Abstract
Many adults in the United States enter primary care late in the course of HIV infection, countering the clinical benefits of timely HIV services and missing opportunities for risk reduction. Our objective was to determine if perceived social support was associated with delay entering care after an HIV diagnosis. Two hundred sixteen patients receiving primary care at a large, university-based HIV outpatient clinic in North Carolina were included in the study. Dimensions of functional social support (emotional/informational, tangible, affectionate and positive social interaction) were quantified with a modified Medical Outcomes Study Social Support Scale and included in proportional hazard models to determine their effect on delays seeking care. The median delay between diagnosis and entry to primary care was 5.9 months. Levels of social support were high but only positive social interaction was moderately associated with delayed presentation in adjusted models. The effect of low perceived positive social interaction on the time to initiation of primary care differed by history of alcoholism (no history of alcoholism, hazard ratio (HR): 1.43, 95% confidence interval (CI): 0.88, 2.34; history of alcoholism, HR: 0.71, 95% CI: 0.40, 1.28). Ensuring timely access to HIV care remains a challenge in the southeastern United States. Affectionate, tangible, and emotional/informational social support were not associated with the time from diagnosis to care. The presence of positive social interaction may be an important factor influencing care seeking behavior after diagnosis.
Keywords: HIV infection, social support, time factors, delivery of health care, southeastern United States
Introduction
Early presentation for medical care among HIV infected persons can improve the length and quality of life by providing access to antiretroviral drugs and prophylactic treatment for opportunistic infections (Egger et al., 2002; Hogg et al., 2001). Further, HIV testing and counseling and knowledge of one’s serostatus has been shown to reduce high-risk behavior, thereby reducing transmission to others (Centers for Disease Control and Prevention, 2000; Weinhardt, Carey, Johnson, & Bickham, 1999). Despite these benefits, many adults enter care late in the course of HIV infection, countering the benefits of timely access to HIV services and missing opportunities for risk reduction (Fleming et al., 2002; Neal & Fleming, 2002; Samet, Freedberg, Savetsky, Sullivan, & Stein, 2001). Consequently, linking HIV positive persons to high-quality care and prevention services has been identified as a priority of the US Centers for Disease Control and Prevention (CDC) (Janssen et al., 2001).
The process of an HIV-infected individual presenting to primary care can be divided into two meaningful time periods: 1) the time between acquisition of infection and testing; and 2) the time between testing and presentation to care (Samet et al., 1998). While delayed testing is common in the US, delayed initiation of medical care after diagnosis is less well-characterized (Centers for Disease Control and Prevention, 2006; Neal & Fleming, 2002). Median reported delays from HIV diagnosis to presentation for care range from 30 days to over a year (Krawczyk et al., 2006; Milberg et al., 2001; Samet et al., 1998; Turner et al., 2000). Demographics, health insurance status, injection drug use, and the testing and counseling history are associated with delayed presentation, but the role of psychosocial factors, such as perceived social support, on care-seeking behavior have not been thoroughly evaluated (Girardi et al., 2004; Milberg et al., 2001; Samet et al., 1998; Turner et al., 2000).
Social support can be subdivided into structural and functional aspects of support. Structural support includes the size, type, contact, and density of social networks and ties (Uchino, 2004). Functional support represents the capacity of relationships to fulfill particular functions, such as providing affection, a sense of belonging, or material aid (Cohen & Syme, 1985). Together, structural and functional support describes the types of resources we receive from other people. Social support can influence health directly by influencing the adoption of healthy (or unhealthy) behaviors and adherence to social norms (Cohen & Syme, 1985). It can also influence health indirectly by buffering the pathogenic effects of stressful events (Cohen & Wills, 1985). Social support may improve coping with HIV and quality of life, improve adherence to antiretroviral therapy, and improve retention in care (Catz, Kelly, Bogart, Benotsch, & McAuliffe, 2000; Derlega, Winstead, Oldfield, & Barbee, 2003; McClure, Catz, & Brantley, 1999; Mostashari, Riley, Selwyn, & Altice, 1998; Ruiz Perez et al., 2005; Tate, Van Den Berg, Hansen, Kochman, & Sikkema, 2006). Our objective was to describe levels of support available to patients in a southeastern HIV clinic and determine if social support was associated with delays between HIV diagnosis and presentation for care.
Methods
Study population
The source population for the study was the University of North Carolina at Chapel Hill Infectious Disease (UNC-ID) Clinic. As a large, university-based medical center, UNC-ID follows approximately 1,300 HIV infected patients per year and provides comprehensive HIV primary care services. We conducted a secondary data analysis of the UNC Clinical and Socio-Demographic Survey, originally designed to collect sociodemographic and behavioral data not available in medical records. Patients receiving care at UNC-ID who were ≥18 years of age, English speaking, and able to provide written informed consent were eligible for the study. Eligible participants were approached for participation by trained research assistants. Consenting patients were interviewed in a quiet and private room, typically after their clinic visit. Interview data were entered into a Microsoft Access database housed on a secured server.
Measurements
The main outcome was the time (months) from HIV diagnosis until entry to HIV care. The date of HIV diagnosis was obtained from the North Carolina (NC) Department of Health and Human Services HIV/AIDS Reporting System. In cases where a match was not obtained with state records or the NC reporting system date was after the diagnosis date in the medical record, the date of diagnosis from the medical record was used. In five cases, only the self-reported date of diagnosis was available. To correctly capture the date of entry to HIV primary care, we defined entry to care as the first UNC-ID outpatient visit for patients who only received care at UNC or, for patients who received care elsewhere, the earliest date of CD4 T-lymphocyte cell count, HIV RNA, antiretroviral therapy, AIDS-defining clinical condition, or outpatient visit. Patients with only self-reported dates of entry to care were excluded.
As the aim of our study was to determine factors associated with the time between diagnosis and care, we excluded patients whose clinical presentation prompted testing and entry to care. These people represent those who significantly delayed testing and the onset of symptoms instigated rapid linkage to primary care. Methodologically, considering delayed testing and delayed presentation to care as distinct outcomes, with overlapping etiologies, is necessary to prevent mixing the effects of late testing with delayed care. We assumed that patients with less than three weeks between diagnosis and entry to care with an AIDS defining illness before, at, or 45 days after diagnosis represented people who were diagnosed due to AIDS related illnesses, and they were excluded from the analysis (n=18). We reviewed medical records of patients with less than three weeks between diagnosis and entry to care and CD4 cell counts ≤200 cells/mm3 without AIDS defining illnesses; those diagnosed as inpatients or diagnosed because of illness were excluded (n=6). We evaluated the time from diagnosis to entry to care for 10 years after diagnosis; patients not in care at this time were censored.
Perceived social support was quantified with a modified version of the Medical Outcomes Study Social Support Scale (MOS-SSS). The MOS-SSS is a brief, multidimensional, 20-item survey. Two items measure structural support and the remaining 18 items measure four functional dimensions of social support: emotional/informational, tangible, affectionate, and positive social interaction (Sherbourne & Stewart, 1991). The modified version used in our study contained items to measure structural support and 13 of the 18 items to measure functional support (all tangible and affectionate indicators, four of eight emotional/informational indicators, and one of four positive social interaction indicators plus an additional positive social interaction indicator evaluated but not included in the MOS-SSS). We included participants who completed ≥80% of the MOS-SSS.
Consistent with the methods for the original MOS-SSS, structural social support is measured by network size, or the total number of close friends and relatives. Reponses to each functional support indicator on the 5-point Likert scale of responses are assigned numeric values (1: support type is never present – 5: support type is always present) and a composite average number is generated for each participant representing the four dimensions of functional social support. This average is transformed to range from 20 to 100.
Confirmatory factor analysis
As our functional support scale was modified from the original 18 item MOS-SSS scale, we used confirmatory factor analysis (CFA) to determine the validity of the model in our overall study population, before exclusions. We evaluated model fit based on a CFA model with 332 patient records, 13 indicators, four latent variables (emotional/informational, affectionate, tangible, and positive interaction domains of functional social support), and a robust weighted least squares fitting function. CFA analyses were done with Mplus software (Muthen & Muthen, 2007).
Statistical analysis
We first performed basic descriptive analyses, including calculating means, standard deviations, medians, and frequencies of the exposure and covariates. Eight participants had missing data in the social support scale; after evaluating multiple methods of handling missing data which yielded similar results, we imputed missing values with the mean of known values. In bivariable analyses, we examined the effect of social support on the time to seeking HIV care using Cox proportional hazards regression. We excluded factors from Cox models that violated the assumption of temporality, for example, events or measurements that took place after HIV diagnosis (e.g., CD4 cell count at entry). We assumed that other factors could be assumed to be valid at the time of diagnosis despite being measured after care was initiated, such as ever being homeless. The proportional hazards assumption was evaluated for all exposures and covariates graphically with the use of a log(−log(S(t))) curve and was tested by adding an interaction with time to the model (Cox test). When necessary, the proportional hazards assumption was relaxed. Equality of survival functions was tested with the log-rank test. We present hazard ratios (HR) and 95% confidence intervals (CI).
In multivariable analysis, we used a manual, backward, change-in-estimate model building strategy. Potential effect measure modification was assessed by adding appropriate product interaction terms and comparing nested models with the likelihood ratio test. Potential confounding variables were assessed by examination of the change in social support estimate; confounding was defined as a ≥10% change in parameter estimate. We first constructed multivariable models for each functional support domain and then created a single multivariable model with all four levels of social support and relevant confounding and modifying covariates. Analysis was conducted with SAS software (Cary, NC).
Human Subjects Protection
Participants provided written informed consent to participate in the interview and HIPAA authorization to access medical information. This study was approved by the UNC Institutional Review Board.
Results
From July 2000 to June 2006, 216 patients completed the interview and met the criteria for inclusion in the analysis. Women comprised 40% of the population, heterosexual men represented about 26% of the population, and men who have sex with men (MSM) were 33% of the population (Table 1). The median age was 36 years at diagnosis and 43 years at the time of interview. At entry to care, the median CD4 T-lymphocyte cell count was 346 cells/mm3 (range: 4–1,428). Ever having spent time in prison was common (30.2%), as was ever having been homeless or living on the streets (28.7%), ever having a drinking problem (34.7%), ever having used illegal drugs on a regular basis (70.1%), and ever having sex for drugs or money (27.4%). Most (67.4%) patients reported having a main partner or spouse.
Table 1.
Sociodemographic, behavioral, and clinical characteristics of 216 patients with HIV infection in North Carolina, July 2000–June 2006.
| Characteristic | N (%)1 | |
|---|---|---|
| SOCIODEMOGRAPHIC CHARACTERISTICS | ||
| Gender and risk behavior | ||
| Women | 87 | (40.3) |
| Man who has sex with men / bisexual | 72 | (33.3) |
| Heterosexual man | 57 | (26.4) |
| Race | ||
| Black | 152 | (70.4) |
| White | 46 | (21.3) |
| Other | 18 | (8.3) |
| Age at diagnosis | ||
| ≤30 | 23 | (10.9) |
| 31–40 | 145 | (68.7) |
| 41 or greater | 43 | (20.4) |
| Year of Diagnosis | ||
| 1981–1989 | 24 | (11.1) |
| 1990–1994 | 71 | (32.9) |
| 1995–1999 | 77 | (35.6) |
| 2000–2005 | 44 | (20.4) |
| Education (highest completed) | ||
| Less than high school | 66 | (30.6) |
| High school graduate or GED | 74 | (34.3) |
| Some college | 47 | (21.8) |
| College graduate or higher | 23 | (10.7) |
| Vocational/technical school | 6 | (2.8) |
| Annual income | ||
| <$5,000 | 54 | (25.4) |
| $5,000 to <$10,000 | 88 | (41.3) |
| $10,000 to <$30,000 | 48 | (22.5) |
| ≥$30,000 | 23 | (10.8) |
| CLINICAL CHARACTERISTICS | ||
| CD4 T-cell count at entry to care2 | ||
| ≤200 cells/mm3 | 71 | (33.5) |
| 201–350 cells/mm3 | 36 | (17.0) |
| >350 cells/mm3 | 105 | (49.5) |
| AIDS defining illness at or before entry to care2 | ||
| Yes | 28 | (13.0) |
| No | 188 | (87.0) |
| Goes to other clinics or doctors for general health care | ||
| Yes | 79 | (36.9) |
| No | 135 | (63.1) |
| SOCIAL SUPPORT | ||
| Structural Social Support (network size) | ||
| 0–3 | 66 | (30.8) |
| 4–7 | 58 | (27.1) |
| 8–12 | 40 | (18.7) |
| 13 or more | 50 | (23.4) |
| Positive Social Interaction | ||
| Most to all of the time | 131 | (60.6) |
| Sometimes | 49 | (22.7) |
| None to little of the time | 36 | (16.7) |
| Tangible Support | ||
| Most to all of the time | 167 | (77.3) |
| Sometimes | 23 | (10.6) |
| None to little of the time | 26 | (12.0) |
| Emotional / Informational Support | ||
| Most to all of the time | 163 | (75.5) |
| Sometimes | 37 | (17.1) |
| None to little of the time | 16 | (7.4) |
| Affectionate Support | ||
| Most to all of the time | 176 | (81.5) |
| Sometimes | 17 | (7.9) |
| None to little of the time | 23 | (10.6) |
Percentages may not add to 100 due to rounding. Numbers may not add to 216 due to missing data.
These factors were not included in Cox proportionate hazards models as they could not reasonably be assumed to be valid at the time of HIV diagnosis.
Confirmatory Factor Analysis
The overall goodness-of-fit indices suggested that the four factor CFA model fit the data reasonably well: χ2(30)=108.1, p<.01, RMSEA=0.089, CFI=0.969, and TLI=0.992 (Brown, 2006). All freely estimated parameters were statistically significant; standardized factor loadings ranged from (0.56–0.97), indicator reliability ranged from 0.32 to 0.92 with eight indicators having reliabilities greater than 0.7, and all reliability estimates were significant (p<.01). We allowed for correlated errors between two similarly worded emotional/informational indicators. The reliability of the four latent variables was 0.72 for emotional/informational support, 0.83 for positive social interaction, 0.86 for tangible support and 0.90 for affectionate support (Jöreskog, 1979). Cronbach’s alpha indicated that the scale was internally consistent (0.93). Higher order analysis did not support the creation of a single construct for functional social support.
Social support
The clinic population had high levels of social support. The median network size of close friends and relatives (structural social support) was six people (range: 0–1,030). Functional support scores were skewed and clustered near levels corresponding to the level of support being present “most to all of the time.” Levels of affectionate support were the highest, followed by tangible support, emotional/information support and positive social interaction (Table 2). Although the median network size was the same for patients with and without a spouse or main partner, those with a spouse or main partner reported higher median levels of affectionate support (p<.01), positive social interaction (p=.08), and emotional/information support (p=.02).
Table 2.
Functional social support definition and scores from the modified MOS Social Support Survey among HIV clinic patients, North Carolina
| Functional Support Type | Definition1 | Scale Items (“How often is this kind of support available?”) | Mean Score (SD)2 |
|---|---|---|---|
| Positive Social Interaction | Shared social activities, a sense of social belonging | Someone to have a good time or hang with Someone to do things with and help you get your mind off things |
71.5 (23.3) |
| Tangible Support | Provision of material aid | Someone to help you if you were confined to bed Someone to take you to the doctor if you needed it Someone to prepare your meals if you were unable to Someone to help with daily chores if you were sick |
79.8 (21.9) |
| Emotional/Information Support | Expressions of comfort and caring, provision of advice and guidance | Someone to give you good advice about a crisis Someone’s whose advice you want Someone you can count on to listen when you need to talk Someone to share your most private worries and fear with |
78.8 (19.4) |
| Affectionate Support | Expressions of love and affection | Someone who shows you love and affection Someone who hugs you Someone to love and make you feel wanted |
83.7 (22.2) |
|
| |||
| Overall Functional Support | 79.1 (18.5) | ||
See references (Sherbourne & Stewart, 1991; Uchino, 2004)
Functional social support scores range from 20 (low) to 100 (high).
Delays between diagnosis and HIV care
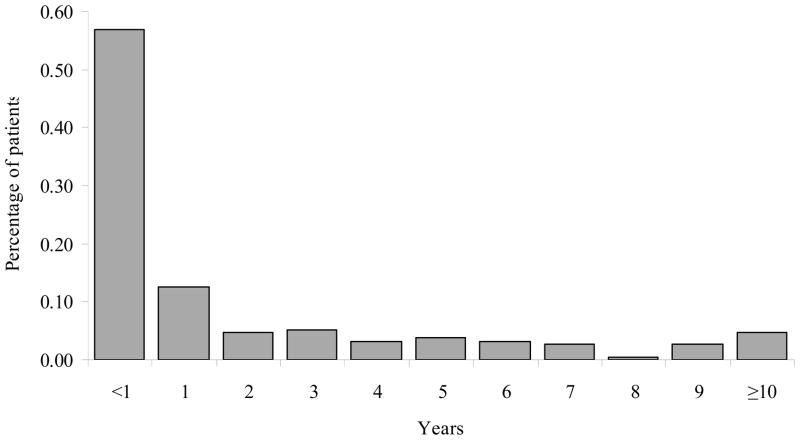
The median delay between diagnosis and entry to care was 5.9 months (inter-quartile range: 1.8 to 38.3 months). Although 57% of patients initiated care within the year after diagnosis, only 12.5% of patients initiated care in the second year. Overall, 69% were in care by two years post diagnosis, 74% were in care by three years, and 79% by four years (Figure 1). Thirty-eight patients (17.6%) delayed entering HIV care for five or more years.
Figure 1.
Distribution of time from HIV diagnosis to the initiation of HIV care among 216 patients in North Carolina.
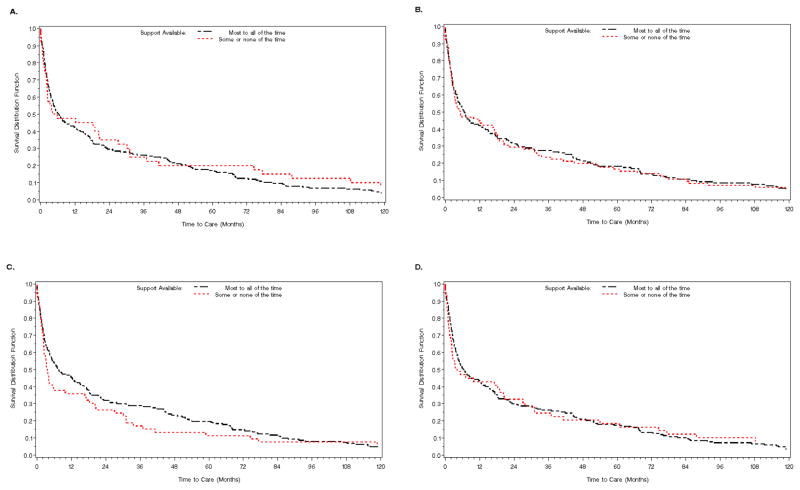
In unadjusted analyses, the time to presentation varied little by functional social support (Figure 2). Across all four domains of functional social support, those with levels of support available “less than most of the time” had shorter median times to care than those with the level of support available “most to all of the time,” although these results were not statistically significant. In multivariable analyses, tangible, emotional/informational, and affectionate support remained unassociated with the time between diagnosis and entry to care (Table 3). The effect of positive social interaction on the outcome differed by a history of alcoholism. In those who had ever had a drinking problem, those with lower levels of positive social interaction had about 0.7 times the hazard of entering care over time compared to those with the highest levels of positive social interaction. However, those without a history of a drinking problem and who had positive social interactions present less than “most of the time” had about 1.4 times the hazard of entering care over time than those with positive social interactions present “most to all of the time.”
Figure 2.
Kaplan-Meier curves of the percent of newly diagnosed patients in care over time by functional social support type. A) Affectionate support, log-rank p=.57; B) Positive social interaction, log-rank p=.84; C) Emotional / Informational support, log-rank p=.31; D) Tangible support, log-rank p=.83.
Table 3.
Multivariable proportional hazards models of functional social support and time to presentation for medical care after HIV diagnosis.
| Characteristic | Median time from diagnosis to care, months (95% CI)1 | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio2 (95% CI) | p-value3 |
|---|---|---|---|---|
| Tangible Support | ||||
| Most to all of the time | 6.35 (4.28, 12.40) | Referent | Referent | 0.80 |
| None of the time or sometimes | 4.01 (2.14, 20.23) | 0.97 (0.69, 1.35) | 0.93 (0.53, 1.63) | |
| Emotional/Informational Support | ||||
| Most to all of the time | 7.14 (4.90, 13.98) | Referent | Referent | 0.44 |
| None of the time or sometimes | 3.55 (2.30, 9.84) | 1.18 (0.86, 1.62) | 1.23 (0.73, 2.09) | |
| Affectionate Support | ||||
| Most to all of the time | 6.20 (4.28, 11.55) | Referent | Referent | 0.87 |
| None of the time or sometimes | 4.87 (2.30, 20.49) | 0.90 (0.63, 1.29) | 1.06 (0.56, 1.98) | |
| Positive Social Interaction | ||||
| Had a drinking problem (ever) | 0.10 | |||
| Most to all of the time | 6.97 (4.15, 17.50) | Referent | Referent | |
| None of the time or sometimes | 21.27 (17.14, 60.76) | 0.67 (0.42, 1.08) | 0.71 (0.40, 1.28) | |
| Never had a drinking problem | ||||
| Most to all of the time | 5.94 (3.03, 13.98) | Referent | Referent | |
| None of the time or sometimes | 3.22 (2.14, 5.20) | 1.54 (1.08, 2.19) | 1.43 (0.88, 2.34) |
Confidence interval
Adjusted for gender and risk behavior, year of diagnosis, income, ever used drugs on a regular basis, ever had a drinking problem (main effect) and number of household members.
Represents the likelihood ratio test for the β coefficient(s) equaling 0 in the adjusted model.
Discussion
Successful efforts to reduce HIV-related morbidity and mortality and improve quality of life are contingent on the timely receipt of primary care services. Although highly active antiretroviral therapy has been the cornerstone of HIV care in the US since its widespread adoption in the late 1990’s, its survival benefit is strongly related to baseline immunosuppression (Hogg et al., 2001). In addition to clinical services, patients in care benefit from a host of social services which can improve the maintenance of care and adherence to therapy, as well as prevention programs to reduce the frequency of transmission to partners. Despite these benefits, prompt linkage to primary care is not always possible. Recently diagnosed individuals must cope with discovering their status, notify at-risk partners, contemplate disclosure to friends and family members, and initiate, establish, and adhere to medical care (Stevens & Tighe Doerr, 1997). It is therefore not surprising that some patients enter care quickly whereas others remain unconnected with the health care system for months or years after diagnosis. In this study, we report a median delay of six months between diagnosis and primary care, slightly longer than other reports (Krawczyk et al., 2006; Milberg et al., 2001; Samet et al., 1998).
Levels of social support were very high in our study. Compared to both the ambulatory patient sample in which the scale was developed and a separate study at an urban hospital-based HIV clinic, patients in our study had higher median levels of tangible, affectionate, and emotional/informational support, despite having similar median network sizes (Burgoyne & Saunders, 2000; Sherbourne & Stewart, 1991). Levels of positive interaction were similar across studies, suggesting that health care systems may have little impact on creating a sense of social belonging. Tangible and emotional/informational support needs, the types of functional support most directly modifiable by HIV primary care and social services, were not being met for only 12% and 7% of the study sample, respectively. Consistent with previous reports, we found that those with a spouse or main partner reported higher median levels of affectionate, emotional/informational, and positive social interaction support, highlighting that quality, not quantity, of support is most meaningful (Burgoyne & Saunders, 2000).
Tangible, affectionate, and emotional/informational support did not have an effect on delays between diagnosis and primary care. This observation is unexpected, given that the presence of some types of social support has been associated with shorter delays, such as having a living mother, spouse or partner (Samet et al., 1998). In addition, social support has been found to improve adherence in most studies (Alfonso, Geller, Bermbach, Drummond, & Montaner, 2006; Catz et al., 2000; Edwards, 2006; Malcolm, Ng, Rosen, & Stone, 2003; Mostashari et al., 1998). However, many of these studies did not use a validated scale – it is recommended that social support should be quantified with at least two of the three main aspects of social support: 1) existence and quantity; 2) aspects of network structure; and 3) functional content and quality of relationships (House & Kahn, 1985). Further, the high levels of support we observe may mask any true effect on the time to care. One study that assessed social support using the MOS-SSS found no relationship between functional social support and the time from diagnosis to care (Burgoyne & Saunders, 2000).
In contrast to the null findings for tangible, affectionate, and emotional/informational support, we found a moderate association between positive social interaction and care initiation. Among those with a history of alcoholism, those with lower levels of positive social interaction were slightly less likely to enter care than persons with high levels of positive social interaction. However, persons who had never had a drinking problem, but had very little positive social interaction entered care about 40% more rapidly than those with the most positive social interaction. This small group (n=22) of persons was less likely to have ever been homeless, spent time in prison, had transactional sex, or have a main partner or spouse than the rest of the study sample. This finding may suggest that some socially isolated individuals who do not abuse alcohol access care rapidly after diagnosis, perhaps to meet their support needs.
Our study, and other similar clinic-based studies of delays between diagnosis and care, must be cautious against over interpretation of data with methodological limitations. As patients must be in care to participate in the study, we do not observe the person-time of patients who delay entering care past the study period. This truncation represents an important selection bias such that later diagnoses (in calendar time) will always appear to be associated with shorter delays. We attempted to account for this bias by including diagnosis year in the multivariable analysis. In addition, our analysis did not support the creation of linear social support terms, so we may have measurement error in our broad categories of perceived social support. Finally, as patients were interviewed once they were in care, we do not know if the levels of social support we observed were present at the time of diagnosis. An optimally designed study to answer questions about presentation to care would follow a cohort of newly diagnosed individuals over time, but as Samet et al. notes, this study design may be biased by the Hawthorne effect and would require a lengthy follow-up period (Samet et al., 1998). However, as CDC recommendations for the adoption of routine HIV screening in all health care settings are implemented, effective linkage to HIV care will likely be a marker of program success, highlighting the need to understand and improve the process of care initiation (Branson et al., 2006).
We have found that ensuring timely access to HIV care remains a challenge in the southeastern U.S. Although much remains to be learned about care-seeking behavior after HIV diagnosis, our findings suggest that social belonging and social interaction may be important elements of the decision to initiate care. Additional research on how to translate this information to practice could have beneficial outcomes for both the patient and the community.
Acknowledgments
The authors are grateful to all of the research assistants who conducted interviews and to the patients who participated in the study. We are also appreciative of Bill Jones at NC DHHS for assistance with matching patients to state records, Sharon Christ at the Odum Institute for her guidance on confirmatory factor analysis, and Sonia Napravnik and Brant Stalzer for oversight and management of data collection. This work was supported in part by a grant from the University of North Carolina Center for AIDS Research (P30 AI50410) and the US Centers for Disease Control and Prevention Grant for Public Health Dissertations (1R36PS000848-01). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the US Centers for Disease Control and Prevention.
References
- Alfonso V, Geller J, Bermbach N, Drummond A, Montaner JS. Becoming a “treatment success”: what helps and what hinders patients from achieving and sustaining undetectable viral loads. AIDS Patient Care STDS. 2006;20(5):326–334. doi: 10.1089/apc.2006.20.326. [DOI] [PubMed] [Google Scholar]
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. quiz CE 11–14. [PubMed] [Google Scholar]
- Brown TA. Confirmatory Factor Analysis for Applied Research. New York: The Guilford Press; 2006. [Google Scholar]
- Burgoyne RW, Saunders DS. Perceived support in newly registered HIV/AIDS clinic outpatients. AIDS Care. 2000;12(5):643–650. doi: 10.1080/095401200750003815. [DOI] [PubMed] [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19(2):124–133. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Adoption of protective behaviors among persons with recent HIV infection and diagnosis--Alabama, New Jersey, and Tennessee, 1997–1998. MMWR Morb Mortal Wkly Rep. 2000;49(23):512–515. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cases of HIV infection and AIDS in the United States and Dependent Areas, 2005. 2006 Retrieved. from http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2005report/default.htm.
- Cohen S, Syme S. Issues in the Study and Application of Social Support. In: Cohen S, Syme S, editors. Social Support and Health. Orlando: Academic Press, Inc; 1985. pp. 3–22. [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357. [PubMed] [Google Scholar]
- Derlega VJ, Winstead BA, Oldfield IE, Barbee AP. Close relationships and social support in coping with HIV: a test of sensitive interaction systems theory. AIDS Behav. 2003;7(2):119–129. doi: 10.1023/a:1023990107075. [DOI] [PubMed] [Google Scholar]
- Edwards LV. Perceived social support and HIV/AIDS medication adherence among African American women. Qual Health Res. 2006;16(5):679–691. doi: 10.1177/1049732305281597. [DOI] [PubMed] [Google Scholar]
- Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- Fleming PL, Byers RH, Sweeney PA, Daniels D, Karon JM, Janssen RS. HIV Prevalence in the United States, 2000. Paper presented at the Conference on Retroviruses and Opportunistic Infections; Seattle, WA.. 2002. [Google Scholar]
- Girardi E, Aloisi MS, Arici C, Pezzotti P, Serraino D, Balzano R, et al. Delayed presentation and late testing for HIV: demographic and behavioral risk factors in a multicenter study in Italy. J Acquir Immune Defic Syndr. 2004;36(4):951–959. doi: 10.1097/00126334-200408010-00009. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O’Shaughnessy MV, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. Jama. 2001;286(20):2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- House JS, Kahn RL. Measures and Concepts of Social Support. In: Cohen S, Syme S, editors. Social Support and Health. Orlando, FL: Academic Press, Inc; 1985. pp. 83–108. [Google Scholar]
- Janssen RS, Holtgrave DR, Valdiserri RO, Shepherd M, Gayle HD, De Cock KM. The Serostatus Approach to Fighting the HIV Epidemic: prevention strategies for infected individuals. Am J Public Health. 2001;91(7):1019–1024. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöreskog KG. Statistical Analysis of Sets of Congeneric Tests. Psychometrika. 1979;36:109–133. [Google Scholar]
- Krawczyk CS, Funkhouser E, Kilby JM, Kaslow RA, Bey AK, Vermund SH. Factors associated with delayed initiation of HIV medical care among infected persons attending a southern HIV/AIDS clinic. South Med J. 2006;99(5):472–481. doi: 10.1097/01.smj.0000215639.59563.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm SE, Ng JJ, Rosen RK, Stone VE. An examination of HIV/AIDS patients who have excellent adherence to HAART. AIDS Care. 2003;15(2):251–261. doi: 10.1080/0954012031000068399. [DOI] [PubMed] [Google Scholar]
- McClure J, Catz S, Brantley P. Early appointment adherence among persons living with HIV. AIDS and Behavior. 1999;3(2):157–165. [Google Scholar]
- Milberg J, Sharma R, Scott F, Conviser R, Marconi K, Parham D. Factors associated with delays in accessing HIV primary care in rural Arkansas. AIDS Patient Care STDS. 2001;15(10):527–532. doi: 10.1089/108729101753205694. [DOI] [PubMed] [Google Scholar]
- Mostashari F, Riley E, Selwyn PA, Altice FL. Acceptance and adherence with antiretroviral therapy among HIV-infected women in a correctional facility. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(4):341–348. doi: 10.1097/00042560-199808010-00005. [DOI] [PubMed] [Google Scholar]
- Muthen & Muthen. Mplus (Version 5.1) Los Angeles, CA: 2007. [Google Scholar]
- Neal JJ, Fleming PL. Frequency and Predictors of Late HIV Diagnosis in the United States, 1994 through 1999. Paper presented at the Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2002. [Google Scholar]
- Ruiz Perez I, Rodriguez Bano J, Lopez Ruz MA, del Arco Jimenez A, Causse Prados M, Pasquau Liano J, et al. Health-related quality of life of patients with HIV: impact of sociodemographic, clinical and psychosocial factors. Qual Life Res. 2005;14(5):1301–1310. doi: 10.1007/s11136-004-4715-x. [DOI] [PubMed] [Google Scholar]
- Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. Aids. 2001;15(1):77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- Samet JH, Freedberg KA, Stein MD, Lewis R, Savetsky J, Sullivan L, et al. Trillion virion delay: time from testing positive for HIV to presentation for primary care. Arch Intern Med. 1998;158(7):734–740. doi: 10.1001/archinte.158.7.734. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Stevens PE, Tighe Doerr B. Trauma of discovery: women’s narratives of being informed they are HIV-infected. AIDS Care. 1997;9(5):523–538. doi: 10.1080/713613201. [DOI] [PubMed] [Google Scholar]
- Tate DC, Van Den Berg JJ, Hansen NB, Kochman A, Sikkema KJ. Race, social support, and coping strategies among HIV-positive gay and bisexual men. Cult Health Sex. 2006;8(3):235–249. doi: 10.1080/13691050600761268. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Cunningham WE, Duan N, Andersen RM, Shapiro MF, Bozzette SA, et al. Delayed medical care after diagnosis in a US national probability sample of persons infected with human immunodeficiency virus. Arch Intern Med. 2000;160(17):2614–2622. doi: 10.1001/archinte.160.17.2614. [DOI] [PubMed] [Google Scholar]
- Uchino B. Social Support and Physical Health: Understanding the Health Consequences of Relationships. New Haven and London: Yale University Press; 2004. [Google Scholar]
- Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health. 1999;89(9):1397–1405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]