2 | 0 | 0% | 3 | 2% | 2 | 1% |
| Dead | 0 | 0% | 18 | 13% | 18 | 13% |
| Total | 140 | 140 | 140 | |||
|
| ||||||
| Improved | NA | NA | 6 | 4% | 22 | 16% |
| Unchanged | NA | NA | 101 | 72% | 87 | 62% |
| Worse | NA | NA | 15 | 11% | 13 | 9% |
| Dead | NA | NA | 18 | 13% | 18 | 13% |
| Total | NA | NA | 140 | 140 | ||
Complications related to bypass resulted from bypass occlusion (7 patients), aneurysm hemorrhage due to incomplete aneurysm occlusion (4 patients), or aneurysm thrombosis with perforator or branch artery occlusion (4 patients). Bypass occlusion resulted in MCA territory infarctions in 2 patients, focal ischemic deficits in 3 patients, and no sequela in 2 patients. Aneurysm re-hemorrhage after bypass occurred after proximal clipping in 1 patient, before endovascular occlusion in 1 patient, after endovascular occlusion from a previously unruptured aneurysm in 1 patient, and after endovascular occlusion while on heparin in 1 patient. Intraluminal aneurysm thrombosis occluded perforators on the basilar trunk in 3 patients with basilar trunk aneurysms and the anterior choroidal artery in 1 patient with an ICA bifurcation aneurysm.
Non-neurologic neurosurgical complications occurred in 7 patients and included five wound infections treated with surgical debridement and antibiotics, and two epidural hematomas treated with surgical evacuation. Medical complications occurred in 6 unruptured aneurysm cases and included two pulmonary emboli, one deep venous thrombosis, and three cases of symptomatic tachyarrythmia, none of which were fatal or caused long term morbidity.
15 patients (11%) were neurologically worse at early follow-up 6 weeks after surgery, but 13 patients (9%) were worse at late follow-up (permanent neurological morbidity, 9%). Overall, good outcomes (GOS 5 or 4) were observed in 114 patients (81%) and poor outcomes (GOS 3 or 2) were observed in 8 patients (5%) (Table 3). Relative to preoperative neurological baseline, 109 patients (78%) were improved or unchanged after therapy. The mean length of post-operative follow-up for all patients was 23±1.9 months.
Patients with giant posterior circulation aneurysms experienced higher rates of ischemic injury and neurologic complications than those with giant anterior circulation aneurysms. Posterior circulation location is an independent risk factor for ischemic injury, controlling for aneurysm morphology (OR 2.86, 95% CI 1.2-7.1, p=0.24). Patients with unruptured giant anterior circulation aneurysms were significantly more likely to have the same or improved neurological function at last follow-up than those treated for unruptured posterior circulation aneurysms (86% vs. 53%, χ2 p=0.007). In general, SAH patients had worse outcomes: with ruptured giant aneurysms, 61% were improved or unchanged, 52% had good outcomes (GOS 5 – 4), and 39% died; with unruptured giant aneurysms, 82% were improved or unchanged, 84% had good outcomes, and 8% died.
DISCUSSION
Trends in Surgical Management
A heavy reliance on bypass techniques (54 bypasses, 38% of aneurysms) distinguishes this contemporary surgical experience with 141 giant aneurysms from earlier ones (Table 4). The increased use of bypasses reflects the impossibility of safely clipping many giant aneurysms. Direct clipping remains the preferred surgical technique for aneurysm occlusion, but it is prevented by dolichoectatic morphology, aberrant branch anatomy, atherosclerotic aneurysm neck, intraluminal thrombus, and in some cases, previous coil deployment. Our experience confirmed that less than half of giant aneurysms (66 aneurysms, 47%) were clippable.
Table 4.
Published experiences with surgical and endovascular treatment of giant aneurysms.
| Author | Year | Patients | Complete Occlusion | Bypass | Excellent/Good | Fair/Poor | Mortality | Morbidity | F/U | Retreatment | Rehemorrhage | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | % | (yrs) | % | % | |||||||
| Endovascular Coiling | ||||||||||||
| Higashida | 1990 | 39 | 63% | 63% | 0/39 | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a |
| Gobin | 1996 | 9 | 5/9 | 56% | 0/9 | 100% | 0% | 0% | 11% | 0.5 | 33% | 0% |
| Gruber | 1999 | 28 | 17/28 | 61% | 0/28 | 73% | 18% | 3.3 | 82% | 4% | ||
| Tateshima | 2000 | 10 | 0/10 | 0% | 0/10 | n/a | n/a | n/a | n/a | 2.6 | 20% | 20% |
| Hallacq | 2002 | 5 | 1/5 | 20% | 0/5 | 60% | 20% | 20% | 20% | 1.0 | 0% | 0% |
| Sluzewski | 2003 | 29 | 5/29 | 17% | 0/29 | 75% | 4% | 21% | 10% | 3.1 | 55% | 7% |
| Henkes | 2004 | 47 | 10/47 | 21% | 0/47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Kolasa | 2004 | 7 | 4/7 | 57% | 0/7 | 85% | 14% | 0% | n/a | n/a | n/a | n/a |
| Murayama | 2006 | 33 | 19/77 | 25% | 0/33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Jahromi | 2008 | 39 | 14/39 | 36% | 0/39 | 63% | 8% | 29% | 20% | 1.3 | 54% | 5% |
| Shi | 2009 | 9 | 9/9 | 100% | 6/9 | 78% | 11% | 11% | 11% | 1.0 | 0% | 0% |
| Lylyk | 2009 | 8 | 4/6 | 67% | 0/8 | n/a | n/a | n/a | 0% | 0.5 | 0% | 0% |
| Microsugical Clipping | ||||||||||||
|
| ||||||||||||
| Peerless, Drake | 1982 | 118 | 97/118 | 82% | 0/118 | 58% | 26% | 14% | n/a | n/a | n/a | n/a |
| Kodama, Suzuki | 1982 | 49 | n/a | n/a | n/a | 61% | 16% | 22% | n/a | n/a | n/a | n/a |
| Yasargil | 1984 | 30 | 26/30 | 87% | 6/30 | 67% | 23% | 10% | n/a | n/a | 0% | 3% |
| Hosobuchi | 1985 | 82 | 80/82 | 98% | 15/82 | 84% | 9% | 7% | 38% | 10.0 | 0% | 0% |
| Heros | 1986 | 25 | 25/25 | 100% | n/a | 72% | 12% | 16% | n/a | n/a | n/a | n/a |
| Sundt | 1990 | 315 | 310/315 | 98% | 81/315 | 80% | 6% | 15% | n/a | n/a | n/a | n/a |
| Ausman | 1990 | 62 | 62/62 | 100% | 23/62 | 84% | 11% | 5% | n/a | n/a | n/a | n/a |
| Tamaki | 1991 | 4 | 4/4 | 100% | 0/4 | 100% | 0% | 0% | 0% | n/a | n/a | n/a |
| Lawton, Spetzler | 1995 | 136 | 132/136 | 97% | 40/136 | 85% | 9% | 6% | 11% | 2.2 | 1% | 0% |
| Shibuya, Sugita | 1996 | 29 | n/a | n/a | n/a | 84% | 7% | 8% | n/a | n/a | n/a | n/a |
| Kattner | 1998 | 29 | 29/29 | 100% | 1/29 | 87% | 10% | 3% | 20% | 7.0 | 0% | 0% |
| Samson | 1999 | 44 | 94% | 94% | n/a | n/a | n/a | n/a | n/a | 0.5 | 0% | 0% |
| Osawa | 2001 | 12 | n/a | n/a | 0/12 | 50% | 16% | 33% | n/a | n/a | 0% | 0% |
| Lawton | 2002 | 28 | 28/28 | 100% | 1/28 | 75% | 11% | 14% | 5% | 1.6 | 0% | 0% |
| Jafar | 2002 | 29 | 29/29 | 100% | 29/29 | 93% | 3% | 3% | 10% | 5.0 | 0% | 0% |
| Lozier | 2004 | 19 | 6/16 | 38% | 2/19 | 36% | 47% | 16% | 89% | 7.4 | 0% | 0% |
| Gonzales | 2004 | 8 | 8/8 | 100% | 1/8 | 63% | 38% | 0% | 50% | 1.0 | 0% | 0% |
| Kolasa | 2004 | 13 | 13/13 | 100% | 0/13 | 76% | 23% | 0% | n/a | n/a | n/a | n/a |
| Krisht | 2007 | 11 | 11/11 | 100% | 0/11 | 88% | 12% | 2% | 27% | 0.5 | 0% | 0% |
| Hauck | 2008 | 62 | 56/62 | 90% | 9/62 | 68% | 32% | 15% | 42% | 1.0 | 0% | 3% |
| Sharma | 2008 | 181 | 106/118 | 90% | 11/181 | 86% | 5% | 9% | 12% | n/a | 0% | 0% |
| Cantore | 2008 | 99 | 99/99 | 100% | 41/99 | 89% | 3% | 8% | 3% | 8.5 | 1% | 1% |
| Xu | 2009 | 51 | 51/51 | 100% | 0/51 | 84% | 14% | 2% | n/a | 0.5 | n/a | n/a |
| Sano | 2010 | 109 | 109/109 | 100% | 0/109 | 63% | 16% | 22% | 4% | n/a | 0% | 0% |
| Current study | 2010 | 140 | 118/140 | 84% | 52/140 | 80% | 7% | 13% | 10% | 1.9 | 1% | 1% |
| Combined Endovascular-Surgical Treatment | ||||||||||||
|
| ||||||||||||
| Hacein-Bey | 1998 | 5 | 4/5 | 80% | 1/5 | 80% | 20% | 0% | 0% | 1.9 | 0% | 0% |
| Arnautovic | 1998 | 8 | 8/8 | 100% | 0/8 | 87% | 13% | 0% | 13% | 2.0 | 0% | 0% |
| Ponce | 2004 | 8 | n/a | n/a | 5/8 | 76% | 0% | 25% | 38% | 1.5 | 0% | 0% |
The failure of conventional clipping requires the use of techniques to indirectly occlude the aneurysm or the use of adjuncts that facilitate direct clipping. Deep hypothermic circulatory arrest is one such adjunct that may transform an unclippable aneurysm into a clippable one by collapsing it, eliminating risk of intraoperative rupture, and permitting aggressive manipulation, even entrance into the aneurysm to remove thrombus and create a supple neck. Giant aneurysms, and in particular those in the posterior circulation, are the most frequent indication for hypothermic circulatory arrest. In the Columbia University experience with 66 procedures in 66 patients, 57 were performed for giant aneurysms 3. In the Barrow Neurological Institute experience between 1985 and 1994 with 62 procedures in 60 patients, 43 were performed for giant aneurysms 6. This adjunct increases the rate of direct clipping, which was 62% in the BNI giant aneurysm experience, with hypothermic circulatory arrest applied in 23% of patients. Direct clipping rates are lower without hypothermic circulatory arrest: 39% in Drake’s experience with posterior circulation giant aneurysms and 49% in Sundt’s overall experience 7.
Deep hypothermic circulatory arrest incurs significant operative morbidity 3. Cannulation of the femoral vessels can cause dissections, occlusions, and compromise of distal circulation 3, 8, 9. Prolonged circulatory arrest is associated with cerebral ischemic injury and poor neurological outcomes 3. Heparinization, slowing of the coagulation cascade by hypothermia, and trauma to red blood cells and platelets by the cardiopulmonary bypass pump contribute to postoperative bleeding complications 3. In the early BNI experience, initial surgical mortality was 8.3% and permanent treatment-associated neurological morbidity was 13.3% 9. As indications narrowed to the most complicated aneurysms, associated risks increased further. The most recent description of the BNI experience in 103 patients and 104 procedures reported 14 perioperative deaths (13.3%) and 9 later deaths (8.6%), for a cumulative mortality rate of 22%. The combined rate of permanent treatment-related morbidity and mortality was 32% 10.
These risks have resulted in declining use of hypothermic circulatory arrest. During a 15-year period at Columbia University, the number of procedures decreased from 34 during the first 5 years to 12 during the last 5 years. Although experiences at other institutions are unpublished, this trend appears widespread (see comments in 3). Our giant aneurysm experience used hypothermic circulatory arrest in just 4 patients (3%).
Indirect aneurysm occlusion with or without a bypass has become a more acceptable alternative, with indirect occlusion consisting of proximal occlusion, distal occlusion, or trapping. This strategy has important advantages. The bypass can be performed with predictable ischemia times, cerebroprotection, and relatively low complication rates. The dangers of direct attack are avoided, including perforator dissection and preservation around clips. Clips are applied to afferent and efferent arteries, avoiding pathological tissues at the neck that can cause clip slippage, intraoperative rupture, and branch artery occlusions. Endovascular techniques can be utilized for indirect aneurysm occlusion, allowing the adequacy of a surgical bypass to be tested before permanent occlusion and also allowing for staged aneurysm occlusion with safe use of heparization or optimization of hemodynamics in the ICU.
Complications Associated with Bypass and Indirect Aneurysm Occlusion
Despite its advantages, indirect aneurysm occlusion introduces unique complications related to bypass graft occlusion and aneurysm thrombosis. Bypass and indirect aneurysm occlusion caused thrombotic occlusion of perforators or branch arteries in 4 patients (7%) with flawless bypasses (Figure 2). These surgical maneuvers are intended to eliminate pathological wall shear stress, high pressure variations, and turbulence that initiate aneurysm development and growth. Malignant hemodynamics are replaced with diminished or reversed intra-aneurysmal flows with more normal wall shear stress. These post-surgical hemodynamics reduce the risk of aneurysm rupture and initiate aneurysm thrombosis that is considered protective. However, unlike non-giant aneurysms that can be trapped safely, giant aneurysm occlusion is often deliberately incomplete because of the presence of perforators or other branch arteries that would otherwise be trapped. Therefore, thrombosis initiated by bypass and aneurysm occlusion can potentially occlude these same arteries too. Giant aneurysm thrombosis is difficult to control temporally or spatially, and can transform a giant sac into a thrombotic and sometimes serpentine aneurysm that can obliterate perforators or small branch arteries at its base (Figure 3).
Figure 2.
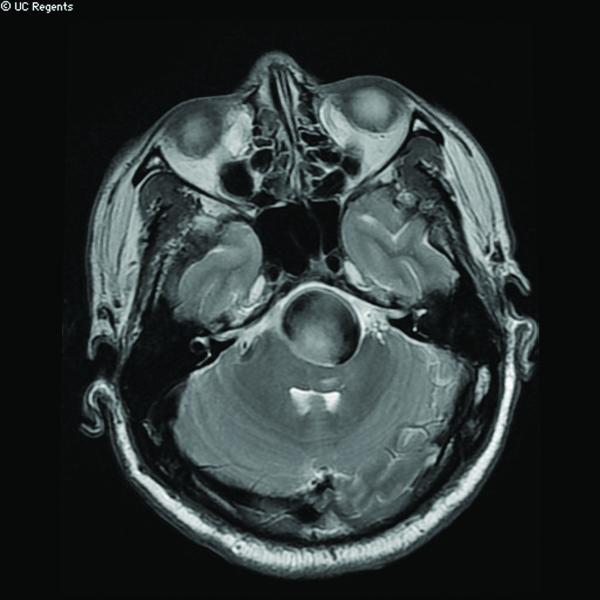
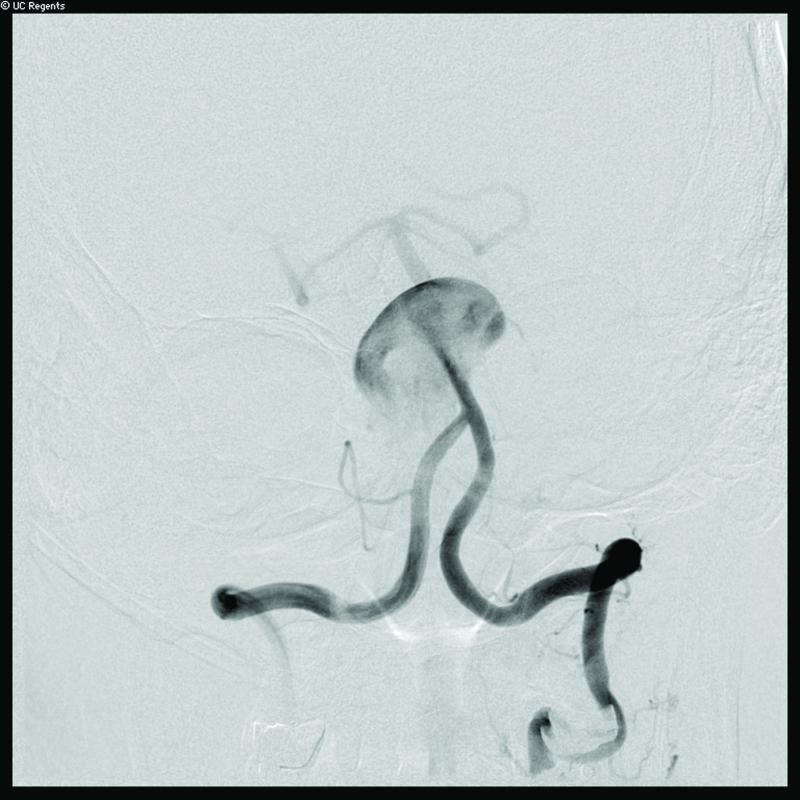
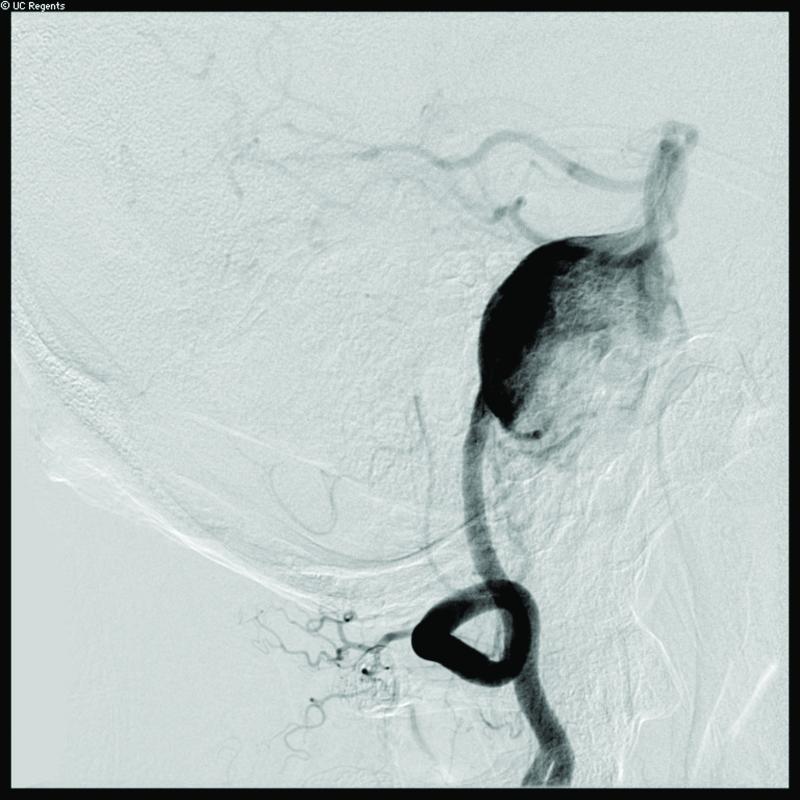
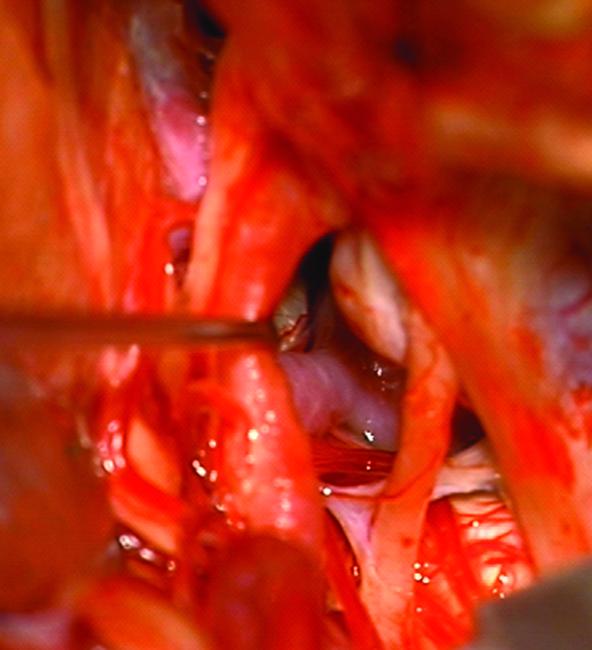
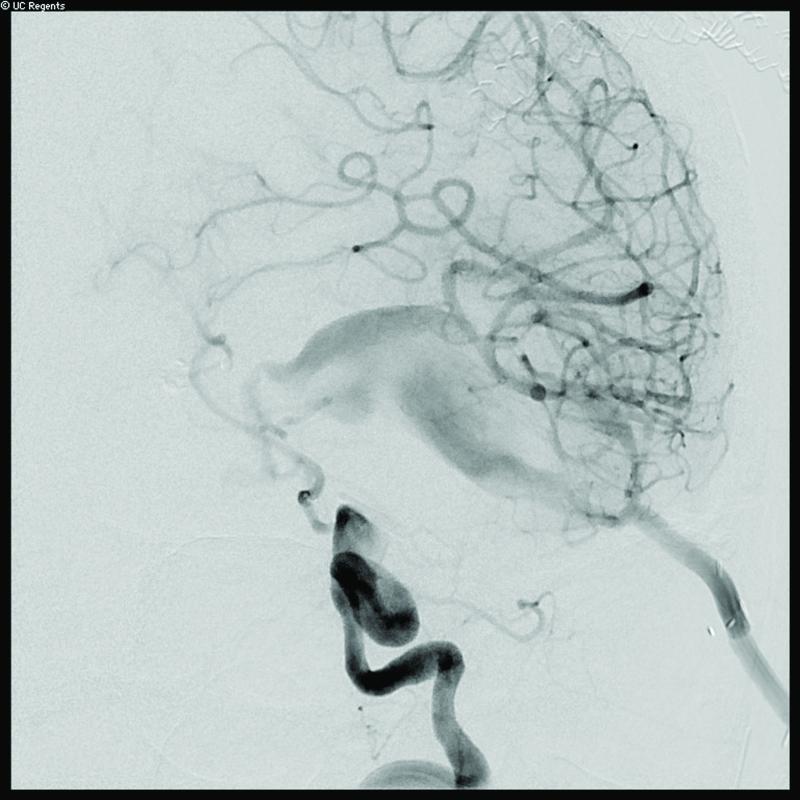
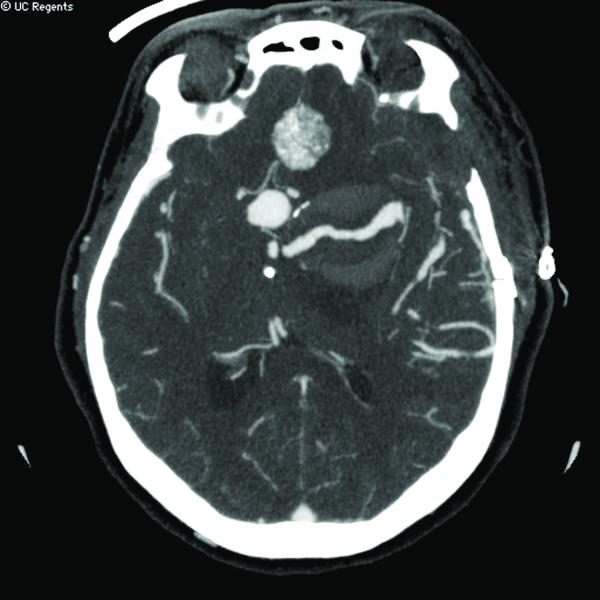
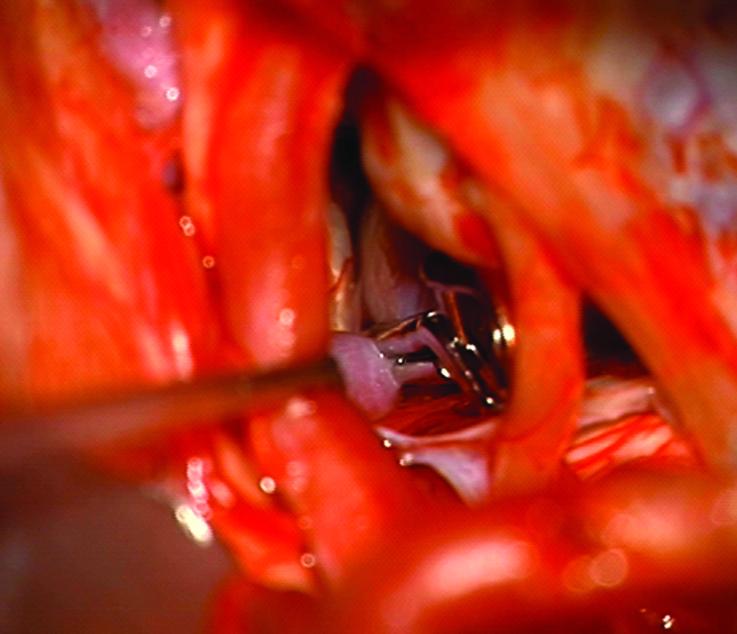
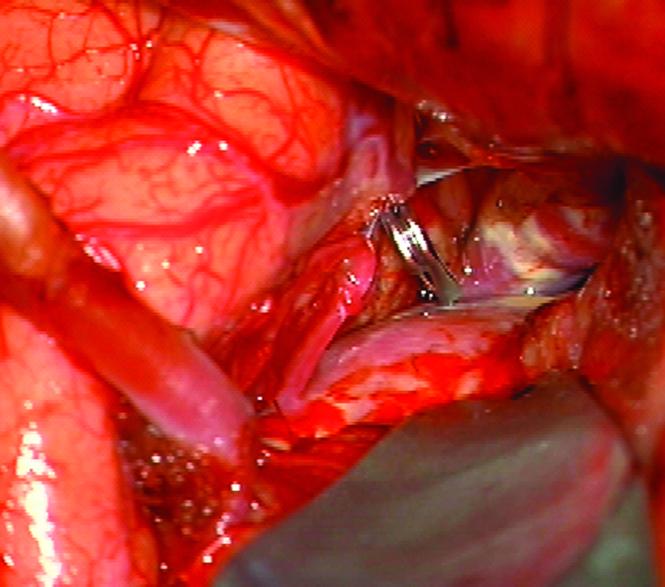
Case 14. (A) Axial T2-weighted magnetic resonance images demonstrated a giant basilar trunk aneurysm with marked pontine compression in this 52 year-old man. The aneurysm had dolichoectatic morphology with separation of afferent and efferent basilar artery, as seen on digital subtraction angiography (left vertebral artery injection, (B) anteroposterior and (C) lateral views). (D) The distal aneurysm was exposed with an orbitozygomatic-pterional craniotomy and dissection through the carotid-oculomotor triangle. (E) A radial artery graft was anastomosed to the P2 segment, (F) as part of an MCA-PCA bypass. Note the proximal donor anastomosis in the Sylvian fissure. (G) The aneurysm was distal occluded with an aneurysm clip. (H) Postoperative angiography demonstrated slow antegrade filling of the aneurysm and brisk filling of the basilar apex through the bypass (right ICA injection, anteroposterior view). Gradual aneurysm thrombosis also occluded basilar perforators and the patient died. This case demonstrates that post-surgical aneurysm thrombosis can occlude perforators, even with distal aneurysm occlusion that preserves antegrade flow.
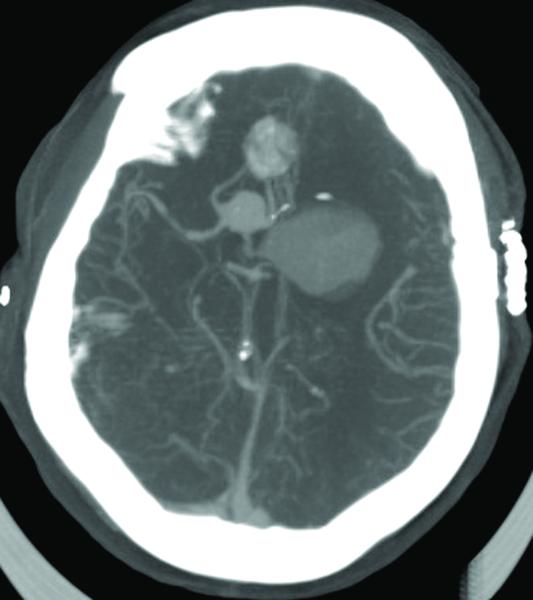
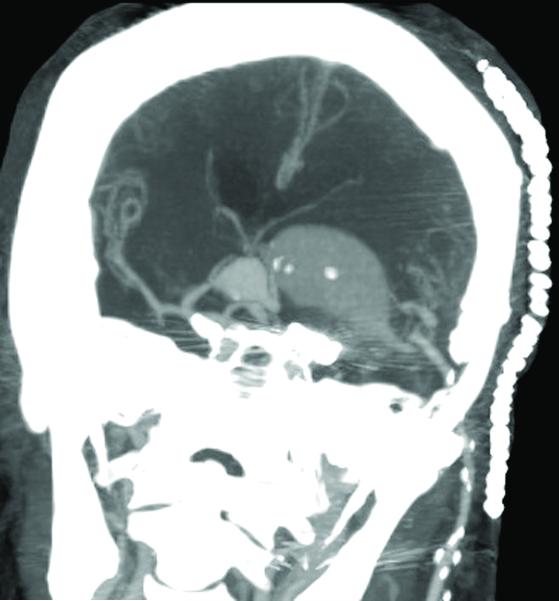
Figure 3.
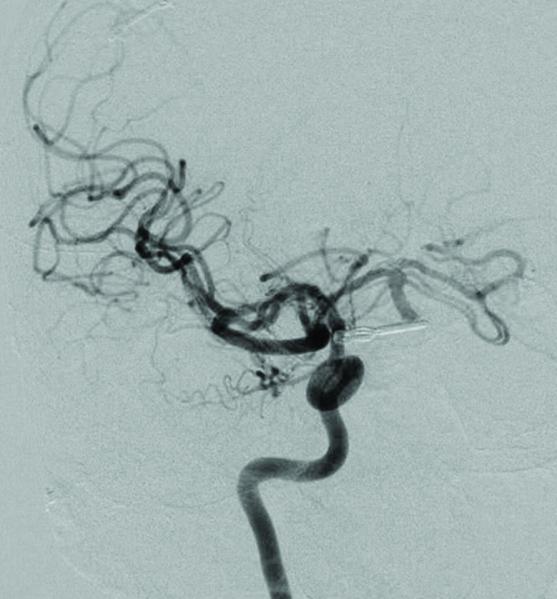
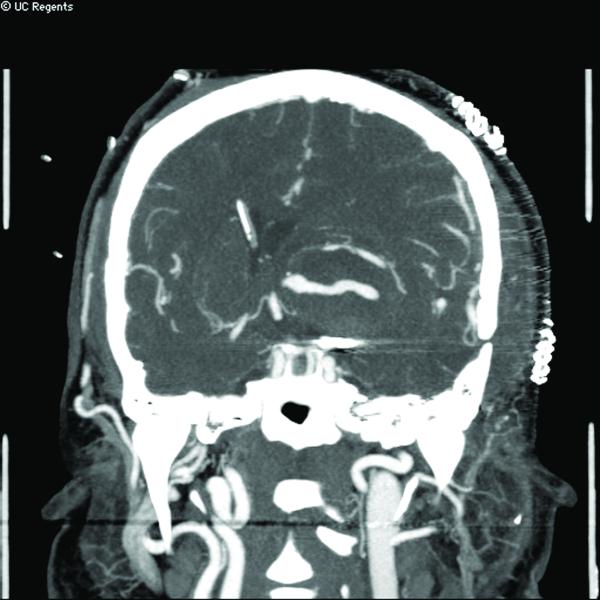
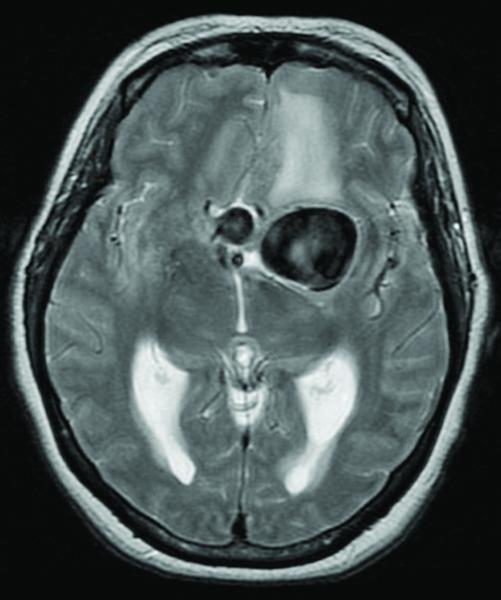
Case 15. (A) Axial T2-weighted MR imaging revealed a giant left ICA bifurcation aneurysm and a large anterior communicating artery aneurysm in this 51 year-old woman. (B) 3D reconstructed angiogram (left ICA injection) demonstrated its dolichoectatic morphology. An end-to-side anastomosis between radial artery and the efferent MCA was part of an ECA-MCA bypass. (C) Supraclinoid ICA was occluded with a clip as it entered the aneurysm, distal to PCoA. Indocyanine green videoangiography demonstrated patency of the bypass graft, filling of distal MCA branches, filling of the supraclinoid ICA up to the clip, and faint flow of dye within the aneurysm. Postoperative CT angiography showed a thin layer of new intra-aneurysmal thrombus anteriorly, posteriorly, and inferiorly on (D) axial and (E) coronal views. (F) Subsequent digital subtraction angiography demonstrated bypass patency and progressive intraluminal thrombosis (left ICA injection, anteroposterior view). CTA on postoperative day 5 revealed further intraluminal thrombosis with two serpentine channels connecting the bypass with the A1 segment on the opposite side of the aneurysm, as seen on (G) axial and (H) coronal views. Post-surgical thrombosis occluded the anterior choroidal artery, and she suffered a capsular infarct. This case demonstrates that post-surgical aneurysm thrombosis after proximal clip occlusion can occlude small branch arteries.
Our computational fluid dynamics (CFD) studies of giant aneurysm patients demonstrate that regions of slow, recirculating blood flow inside aneurysms are associated with abnormally low wall shear stress and aneurysm thrombosis 11. Using high resolution gadolinium contrast-enhanced MR angiography and phase mapping velocimetry, regions predicted by CFD to have low velocities and low wall shear stresses correlated with regions of thrombus deposition in longitudinal studies of patients who initially had thrombus-free aneurysms and later developed intraluminal thrombus 12. Using a “virtual ink” technique to inject aneurysms and follow its wash-out over several cardiac cycles of ink-free flow, estimates of flow residence times in giant aneurysms were elevated in regions where thrombus deposition was observed to occur in vivo 13. Slow or stagnant flows appear to cause platelet aggregation, activation, and adhesion to aneurysm wall in these recirculation zones. Platelet activity induces thrombus formation, which also attaches to arterial walls, with potential peril to perforators after bypass.
Patency of branch arteries after bypass appears to be caliber-dependent. Thrombotic occlusions resulting in death or neurological deterioration after treatment involved basilar trunk perforators (3 patients) and anterior choroidal artery (1 patient). Flow reversal seems more thrombogenic than flow reduction, but direction of post-surgical blood flow did not correlate with outcomes. Thrombotic perforator occlusion occurred in 2 of 4 cases with preserved anterograde flow in the aneurysm and distal basilar artery occlusion. Thrombotic complications were not observed after bypass and occlusion of cavernous ICA aneurysms, an arterial segment that lacks branches supplying brain.
Postoperative anticoagulation might address this complication in some cases. Patients undergoing endovascular aneurysm occlusion can be anticoagulated 2-3 days after bypass surgery as part of a staged surgical-endovascular treatment. However, we did encounter heparin-induced hemorrhage after such a treatment (1 patient), and even a short delay between bypass and staged aneurysm occlusion by endovascular means can invite rehemorrhage (1 patient). Anticoagulation is problematic in patients immediately after surgical aneurysm occlusion, when most of our thrombotic complications occurred. Post-operative anti-platelet agents may be a remedy for thrombotic perforator occlusion.
CFD may be useful in guiding surgical strategy. Post-bypass hemodynamics can be simulated with both proximal and distal occlusion options and the regions of aneurysm thrombosis can be estimated with wall shear stress maps and virtual ink injections (Figure 4). The preferred treatment strategy will maintain robust flow with normal wall shear stresses in regions where branch arteries originate, while accepting stagnation in perforator-free zones like the dome and fundus. It is conceivable that stents, flow diverters, or other endovascular devices might direct blood flow to perforator zones and shape intra-luminal thrombosis. This technology is in its infancy, and currently the problem of thrombotic perforator occlusion remains unsolved.
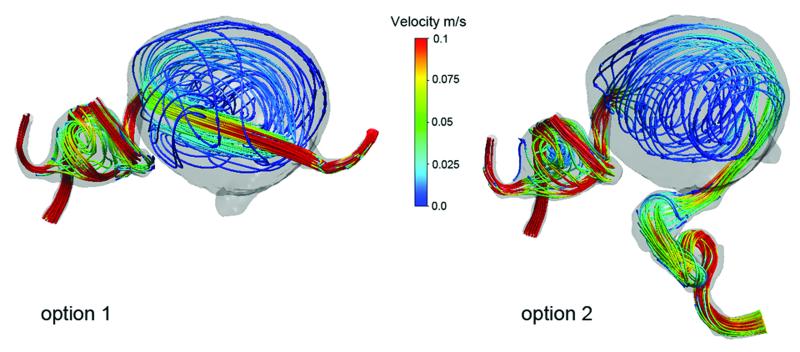
Figure 4.
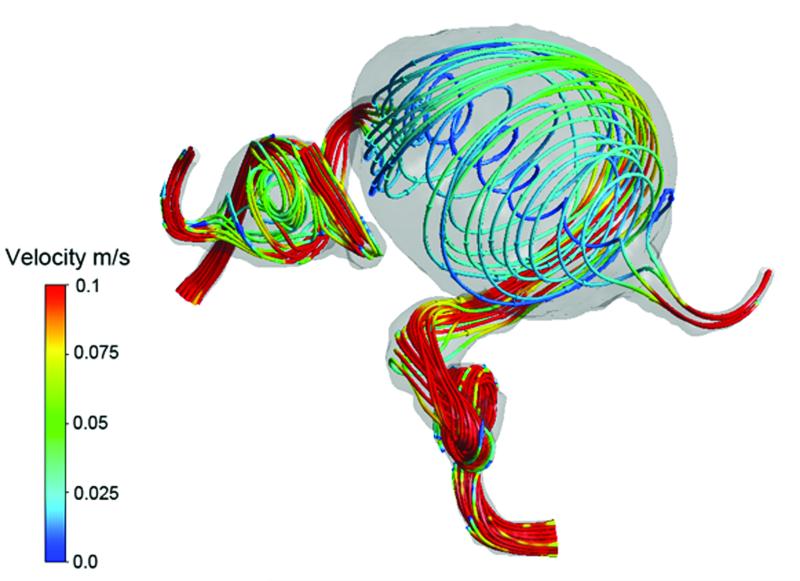
Computational fluid dynamics explains the post-surgical aneurysm thrombosis observed in Case 15. (A) Preoperative velocity streamlines demonstrate the hemodynamics of this giant MCA aneurysm,. Following ECA-MCA bypass with radial artery graft, two surgical options were considered: proximal aneurysm occlusion with the clip on the ICA distal to PCoA (option 1); or distal aneurysm occlusion with the clip on the MCA as it exits the aneurysm (option 2). (B) Proximal aneurysm occlusion results in flow through the center of the aneurysm and stagnation near the walls. Distal aneurysm occlusion results in flow along the walls of the aneurysm and stagnation in the center. Proximal aneurysm occlusion (option 1) was selected because it more dramatically altered intra-aneurysmal flow, and CFD predicted the pattern of peripheral aneurysm thrombosis based on stagnant flow and low shear stress near the walls. These simulations demonstrate that CFD can predict postoperative thrombotic complications and might be useful in planning surgical strategy.
Superiority of Surgical Management
This experience demonstrates that good results can be achieved with surgery in the majority of patients with giant aneurysms (GOS 5 or 4 in 81%, and improved or unchanged in 78% of patients). Treatment risks were significant, with a surgical mortality rate of 13% and treatment-associated neurological morbidity rate of 9%. 77% of aneurysms were occluded completely, there were no late rehemorrhages, and the re-treatment rate was 3.5%. These results are comparable to other surgical series in the literature, where mortality rates range between 5% and 30% and good outcomes range between 60% and 90% 1, 6, 7, 14-37 (Table 6).
Giant aneurysms have not been favorable lesions for endovascular therapy 1, 8, 20, 38-46 (Table 6) because they frequently widen the neck, distort the anatomy of parent and branch arteries at the base, and induce intraluminal thrombosis. These anatomical factors make giant aneurysms dangerous to coil and difficult to obliterate completely, leading to recurrent aneurysm, multiple retreatments, occasional rehemorrhages, and neurological deterioration from progressive aneurysm enlargement. Giant aneurysms require techniques like stent- or balloon-assistance, which increase intraprocedural risks 41. New technologies and techniques have advanced the efficacy and scope of endovascular therapy. In one of the largest endovascular experiences with 39 giant aneurysms, Jahromi et al. reported a complete occlusion rate of 36%, stent-assistance in 66%, and an average of 2 sessions to treat each aneurysm 40. Cumulative treatment morbidity occurred in 12 patients (32%), and treatment mortality occurred in 6 patients (16%). Overall, 11 patients died at late follow-up (29%). Follow-up was short (mean duration, 25 months), which can result in underestimates of rates of recurrence, retreatment, and complications.
Endovascular therapy in its current state still transforms a significant number of giant aneurysms into a chronic disease requiring extensive surveillance, multiple retreatments, repeated risk exposure, and a relapsing clinical course. Flow diversion may yet offer some meaningful treatment options, particularly with basilar trunk and some basilar apex aneurysms where surgical morbidity rates are particularly high. Microsurgical aneurysm occlusion still appeals to many patients who prefer a single, definitive, and durable therapy. This contemporary experience with 141 giant aneurysms is a retrospective review and is limited by selection bias, lack of a control group, and short follow-up duration. Nonetheless, it demonstrates that experienced neurosurgeons can achieve excellent results with surgery as the “first-line” management approach, heavy reliance on bypass plus indirect aneurysm occlusion when conventional clipping fails, limited (if any) use of hypothermic circulatory arrest, and liberal use of endovascular techniques as adjuncts to surgery.
Acknowledgements
Dr. Sughrue is supported by the AANS NREF
Footnotes
Disclosures: The authors declare that they are not involved in any relationships with companies that make products related to this study.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gobin YP, Vinuela F, Gurian JH, et al. Treatment of large and giant fusiform intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 1996 Jan;84(1):55–62. doi: 10.3171/jns.1996.84.1.0055. [DOI] [PubMed] [Google Scholar]
- 2.Fiorella D, Kelly ME, Albuquerque FC, Nelson PK. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009 Feb;64(2):212–217. doi: 10.1227/01.NEU.0000337576.98984.E4. discussion 217. [DOI] [PubMed] [Google Scholar]
- 3.Mack WJ, Ducruet AF, Angevine PD, et al. Deep hypothermic circulatory arrest for complex cerebral aneurysms: lessons learned. Neurosurgery. 2007 May;60(5):815–827. doi: 10.1227/01.NEU.0000255452.20602.C9. [DOI] [PubMed] [Google Scholar]
- 4.Hosmer DW, Hjort NL. Goodness-of-fit processes for logistic regression: simulation results. Stat Med. 2002 Sep 30;21(18):2723–2738. doi: 10.1002/sim.1200. [DOI] [PubMed] [Google Scholar]
- 5.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed John Wiley; New York: 2000. [Google Scholar]
- 6.Lawton MT, Spetzler RF. Surgical management of giant intracranial aneurysms: experience with 171 patients. Clin Neurosurg. 1995;42:245–266. [PubMed] [Google Scholar]
- 7.Sundt TM., Jr. Piepgras DG, Fode NC, Meyer FB. Giant intracranial aneurysms. Clin Neurosurg. 1991;37:116–154. [PubMed] [Google Scholar]
- 8.Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery. 1999 Oct;45(4):793–803. doi: 10.1097/00006123-199910000-00013. discussion 803-794. [DOI] [PubMed] [Google Scholar]
- 9.Lawton MT, Raudzens PA, Zabramski JM, Spetzler RF. Hypothermic circulatory arrest in neurovascular surgery: evolving indications and predictors of patient outcome. Neurosurgery. 1998 Jul;43(1):10–20. doi: 10.1097/00006123-199807000-00009. discussion 20-11. [DOI] [PubMed] [Google Scholar]
- 10.Ponce FA, Spetzler RF, Han PP, et al. Cardiac standstill for cerebral aneurysms in 103 patients: an update on the experience at the Barrow Neurological Institute. J Neurosurg. Oct 15; doi: 10.3171/2010.9.JNS091178. [DOI] [PubMed] [Google Scholar]
- 11.Boussel L, Rayz V, McCulloch C, et al. Aneurysm growth occurs at region of low wall shear stress: patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke. 2008 Nov;39(11):2997–3002. doi: 10.1161/STROKEAHA.108.521617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rayz VL, Boussel L, Lawton MT, et al. Numerical modeling of the flow in intracranial aneurysms: prediction of regions prone to thrombus formation. Ann Biomed Eng. 2008 Nov;36(11):1793–1804. doi: 10.1007/s10439-008-9561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayz VL, Boussel L, Ge L, et al. Flow residence time and regions of intraluminal thrombus deposition in intracranial aneurysms. Ann Biomed Eng. Oct;38(10):3058–3069. doi: 10.1007/s10439-010-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnautovic KI, Al-Mefty O, Angtuaco E. A combined microsurgical skull-base and endovascular approach to giant and large paraclinoid aneurysms. Surg Neurol. 1998 Dec;50(6):504–518. doi: 10.1016/s0090-3019(97)80415-6. discussion 518-520. [DOI] [PubMed] [Google Scholar]
- 15.Cantore G, Santoro A, Guidetti G, Delfinis CP, Colonnese C, Passacantilli E. Surgical treatment of giant intracranial aneurysms: current viewpoint. Neurosurgery. 2008 Oct;63(4 Suppl 2):279–289. doi: 10.1227/01.NEU.0000313122.58694.91. discussion 289-290. [DOI] [PubMed] [Google Scholar]
- 16.Hacein-Bey L, Connolly ES, Jr., Mayer SA, Young WL, Pile-Spellman J, Solomon RA. Complex intracranial aneurysms: combined operative and endovascular approaches. Neurosurgery. 1998 Dec;43(6):1304–1312. doi: 10.1097/00006123-199812000-00020. discussion 1312-1303. [DOI] [PubMed] [Google Scholar]
- 17.Hauck EF, Wohlfeld B, Welch BG, White JA, Samson D. Clipping of very large or giant unruptured intracranial aneurysms in the anterior circulation: an outcome study. J Neurosurg. 2008 Dec;109(6):1012–1018. doi: 10.3171/JNS.2008.109.12.1012. [DOI] [PubMed] [Google Scholar]
- 18.Jafar JJ, Russell SM, Woo HH. Treatment of giant intracranial aneurysms with saphenous vein extracranial-to-intracranial bypass grafting: indications, operative technique, and results in 29 patients. Neurosurgery. 2002 Jul;51(1):138–144. doi: 10.1097/00006123-200207000-00021. discussion 144-136. [DOI] [PubMed] [Google Scholar]
- 19.Kattner KA, Bailes J, Fukushima T. Direct surgical management of large bulbous and giant aneurysms involving the paraclinoid segment of the internal carotid artery: report of 29 cases. Surg Neurol. 1998 May;49(5):471–480. doi: 10.1016/s0090-3019(97)00374-1. [DOI] [PubMed] [Google Scholar]
- 20.Kolasa PP, Kaurzel Z, Lewinski A. Treatment of giant paraclinoid aneurysms. Own experience. Neuro Endocrinol Lett. 2004 Aug;25(4):287–291. [PubMed] [Google Scholar]
- 21.Krisht AF, Krayenbuhl N, Sercl D, Bikmaz K, Kadri PA. Results of microsurgical clipping of 50 high complexity basilar apex aneurysms. Neurosurgery. 2007 Feb;60(2):242–250. doi: 10.1227/01.NEU.0000249265.88203.DF. discussion 250-242. [DOI] [PubMed] [Google Scholar]
- 22.Lawton MT. Basilar apex aneurysms: surgical results and perspectives from an initial experience. Neurosurgery. 2002 Jan;50(1):1–8. doi: 10.1097/00006123-200201000-00002. discussion 8-10. [DOI] [PubMed] [Google Scholar]
- 23.Lozier AP, Kim GH, Sciacca RR, Connolly ES, Jr., Solomon RA. Microsurgical treatment of basilar apex aneurysms: perioperative and long-term clinical outcome. Neurosurgery. 2004 Feb;54(2):286–296. doi: 10.1227/01.neu.0000103222.13642.00. discussion 296-289. [DOI] [PubMed] [Google Scholar]
- 24.Osawa M, Hongo K, Tanaka Y, Nakamura Y, Kitazawa K, Kobayashi S. Results of direct surgery for aneurysmal subarachnoid haemorrhage: outcome of 2055 patients who underwent direct aneurysm surgery and profile of ruptured intracranial aneurysms. Acta Neurochir (Wien) 2001;143(7):655–663. doi: 10.1007/s007010170043. discussion 663-654. [DOI] [PubMed] [Google Scholar]
- 25.Ponce FA, Albuquerque FC, McDougall CG, Han PP, Zabramski JM, Spetzler RF. Combined endovascular and microsurgical management of giant and complex unruptured aneurysms. Neurosurg Focus. 2004 Nov 15;17(5):E11. doi: 10.3171/foc.2004.17.5.11. [DOI] [PubMed] [Google Scholar]
- 26.Samson D, Batjer HH, Kopitnik TA., Jr. Current results of the surgical management of aneurysms of the basilar apex. Neurosurgery. 1999 Apr;44(4):697–702. doi: 10.1097/00006123-199904000-00001. discussion 702-694. [DOI] [PubMed] [Google Scholar]
- 27.Sano H. Treatment of complex intracranial aneurysms of anterior circulation using multiple clips. Acta Neurochir Suppl. 107:27–31. doi: 10.1007/978-3-211-99373-6_4. [DOI] [PubMed] [Google Scholar]
- 28.Sharma BS, Gupta A, Ahmad FU, Suri A, Mehta VS. Surgical management of giant intracranial aneurysms. Clin Neurol Neurosurg. 2008 Jul;110(7):674–681. doi: 10.1016/j.clineuro.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Tamaki N, Kim S, Ehara K, et al. Giant carotid-ophthalmic artery aneurysms: direct clipping utilizing the “trapping-evacuation” technique. J Neurosurg. 1991 Apr;74(4):567–572. doi: 10.3171/jns.1991.74.4.0567. [DOI] [PubMed] [Google Scholar]
- 30.Xu BN, Sun ZH, Romani R, et al. Microsurgical management of large and giant paraclinoid aneurysms. Surg Neurol. 2009 Oct 13; doi: 10.1016/j.surneu.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 31.Ausman JI, Diaz FG, Sadasivan B, Gonzeles-Portillo M, Jr., Malik GM, Deopujari CE. Giant intracranial aneurysm surgery: the role of microvascular reconstruction. Surg Neurol. 1990 Jul;34(1):8–15. doi: 10.1016/0090-3019(90)90166-m. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez LF, Alexander MJ, McDougall CG, Spetzler RF. Anteroinferior cerebellar artery aneurysms: surgical approaches and outcomes--a review of 34 cases. Neurosurgery. 2004 Nov;55(5):1025–1035. doi: 10.1227/01.neu.0000141083.00866.82. [DOI] [PubMed] [Google Scholar]
- 33.Hosobuchi Y. Direct surgical treatment of giant intracranial aneurysms. J Neurosurg. 1979 Dec;51(6):743–756. doi: 10.3171/jns.1979.51.6.0743. [DOI] [PubMed] [Google Scholar]
- 34.Morcos J, Heros R. Distal basilar artery aneurysm: Surgical techniques. In: Batjer H, Caplan L, Friberg L, Greenlee R, Kopitnik T, Young W, editors. Cerebrovascular Disease. Lippincott-Raven; Philadelphia: 1997. pp. 1055–1077. [Google Scholar]
- 35.Peerless S, Drake C. Management of aneurysms of the posterior circulation. In: Youmans J, editor. Neurological Surgery. W.B. Saunders Co; Philadelphia: 1990. pp. 1764–1806. [Google Scholar]
- 36.Shibuya M, Sugita K. Intracranial Giant Aneurysms. In: Youmans J, editor. Neurological Surgery. WB Saunders Co; Philadelphia: 1996. pp. 1310–1319. [Google Scholar]
- 37.Yasargil MG. Microneurosurgery. Vol II. Thieme-Stratton; New York: 1984. [Google Scholar]
- 38.Henkes H, Fischer S, Weber W, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004 Feb;54(2):268–280. doi: 10.1227/01.neu.0000103221.16671.f0. discussion 280-265. [DOI] [PubMed] [Google Scholar]
- 39.Higashida RT, Halback VV, Dormandy B, Bell JD, Hieshima GB. Endovascular treatment of intracranial aneurysms with a new silicone microballoon device: technical considerations and indications for therapy. Radiology. 1990 Mar;174(3 Pt 1):687–691. doi: 10.1148/radiology.174.3.2305050. [DOI] [PubMed] [Google Scholar]
- 40.Jahromi BS, Mocco J, Bang JA, et al. Clinical and angiographic outcome after endovascular management of giant intracranial aneurysms. Neurosurgery. 2008 Oct;63(4):662–674. doi: 10.1227/01.NEU.0000325497.79690.4C. discussion 674-665. [DOI] [PubMed] [Google Scholar]
- 41.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009 Apr;64(4):632–642. doi: 10.1227/01.NEU.0000339109.98070.65. discussion 642-633; quiz N636. [DOI] [PubMed] [Google Scholar]
- 42.Murayama Y, Vinuela F, Ishii A, et al. Initial clinical experience with matrix detachable coils for the treatment of intracranial aneurysms. J Neurosurg. 2006 Aug;105(2):192–199. doi: 10.3171/jns.2006.105.2.192. [DOI] [PubMed] [Google Scholar]
- 43.Shi ZS, Ziegler J, Duckwiler GR, et al. Management of giant middle cerebral artery aneurysms with incorporated branches: partial endovascular coiling or combined extracranial-intracranial bypass--a team approach. Neurosurgery. 2009 Dec;65(6 Suppl):121–129. doi: 10.1227/01.NEU.0000335173.80605.1D. discussion 129-131. [DOI] [PubMed] [Google Scholar]
- 44.Sluzewski M, Menovsky T, van Rooij WJ, Wijnalda D. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003 Feb;24(2):257–262. [PMC free article] [PubMed] [Google Scholar]
- 45.Tateshima S, Murayama Y, Gobin YP, Duckwiler GR, Guglielmi G, Vinuela F. Endovascular treatment of basilar tip aneurysms using Guglielmi detachable coils: anatomic and clinical outcomes in 73 patients from a single institution. Neurosurgery. 2000 Dec;47(6):1332–1339. discussion 1339-1342. [PubMed] [Google Scholar]
- 46.Hallacq P, Piotin M, Moret J. Endovascular occlusion of the posterior cerebral artery for the treatment of p2 segment aneurysms: retrospective review of a 10-year series. AJNR Am J Neuroradiol. 2002 Aug;23(7):1128–1136. [PMC free article] [PubMed] [Google Scholar]